Abstract
Background
Circulating tumor cells (CTCs) have been regarded as a promising biomarker for colorectal cancer (CRC); however, the prognostic value of post-operative (op) CTCs is still unclear. This study aimed to compare the recurrence prediction value of pre- and post-op CTCs in CRC patients treated with curative resection.
Patients and methods
Consecutive CRC patients treated with curative resection from January 2014 to March 2015 were identified. CTCs from 2.5 mL peripheral blood were enumerated with an ISETdevice-CTCBIOPSY® before and after surgery. Based on the status of pre- and post-op CTCs, the included patients were grouped into four cohorts: pre- and post-op CTCs−, pre-op CTCs− but post-op CTCs+, pre-op CTCs+ but post-op CTCs−, and pre- and post-op CTCs+. The 3-year recurrence-free survival (RFS) rate of patients was analyzed.
Results
A total of 138 patients (79 [57.2%] male; median age=62 [43–75] years) were enrolled. Patients with pre-op CTCs− had a 19.2% higher 3-year RFS rate (86.2%) than the combined cohorts with pre-op CTCs+ (67.0%) (P=0.038). Patients with post-op CTCs+ had aa 25.6% lower 3-year RFS rate (57.1%) than the combined cohorts with post-op CTCs− (82.7%) (P=0.001). Moreover, patients with pre- and post-op CTCs+ had a 25.1% lower 3-year RFS rate (53.8%) than patients with pre-op CTCs+ but post-op CTCs− (78.9%) (P=0.004). Multivariate analyses confirmed that post-op CTCs+ (HR=2.82, 95% CI=1.39–5.75, P=0.004), but not but pre-op CTCs+ (HR=2.17, 95% CI=0.75–6.31, P=0.153), was independently associated with shorter 3-year RFS rate.
Conclusion
Post-op CTCs+, but not pre-op CTCs+, is an independent indicator of poor prognosis for CRC patients treated with curative resection. Patients with post-op CTCs+ have a higher risk of recurrence those with pre-op CTCs+. Evaluation of post-op, rather than pre-op, CTCs is warranted.
Keywords: circulating tumor cells, colorectal cancer, preoperative, postoperative, recurrence
Introduction
The metastatic process of a malignant tumor can be divided into two phases: cancer cells translocate from a primary tumor or metastasis site to distant organs and form a metastatic tumor through cloning and proliferation.1 In the first phase, blood is an important translocation pathway of cancer cells and these tumor- or metastasis-derived cells that are present in the peripheral blood (PB), termed circulating tumor cells (CTCs).2 With years of efforts, CTC detection from patients with most solid cancers has recently become a reality.3 As a new “liquid biopsy” technology, CTC detection has the advantages of convenience, real-time, and minimally invasive, and had been gradually proven to have great clinical potential for better identification of early-stage patients with high-risk of recurrence or metastasis who could need tailored, more effective treatments after curative resection.4,5 In addition, the clinical value of CTC detection in prognostic evaluation and therapeutic monitoring has been confirmed by a series of clinical studies6,7 and meta-analysis.8–10 However, due to a certain degree of heterogeneity between different detection methods and included patients among studies, the prognostic value of CTC detection is still controversial. Particularly, regarding the change and prognostic value of CTC counts before and after curative resection in patients with colorectal cancer (CRC), relative researches were limited, but the results were different due to different detection methods.11,12 Moreover, the defects of the detection method may greatly affect the accuracy and credibility of the above research results.
For decades, the lack of a high-sensitivity detection technique has greatly limited the clinical utility of CTC evaluation.13 Current techniques of isolating and identifying CTCs can be classified into tumor-marker-dependent and -independent technologies. Among these, immunocytology/cytometry- and PCR-based methods are the most widely used techniques for CTC detection; however, as two tumor-marker-dependent methods, both of them show low sensitivity and specificity.14 The CellSearch™ system (Veridex, LLC, Raritan, NJ, USA), the only method approved by both the FDA and CFDA, is an epithelium-associated marker-dependent method (co-expressing EpCAM and CK); therefore, it misses some epithelial–mesenchymal transition (EMT) induced CTCs, leading to false-negative results.15 Recently, isolation by size of epithelial tumor cells (ISET), as a tumor-marker-independent technology, has attracted more attention in the field of CTC research.16 Compared with the aforementioned technologies, ISET offers a number of advantages, including retention of cell morphology, non-antigen dependence, the ability to capture EMT-induced CTCs, high sensitivity with high specificity, and it is more reliable.16 Additionally, use of ISET for CTC isolation and identification requires no expensive or special laboratory equipment. Therefore, morphological-analysis-based and antigen-independent ISET methodology is widely being accepted for the clinical management of cancer patients. CTCBIOPSY® (Wuhan YZY Medical Science and Technology Co., Ltd., Wuhan, China) is a novel one-stop ISET device, independently developed in the People’s Republic of China, which can automatically isolate and identify CTCs. Due to the excellent performance in capturing patient CTC, CTCBIOPSY® had been approved by the CFDA for clinical application in cancer management. Also, its potential clinical value is under evaluation by emerging evidences.17–20
In this prospective cohort study, we sought to assess whether pre- or post-op CTC is more prognostic in CRC by using an inexpensive automatic one-stop ISET device-CTCBIOPSY® to detect CTCs from the peripheral blood of 138 stage I-III CRC patients pre- and post-operatively.
Patients and methods
Patient recruitment and cohort design
With approval of the Ethics Committee of Zhongnan Hospital of Wuhan University, a total of 211 consecutive patients with stage I–III CRC from January 2014 to March 2015 at Zhongnan Hospital of Wuhan University were prospectively recruited in this study, and all patients provided written informed consent. The exclusion criteria were a history of cancer <5 years, pre-op chemotherapy or radiotherapy, no pre-op CTC data available, noncurative resection, and lack of post-op CTC data. All data on patient demographics, laboratory assessment results, and pathologic outcomes were collected from the electronic medical record system. The TNM stage of CRC was based on the seventh edition of the American Joint Committee on Cancer. PB samples (2.5 mL) were collected from all included patients in EDTA-containing tubes (BD, Franklin Lakes, NJ) before and after surgery and processed within 2 hours to evaluate CTCs. Pre-op CTC was defined as the number of CTC evaluations closest to the time of surgery, and post-op CTC was defined as the CTC evaluation at the time of reviewing 1 month after surgery or before starting adjuvant therapy.
Based on the pre- and post-op CTCs status, included CRC patients were grouped into four cohorts as follows: (1) patients whose pre- and post-op CTCs were both negative (pre- and post-op CTCs−); (2) patients with pre-op CTCs negative but post-op CTCs positive (pre-op CTCs− but post-op CTCs+); (3) patients with pre-op CTCs positive but post-op CTCs negative (pre-op CTCs+ but post-op CTCs−); and (4) patients whose pre- and post-op CTCs were both positive (pre- and post-op CTCs+).
CTC isolation and identification
CTCs were enriched using the above mentioned CTCBI-OPSY® device, as described in our previous study.17 In brief, a 2.5 mL blood sample of the included patient was diluted up to 8 mL with 0.9% physiological saline containing 0.2% paraformaldehyde, then transferred to ISET tubes with an 8 µm diameter aperture membrane. After being filtered by positive pressure from 12 mmHg to 20 mmHg, candidate CTCs were distinguished from the captured cells on the membrane (including abnormal cells and residual hemocytes) by Wright’s staining. Then, all candidate CTCs were reviewed and identified independently by three senior cytopathologists without knowledge of the patients’ clinical status and pathologic diagnosis, and any discrepancies among the three cytopathologists were resolved by discussion or consulting a fourth senior one. The reference threshold for this device was 1 CTCs/2.5 mL, that is, 0 CTCs per 2.5 mL blood means CTC negative while ≥ 1 CTCs means CTC positive.
Staging, surveillance, and follow-up
Following the recommended recognized guidelines,21–23 pre-op tumor staging was evaluated by colonoscopy and contrast-enhanced computed tomography (CT) of the thorax, abdomen, and pelvis, especially for rectal cancer, contrast-enhanced CT of the pelvis was replaced by contrast-enhanced MRI. Adjuvant chemotherapy was administered to patients with high-risk stage II and stage III disease after histological evaluation of the surgical specimen, and adjuvant radiotherapy was additionally supplemented for patients with stage III rectal cancer. The general practice for post-op surveillance of stage I to III CRC included physical examination, interval history, and serum carcinoembryonic antigen (CEA) testing at 3–6-month intervals for the first 2–3 years and at 6-month intervals thereafter for 5 years. Imaging, most frequently CT of the thorax, abdomen, and pelvis, was performed at a minimum of every 12 months for at least 3 years. Colonoscopy was typically performed within the first year after surgery and then repeated every 3–5 years unless advanced tumors were identified. The last follow-up time was April 2018.
End-points
The primary end-point was 3-year recurrence-free survival (RFS) rate, which was defined as the proportion of relapsed patients within 3 years after surgery and was used to investigate the relationship between pre- and post-op CTCs status and tumor recurrence. The secondary end-point included the relationship between pre- and post-op CTCs status and other clinicopathological prognostic factors. Tumor recurrence was definitively diagnosed based on the appearance of new lesions on CT, MRI, and/or positron emission tomography (PET) images and/or histological confirmation through biopsy through reviewing the radiographic reports.
Statistical analysis
All statistical analyses were carried out using SPSS 23.0 statistic software (SPSS Inc., Chicago, IL). Categorical variables were compared using the chi-squared test. Continuous variables were compared using the Mann-Whitney U-test. The 3-year RFS rate was defined as the ratio of the number of patients who did not relapse to the total number of patients within 3 years of follow-up, which was calculated using the Kaplan–Meier method and compared via the log-rank test. Patients who were alive without recurrence at last follow-up were censored. Cox’s proportional hazards model was used to identify factors influencing 3-year RFS. Factors significantly associated with 3-year RFS on univariate analysis (P<0.05) were subjected to the multivariate model. HR and 95% CI were assessed by the Wald test. The cumulative hazard function was used to compare the risk of recurrence of different patients. All statistical tests were 2-sided, and a P-value <0.05 was considered statistically significant for all analyses.
Results
Patient characteristics
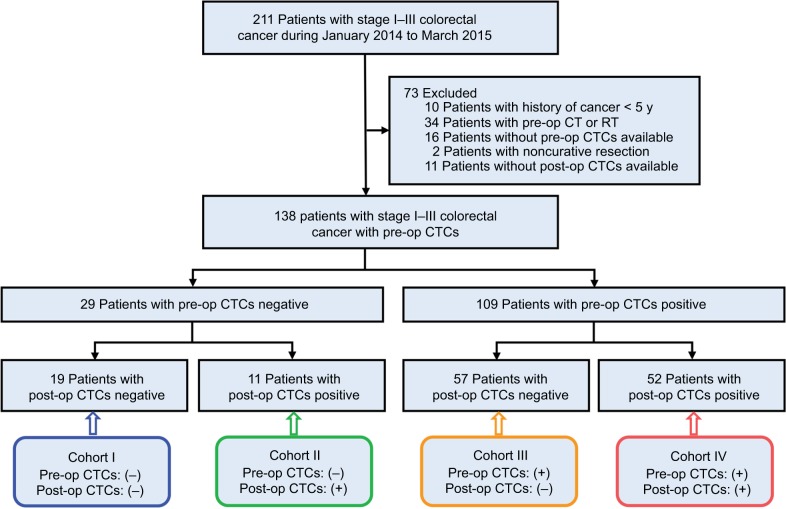
A total of 211 consecutive patients (123 males, 88 females; Median age=61 [39–76] years) with stage I–III CRC were identified in this study. Based on the exclusion criteria, 73 patients were excluded. Finally, 138 patients (79 [57.2%] male; median age=62 [43–75] years) who underwent curative resection with pre- and post-op CTCs data were enrolled, as outlined in Figure 1. The clinical and pathologic characteristics of the included patients are summarized in Table 1. Among all the included patients, tumors were located in the colon in 74 patients (53.6%) and in the rectum in 64 patients (46.4%); tumors with poor, moderate, and well grade were 41 (29.7%), 59 (42.8%), and 38 (27.5%), respectively; lymphovascular invasion (LVI) was absent in 77 patients (55.8%) and present in 61 patients (44.2%); patients for T1–2 and T3–4 were 20 (14.5%) and 118 (85.5%), respectively; patients for N0 and N+ were 46 (33.3%) and 92 (66.7%), respectively; patients for stage I, II, and III were 13 (9.4%), 67 (48.6%), and 58 (42.0%), respectively; patients with normal CEA level (<5 ng/mL) and elevated CEA level (≥5 ng/mL) were 98 (71.0%) and 40 (29.0%), respectively.
Figure 1.
Flowchart of study design.
Abbreviations: CT, computed tomography; RT, reverse transcriptase; CTCs, circulating tumor cells; pre-op, pre-operative; post-op, post-operative.
Table 1.
Clinical and pathologic characteristics of included CRC patients (n=138)
| Characteristics | No. of patients | No. of pre-op CTCs (+) | χ2 value | P-value | No. of post-op CTCs (+) | χ2 value | P-value |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Gender [n (%)] | 0.028 | 0.866 | 0.002 | 0.966 | |||
| Male | 79 (57.2) | 62 (78.5) | 39 (49.4) | ||||
| Female | 59 (42.8) | 47 (79.7) | 24 (40.7) | ||||
| Age [n (%)] | 2.686 | 0.101 | 0.294 | 0.587 | |||
| <60 years | 71 (51.4) | 60 (84.5) | 34 (47.9) | ||||
| ≥60 years | 67 (48.6) | 49 (73.1) | 29 (43.3) | ||||
| Tumor location [n (%)] | 2.089 | 0.148 | 1.216 | 0.270 | |||
| Colon | 74 (53.6) | 55 (74.3) | 37 (50.0) | ||||
| Rectal | 64 (46.4) | 54 (84.4) | 26 (40.6) | ||||
| Grade [n (%)] | 7.838 | 0.020* | 8.154 | 0.017* | |||
| Poor | 41 (29.7) | 38 (92.3) | 25 (61.0) | ||||
| Moderate | 59 (42.8) | 41 (69.5) | 27 (45.8) | ||||
| Well | 38 (27.5) | 30 (78.9) | 11 (28.9) | ||||
| LVI [n (%)] | 0.586 | 0.444 | 1.657 | 0.196 | |||
| Absence | 77 (55.8) | 59 (76.6) | 31 (40.3) | ||||
| Presence | 61 (44.2) | 50 (82.0) | 32 (52.5) | ||||
| TI [n (%)] | 0.015 | 0.904 | 0.004 | 0.950 | |||
| T1–2 | 20 (14.5) | 16 (80.0) | 9 (45.0) | ||||
| T3–4 | 118 (85.5) | 93 (78.8) | 54 (45.8) | ||||
| LNM [n (%)] | 1.070 | 0.376 | 1.183 | 0.365 | |||
| N0 | 46 (33.3) | 34 (73.9) | 18 (39.1) | ||||
| N+ | 92 (66.7) | 75 (81.5) | 45 (48.9) | ||||
| TNM stagea [n (%)] | 2.133 | 0.344 | 3.553 | 0.169 | |||
| I | 13 (9.4) | 9 (69.2) | 3 (23.1) | ||||
| II | 67 (48.6) | 51 (76.1) | 30 (44.8) | ||||
| III | 58 (42.0) | 49 (84.5) | 30 (51.7) | ||||
| CEA level [n (%)] | 4.117 | 0.042* | 1.984 | 0.159 | |||
| <5 ng/mL | 98 (71.0) | 73 (74.5) | 41 (41.8) | ||||
| ≥5 ng/mL | 40 (29.0) | 36 (90.0) | 22 (55.0) | ||||
| Overall | 138 (100.0) | 109 (79.0) | — | — | 63 (45.7) | — | — |
Notes:
The seventh edition of American Joint Committee on Cancer staging system;
P<0.05.
Abbreviations: CEA, carcinoembryonic antigen; CRC, colorectal cancer; CTCs, circulating tumor cells; LVI, lymphovascular invasion; LNM, lymph node metastasis; n, number; op, operative; TI, tumor invasion; TNM, tumor-node-metastasis.
Pre-op CTCs, post-op CTCs, and clinicopathological characteristics
As shown in Figure 2, pre-op CTCs were negative in 29 patients (21.0%) and positive in 109 patients (79.0%), while post-op CTCs were negative in 75 patients (54.3%) and positive in 63 patients (45.7%). Of the 29 patients with pre-op CTCs−, 18 patients (62.1%) were post-op CTCs− and 11 patients (37.9%) were post-op CTCs+; while, of the 109 patients with pre-op CTCs+, 57 patients (52.3%) were post-op CTCs− and 52 patients (47.7%) were post-op CTCs+. The relationship between pre- and post-op CTCs status and clinicopathological characteristics of 138 included CRC patients are shown in Table 1. Pre-op CTCs are positively correlated with tumor grade (P=0.020) and serum CEA level (P=0.042), in contrast, no significant association was found between pre-op CTCs+ and other clinicopathological characteristics (P>0.05 for all others), such as gender, age, tumor location, LVI, tumor invasion (TI), lymph node metastasis (LNM) and tumor-node-metastasis (TNM) stage. Post-op CTCs+ were only positively correlated with tumor grade (P=0.017) and not significantly correlated with gender, age, tumor location, LVI, TI, lymph LNM, TNM stage, or serum CEA level (P>0.05 for all others).
Figure 2.
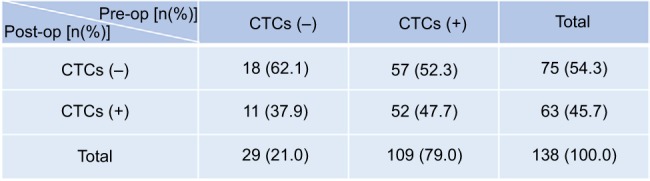
The number and change of patients with CTCs negative and positive before and after curative resection.
Abbreviations: CTCs, circulating tumor cells; Post-op, post-operative; Pre-op, pre-operative.
Pre-op CTCs, post-op CTCs, and 3-year RFS rate
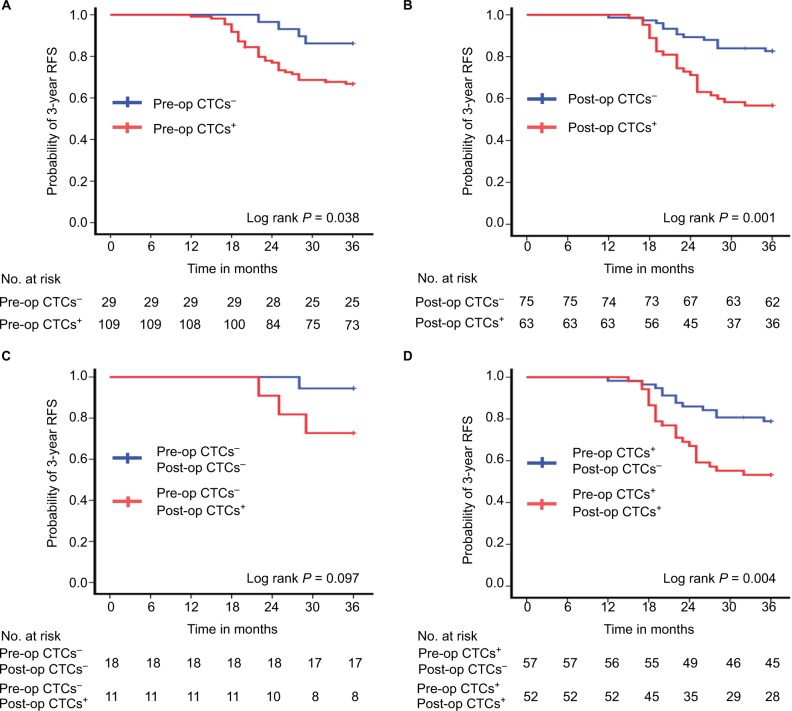
The median follow-up was 41 months (range=20–49 months). During the follow-up period, a total of 40 patients (29.0%) experienced recurrences, and the 3-year RFS rate for all patients was 71.0%. The 3-year RFS rate for the 109 patients with pre-op CTCs+ was 67.0%, which was significantly lower than the 86.2% 3-year RFS rate in the 29 patients with pre-op CTCs− (P=0.049) (Figure 3A). The 3-year RFS rate was 82.7% for the 75 patients with post-op CTCs− compared with 57.1% for the 63 patients with post-op CTCs+ (P=0.001) (Figure 3B). The 3-year RFS rate was 94.4% for the 18 patients with pre- and post-op CTCs−, compared with 72.7% for the 11 patients with pre-op CTCs− but post-op CTCs+ (P=0.141) (Figure 3C). The 3-year RFS rate was 78.9% for the 57 patients with pre-op CTCs+, but post-op CTCs−, compared with 53.8% for the 52 patients with pre-and post-op CTCs+ (P=0.006) (Figure 3D).
Figure 3.
Three-year RFS rate by pre-op and post-op CTCs status. (A) Patients with pre-op CTCs− (n=29) vs pre-op CTCs+ (n=109). (B) Patients with post-op CTCs− (n=75) vs post-op CTCs+ (n=63). (C) Patients with pre- and post-op CTCs− (n=18) vs pre-op CTCs−, but post-op CTCs+ (n=11). (D) Patients with pre-op CTCs+, but post-op CTCs− (n=57) vs pre- and post-op CTCs+ (n=52).
Abbreviations: CTCs, circulating tumor cells; Post-op, post-operative; Pre-op, pre-operative; RFS, recurrence-free survival.
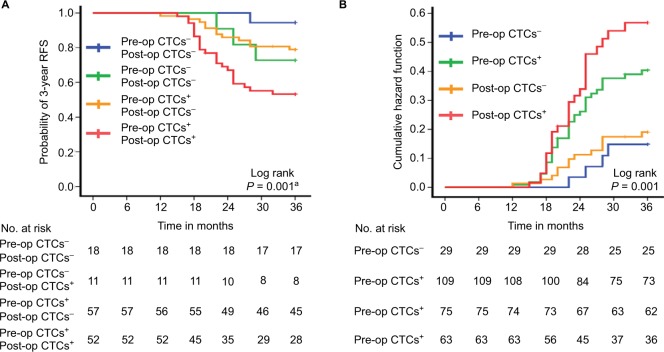
Furthermore, the 3-year RFS rate of the 52 patients with pre- and post-op CTCs+ was 53.8%, which was significantly lower than that of the pre- and post-op CTCs− or pre-op CTCs+, but post-op CTCs− cohort (pre- and post-op CTCs+ vs pre- and post-op CTCs−: P=0.019; pre- and post-op CTCs+ vs pre-op CTCs+, but post-op CTCs−: P=0.006), but similar to the cohort of pre-op CTCs− but post-op CTCs+ (P=0.220) (overall log-rank P=0.001) (Figure 4A). The cumulative hazard function for different pre- or post-op CTCs status group indicated that the risk of recurrence was higher in the post-op CTCs+ group compared with pre-op CTCs−, post-op CTCs−, and pre-op CTCs+ group (overall log-rank P=0.001) (Figure 4B).
Figure 4.
Three-year RFS rate of the four patient cohorts. (A) Patients with pre- and post-op CTCs− (n=18), pre-op CTCs−, but post-op CTCs+ (n=11), pre-op CTCs+, but post-op CTCs− (n=57), and pre- and post-op CTCs+ (n=52). (B) Hazard functions for disease recurrence of different pre- and post-CTCs status.
Note: a Pre- and post-op CTCs+ vs pre- and post-op CTCs−, P=0.019; pre- and post-op CTCs+ vs pre-op CTCs−, but post-op CTCs+, P=0.220; pre- and post-op CTCs+ vs pre-op CTCs+, but post-op CTCs−, P=0.006.
Abbreviations: CTCs, circulating tumor cells; Post-op, post-operative; Pre-op, pre-operative; RFS, recurrence-free survival.
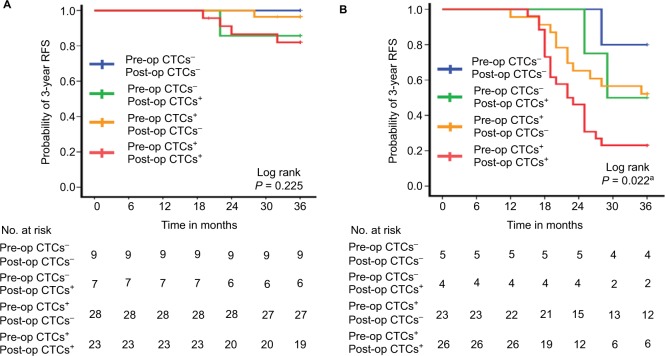
The stage-specific 3-year RFS rate of the four patient cohorts is shown in Figure 5. In patients with stage I or stage II (Figure 5A) disease, the 3-year RFS rate of the four cohorts was not significantly different (overall log-rank: stage I: P=0.325; stage II: P=0.225). However, among patients with stage III disease, the 3-year RFS rate in the pre- and post-op CTCs+ cohort was significantly lower than that of the pre- and post-op CTCs− or pre-op CTCs+, but post-op CTCs− cohort (pre- and post-op CTCs+ vs pre- and post-op CTCs−: P=0.049; pre- and post-op CTCs+ vs pre-op CTCs+, but post-op CTCs−: P=0.039), but similar to the cohort of pre-op CTCs−, but post-op CTCs+ (P=0.194) (overall log-rank P=0.022) (Figure 5B).
Figure 5.
Stage-specific three-year RFS rate of the four patient cohorts. (A) Stage II patients with pre- and post-op CTCs− (n=9), pre-op CTCs−, but post-op CTCs+ (n=7), pre-op CTCs+, but post-op CTCs− (n=28), and pre- and post-op CTCs+ (n=23). (B) Stage III patients with pre- and post-op CTCs− (n=5), pre-op CTCs−, but post-op CTCs+ (n=4), pre-op CTCs+, but post-op CTCs− (n=23), pre- and post-op CTCs+ (n=26).
Note: aPre- and post-op CTCs+ vs pre- and post-op CTCs−, P=0.049; pre- and post-op CTCs+ vs pre-op CTCs−, but post-op CTCs+, P=0.194; pre- and post-op CTCs+ vs pre-op CTCs+, but post-op CTCs−, P=0.039.
Abbreviations: CTCs, circulating tumor cells; Post-op, post-operative; Pre-op, pre-operative; RFS, recurrence-free survival.
Univariate and multivariate analyses of factors associated with 3-year RFS rate are shown in Table 2. In univariate analysis, the presence of LVI, deeper of TI, more LNM, higher AJCC stage, pre-op CTCs+, and post-op CTCs+ were associated with lower 3-year RFS rate. Multivariate analyses revealed that post-op CTCs+ (HR=2.82, 95% CI=1.39–5.75, P=0.004), but not pre-op CTCs+ (HR=2.17, 95% CI=0.75–6.31, P=0.153), was independently associated with shorter 3-year RFS rate as well as with the presence of LVI (HR=2.26, 95% CI=1.11–4.62, P=0.026) and higher TNM stage (HR=11.25, 95% CI=4.04–31.34, P<0.001).
Table 2.
Univariate and multivariate analysis for predictors of 3-year recurrence-free survival
| Predictor | Univariate analysis
|
Multivariate analysis
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
|
| ||||||
| Clinical predictors | ||||||
| Gender (Male vs Female) | 0.93 | 0.49–1.75 | 0.816 | — | — | — |
| Age (<60 years vs ≥60 years) | 0.62 | 0.33–1.16 | 0.136 | — | — | — |
| Tumor location (Colon vs Rectal) | 1.02 | 0.55–1.89 | 0.955 | — | — | — |
| Pathological predictors | ||||||
| Grade (Poor vs Moderate vs Well) | 0.69 | 0.45–1.04 | 0.077 | — | — | — |
| LVI (Absence vs Presence) | 4.04 | 2.02–8.10 | <0.001* | 2.26 | 1.11–4.62 | 0.026* |
| TI (T1–2 vs T3–4) | 1.87 | 1.13–3.08 | 0.014* | 0.93 | 0.51–1.68 | 0.803 |
| LNM (N0 vs N+) | 1.53 | 1.09–2.15 | 0.015* | 0.68 | 0.43–1.09 | 0.106 |
| TNM stagea (I–II vs III) | 9.56 | 4.08–22.37 | <0.001* | 11.25 | 4.04–31.34 | <0.001* |
| Laboratory predictors | ||||||
| CEA level (<5 ng/mL vs ≥5 ng/mL) | 1.39 | 0.73–2.66 | 0.321 | — | — | — |
| Pre-op CTCs (Negative vs Positive) | 2.82 | 1.01–7.92 | 0.049* | 2.17 | 0.75–6.31 | 0.153 |
| Post-op CTCs (Negative vs Positive) | 2.97 | 1.53–5.76 | 0.001* | 2.82 | 1.39–5.75 | 0.004* |
Notes:
The seventh edition of American Joint Committee on Cancer staging system;
P<0.05.
Abbreviations: CEA, carcinoembryonic antigen; CTCs, circulating tumor cells; LVI, lymphovascular invasion; LNM, lymph node metastasis; op, operative; TI, tumor invasion; TNM, tumor-node-metastasis.
Discussion
Currently, new diagnostic methods that can predict risk and early identification of metastasis are eagerly needed to improve the survival of CRC patients. CTC, as the important medium for hematogenous metastasis of cancer, has been proving to provide a mass of relevant information about tumor metastasis.2 In the present study, our results showed that post- op CTC is more informative than pre-op CTC. Patients with pre-op CTCS+ had an absolute 19.2% lower 3-year RFS rate than those with pre-op CTC−. However, CTCs normalized in >50% of patients with pre-op CTCS+ following surgery, and the 3-year RFS rate of patients with pre-op CTCS+ but post-op CTC− was similar to that of patients with pre-op CTCs−. Conversely, those patients with pre- and post-op CTCS+ had at least 25.1% lower 3-year RFS rate than those with either pre- and post-op CTCs− or pre-op CTCS+, but post-op CTCs−. Additionally, patients with post-op CTCS+ had an absolute 25.6% lower 3-year RFS rate than those with post-op CTCs−. Furthermore, the impact of post-op CTCS+ was demonstrated by the hazard function curves over time, which showed a higher hazard rate in the post-op CTCS+ cohort compared with pre-op CTCs−, pre-op CTCS+ and post-op CTCS+ cohorts. Multivariate analyses also confirmed that post-op CTCS+, but not pre-op CTCS+, is an independent indicator of poor prognosis, which further demonstrated post-op CTCS+ was more prognostic than pre-op CTCS+. When evaluating the results by tumor stage, it was clear that pre- and post-op CTCS+ was able to stratify patients with stage III rather than stage I and II, probably because of limited recurrence in the latter two groups, with a 3-year RFS rate >92.3% and 91.0% for stage I and II, respectively. These results were consistent with the previous published literatures.12,24 All above findings suggested that, with regard to recurrence risk judgment, measuring post-op CTCs rather than pre-op CTCs is more instructive.
Theoretically, post-op evaluation has been suggested to better reflect the most relevant CTC status because it reflects the combined information of preoperative CTC, intraoperative tumor cell release by surgical manipulation, and rapid apoptotic death of shed cells.25 Previously, Patel et al24 detected the change of pre-op and post-op CTC count based on reverse transcriptase-polymerase chain reaction (RT-PCR) method, and the results showed that the positive rate of CTCs was significantly decreased at 24 hours after surgery compared with before surgery in patients with CRC, and the clearance of CTCs within 24 hours of CRC excision was the greatest in tumors with the best prognosis. Bessa et al11 reported that the presence of CTC detected by RT-PCR targeting CEA mRNA 24 hours after surgery did not correlate with tumor recurrence or survival in 66 CRC patients operated on for cure. Ikeguchi and Kaibara26 detected pre- and post-op CTCs of 59 gastric cancer patients by RT-PCR method, and found that gastrectomy could spread gastric cancer cells into the PB from primary tumors; however, such circulating gastric cancer cells might be destroyed within a short time. Furthermore, post-op CTCS+ patients might have better disease-free survival and overall survival than post-op CTCs− ones if the blood samples were post-operatively collected within 48 hours. In contrast, Galizia et al12 recently used flow cytometry to isolate the pre- and post-op CTCs of 76 CRC patients who underwent surgical resection and identified CTCs based on epithelial surface antigen (EpCAM/CD326). The results demonstrated that a high level of post-op CTCs was the only independent factor related to cancer relapse which accurately predicted tumor recurrence. Our results also found that post-op CTCS+, but not pre-op CTCS+, was the independent factor related to cancer relapse. Moreover, our study applied a one-stop ISET device to identify CTCs preoperatively and postoperatively, compared with the detection methods mentioned in the above study (RT-PCR or flow cytometry based on EpCAM/CD326). This method has the advantages of being inexpensive, automatic, higher sensitivity, and reliability.16 Therefore, our results provided more reliable information on the prognostic value of post-op CTCs.
Our results also indicate that post-op CTC may inform the frequency of surveillance. Patients with post-op CTCS+ had a higher risk of recurrence compared with the pre-op CTCs−, post-op CTCs−, and pre-op CTCS+ groups. This might support the argument that more aggressive adjuvant therapy and more frequent follow-up are worth considering for these patients, as reported by a previous study.12 However, we cannot conclude from these data sets if additional treatment and imaging would be beneficial in patients with post-op CTCS+. Maybe, a short interval follow-up CT scan is feasible for stage III CRC patients because we found that the 3-year RFS rate for stage III patients with pre- and post-op CTCS+ was 23.1%, which was significantly lower than the pre-op CTCS+, but post-op CTCs− cohort. However, we did not have the power to determine the significance of post-op CTCs in patients with stage I and II disease, due to the 100% and 82.6% 3-year RFS rate for stage I and II patients with pre-and post-op CTCs+, respectively. In addition, we also found that there were 11 patients (37.9%) turned to positive after surgery in the 29 patients with pre-op CTCs− in our study, and the 3-year RFS rate of this group was not significantly different compared with that of persistent negative before and after surgery group. This change of CTC in PB before and after surgery was intriguing. As we all known, CTCs refer to tumor cells that released from the tumor lesion into the blood. In the process of curative resection, the internal environment changes caused by anesthesia and surgical stress, the inadvertent squeezing of tumor by the surgeon, and the pneumoperitoneum pressure during laparoscopic surgery may induce the primary tumor to release tumor cells into the bloodstream, which might lead to the patients having a negative pre-op CTC that turned out to become positive after operation. Presumably, this may be the reason why some doctors have observed that surgery may induce distant metastasis of tumors. However, due to the small number of patients and the relatively short follow-up time, our results should be interpreted with caution, and larger-scale, longer-term follow-up studies are requires to verify this.
In our study, we used the CTCBIOPSY® combined with Wright’s staining to isolate and identify CTCs. As an ISET device independently developed by China, it has the advantages of being inexpensive, automatic, and efficient. It also has been demonstrated to have high capture efficiency for both EpCAM positive and negative cells in our previous study,17 which showed that it has the potential of capturing both epithelial and non-epithelial tumor cells. Subsequently, a series of clinical studies have gradually confirmed that it can efficiently capture epithelial, epithelial/mesenchymal, and mesenchymal CTCs from PB of multiple tumor patients and exhibits an important clinical application value in tumor efficacy monitoring and prognosis evaluation.18–20
To our knowledge, this is the first prospective cohort study utilizing the ISET technology to evaluate the prognostic influence of pre- and post-op CTCs in CRC patients who underwent curative resection, and, more importantly, it is also the largest study to date. Although the detection technology advantages and larger sample size improve the reliability and persuasiveness of results, this study was still subject to several limitations. First, although the CTCBIOPSY® device used in our study has many advantages in the capture of CTCs, its reliability has only been validated in a few small studies from the People’s Republic of China previously, and multi-center, large-scale studies from different countries are need in the future. Second, timing of post-op CTC measurement was not strictly uniform, although it was limited to CTC detection at the time of reviewing 1 month after surgery or before starting adjuvant therapy. Third, we did not evaluate the optimal CTC cut-off value of this data set, but only used one as the cut-off point based on previous studies. Fourth, the limited included patients of every cohort and the single center reduced the persuasiveness of this prospective cohort study.
Conclusion
In summary, we designed a prospective cohort study which used a one-stop ISET device able to identify CTCs in an inexpensive, automatic, and reliable fashion to compare the prognostic value of pre- and post-op CTCs in patients with stage I–III CRC who underwent curative resection. The results showed post-op CTCS+, but not pre-op CTCS+, is an independent indicator of poor prognosis. After curative resection, CTC can normalize and the patients with pre-op CTCS+ that normalized after surgery have similar outcomes to patients with pre-op CTCs−. Evaluation of post-op, rather than pre-op CTC is warranted. Furthermore, patients with post-op CTCS+ tend to have a higher risk of recurrence after 3 years of surgery, which might justify a risk-adjusted and individualized surveillance strategy. Confirmation of these results using a multicenter, large scale trial with a uniform detection time and optimal cut-off value would be beneficial.
Acknowledgments
This work was supported in part by the following funds: (1) Science Fund of the National Natural Science Foundation of China (grant numbers: 81572874 and 81702411); (2) Zhongnan Hospital of Wuhan University, Technology and Innovation Seed Found (grant number: znpy2016058). The above funding body was not involved in the design of the study and collection, analysis, and interpretation of data or in writing the manuscript. The authors also appreciate Wuhan YZY Medical Science and Technology Co., Ltd. for providing equipment and excellent technical support.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 2.Plaks V, Koopman CD, Werb Z, Cancer WZ. Cancer. Circulating tumor cells. Science. 2013;341(6151):1186–1188. doi: 10.1126/science.1235226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10(20):6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 4.Gazzaniga P, Gianni W, Raimondi C, et al. Circulating tumor cells in high-risk nonmetastatic colorectal cancer. Tumour Biol. 2013;34(5):2507–2509. doi: 10.1007/s13277-013-0752-9. [DOI] [PubMed] [Google Scholar]
- 5.Hardingham JE, Grover P, Winter M, Hewett PJ, Price TJ, Thierry B. Detection and clinical significance of circulating tumor cells in colorectal cancer—20 years of progress. Mol Med. 2015;211(Suppl):1–31. doi: 10.2119/molmed.2015.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen SJ, Punt CJ, Iannotti N, et al. Prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Ann Oncol. 2009;20(7):1223–1229. doi: 10.1093/annonc/mdn786. [DOI] [PubMed] [Google Scholar]
- 7.Akagi Y, Kinugasa T, Adachi Y, Shirouzu K. Prognostic significance of isolated tumor cells in patients with colorectal cancer in recent 10-year studies. Mol Clin Oncol. 2013;1(4):582–592. doi: 10.3892/mco.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang X, Gao P, Song Y, et al. Relationship between circulating tumor cells and tumor response in colorectal cancer patients treated with chemotherapy: a meta-analysis. BMC Cancer. 2014;14(1):976. doi: 10.1186/1471-2407-14-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang X, Gao P, Song Y, et al. Meta-analysis of the prognostic value of circulating tumor cells detected with the CellSearch System in colorectal cancer. BMC Cancer. 2015;15(1):202. doi: 10.1186/s12885-015-1218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang C, Zou K, Zheng L, Xiong B. Prognostic and clinicopathological significance of circulating tumor cells detected by RT-PCR in non-metastatic colorectal cancer: a meta-analysis and systematic review. BMC Cancer. 2017;17(1):725. doi: 10.1186/s12885-017-3704-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bessa X, Piñol V, Castellví-Bel S, et al. Prognostic value of postoperative detection of blood circulating tumor cells in patients with colorectal cancer operated on for cure. Ann Surg. 2003;237(3):368–375. doi: 10.1097/01.SLA.0000055223.27623.F3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galizia G, Gemei M, Orditura M, et al. Postoperative detection of circulating tumor cells predicts tumor recurrence in colorectal cancer patients. J Gastrointest Surg. 2013;17(10):1809–1818. doi: 10.1007/s11605-013-2258-6. [DOI] [PubMed] [Google Scholar]
- 13.Negin BP, Cohen SJ. Circulating tumor cells in colorectal cancer: past, present, and future challenges. Curr Treat Options Oncol. 2010;11(1-2):1–13. doi: 10.1007/s11864-010-0115-3. [DOI] [PubMed] [Google Scholar]
- 14.Harouaka R, Kang Z, Zheng SY, Cao L. Circulating tumor cells: advances in isolation and analysis, and challenges for clinical applications. Pharmacol Ther. 2014;141(2):209–221. doi: 10.1016/j.pharmthera.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grover PK, Cummins AG, Price TJ, Roberts-Thomson IC, Hardingham JE. Circulating tumour cells: the evolving concept and the inadequacy of their enrichment by EpCAM-based methodology for basic and clinical cancer research. Ann Oncol. 2014;25(8):1506–1516. doi: 10.1093/annonc/mdu018. [DOI] [PubMed] [Google Scholar]
- 16.Ma YC, Wang L, Yu FL. Recent advances and prospects in the isolation by size of epithelial tumor cells (ISET) methodology. Technol Cancer Res Treat. 2013;12(4):295–309. doi: 10.7785/tcrt.2012.500328. [DOI] [PubMed] [Google Scholar]
- 17.Chen F, Wang S, Fang Y, et al. Feasibility of a novel one-stop ISET device to capture CTCs and its clinical application. Oncotarget. 2016 doi: 10.18632/oncotarget.13823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng X, Fan L, Zhou P, et al. Detection of circulating tumor cells and circulating tumor microemboli in gastric cancer. Transl Oncol. 2017;10(3):431–441. doi: 10.1016/j.tranon.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang D, Zhao L, Zhou P, et al. Circulating tumor microemboli (CTM) and vimentin+ circulating tumor cells (CTCs) detected by a size-based platform predict worse prognosis in advanced colorectal cancer patients during chemotherapy. Cancer Cell Int. 2017;17(1):6. doi: 10.1186/s12935-016-0373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng L, Zou K, Yang C, Chen F, Guo T, Xiong B. Inflammation-based indexes and clinicopathologic features are strong predictive values of preoperative circulating tumor cell detection in gastric cancer patients. Clin Transl Oncol. 2017;19(9):1125–1132. doi: 10.1007/s12094-017-1649-7. [DOI] [PubMed] [Google Scholar]
- 21.Benson AB, Venook AP, Bekaii-Saab T, et al. Rectal cancer, version 2.2015. J Natl Compr Canc Netw. 2015;13(6):719–728. doi: 10.6004/jnccn.2015.0087. [DOI] [PubMed] [Google Scholar]
- 22.Benson AB, Venook AP, Cederquist L, et al. Colon cancer, version 1.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(3):370–398. doi: 10.6004/jnccn.2017.0036. [DOI] [PubMed] [Google Scholar]
- 23.Glynne-Jones R, Wyrwicz L, et al. ESMO Guidelines Committee et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(Suppl 4):iv22–iv40. doi: 10.1093/annonc/mdx224. [DOI] [PubMed] [Google Scholar]
- 24.Patel H, Le Marer N, Wharton RQ, et al. Clearance of circulating tumor cells after excision of primary colorectal cancer. Ann Surg. 2002;235(2):226–231. doi: 10.1097/00000658-200202000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vincent-Salomon A, Bidard FC, Pierga JY. Bone marrow micrometastasis in breast cancer: review of detection methods, prognostic impact and biological issues. J Clin Pathol. 2008;61(5):570–576. doi: 10.1136/jcp.2007.046649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikeguchi M, Kaibara N. Detection of circulating cancer cells after a gastrectomy for gastric cancer. Surg Today. 2005;35(6):436–441. doi: 10.1007/s00595-004-2978-z. [DOI] [PubMed] [Google Scholar]