Abstract
Secretion of bacterial signaling proteins and adaptation to the host, especially during infection, are processes that are often linked in pathogenic bacteria. The human pathogen Staphylococcus aureus is equipped with a large arsenal of immune-modulating factors, allowing it to either subvert the host immune response or to create permissive niches for its survival. Recently, we showed that one of the low-molecular-weight protein tyrosine phosphatases produced by S. aureus, PtpA, is secreted during growth. Here, we report that deletion of ptpA in S. aureus affects intramacrophage survival and infectivity. We also observed that PtpA is secreted during macrophage infection. Immunoprecipitation assays identified several host proteins as putative intracellular binding partners for PtpA, including coronin-1A, a cytoskeleton-associated protein that is implicated in a variety of cellular processes. Of note, we demonstrated that coronin-1A is phosphorylated on tyrosine residues upon S. aureus infection and that its phosphorylation profile is linked to PtpA expression. Our results confirm that PtpA has a critical role during infection as a bacterial effector protein that counteracts host defenses.
Keywords: Staphylococcus aureus (S. aureus), bacterial protein phosphatase, host-pathogen interaction, bacterial pathogenesis, protein phosphorylation, coronin
Introduction
The success of Staphylococcus aureus as a pathogen and its ability to cause a wide range of disease patterns are the result of its large arsenal of virulence factors that is controlled by a sophisticated network of regulatory molecules (reviewed in Refs. 1 and 2). A number of experiments assessing the invasion and the intracellular survival of S. aureus in endothelial and epithelial cells as well as osteoblasts suggest that such events may contribute to the persistence of S. aureus during infections such as endocarditis, bovine mastitis, and osteomyelitis (3). Other work demonstrated that S. aureus bacteria possess a high level resistance to neutrophil (4) and macrophage (5) mediated killing, and it has been proposed that professional phagocytes may serve as intracellular reservoirs of S. aureus (5, 6). It is nowadays well accepted that the facultative intracellular lifestyle of S. aureus contributes to recurrent infections that are frequently observed with this species (7). The pathogen is able to replicate in the phagosome or freely in the cytoplasm of its host cells, and may escape the phagolysosome of professional and nonprofessional phagocytes, subvert autophagy, induce cell death mechanisms, such as apoptosis and pyronecrosis, or may induce anti-apoptotic programs in phagocytes (reviewed in Ref. 8). Earlier work demonstrated that a subpopulation of ingested S. aureus bacteria can survive for up to 7 days within macrophages (5, 9), and a number of S. aureus global regulators and secreted virulence factors have been identified that contribute to this ability (5, 6, 10–13). However, one strategy utilized by a number of pathogenic bacteria, the secretion of bacterial signaling proteins into target host cells (14, 15), thereby directly modulating host signaling networks, has not yet been studied with S. aureus.
Recently, numerous host-pathogen interactions were found to be dependent on pathogen-secreted phosphatases (16–20). Bacterial tyrosine phosphatases catalyze the dephosphorylation of tyrosyl-phosphorylated proteins, which in turn can result in either the propagation or inhibition of phospho-dependent signaling. Whereas bacterial tyrosine phosphatases can be intimately involved in a number of cellular processes, one major theme has become apparent with the involvement of tyrosine phosphatases as secreted effectors with the potential for manipulation of host cell signal transduction pathways (18). Although a detailed picture is yet unavailable, a role of secreted bacterial protein-tyrosine phosphatases during host infection has been identified in different facultative and obligate intracellular pathogens, and the strategies employed by them are currently being elucidated. For instance, the protein-tyrosine phosphatase YopH is a major virulence factor of Yersinia spp. that is injected into epithelial cells by the type III secretion machinery of the pathogen. YopH can uncouple multiple signal transduction pathways (21), and in human epithelial cells, YopH dephosphorylates several focal adhesion proteins, including p130Cas (Cas), focal adhesion kinase, and paxillin (22–24). Similarly, Salmonella typhimurium translocates the low-molecular-weight (LMW)5 protein-tyrosine phosphatase (PTP) SptP into epithelial cells to reverse mitogen-activated protein kinase activation (25). Moreover, SptP is required for full virulence in murine models of disease (26). Mycobacterium tuberculosis (Mtb) secretes two LMW-PTPs, termed PtpA and PtpB (27). Expression of PtpA in Mtb is up-regulated within monocytes, and PtpA is secreted from Mtb into the host macrophage cytosol and disrupts key components of the endocytic pathway, resulting in the arrest of phagosome maturation (20, 28). Human vacuolar protein sorting 33B (VPS33B), a regulator of membrane fusion, was identified as the cognate substrate of PtpA, and it is assumed that PtpA impairs phagolysosomal fusion in Mtb-infected macrophages by dephosphorylation of VPS33B (20). A Mtb ptpB mutant was shown to be impaired in its ability to grow in human macrophages (20), and to display a decreased survival rate in a guinea pig model (29).
A wealth of information has been gained from studies aimed at deciphering the pathophysiological events during S. aureus-macrophage infection (reviewed in Ref. 30), but the signaling pathways leading to these adaptations are still poorly understood. The Gram-positive pathogen is known to produce two LMW-PTPs, PtpA and PtpB (31). Earlier work demonstrated that the S. aureus PtpA dephosphorylates not only protein tyrosine phosphates, but also protein ribulosamine 5-phosphates as well as free ribuloselysine 5-phosphate and erythruloselysine 4-phosphate (32). However, deletion of ptpA and/or ptpB in S. aureus did neither affect the in vitro growth kinetics nor the cell wall integrity of the mutants, which led to the assumption that the S. aureus Ptp homologs might have some specialized functions during infection (33). Support for this hypothesis is given by our recent observations, indicating that PtpA is secreted during growth of S. aureus, albeit of the fact that the protein lacks a clear export pathway signal sequence (34).
Here we demonstrate that PtpA contributes to the intracellular survival capacity of S. aureus within macrophages, and participates in the infectivity of this pathogen. Additionally, we show that PtpA is secreted by S. aureus upon ingestion by macrophages, and identify potential PtpA interaction partners within this host cell type.
Results
S. aureus PtpA is required for intramacrophage survival
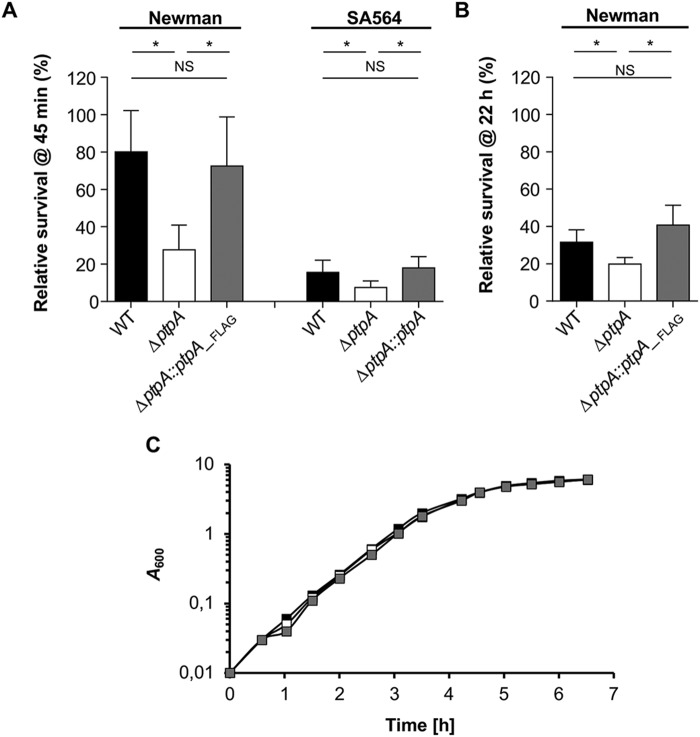
Given the impact of the Mtb PtpA homolog on the persistence capacity of this pathogen within macrophages (35, 36) and the findings that S. aureus persists readily within this host immune cell type we wondered whether the PtpA homolog of S. aureus might fulfill similar function(s). For this purpose, ptpA deletion mutants in S. aureus strains Newman, a frequently used laboratory strain, and SA564, a low passage clinical isolate, were generated. First, we determined the survival rates of S. aureus WT and ptpA mutants within RAW 264.7 cells at 45 min post-gentamicin treatment (pGt) (Fig. 1A). Already after this short period of time, a significantly smaller proportion of intracellular surviving cells were observed in RAW 264.7 cells infected with the ptpA mutants of Newman and SA564, respectively. Cis-complementation of the ptpA mutants with a functional ptpA locus reverted in both cases the intracellular survival rates to levels comparable with WT strains (Fig. 1A). Notably, ingested SA564 bacteria were killed much faster by RAW 264.7 cells than Newman cells. After 45 min pGT, about 80% of the ingested Newman bacteria were still viable and cultivable, whereas this was only the case for 16% of the ingested SA564 bacteria (Fig. 1A). Next, the effect of PtpA on intracellular survival of S. aureus Newman in macrophages was determined at a later infection stage (Fig. 1B). Similar to the situation seen at 45 min pGt, significantly reduced survival rates were observed in macrophages infected with the Newman ΔptpA mutant at 22 h pGt when compared with the WT and the cis-complemented derivative, respectively, indicating that the survival defect of the ptpA mutant in macrophages is maintained over time. In vitro growth curves performed with WT and mutant Newman strains excluded that deletion of ptpA in S. aureus might affect the bacterial growth in suspension (Fig. 1C).
Figure 1.
PtpA promotes S. aureus survival in macrophages. A, S. aureus short-term survival in infected macrophages. Cells of S. aureus strains SA564 and Newman and their isogenic ΔptpA mutants and cis-complemented derivatives, respectively, were used to infect RAW 264.7 macrophages (5 × 105 cells/ml) at a m.o.i. of 20 for 1 h at 37 °C followed by 30 min incubation with gentamicin. Macrophages were then incubated for 45 min in complete media supplemented with lysostaphin to kill extracellular bacteria that might be released from lysed macrophages during the successive incubation time, and subsequently lysed in 0.1% Triton X-100. Survival rates are given in relationship to the intracellular bacterial cell numbers seen pGt treatment. Results represent the mean ± S.D. (n = 5). *, p < 0.05; NS, not significant (Mann-Whitney-U test). B, S. aureus long-term survival in infected macrophages. RAW 264.7 macrophages (5 × 105 cells/ml) were infected with a m.o.i. of 20 of S. aureus strains Newman (black bar), Newman ΔptpA (white bar), and Newman ΔptpA::ptpA_Flag (gray bar), respectively, and co-incubated for 2 h at 37 °C, followed by a gentamicin/lysostaphin treatment to eliminate external bacteria. Macrophages were lysed with Triton X-100 (0.1%) at 22 h pGt, and bacteria enumerated by plate counting. Survival rates are given in relationship to the intracellular bacterial cell numbers seen immediately after gentamicin treatment. Data represent the mean ± S.D. (n = 5). *, p < 0.05; NS, not significant (Mann-Whitney-U test). C, in vitro growth kinetics of the S. aureus Newman/ΔptpA/ΔptpA::ptpA_Flag strain triplet. Growth of S. aureus strains Newman (black symbols), Newman ΔptpA (white symbols), and Newman ΔptpA::ptpA_Flag (gray symbols) was measured in TSB at 37 °C and 150 rpm. Data represent the mean A600 readings at the time points indicated (n = 3).
S. aureus PtpA contributes to infectivity of S. aureus in a murine abscess model
Because PtpA enhances the survival capacity of S. aureus within macrophages (Fig. 1, A and B), we hypothesized that PtpA may affect the infectivity of S. aureus in vivo. To address this hypothesis, we next assessed the ability of the strain triplet SA564/SA564 ΔptpA/SA564 ΔptpA::ptpA to cause disease in a murine abscess model (37). Consistent with our intramacrophage survival findings (Fig. 1), a significant decrease (about 2-log) in the bacterial loads in liver was detected in mice infected with the ΔptpA mutant as compared with mice challenged with the WT strain (Fig. 2). Mice infected with the cis-complemented ptpA+ derivative SA564 ΔptpA::ptpA displayed significantly higher bacterial loads in liver than in ΔptpA mutant-challenged mice, although the levels remained lower as seen in WT-challenged animals, however, this effect was not statistically significant (p = 0.215). These data suggest that PtpA positively contributes to the infectivity of S. aureus in mice.
Figure 2.
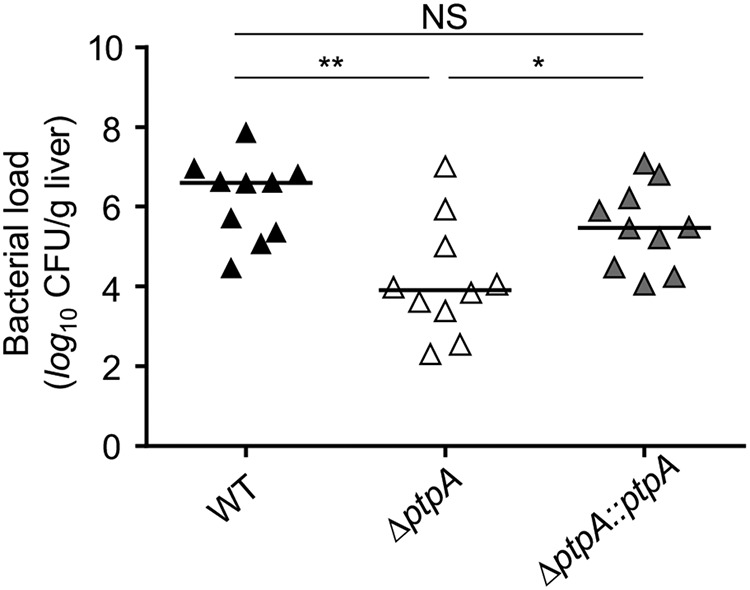
Effect of ptpA deletion on infectivity of S. aureus SA564 in a murine abscess model. C57BL/6N mice were infected via retroorbital injection with 1 × 107 cells of S. aureus strain SA564 (black symbols), SA564 ΔptpA (white symbols), and the cis-complemented derivative SA564 ΔptpA::ptpA (gray symbols), respectively (n = 10 per group). Mice were euthanized 4 days post-infection, the livers were removed and homogenized in PBS to determine the bacterial loads. Each symbol represents an individual mouse. Horizontal bars indicate the median of all observations. *, p < 0.05; **, p < 0.01; NS, not significant (Mann-Whitney-U test).
PtpA is secreted intracellularly upon S. aureus macrophage infection
We recently demonstrated that PtpA was secreted into the extracellular milieu by S. aureus under in vitro growth conditions (34). To test whether PtpA might be also secreted by S. aureus into the host macrophage, we used the cis-complemented S. aureus derivative Newman ΔptpA::ptpA_FLAG (34), which facilitates detection of the expressed PtpA by anti-FLAG immunoblotting. Macrophages were infected with the Newman ΔptpA mutant or the cis-complemented Newman ΔptpA::ptpA_Flag derivative expressing PtpA_FLAG, lysed at different time points pGt, separated from the intracellular bacteria and processed for anti-FLAG immunoprecipitation and Western blot analysis. PtpA_FLAG could be detected in increasing amounts in macrophage lysates over time upon cell infection with the ptpA complemented Newman strain ΔptpA::ptpA_FLAG, whereas no such signal was observed in Newman ΔptpA-infected macrophages (Fig. 3A).
Figure 3.
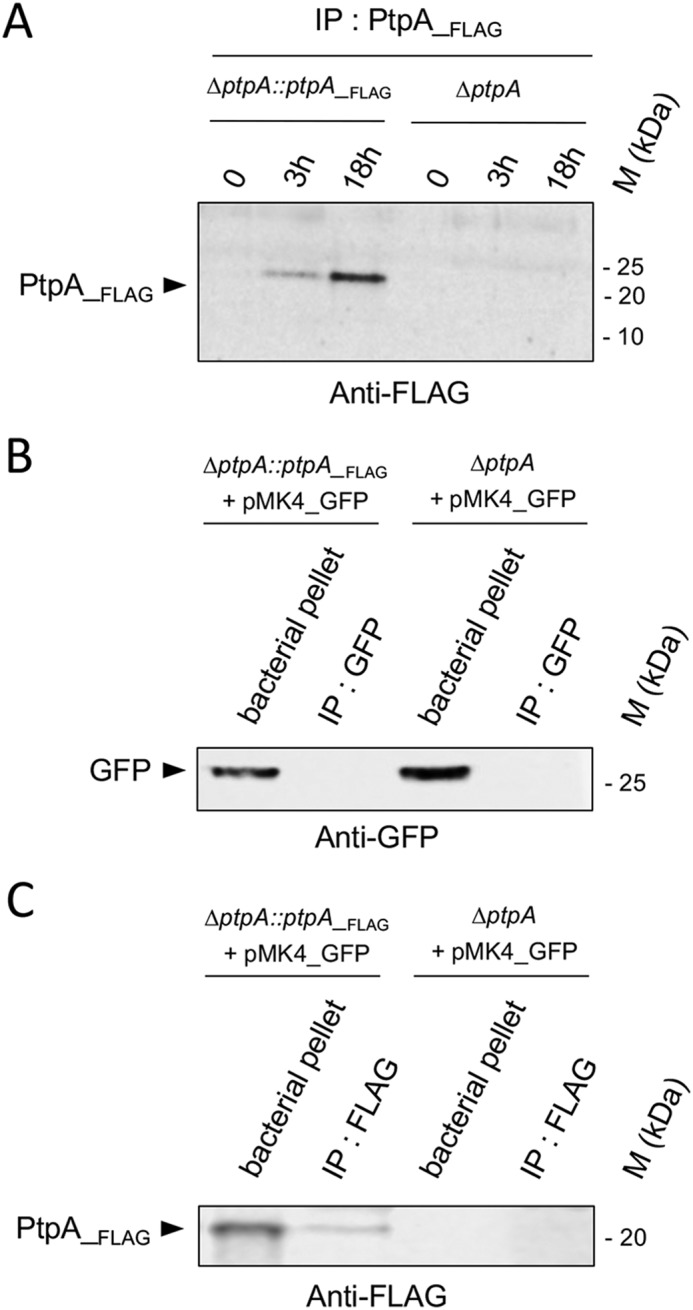
PtpA is secreted in macrophages during infection. A, PtpA secretion in macrophage lysates. RAW 264.7 macrophages (5 × 105 cells/ml) were incubated for 2 h with either S. aureus Newman ΔptpA cells or S. aureus ΔptpA cells complemented with a FLAG-tagged ptpA (Newman ΔptpA::ptpA_Flag) at a m.o.i. of 20, and nonphagocytosed bacteria were subsequently removed by gentamicin/lysostaphin treatment. Macrophages were lysed at the time points indicated, centrifuged to eliminate intracellular bacteria, and macrophage lysates were subjected to immunoprecipitation and Western blotting analyses using anti-FLAG antibodies. B and C, control immunoprecipitations to rule out bacterial proteins leaking in macrophage lysates. RAW 264.7 macrophages (5 × 105 cells/ml) were infected with Newman ΔptpA::ptpA_FLAG harboring plasmid pMK4_GFP and Newman ΔptpA harboring plasmid pMK4_GFP at a m.o.i. of 20, respectively. At 3 h pGt, infected macrophages were lysed in 0.1% Triton X-100, and centrifuged at 14,000 × g. The obtained supernatants corresponding to macrophage lysates were immunoprecipitated with GFP-trap beads (Chromoteck) (B) or with anti-FLAG antibodies coated on agarose-beads (C), whereas the pellets containing intracellular bacteria were resuspended in an equal amount of PBS with protease inhibitor mixture and lysed in a bead-beater (Retsch, MM400). Immunoprecipitated proteins and bacterial pellets were resolved on SDS-PAGE and detected with an anti-GFP (B) or anti-FLAG (C) antibody. M kDa, molecular markers.
To exclude that the PtpA_FLAG signal might originate from lysed bacteria, we transformed the Newman ΔptpA mutant and the cis-complemented Newman ΔptpA::ptpA_FLAG derivative with the green fluorescent protein (GFP) expressing vector pMK4_GFP. Following macrophage infection with these pMK4_GFP harboring derivatives, no GFP signal could be detected in the cleared macrophage lysates after concentration by GFP immunoprecipitation (GFP-trap, Chrommoteck), whereas strong GFP signals were detected in the bacterial pellets that were obtained from the macrophage lysates by centrifugation (Fig. 3B). In the same samples, PtpA_FLAG was detected in the bacterial pellet and after FLAG-immunoprecipitation from the cleared lysates of the Newman ΔptpA::ptpA_FLAG + pMK4_GFP-infected macrophages, whereas this signal was not seen in the cleared lysates of macrophages that were challenged with the pMK4_GFP-transformed ΔptpA mutant (Fig. 3C). Taken together, these findings indicate that the PtpA_FLAG signal detected in the lysate fractions of macrophages infected with the Newman ΔptpA::ptpA_Flag derivative was not substantially caused by intracellular bacterial lysis, suggesting a secretion of PtpA into macrophages.
PtpA interacts with several host cell proteins in pulldown experiments
Because PtpA is most likely secreted into host cells, we next assessed whether this bacterial derived phosphatase might interfere with host cell signal transduction pathways. To identify host cell proteins that might interact with PtpA, we developed a strategy combining the use of the slime mold Dictyostelium discoideum, and our PtpA functional mutants. D. discoideum is a eukaryotic professional phagocyte amenable to genetic and biochemical studies, and in our case allowing the ectopic expression of PtpA in large culture volumes. In a second approach, we used a “substrate trapping” strategy, based on a methodology used to identify substrates for the Yersinia phosphatase YopH in HeLa cells (38), or Mtb PtpA host interactants (20). The latter mechanism-based approach utilized a catalytically defective mutant of PtpA to trap substrate complexes. PTPs contain a cysteine nucleophile (Cys-8 within the highly conserved sequence C-X5-R) that forms a phosphocysteinyl intermediate during catalysis (39). We hypothesized that a cysteine to serine mutation at position 8 of the S. aureus Newman PtpA ORF (PtpA_C8S) would result in a catalytically defective PtpA variant that, like other similar PTP family mutants, might “trap” host substrate proteins by stabilizing the covalent enzyme-substrate complexes. To confirm that the C8S mutation in S. aureus PtpA affects its activity, we first tested the recombinant His-tagged versions of PtpA and PtpA-C8S with the substrate p-nitrophenylphosphate. Our results demonstrate that phosphatase activity of the S. aureus C8S mutant was indeed abrogated (Fig. S1).
Lysates from D. discoideum overexpressing PtpA_C8S_FLAG, or expressing the FLAG alone as a control, were next incubated with beads coupled to an anti-FLAG antibody. Afterward, beads were extensively washed, and bound proteins were subsequently stripped off and separated by SDS-PAGE before MS analysis. The proteins identified under each condition were compared and purged of those interacting with FLAG alone (Table 1).
Table 1.
Major PtpA interactants identified by mass spectrometry
| Protein name | Accession number | Sum PEP scorea | Coverageb | Number of peptidesc,d | Mass |
|---|---|---|---|---|---|
| % | kDa | ||||
| V-type proton ATPase subunit A | P54647 | 3216 | 65 | 30 | 68.16 |
| V-type proton ATPase subunit B | Q76NU1 | 454 | 35 | 12 | 54.84 |
| Coronin-A | P27133 | 435 | 33 | 10 | 49.18 |
| Cathepsin D | O76856 | 421 | 19 | 6 | 41.09 |
| Vacuolin-A | O15706 | 196 | 13 | 6 | 66.25 |
| Lysosomal α-mannosidase | P34098 | 131 | 7 | 4 | 113.36 |
| Arf GTPase-activating protein | Q9Y2X7 | 110 | 10 | 3 | 64.89 |
| Phox domain-containing protein vps5 | Q86IF6 | 26 | 9 | 2 | 61.92 |
a Sum PEP score: sum of –log(PEP) (PEP: posterior error probability), which is a probability that a Peptide Spectra Matches (PSM) is incorrect. The lower the PEP, the higher the sum PEP score.
b % of protein sequence coverage.
c A peptide is identified by one or more PSM corresponding to relevant MS-MS mass spectra leading to the identification of a peptide. A protein is identified by several peptides (# Peptides).
d The cut-off is validated by the SEQUEST HT algorithm and corresponds to at least two peptides to identify the protein.
The putative interactants of PtpA identified by this approach are involved in different cellular pathways involving cell adherence or endosomal trafficking. Two of them, lysosomal-α-mannosidase ManA and Arf-GTPase–activating protein DDB0167328, likely play a role in cell adherence (40, 41). Lysosomal-α-mannosidases are members of the glycoside hydrolase family 38. They are involved in the catabolism of Asn-linked glycans of glycoproteins and play a vital role in maintaining cellular homeostasis, cell adhesion during development, viral infection, or immune response (40). Among several functions, Arf-GTPase activating proteins are notably regulators of specialized membrane surfaces implicated in cell migration involving adhesive structures in which the cell membrane is integrated with the actin cytoskeleton (41). Additionally, proteins related to endosome function and trafficking have been co-immunoprecipitated with PtpA: Vacuolin A, Cathepsine D, and Phox domain-containing protein Vps5. Vacuolin A is a flotillin/reggie-related protein from Dictyostelium that oligomerizes for endosome association (42), and Cathepsin D is an aspartic endoprotease that is ubiquitously distributed in lysosomes to degrade proteins and activate precursors of bioactive proteins in pre-lysosomal compartments (43). Vps5 belongs to the family of sorting nexins containing a Phox homology domain and might be a component of the retromer complex. These proteins are involved in regulating membrane traffic and protein sorting in the endosomal system (44). Interestingly, V-ATPase, previously identified as an interactant of M. tuberculosis PtpA (28), was captured also in our substrate-trapping assay, suggesting that in S. aureus a similar interaction might occur. Moreover, the host protein coronin-A (CorA) was identified as putative PtpA interactant (Table 1). Interestingly, the mammalian homologue coronin-1A (Coro-1A) was reported as being retained on phagosomes containing living Mtb, while being rapidly released from phagosomes containing inactive mycobacteria (45). Furthermore, genetic depletion or RNAi-mediated gene silencing of Coro-1A were later reported to inhibit the survival of mycobacteria within macrophages (46–48).
PtpA interacts with coronin in vitro
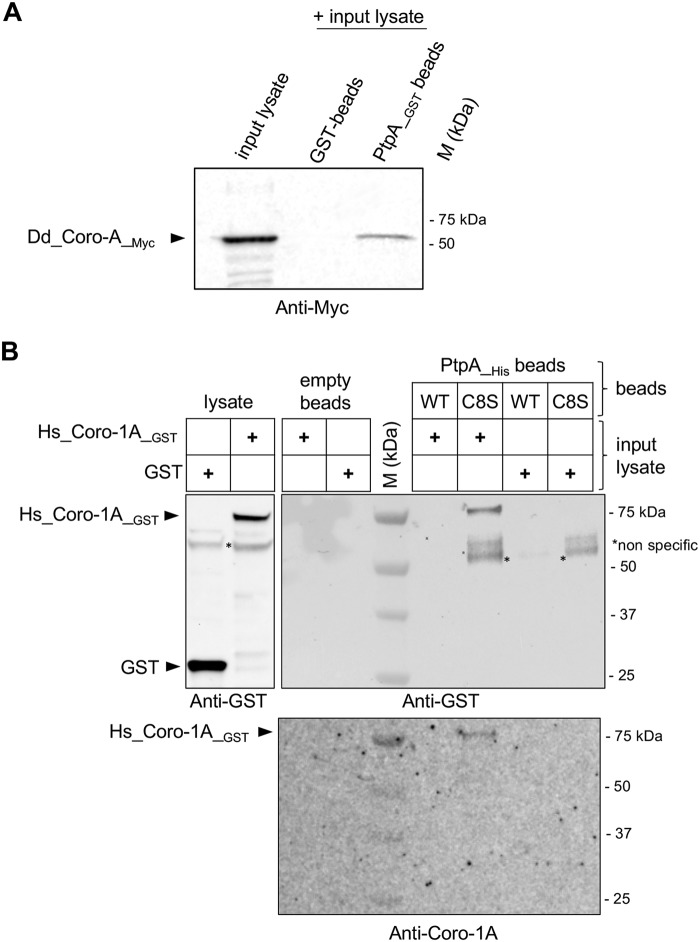
As Coro-1A was shown to be important for the survival of mycobacteria in infected macrophages (45–49), we decided to investigate the putative interaction of S. aureus PtpA and Coro-1A in more detail. First, we performed GST pulldown assays to verify the interaction between PtpA and D. discoideum CorA (Dd_Coro-A) (Fig. 4A). Fusion proteins combining GST and PtpA (PtpA_GST) were immobilized onto GSH-agarose beads and incubated with D. discoideum cell lysates expressing a myc-tagged-Dd_Coro-A (myc-Dd_Coro-A). Bound proteins were stripped off the beads and subjected to Western blotting analyses with an anti-myc antibody. As displayed in Fig. 4A, myc-Dd_Coro-A was pulled down with the PtpA_GST fusion, but not with GST alone. This observation strongly suggests that PtpA interacts with CorA from D. discoideum, thus supporting our MS findings.
Figure 4.
PtpA interacts with Coro-1A in vitro. A, the interaction between PtpA and D. discoideum CorA was confirmed by GST pulldown assay. The indicated GST fusion proteins were expressed in E. coli, bound to GSH beads, and then incubated with D. discoideum cell lysates expressing myc-tagged-Dd_Coro-1 (Dd_Coro-A_myc). Beads were washed, eluted by boiling, and bound proteins were revealed by Western blot analysis with an anti-myc antibody. B, the interaction between PtpA and human Coro-1A was tested by pulldown analysis. His-tagged versions of PtpA (WT) and the catalytically inactive PtpA_C8S (C8S) were constructed, produced. and purified as described under “Experimental procedures.” BL21 lysates expressing Hs_Coro-1A_GST or GST alone were prepared and incubated with the PtpA_His derivatives immobilized on Ni-NTA-agarose beads. The bound proteins were eluted and resolved by SDS-PAGE followed by immunoblotting using anti-GST and anti-Coro-1A antibodies. Empty Ni-NTA-agarose beads were used as a control. *, nonspecific signals co-precipitated with PtpA_C8S_His. M kDa, molecular markers.
Next, we assayed the interaction of the S. aureus PtpA with human Coro-1A (Hs_Coro-1A) by using a pulldown assay (Fig. 4B). His-tagged versions of PtpA (PtpA_His) and the catalytically inactive PtpA_C8S (PtpA_C8S_His) were overexpressed and purified as previously described (34), bound on Ni-NTA-agarose beads and incubated with BL21 lysates expressing the recombinant Hs_Coro-1A protein harboring a GST tag (Hs_Coro-1A_GST). As control, PtpA_His beads were incubated with BL21 lysates expressing GST alone. Ni-NTA-agarose beads without PtpA were additionally incubated with GST or Hs_Coro-1A _GST to confirm that beads alone could not interact with GST fusion proteins (Fig. 4B, upper panel). Protein complexes were pulled down, separated by SDS-PAGE, and transferred onto a nitrocellulose membrane before detection by anti-GST and anti-coronin-1A antibodies, respectively. In this assay, Hs_Coro-1A_GST was retained on beads when PtpA_C8S_His was present, whereas no signal was seen with the PtpA_His version (Fig. 4B, lower panel). Additionally, GST alone did neither in absence nor presence of the His-tagged PtpA fusion proteins bind to the beads (Fig. 4B). Taken together, these data suggest that specific complexes were formed between S. aureus PtpA and Coro-1A either with the D. discoideum CorA or the human homologue Hs_Coro-1A.
Coronin-1A is phosphorylated on tyrosine residues upon infection
To test whether Coro-1A might serve as a tyrosine-phosphorylated substrate for PtpA in vivo, we infected RAW 264.7 cells with S. aureus Newman and its isogenic ΔptpA mutant, respectively, and lysed the macrophages for 30 min and 3 h pGt. The murine macrophage Coro-1A homolog (Mm_Coro-1A) was subsequently immunoprecipitated with an anti-Coro-1A antibody, and the tyrosine phosphorylation status of Mm_Coro-1A was determined by Western blotting analyses using anti-phosphotyrosine antibodies. We observed that phosphorylation of Mm_Coro-1A on tyrosine residues was increased upon infection, and this effect seemed to be influenced by PtpA (Fig. 5A). Infection of RAW 264.7 cells with S. aureus clearly enhanced the tyrosine phosphorylation signal of Mm_Coro-1A at both time points analyzed. Interestingly, in lysates of Newman ΔptpA-infected macrophages, about 2-fold higher phosphotyrosine signals (2.2 ± 0.7; n = 6) were observed at 3 h pGt than in lysates of RAW 264.7 cells that were challenged with the parental strain Newman. Therefore, our results reveal for the first time a tyrosine phosphorylation of Coro-1A, as previous records of phosphorylation for this protein were related only to Ser-Thr residues (50–52), as well as its PtpA-mediated dephosphorylation.
Figure 5.
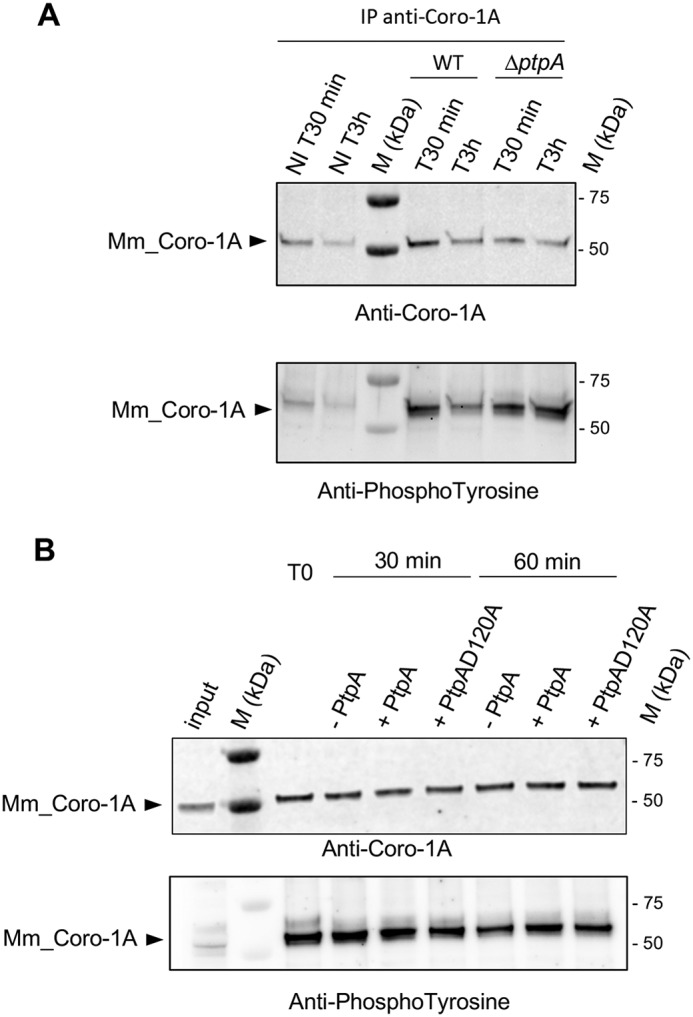
Coro-1A is phosphorylated on tyrosines in vivo but is not dephosphorylated by PtpA in vitro. A, immunoblot analysis with anti-Coro-1A (upper panel) or anti-phosphotyrosine antibodies (bottom panel) of immunoprecipitated (IP) endogenous Coro-1A (Mm_Coro-1A) from lysates of RAW 264.7 macrophages infected with cells of S. aureus Newman (WT) or the isogenic ΔptpA mutant. Noninfected macrophages and infected macrophages were treated with gentamicin for 30 min and subsequently incubated with lysostaphin to kill extracellular bacteria that might be released from lysed macrophages during the successive incubation time. Noninfected and infected macrophages were lysed 30 min (NI T30 min and T30 min) and 3 h (NI T3 h and T3 h) pGt treatment. B, immunoprecipitated Mm_Coro-1A from lysates of macrophages infected with strain Newman for 30 min pGt were incubated in the absence (−) or presence (+) of 2 μg of PtpA_His or PtpA-D120A_His at 37 °C for the time points indicated (T0, 30 and 60 min). Proteins were resolved by SDS-PAGE and probed with anti-Coro-1A (upper panel) or anti-phosphotyrosine (bottom panel) antibodies on the same blot. Contents of Mm_Coro-1A and Tyr-phosphorylated Mm_Coro-1A in lysates of S. aureus-infected macrophages prior to concentration by IP are indicated in the input lane (input). M kDa, molecular markers.
Coronin-1A is not dephosphorylated in vitro by recombinant PtpA
The observation that Coro-1A can be Tyr-phosphorylated, and that its Tyr-phosphorylation status seemed to be influenced by PtpA, prompted us to assess whether Coro-1A might be directly dephosphorylated by PtpA, as previously reported for some Mtb PtpA substrates (20). Thus we tested the ability of PtpA and of the catalytically inactive PtpA derivative PtpA_D120A (34) to dephosphorylate the murine variant of Coro-1A. PtpA_D120A was chosen for this dephosphorylation assay as it corresponds to the commonly used phosphatase-defective PtpA mutant PtpA_D126A in Mtb (20, 28, 53, 54). First, Mm_Coro-1A was purified by immunoprecipitation from S. aureus Newman-infected macrophages. Equal amounts of the immunoprecipitated Mm_Coro-1A were then incubated with recombinant PtpA derivatives for 30 min and 1 h, respectively. Proteins were subsequently separated by SDS-PAGE and electrotransferred to a membrane. The Coro-1A and Tyr-phosphorylation signals were revealed using anti-Coro-1A and anti-phosphotyrosine antibodies, respectively (Fig. 5). However, no clear reductions in the phosphorylation levels of Mm_Coro-1A were observed after incubation with either PtpA or the catalytically inactive PtpA_D120A. All together, these data indicate that Mm_Coro-1A is a bona fide substrate for tyrosine phosphorylation, and that PtpA seems to affect Coro-1A Tyr-phosphorylation in an indirect manner. Notably, a similar observation was made with the Mtb PtpA, which is able to interact with the V-ATPase subunit H without using this protein as a catalytic substrate (28).
To identify putative Tyr-phosphorylation sites of Coro-1A, we next performed an in silico analysis with the ORFs of the Coro-1A variants used in this study. The alignment of the amino acid sequences of the three Coro-1A homologues identified 94% of homology between the Mm_Coro-1A and Hs_Coro-1A ORFs, whereas the Dd_Coro-A homologue shared only 38% of homology with the human Coro-1A (Fig. S2). Despite the comparably low degree of conservation between Dd_Coro-A and the two mammalian Coro-1A variants, four Tyr-residues were identified as conserved among the three species. A subsequent alignment of 25 Coro-1A homologs of various eukaryotes including protists, fungi, and animalia confirmed a very high conservation of these four Tyr residues (Fig. S3), supporting the idea that these sites might be involved in the Tyr-mediated phosphorylation of this protein.
In a first attempt to identify the host kinase responsible for Coro-1A Tyr-phosphorylation upon S. aureus infection, we tested the spleen tyrosine kinase Syk on Mm_Coro-1A Tyr-phosphorylation, as Syk is highly expressed in RAW 264.7 cells (55), and implicated to play a pivotal role in macrophage-mediated inflammatory responses (56). Moreover, one of the human coronin homologs, coronin-1C (sharing 62% of identity to Hs_Coro-1A at the amino acid level), was previously identified as ligand of Syk in B-cells (57). However, we failed to detect any tyrosine phosphorylation signal on Coro-1A after co-incubation with Syk in our in vitro phosphorylation assays (data not shown), indicating that Syk is not the kinase responsible for Tyr-phosphorylation of Coro-1A.
Discussion
Our results provide the first interactor candidate identification of host partners of the secreted phosphatase PtpA, and its involvement in the process of infection and intracellular survival of S. aureus. The macrophage survival and infection data suggest an important role for PtpA during infection. Our co-immunoprecipitation studies indicate PtpA interacts with a number of host factors including Coro-1A, and revealed that this actin-binding protein can be phosphorylated at tyrosine residues. Together, these findings suggest a role for PtpA as modulator of the host immune response, particularly after uptake of S. aureus by macrophages. As the impact of Tyr-phosphorylation on Coro-1A function/activity was not studied yet, we can only hypothesize that phosphorylation of the Coro-1A Tyr residue(s) might affect the intracellular distribution of this actin-binding protein within macrophages, as it has been suggested for serine/threonine-mediated phosphorylation of coronin-1. Indeed, protein kinase C-dependent coronin-1 phosphorylation on Ser and Thr residues was identified as an important mechanism to modulate the intracellular distribution of this protein during phagolysosome maturation. Earlier work studying the phagocytosis of opsonized zymosan particles by HL-60 leukemia cells demonstrated that phosphorylation of coronin-1 by protein kinase C triggered the dissociation of the actin-binding protein from nascent phagosomes (51). Another study performed with mycobacteria-infected macrophages showed that coronin-1 accumulated on bacteria-loaded phagosomes, and that this protein was actively retained by viable mycobacteria residing inside phagosomes (45) suggesting that the retention of coronin-1 on mycobacteria-loaded phagosomes is responsible for suppressing phagosome-lysosome formation (58). More recent work demonstrated that trimerization of coronin-1 was essential for mycobacterial survival, and that the transition from the trimer to the monomer form was regulated by serine phosphorylation (59). In the light of the latter findings one may speculate that Tyr-phosphorylation of Coro-1A might also affect the spatial distribution of this protein in S. aureus-infected macrophages, and that the bacterium attempts to modulate this process via PtpA. However, it is unclear yet whether Tyr-phosphorylation of Coro-1A affects the trimerization of this protein, and further work is required to understand how PtpA recognizes Coro-1A and/or other putative interactors identified by our co-immunoprecipitation studies, and whether and how they participate in the establishment of S. aureus survival and virulence.
Experimental procedures
Bacterial strains and culture conditions
The bacterial strains and plasmids used in this study are listed in Table 2. Escherichia coli strains were grown at 37 °C in LB medium supplemented with antibiotic when required. S. aureus isolates were plated on tryptic soy agar (BD Bioscience) supplemented with antibiotic when required, or grown in tryptic soy broth (TSB) (BD Bioscience) medium at 37 °C and 150 rpm. The Newman ΔptpA mutant derivatives were previously constructed (34). The SA564 ΔptpA mutant was obtained by phage transducing the aphAIII-lox–tagged ΔptpA mutation from Newman ΔptpA into the low passage clinical isolate SA564 (60).
Table 2.
Strains, plasmids, and primers used in this study
| Strain or plasmid | Relevant genotype or characteristic(s)a | Ref. or source | |||
|---|---|---|---|---|---|
| S. aureus | |||||
| Newman | Laboratory strain (ATCC 25904), wildtype | ||||
| Newman ΔptpA | Newman ΔptpA::lox66-aphAIII-lox71; Kanr | 34 | |||
| Newman ΔptpA::ptpA_Flag | Newman ΔptpA derivative cis-complemented with pEC1_ptpA_Flag; Emr | 34 | |||
| SA564 | Clinical isolate, wildtype | 60 | |||
| SA564 ΔptpA | SA564 ΔptpA::lox66-aphAIII-lox71; Kanr | This study | |||
| SA564 ΔptpA::ptpA | SA564 ΔptpA derivative cis-complemented with pEC1_ptpAc; Emr | This study | |||
| E. coli | |||||
| IM08B | SA08BΩPN25-hsdS (CC8–1) (SAUSA300_0406) of NRS384 integrated between the essQ and cspB genes | 62 | |||
| D. discoideum | |||||
| DH1–10 | Axenic, uracil auxotroph D. discoideum strain | 64 | |||
| Plasmids | |||||
| pDXA | D. discoideum vector | 63 | |||
| pFL1290 | pDXA-3 × myc-Coronin-A corresponding to pDXA vector with a 1338-kb fragment covering the ORF for the D. discoideum coronin-A (corA; Gene ID: DDB_G0267382; NCBI reference sequence: XM_642251.1) fused to 3 × myc tag. | This study | |||
| pDXA_PtpA-C8S-Flag | pDXA_PtpA-C8S-FLAG corresponding to pDXA vector with a 0.8-kb fragment covering the ORF for the S. aureus ptpA gene fused to 3 × FLAG tag. | This study | |||
| pEC1 | pUC19 derivative containing the 1.45-kb ClaI erm(B) fragment of Tn551 | 61 | |||
| pEC1_ptpAc | pEC1 with a 0.8-kb fragment covering the C-terminal part of ORF RT87_RS09705 and the putative terminator region (NCBI reference sequence: NZ_CP010890.1). | This study | |||
| pETPhos_PtpA_C8S | Modified from pETPhos_PtpA (34) | This study | |||
| pCDFDuet GST Cter up1 | Modified from Novagen pCDFDuet-1 | This study | |||
| pCDFDuet-Coro-1A-GST | pCDFDuet-GST Cter Up1 with a 1.4-kb fragment covering the ORF for the human Coro-1A (NCBI reference sequence: NC_000016.10) optimized for bacterial expression and fused to GST. | This study | |||
| Primers | |||||
| OL989–5′-BamHIx2 (forward) | GGATCCGGATCCATGTCTAAAGTAGTCCGTAGTAGTAAA | This study | |||
| OL990–3′-XhoIx2-stop (reverse) | CTCGAGCTCGAGTTAGTTGGTGAGTTCTTTGATTTTGGC | This study | |||
| Nterm_PtpA_C8S | ATGGTAGATGTAGCATTTGTCAGTCTTGGCAATATATGTCG | This study | |||
| Nter_PtpA_flag_C8S_IF_Bam | GAATTCCCGGGGATCCATGGTAGATGTAGCATTTGTCAGTCTTGGCAATATATGTCG | This study | |||
| Cter_PtpAflag_Xho_pDXA | ATCTATCTCGAGTTATTTATCATCATCATCTTTGTA | This study | |||
| Nterm PtpA Bam_pGEX | TATGGATCCATGGTAGATGTAGCATTTGTCTGT | This study | |||
| Cterm PtpA Hind IF_pGEX | TATCATCGATAAGCTTCTACCCCTCTTTCAAATTTGCATC | This study | |||
| MBH425 | GCAATTATGAATTCTTTCAATGTTGC | This study | |||
| MBH426 | GCTGGTACCGAATTAAGAAAAGTTACTTACGCC | This study | |||
a Emr, Erythromycin resistant; Kanr, kanamycin resistant.
Construction of the S. aureus ptpA cis-complementation strain SA564 ΔptpA::ptpA
For cis-complementation of the ptpA mutation in strain SA564 ΔptpA, a 0.8-kb fragment containing the C-terminal part of ORF RT87_RS09705 located downstream of ptpA (RT87_RS09695) was amplified by PCR from chromosomal DNA of S. aureus strain SA564 using the primer pair MBH425/MBH426 (Table 2). The resulting PCR product was digested with KpnI/EcoRI, and subsequently cloned into KpnI/EcoRI-digested vector pEC1 (61) to generate the suicide plasmid pEC1_ptpAc. E. coli IM08B (62)-derived plasmid pEC1_ptpAc was directly electroporated into S. aureus strain SA564. A SA564 derivative that integrated pEC1_ptpAc was subsequently used as a donor for transducing the cis-integrated pEC1_ptpAc into SA564 ΔptpA, thereby replacing the aph-tagged ptpA deletion with the WT ptpA genome region.
Animal studies statement
Animal experiments were approved by the local State Review Boards of Saarland and conducted according to the regulations of German veterinary law.
Murine abscess model
Preparation of the bacterial inoculum and infection of the animals were carried out as described (37), with minor modifications. Briefly, 100-μl bacterial suspensions containing ∼107 colony forming unit (cfu) were administered intravenously by retro-orbital injection into female, 8–10–week-old C57/BL6N mice that were anesthetized by isoflurane inhalation (5%; Baxter). Immediately after infection, mice were treated with a dose of caprofen (5 mg/kg; Pfizer), and at 4 days post-infection, mice were sacrificed, and livers were removed. The organs were weight adjusted and homogenized in PBS, and serial dilutions of the homogenates were plated on blood agar plates to enumerate the cfu.
Cloning and expression of PtpA in D. discoideum
For S. aureus PtpA-C8S overexpression in D. discoideum, the coding sequence of PtpA-C8S was amplified by PCR using the set of primer Nter_PtpA_flag_C8S_IF_Bam and Cter_PtpAflag_Xho_pDXA (Table 2). The amplified product was digested with BamHI and XhoI restriction enzymes and cloned into D. discoideum expression vector pDXA vector (63). The pDXA_PtpA-C8S-Flag plasmid was transfected in D. discoideum cells as described (64).
Cloning, expression, and purification of recombinant PtpA derivatives
GST-PtpA recombinant protein was obtained by cloning the ptpA fragment generated by PCR using S. aureus N315 genomic DNA as a template with the primers Nterm PtpA Bam_pGEX and Cterm PtpA Hind_pGEX (Table 2) into the BamHI-HindIII–digested pGEX vector. PtpA_C8S_His harboring cysteine to serine substitution was generated by using the QuikChange Site-directed Mutagenesis Kit (Agilent Technologies) on pETPhos_PtpA template (34) using the primer Nterm PtpA_C8S (Table 2) thus generating pETPhos_PtpA_C8S. PtpA-His and PtpA-GST derivatives were purified as previously described (34, 65).
Cloning, expression, and purification of coronin derivatives
The coding sequence of the D. discoideum CorA homolog was amplified by PCR from D. discoideum genomic DNA using the appropriate primers introducing BamHI and XhoI restriction sites, respectively (Table 2). The PCR product was digested with BamHI and XhoI, and ligated into the D. discoideum expression vector pDXA that was digested with the same restriction enzymes. The pDXA 3xmyc-CorA plasmid (pFL1290) was linearized with ScaI and transfected in D. discoideum strain DH1–10. Cells were grown at 22 °C in HL5 medium as previously described (64). Human coronin-1A (Hs-Coro-1A) (gi: 300934762, NP_001180262.1) was synthesized, codon optimized for bacterial production by GenScript, and cloned into pUC57 vector. The coding sequence was amplified by PCR from pUC57 vector, digested by NcoI-HindIII restriction enzymes, and ligated into pCDFDuet-GSTCter-up1 expression vector, thus generating pCDFDuet-Coro-1A-GST. The construct was verified by DNA sequencing. For pulldown assays, lysates of BL21 Star expressing GST-Hs-coronin-1A or GST alone were prepared as follows. Transformed E. coli BL21 Star cells with human coronin-1A codon optimized were grown at 16 °C in LB medium containing 1 g/liter of glucose and 50 μg/ml of spectinomycin and protein synthesis induced with 0.5 mm isopropyl 1-thio-β-d-galactopyranoside overnight. Bacteria expressing GST-Hs-coronin-1A or GST alone were disrupted by sonication (Branson, digital sonifier) and centrifuged at 14,000 rpm for 25 min. Protein concentration was determined using BCA reagent (ThermoFisher Scientific, France).
Macrophage culture and infection
The murine macrophage cell line RAW 264.7 (mouse leukemic monocyte macrophage, ATCC TIB-71) was cultured in Dulbecco's modified Eagle's medium (ThermoFisher Scientific, France) supplemented with 10% fetal calf serum (ThermoFisher Scientific, France) in a humidified atmosphere at 5% CO2 at 37 °C. For macrophage infection, S. aureus Newman and SA564 strains were grown to the midexponential growth phase (A600 = 0.7–0.9) in TSB medium. The bacteria were then collected by centrifugation at 10,000 rpm for 5 min and resuspended in sterile PBS. The RAW 264.7 cells (5 × 105 cells/ml, in 24-well plates) were inoculated with S. aureus at the m.o.i. of 20:1 (bacteria:cells) and incubated at 37 °C and 5% CO2 for the indicated time. Subsequently, cells were washed once with PBS and the remaining extracellular bacteria were killed by incubation with gentamicin (100 μg/ml) for 30 min. After gentamicin treatment, macrophages were rinsed twice with PBS (T0), and then further incubated in Dulbecco's modified Eagle's medium containing 5 μg/ml of lysostaphin for 45 min and 22 h, respectively. Enumeration of intracellular bacteria was performed by lysing infected macrophages with 0.1% Triton X-100 in PBS. Macrophages lysates were serially diluted and plated on TSB agar plates that were subsequently cultivated at 37 °C for 16 h. The survival rate of bacteria was defined as follows: number of bacterial colonies at time post gentamicin/number of bacterial colonies at T0 × 100%.
Immunoprecipitation of PtpA from D. discoideum
For immunoprecipitations, 2 × 107 D. discoideum cells expressing flagged PtpA (PtpA_FLAG), were lysed in lysis buffer (50 mm Tris-HCl, pH 7.5, 300 mm NaCl, 0.5% Nonidet P-40, protease inhibitors (Roche Applied Science), and cleared by centrifugation for 15 min at 14,000 rpm. Lysate supernatants were incubated overnight at 4 °C with a monoclonal anti-FLAG antibody coated on agarose beads (Genscript). The beads were then washed five times in wash buffer (50 mm Tris-HCl, pH 7.5, 300 mm NaCl, 0.1% Nonidet P-40) and once in PBS. Bound proteins were migrated on SDS-PAGE and analyzed by immunoblotting or MS when required.
PtpA phosphatase activity assay
Phosphatase activity of PtpA-wt and -C8S His-tagged recombinant proteins was determined using p-nitrophenylphosphate as chromogenic substrate as previously published (34).
Immunoprecipitation of Coro-1A from infected macrophages
Murine macrophage coronin-1A (Mm_Coro-1A) was obtained from S. aureus-infected RAW 264.7 macrophage extracts by immunoprecipitation with anti-Coro-1A antibody (5 μg, rabbit anti-coronin-1A; SAB4200078, Sigma). Briefly, the anti-Coro-1A antibody was first coupled to 50 μl of Dynabeads-Protein G (10003D, Life Technologies) in a rotator for 10 min at room temperature and then covalently cross-linked using 5 mm BS3 (21580, Thermo Fisher Scientific) for 30 min at room temperature. Infected macrophages (m.o.i. = 20) were lysed at different time points pGt in 0.1% Triton X-100 in PBS containing a protease inhibitor mixture (complete EDTA-free protease inhibitor mixture, Roche). Antibody-coupled beads were added to these lysates and incubated in a rotator for 1 h at room temperature. Thereafter, the beads were washed twice in PBS, 0.02% Tween 20 and 3 times in phosphatase buffer (Tris-HCl 20 mm, pH 7.5, MgCl2 5 mm, DTT 5 mm). Immunoprecipitated samples were washed 3 times in PBS, 0.02% Tween 20 followed by elution in Laemmli sample buffer and analyzed by SDS-PAGE and immunoblotting using the mouse anti-phosphotyrosine (clone 4G10, Millipore) or the rabbit anti-coronin-1A antibodies.
In vitro dephosphorylation of Mm_Coro-1A
Immunoprecipitated Mm_Coro-1A from infected macrophages was obtained as described above. To examine if phosphorylated Mm_Coro-1A is a suitable substrate of PtpA, equal amounts of beads were incubated with 2 μg of purified PtpA. After 30 min and 1 h at 37 °C at 650 rpm, respectively, the reaction was stopped by the addition of sample buffer and proteins bound to the beads were resolved in a 4–20% SDS-PAGE. Mm_Coro-1A contents and its phosphorylation status were revealed by immunoblotting using anti-Coro-1A and anti-phosphotyrosine antibodies on the same blot.
Pulldown assays
For GST pulldown assays, GST-PtpA fusion proteins were produced as described above and bound to GSH-Sepharose 4B beads according to manufacturer's instructions (GE Heathcare). To prepare cell lysates, D. discoideum cells, expressing 3xmyc-Dd_Coro-A (2 × 107) were incubated 15 min in lysis buffer (20 mm HEPES buffer, pH 7.0, 100 mm NaCl, 5 mm MgCl2, 1% Triton X-100; complete protease inhibitor mixture, Roche) and centrifuged for 15 min at 14,000 rpm in a refrigerated microcentrifuge. GST beads were then incubated with cell lysates (800 μg) overnight at 4 °C on a wheel. After three washes in lysis buffer, beads were heated at 95 °C for 10 min. Bound proteins were separated by SDS-PAGE and transferred to nitrocellulose before incubation with an anti-myc antibody (clone 9E10, Sigma). Blots were revealed with the Odyssey Western Detection System (Bio-Rad).
For His-pulldown assays, His-tagged PtpA fusion proteins were produced as described (34). Equal amounts of PtpA_His Ni-NTA beads were incubated with BL21 Star lysates expressing GST-Hs Coro-1A or GST alone for 30 min at 4 °C with gentle agitation in coupling buffer (HEPES 20 mm, NaCl 100 mm, MgCl2 5 mm, Nonidet P-40 0.5%, glycerol 10%, imidazole 10 mm, pH 7.4). In parallel, as a control for unspecific binding, the same amount of Ni-NTA without immobilized PtpA was incubated in the same buffer with both lysates. For each Coro-1A assay, 100 μl of the matrix with or without immobilized PtpA was incubated with 5 mg of BL21 Star lysates diluted to 1 mg/ml. The matrix was collected by low speed centrifugation and then washed three times with the coupling buffer containing 50 mm imidazole. Proteins bound to the beads were recovered by the treatment with 40 μl of Laemmli sample buffer at 95 °C for 5 min. Samples were then resolved on SDS-PAGE gel and subjected to immunoblotting with anti-GST and anti-Coro-1A antibodies, respectively.
Statistical analyses
Statistical significance was assessed using the Mann-Whitney U test. p values <0.05 were considered significant.
Author contributions
L. G.-Z., S. H.-B., M. B., and V. M. conceptualization; L. G.-Z., M. B., and V. M. data curation; L. G.-Z., L. P., S. H.-B., R. G., M. M., M. B., and V. M. formal analysis; L. G.-Z., M. B., and V. M. supervision; L. G.-Z., M. B., and V. M. validation; L. G.-Z., L. P., S. H.-B., G. B., M. I. E., R. G., M. M., A.-B. B.-P., F. L., M. B., and V. M. investigation; L. G.-Z. visualization; L. G.-Z., S. H.-B., G. B., M. I. E., R. G., M. B., and V. M. methodology; L. G.-Z., M. B., and V. M. project administration; L. G.-Z., L. P., S. H.-B., M. I. E., R. G., M. M., A.-B. B.-P., F. L., M. B., and V. M. writing-review and editing; G. B. and V. M. writing-original draft; M. B. software; V. M. resources; V. M. funding acquisition.
Supplementary Material
Acknowledgment
We thank Tim Foster, Dublin, for providing strain IM08B.
This work was supported by grants from the ATIP/AVENIR Program (to V. M. and L. G.-Z.). The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1–S3.
- LMW
- low-molecular-weight
- PTP
- protein-tyrosine phosphatase
- VPS
- vacuolar protein sorting
- pGt
- post-gentamicin treatment
- GFP
- green fluorescent protein
- GST
- glutathione S-transferase
- CorA
- coronin-A
- Ni-NTA
- nickel-nitrilotriacetic acid
- TSB
- tryptic soy broth
- cfu
- colony forming unit
- m.o.i.
- multiplicity of infection.
References
- 1. Bischoff M. R., and Romby P. (2016) Genetic regulation in Staphylococcus: Genetics and Physiology (Somerville G. A. S., ed) pp. 301–334, Caister Academic Press, Wymondham, United Kingdom [Google Scholar]
- 2. Schlievert P. M. (2016) Staphylococcal virulence factors in Staphylococcus: Genetics and Physiology (Somerville G. A. S., ed) pp. 81–106, Caister Academic Press, Wymondham, United Kingdom [Google Scholar]
- 3. Lowy F. D. (2000) Is Staphylococcus aureus an intracellular pathogen? Trends Microbiol. 8, 341–343 10.1016/S0966-842X(00)01803-5 [DOI] [PubMed] [Google Scholar]
- 4. Voyich J. M., Braughton K. R., Sturdevant D. E., Whitney A. R., Saïd-Salim B., Porcella S. F., Long R. D., Dorward D. W., Gardner D. J., Kreiswirth B. N., Musser J. M., and DeLeo F. R. (2005) Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J. Immunol. 175, 3907–3919 10.4049/jimmunol.175.6.3907 [DOI] [PubMed] [Google Scholar]
- 5. Kubica M., Guzik K., Koziel J., Zarebski M., Richter W., Gajkowska B., Golda A., Maciag-Gudowska A., Brix K., Shaw L., Foster T., and Potempa J. (2008) A potential new pathway for Staphylococcus aureus dissemination: the silent survival of S. aureus phagocytosed by human monocyte-derived macrophages. PLoS ONE 3, e1409 10.1371/journal.pone.0001409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koziel J., Maciag-Gudowska A., Mikolajczyk T., Bzowska M., Sturdevant D. E., Whitney A. R., Shaw L. N., DeLeo F. R., and Potempa J. (2009) Phagocytosis of Staphylococcus aureus by macrophages exerts cytoprotective effects manifested by the upregulation of antiapoptotic factors. PLoS ONE 4, e5210 10.1371/journal.pone.0005210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Horn J., Stelzner K., Rudel T., and Fraunholz M. (2018) Inside job: Staphylococcus aureus host-pathogen interactions. Int. J. Med. Microbiol. 308, 607–624 [DOI] [PubMed] [Google Scholar]
- 8. Fraunholz M., and Sinha B. (2012) Intracellular Staphylococcus aureus: live-in and let die. Front. Cell. Infect. Microbiol. 2, 43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hamza T., and Li B. (2014) Differential responses of osteoblasts and macrophages upon Staphylococcus aureus infection. BMC Microbiol. 14, 207 10.1186/s12866-014-0207-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Surewaard B. G., Deniset J. F., Zemp F. J., Amrein M., Otto M., Conly J., Omri A., Yates R. M., and Kubes P. (2016) Identification and treatment of the Staphylococcus aureus reservoir in vivo. J. Exp. Med. 213, 1141–1151 10.1084/jem.20160334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tranchemontagne Z. R., Camire R. B., O'Donnell V. J., Baugh J., and Burkholder K. M. (2016) Staphylococcus aureus strain USA300 perturbs acquisition of lysosomal enzymes and requires phagosomal acidification for survival inside macrophages. Infect. Immun. 84, 241–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jubrail J., Morris P., Bewley M. A., Stoneham S., Johnston S. A., Foster S. J., Peden A. A., Read R. C., Marriott H. M., and Dockrell D. H. (2016) Inability to sustain intraphagolysosomal killing of Staphylococcus aureus predisposes to bacterial persistence in macrophages. Cell Microbiol. 18, 80–96 10.1111/cmi.12485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koziel J., Chmiest D., Bryzek D., Kmiecik K., Mizgalska D., Maciag-Gudowska A., Shaw L. N., and Potempa J. (2015) The Janus face of α-toxin: a potent mediator of cytoprotection in staphylococci-infected macrophages. J. Innate Immun. 7, 187–198 10.1159/000368048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alto N. M., and Orth K. (2012) Subversion of cell signaling by pathogens. Cold Spring Harb. Perspect. Biol. 4, a006114, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cornejo E., Schlaermann P., and Mukherjee S. (2017) How to rewire the host cell: a home improvement guide for intracellular bacteria. J. Cell Biol. 216, 3931–3948 10.1083/jcb.201701095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Heneberg P. (2012) Finding the smoking gun: protein tyrosine phosphatases as tools and targets of unicellular microorganisms and viruses. Curr. Med. Chem. 19, 1530–1566 10.2174/092986712799828274 [DOI] [PubMed] [Google Scholar]
- 17. Wong D., Chao J. D., and Av-Gay Y. (2013) Mycobacterium tuberculosis-secreted phosphatases: from pathogenesis to targets for TB drug development. Trends Microbiol. 21, 100–109 [DOI] [PubMed] [Google Scholar]
- 18. Whitmore S. E., and Lamont R. J. (2012) Tyrosine phosphorylation and bacterial virulence. Int. J. Oral Sci. 4, 1–6 10.1038/ijos.2012.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cozzone A. J. (2005) Role of protein phosphorylation on serine/threonine and tyrosine in the virulence of bacterial pathogens. J. Mol. Microbiol. Biotechnol. 9, 198–213 10.1159/000089648 [DOI] [PubMed] [Google Scholar]
- 20. Bach H., Papavinasasundaram K. G., Wong D., Hmama Z., and Av-Gay Y. (2008) Mycobacterium tuberculosis virulence is mediated by PtpA dephosphorylation of human vacuolar protein sorting 33B. Cell Host Microbe 3, 316–322 10.1016/j.chom.2008.03.008 [DOI] [PubMed] [Google Scholar]
- 21. Bliska J. B. (2000) Yop effectors of Yersinia spp., and actin rearrangements. Trends Microbiol. 8, 205–208 10.1016/S0966-842X(00)01738-8 [DOI] [PubMed] [Google Scholar]
- 22. Black D. S., Montagna L. G., Zitsmann S., and Bliska J. B. (1998) Identification of an amino-terminal substrate-binding domain in the Yersinia tyrosine phosphatase that is required for efficient recognition of focal adhesion targets. Mol. Microbiol. 29, 1263–1274 10.1046/j.1365-2958.1998.01014.x [DOI] [PubMed] [Google Scholar]
- 23. Persson C., Nordfelth R., Andersson K., Forsberg A., Wolf-Watz H., and Fällman M. (1999) Localization of the Yersinia PTPase to focal complexes is an important virulence mechanism. Mol. Microbiol. 33, 828–838 10.1046/j.1365-2958.1999.01529.x [DOI] [PubMed] [Google Scholar]
- 24. Persson C., Carballeira N., Wolf-Watz H., and Fällman M. (1997) The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130Cas and FAK, and the associated accumulation of these proteins in peripheral focal adhesions. EMBO J. 16, 2307–2318 10.1093/emboj/16.9.2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lin S. L., Le T. X., and Cowen D. S. (2003) SptP, a Salmonella typhimurium type III-secreted protein, inhibits the mitogen-activated protein kinase pathway by inhibiting Raf activation. Cell Microbiol. 5, 267–275 10.1046/j.1462-5822.2003.t01-1-00274.x [DOI] [PubMed] [Google Scholar]
- 26. Kaniga K., Uralil J., Bliska J. B., and Galan J. E. (1996) A secreted protein tyrosine phosphatase with modular effector domains in the bacterial pathogen Salmonella typhimurium. Mol. Microbiol. 21, 633–641 10.1111/j.1365-2958.1996.tb02571.x [DOI] [PubMed] [Google Scholar]
- 27. Koul A., Choidas A., Treder M., Tyagi A. K., Drlica K., Singh Y., and Ullrich A. (2000) Cloning and characterization of secretory tyrosine phosphatases of Mycobacterium tuberculosis. J. Bacteriol. 182, 5425–5432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wong D., Bach H., Sun J., Hmama Z., and Av-Gay Y. (2011) Mycobacterium tuberculosis protein tyrosine phosphatase (PtpA) excludes host vacuolar-H+-ATPase to inhibit phagosome acidification. Proc. Natl. Acad. Sci. U.S.A. 108, 19371–19376 10.1073/pnas.1109201108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Singh B., Singh G., Trajkovic V., and Sharma P. (2003) Intracellular expression of Mycobacterium tuberculosis-specific 10-kDa antigen down-regulates macrophage B7.1 expression and nitric oxide release. Clin. Exp. Immunol. 134, 70–77 10.1046/j.1365-2249.2003.02258.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Flannagan R. S., Heit B., and Heinrichs D. E. (2015) Antimicrobial mechanisms of macrophages and the immune evasion strategies of Staphylococcus aureus. Pathogens 4, 826–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Soulat D., Vaganay E., Duclos B., Genestier A. L., Etienne J., and Cozzone A. J. (2002) Staphylococcus aureus contains two low-molecular-mass phosphotyrosine protein phosphatases. J. Bacteriol. 184, 5194–5199 10.1128/JB.184.18.5194-5199.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gemayel R., Fortpied J., Rzem R., Vertommen D., Veiga-da-Cunha M., and Van Schaftingen E. (2007) Many fructosamine 3-kinase homologues in bacteria are ribulosamine/erythrulosamine 3-kinases potentially involved in protein deglycation. FEBS J. 274, 4360–4374 10.1111/j.1742-4658.2007.05948.x [DOI] [PubMed] [Google Scholar]
- 33. Vega C., Chou S., Engel K., Harrell M. E., Rajagopal L., and Grundner C. (2011) Structure and substrate recognition of the Staphylococcus aureus protein-tyrosine phosphatase PtpA. J. Mol. Biol. 413, 24–31 10.1016/j.jmb.2011.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brelle S., Baronian G., Huc-Brandt S., Zaki L. G., Cohen-Gonsaud M., Bischoff M., and Molle V. (2016) Phosphorylation-mediated regulation of the Staphylococcus aureus secreted tyrosine phosphatase PtpA. Biochem. Biophys. Res. Commun. 469, 619–625 10.1016/j.bbrc.2015.11.123 [DOI] [PubMed] [Google Scholar]
- 35. Hingley-Wilson S. M., Sambandamurthy V. K., and Jacobs W. R. Jr. (2003) Survival perspectives from the world's most successful pathogen, Mycobacterium tuberculosis. Nat. Immunol. 4, 949–955 10.1038/ni981 [DOI] [PubMed] [Google Scholar]
- 36. Clemens D. L., and Horwitz M. A. (1995) Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J. Exp. Med. 181, 257–270 10.1084/jem.181.1.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gaupp R., Wirf J., Wonnenberg B., Biegel T., Eisenbeis J., Graham J., Herrmann M., Lee C. Y., Beisswenger C., Wolz C., Tschernig T., Bischoff M., and Somerville G. A. (2016) RpiRc is a pleiotropic effector of virulence determinant synthesis and attenuates pathogenicity in Staphylococcus aureus. Infect. Immun. 84, 2031–2041 10.1128/IAI.00285-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bliska J. B., Clemens J. C., Dixon J. E., and Falkow S. (1992) The Yersinia tyrosine phosphatase: specificity of a bacterial virulence determinant for phosphoproteins in the J774A.1 macrophage. J. Exp. Med. 176, 1625–1630 10.1084/jem.176.6.1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barford D., Das A. K., and Egloff M. P. (1998) The structure and mechanism of protein phosphatases: insights into catalysis and regulation. Annu. Rev. Biophys. Biomol. Struct. 27, 133–164 10.1146/annurev.biophys.27.1.133 [DOI] [PubMed] [Google Scholar]
- 40. Dennis J. W., Granovsky M., and Warren C. E. (1999) Protein glycosylation in development and disease. Bioessays 21, 412–421 10.1002/(SICI)1521-1878(199905)21:5%3C412::AID-BIES8%3E3.0.CO%3B2-5 [DOI] [PubMed] [Google Scholar]
- 41. Lauffenburger D. A., and Horwitz A. F. (1996) Cell migration: a physically integrated molecular process. Cell 84, 359–369 10.1016/S0092-8674(00)81280-5 [DOI] [PubMed] [Google Scholar]
- 42. Wienke D., Drengk A., Schmauch C., Jenne N., and Maniak M. (2006) Vacuolin, a flotillin/reggie-related protein from Dictyostelium oligomerizes for endosome association. Eur. J. Cell Biol. 85, 991–1000 10.1016/j.ejcb.2006.04.010 [DOI] [PubMed] [Google Scholar]
- 43. Diment S., Martin K. J., and Stahl P. D. (1989) Cleavage of parathyroid hormone in macrophage endosomes illustrates a novel pathway for intracellular processing of proteins. J. Biol. Chem. 264, 13403–13406 [PubMed] [Google Scholar]
- 44. Seaman M. N., and Williams H. P. (2002) Identification of the functional domains of yeast sorting nexins Vps5p and Vps17p. Mol. Biol. Cell 13, 2826–2840 10.1091/mbc.02-05-0064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ferrari G., Langen H., Naito M., and Pieters J. (1999) A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell 97, 435–447 10.1016/S0092-8674(00)80754-0 [DOI] [PubMed] [Google Scholar]
- 46. Jayachandran R., Sundaramurthy V., Combaluzier B., Mueller P., Korf H., Huygen K., Miyazaki T., Albrecht I., Massner J., and Pieters J. (2007) Survival of mycobacteria in macrophages is mediated by coronin L-dependent activation of calcineurin. Cell 130, 37–50 10.1016/j.cell.2007.04.043 [DOI] [PubMed] [Google Scholar]
- 47. Jayachandran R., Gatfield J., Massner J., Albrecht I., Zanolari B., and Pieters J. (2008) RNA interference in J774 macrophages reveals a role for coronin 1 in mycobacterial trafficking but not in actin-dependent processes. Mol. Biol. Cell 19, 1241–1251 10.1091/mbc.e07-07-0640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kumar D., Nath L., Kamal M. A., Varshney A., Jain A., Singh S., and Rao K. V. (2010) Genome-wide analysis of the host intracellular network that regulates survival of Mycobacterium tuberculosis. Cell 140, 731–743 [DOI] [PubMed] [Google Scholar]
- 49. Seto S., Tsujimura K., and Koide Y. (2012) Coronin-1a inhibits autophagosome formation around Mycobacterium tuberculosis-containing phagosomes and assists mycobacterial survival in macrophages. Cell Microbiol. 14, 710–727 10.1111/j.1462-5822.2012.01754.x [DOI] [PubMed] [Google Scholar]
- 50. Bosedasgupta S., and Pieters J. (2014) Inflammatory stimuli reprogram macrophage phagocytosis to macropinocytosis for the rapid elimination of pathogens. PLoS Pathog. 10, e1003879 10.1371/journal.ppat.1003879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Itoh S., Suzuki K., Nishihata J., Iwasa M., Oku T., Nakajin S., Nauseef W. M., and Toyoshima S. (2002) The role of protein kinase C in the transient association of p57, a coronin family actin-binding protein, with phagosomes. Biol. Pharmaceut. Bull. 25, 837–844 [DOI] [PubMed] [Google Scholar]
- 52. Oku T., Nakano M., Kaneko Y., Ando Y., Kenmotsu H., Itoh S., Tsuiji M., Seyama Y., Toyoshima S., and Tsuji T. (2012) Constitutive turnover of phosphorylation at Thr-412 of human p57/coronin-1 regulates the interaction with actin. J. Biol. Chem. 287, 42910–42920 10.1074/jbc.M112.349829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang J., Ge P., Qiang L., Tian F., Zhao D., Chai Q., Zhu M., Zhou R., Meng G., Iwakura Y., Gao G. F., and Liu C. H. (2017) The mycobacterial phosphatase PtpA regulates the expression of host genes and promotes cell proliferation. Nat. Commun. 8, 244 10.1038/s41467-017-00279-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang J., Teng J. L., Zhao D., Ge P., Li B., Woo P. C., and Liu C. H. (2016) The ubiquitin ligase TRIM27 functions as a host restriction factor antagonized by Mycobacterium tuberculosis PtpA during mycobacterial infection. Sci. Rep. 6, 34827 10.1038/srep34827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lee Y. G., Chain B. M., and Cho J. Y. (2009) Distinct role of spleen tyrosine kinase in the early phosphorylation of inhibitor of κBα via activation of the phosphoinositide-3-kinase and Akt pathways. Int. J. Biochem. Cell Biol. 41, 811–821 10.1016/j.biocel.2008.08.011 [DOI] [PubMed] [Google Scholar]
- 56. Yi Y. S., Son Y. J., Ryou C., Sung G. H., Kim J. H., and Cho J. Y. (2014) Functional roles of Syk in macrophage-mediated inflammatory responses. Mediators Inflamm. 2014, 270302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bohnenberger H., Oellerich T., Engelke M., Hsiao H. H., Urlaub H., and Wienands J. (2011) Complex phosphorylation dynamics control the composition of the Syk interactome in B cells. Eur. J. Immunol. 41, 1550–1562 10.1002/eji.201041326 [DOI] [PubMed] [Google Scholar]
- 58. Nguyen L., and Pieters J. (2005) The Trojan horse: survival tactics of pathogenic mycobacteria in macrophages. Trends Cell Biol. 15, 269–276 10.1016/j.tcb.2005.03.009 [DOI] [PubMed] [Google Scholar]
- 59. BoseDasgupta S., and Pieters J. (2014) Coronin 1 trimerization is essential to protect pathogenic mycobacteria within macrophages from lysosomal delivery. FEBS Lett. 588, 3898–3905 10.1016/j.febslet.2014.08.036 [DOI] [PubMed] [Google Scholar]
- 60. Somerville G. A., Beres S. B., Fitzgerald J. R., DeLeo F. R., Cole R. L., Hoff J. S., and Musser J. M. (2002) In vitro serial passage of Staphylococcus aureus: changes in physiology, virulence factor production, and agr nucleotide sequence. J. Bacteriol. 184, 1430–1437 10.1128/JB.184.5.1430-1437.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bruckner R. (1997) Gene replacement in Staphylococcus carnosus and Staphylococcus xylosus. FEMS Microbiol. Lett. 151, 1–8 10.1016/S0378-1097(97)00116-X [DOI] [PubMed] [Google Scholar]
- 62. Monk I. R., Tree J. J., Howden B. P., Stinear T. P., and Foster T. J. (2015) Complete bypass of restriction systems for major Staphylococcus aureus lineages. mBio 6, e00308–00315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Manstein D. J., Schuster H. P., Morandini P., and Hunt D. M. (1995) Cloning vectors for the production of proteins in Dictyostelium discoideum. Gene 162, 129–134 [DOI] [PubMed] [Google Scholar]
- 64. Alibaud L., Cosson P., and Benghezal M. (2003) Dictyostelium discoideum transformation by oscillating electric field electroporation. BioTechniques 35, 78–80 [DOI] [PubMed] [Google Scholar]
- 65. Molle V., Kremer L., Girard-Blanc C., Besra G. S., Cozzone A. J., and Prost J. F. (2003) An FHA phosphoprotein recognition domain mediates protein EmbR phosphorylation by PknH, a Ser/Thr protein kinase from Mycobacterium tuberculosis. Biochemistry 42, 15300–15309 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.