Abstract
The β3‐adrenoceptor was initially an attractive target for several pharmaceutical companies due to its high expression in rodent adipose tissue, where its activation resulted in decreased adiposity and improved metabolic outputs (such as glucose handling) in animal models of obesity and Type 2 diabetes. However, several drugs acting at the β3‐adrenoceptor failed in clinical trials. This was thought to be due to their lack of efficacy at the human receptor. Recently, mirabegron, a β3‐adrenoceptor agonist with human efficacy, was approved in North America, Europe, Japan and Australia for the treatment of overactive bladder syndrome. There are indications that mirabegron may act at other receptors/targets, but whether they have any clinical relevance is relatively unknown. Besides overactive bladder syndrome, mirabegron may have other uses such as in the treatment of heart failure or metabolic disease. This review gives an overview of the off‐target effects of mirabegron and its potential use in the treatment of other diseases.
Linked Articles
This article is part of a themed section on Molecular Pharmacology of GPCRs. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v175.21/issuetoc
Abbreviations
- BAT
brown adipose tissue
- CYP
cytochrome P450
- OAB
overactive bladder
- OCT
organic cation transporter
- PET
positron emission tomography
- UCP1
uncoupling protein‐1
Introduction
http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=694&familyType=GPCR are attractive pharmacological targets and are highly druggable due to their cell surface location. Drugs targeting them make up ~30% of the current pharmaceutical market, despite comprising only ~4% of the human genome (or ~800 genes). One important GPCR family are the β‐adrenoceptors, which comprise three subtypes: http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=28&familyId=4&familyType=GPCR‐, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=29&familyId=4&familyType=GPCR‐ and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=30&familyId=4&familyType=GPCR. While there are numerous drugs (both agonists and antagonists) on the market acting at β1‐ and β2‐adrenoceptors, the therapeutic potential of the β3‐adrenoceptor has only recently been shown.
The β3‐adrenoceptor is a 7 transmembrane GPCR, with an extracellular N‐terminal tail and an intracellular C‐terminal tail, comprising 408 amino acids for the human β3‐adrenoceptor, but 400 amino acids for the mouse β3‐adrenoceptor. It primarily couples to Gs to activate http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=257#1279, resulting in increased intracellular levels of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2352, although promiscuous coupling to other effectors such as Gi have been reported. It is distinct from the β1‐ and β2‐adrenoceptors in several aspects: its gene contains an intron with the second exon encoding the last 7 (human) or 13 (mouse) amino acids; and it is resistant to agonist promoted desensitization due to a lack of phosphorylation sites in its C‐terminal tail. The mouse β3‐adrenoceptor gene also undergoes alternative splicing that results in the generation of a splice variant, termed the β3b‐adrenoceptor (Evans et al., 1999), that exhibits a similar pharmacological profile as the mouse β3a‐adrenoceptor but can couple to both Gs and Gi proteins (Hutchinson et al., 2002). The β3‐adrenoceptor was originally identified from the lack of effect of several β‐adrenoceptor antagonists on β‐adrenoceptor‐mediated relaxation of the gastrointestinal tract and β‐adrenoceptor‐mediated lipolysis (see Arch and Kaumann, 1993). This led to the identification of the first series of selective β3‐adrenoceptor agonists (such as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=567) that were more potent at increasing adipose tissue lipolysis versus atrial contraction or tracheal/uterine relaxation (Arch et al., 1984). Because of these activities, the β3‐adrenoceptor was a target for several pharmaceutical companies as an anti‐obesity and anti‐diabetic agent. These programs failed in clinical trials, as these compounds (such as BRL37344), while being active at the rodent β3‐adrenoceptor, lacked efficacy at the human receptor, although they can have some effects at the human β3‐adrenoceptor when the receptor is overexpressed (Wilson et al., 1996). This is thought to be due to subtle differences in the ligand‐binding pocket of the rodent versus human β3‐adrenoceptor, lack of bioavailability and the differential pattern of expression of the β3‐adrenoceptor between rodents and humans. In addition to its roles in adipose tissue (thermogenesis and lipolysis), this receptor has demonstrated roles in the brain (memory, learning and regulation of appetite), heart (cardioprotection), throughout the gastrointestinal tract (regulation of motility) and the genitourinary system (regulation of bladder function). A brief overview of the structure, agonist/antagonist profile and roles for the β3‐adrenoceptor is shown in Figure 1.
Figure 1.
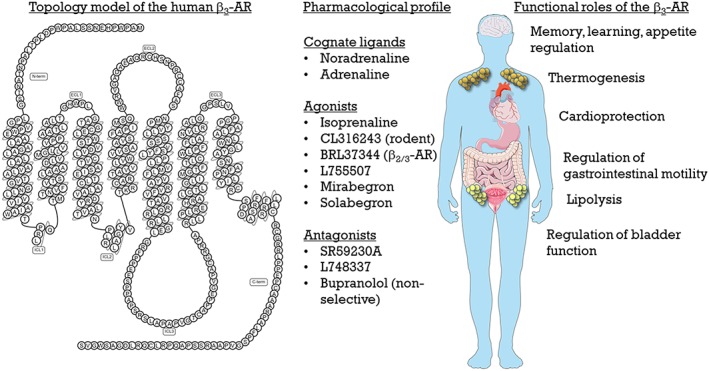
Structure, pharmacology and roles of the β3‐adrenoceptor (AR).
Several newer human‐selective, or at least human‐potent, β3‐adrenoceptor agonists have been described, with most of their development being carried out in transfected cell lines expressing the human β3‐adrenoceptor to determine potency and affinity. http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7445 is a highly selective β3‐adrenoceptor agonist. In CHO‐K1 cells expressing the human or monkey β1‐ or β2‐adrenoceptor, it fails to increase cAMP levels, whereas in CHO‐K1 cells expressing the human or monkey β3‐adrenoceptor, it increases cAMP levels with a potency and efficacy nearly identical to that of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=536 (Takasu et al., 2007; Hatanaka et al., 2013a,b). While mirabegron was a partial agonist for cAMP accumulation at the cloned human (intrinsic activity 0.8; Takasu et al., 2007; Hatanaka et al., 2013b) or cynomolgus monkey β3‐adrenoceptors (intrinsic activity 0.8; Hatanaka et al., 2013a) compared to isoprenaline, this may not explain fully its activity in detrusor muscle in vitro or in vivo, as in parallel experiments, mirabegron was a full agonist for relaxation of human or cynomolgus monkey detrusor muscle (Hatanaka et al., 2013a,b). Studies of CHO‐K1 cells transfected with the rat β‐adrenoceptors showed that mirabegron has no agonist activity at the β2‐adrenoceptor, has weak low affinity partial agonist activity at the β1‐adrenoceptor, and is a full agonist at the β3‐adrenoceptor (Hatanaka et al., 2013b).
As a result, several β3‐adrenoceptor agonists have entered clinical trials, including ritobegron (Kissei Pharmaceutical Co Ltd; Phase 3 terminated) and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=9512 (Velicept Therapeutics Inc; Phase 2 on‐going). Mirabegron (Astellas Pharma Inc) was approved for the treatment of overactive bladder (OAB) syndrome via relaxation of detrusor smooth, firstly in Japan (2011), followed by other countries in Europe, North America and Australia (2014). The standard dosing for mirabegron is initially 25 mg orally once a day, although this can be increased to 50 mg based on a patients' tolerability and efficacy. A summary of the pharmacology, pharmacokinetics and uses for mirabegron is shown in Figure 2.
Figure 2.
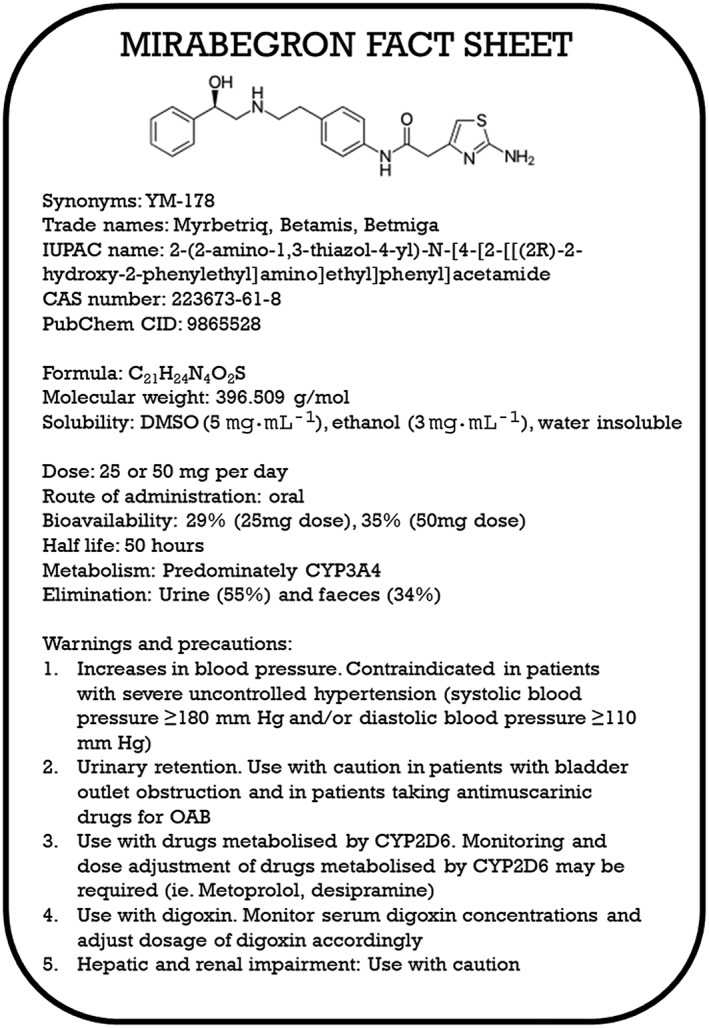
Brief pharmacological profile, approved use, warnings and precautions for mirabegron.
Mirabegron for the treatment of overactive bladder
OAB syndrome is characterized by an increase in the frequency and urgency of urination, with or without urge incontinence. The main therapeutic treatment for OAB are http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=2 antagonists that include http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7483, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=359, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=360, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7480, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=321 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7473. While effective in the treatment of OAB, their side effects (such as dry mouth, constipation, voiding dysfunction and cognitive dysfunction especially in the elderly) often leads to discontinuation of treatment, and their use is also contraindicated with other diseases such as glaucoma. Hence, a non‐muscarinic target for the treatment of OAB is clearly needed.
The mRNA and protein of the β3‐adrenoceptor has been detected in human bladder smooth muscle by several groups (Fujimura et al., 1999; Igawa et al., 1999; Takeda et al., 1999; Yamaguchi, 2002; Nomiya and Yamaguchi, 2003; Silva et al., 2017). In human detrusor muscle, the β3‐adrenoceptor accounts for 97% of all total β‐adrenoceptor mRNA transcripts (Yamaguchi, 2002; Nomiya and Yamaguchi, 2003), and activation of β3‐adrenoceptors (and not β1‐ or β2‐ adrenoceptors) are responsible for β‐adrenoceptor‐mediated relaxation (Igawa et al., 1999; Takeda et al., 1999). There are several other reviews of the effect of β3‐adrenoceptors on bladder function for further reading (Yamaguchi and Chapple, 2007; Michel and Korstanje, 2016), including reviews on the outcomes of the clinical trials resulting in the approval of mirabegron for the treatment of OAB (Cui et al., 2014; Warren et al., 2016). In brief, mirabegron was found to be an effective treatment for OAB, with improvements in primary end points such as the mean number of incontinence periods per 24 h, and mean number of micturition per 24 h, while also improving several secondary end points, including mean volume voided per micturition, and mean number of urgency episodes per 24 h as compared to placebo. Mirabegron was well tolerated and proposed to have increased patient compliance due to the lack of dry mouth side effects, a common cited reason for non‐compliance when anti‐cholinergics are used. There are relatively few studies directly comparing the efficacy of mirabegron versus anti‐cholinergic drugs. One study (Maman et al., 2014) reviewed 44 randomized controlled trials that studied the efficacy or safety of a wide range of drugs used clinically to treat OAB that included anti‐muscarinic drugs as well as mirabegron. There was no difference in micturition frequency or incontinence episodes between mirabegron and other drugs used to treat OAB; however, solifenacin (as compared to mirabegron) was considered to be the most efficacious for improvement of micturitions in a 24 h period, or urinary urge incontinence. Currently, the American Urological Association recommends anti‐cholinergic drugs or mirabegron as first‐line treatments of OAB, whereas the European Association of Urology recommends anti‐cholinergic drugs as first‐line treatment, with mirabegron to be recommended only in patients with urgency urinary incontinence who have inadequate responses to other treatments.
It was assumed that mirabegron has its effects on urinary incontinence by acting at receptors located on the detrusor muscle of the urinary bladder. However, this assumption is being increasingly questioned, since the maximal plasma levels measured in patients upon therapeutic dosing is 83–167 nM (Krauwinkel et al., 2012), in comparison to its EC50 values that typically range in the high nM to low mM range in human or rodent isolated detrusor muscle (see Michel and Korstanje, 2016). This discrepancy may be due to the pharmacokinetics and the clearance of tissue bound mirabegron. Mirabegron, in adults dosed with 50 mg·day−1 for 7 days, is rapidly absorbed following oral administration, with a Tmax (time to maximal plasma concentration) of 3–4 h and a terminal plasma half‐life of ~60 h (Krauwinkel et al., 2012), of which ~71% is bound to plasma proteins (Department of Health Therapeutic Goods Administration: Australian Public Assessment Report for Mirabegron, 2014). After a single oral dose of [14C]‐mirabegron in rats, the tissue : plasma radioactivity levels are the highest in the alimentary canal and excretory organs (with ratios increasing to 20 in some tissues such as the liver with repeated dosing), and mirabegron shows slow elimination from some tissues including the kidney (Department of Health Therapeutic Goods Administration: Australian Public Assessment Report for Mirabegron, 2014). While this report did not state the tissue : plasma radioactivity levels or elimination rates of [14C]‐mirabegron in the bladder, it is possible that the levels of mirabegron in the bladder are higher than that in the plasma. Coupled with the highly lipophilic chemistry of mirabegron, this could assist in reconciling the differences in the plasma levels reached with therapeutic dosing and the concentrations required to produce effects in human or rodent isolated detrusor.
The β3‐adrenoceptor is also expressed (or mediates responses) in the urothelium, interstitial cells of Cajal, afferent nerves, and the bladder vasculature (reviewed in Michel and Korstanje, 2016). Can the β3‐adrenoceptor in these locations contribute to the actions of mirabegron in the bladder? Or can mirabegron act at other targets/receptors to elicit its effects? For instance, recently mirabegron was shown to be taken up into cardiac sympathetic nerve terminals through noradrenaline transporters, resulting in increased noradrenaline release and subsequent activation of β1‐adrenoceptors (Mo et al., 2017). Whether mirabegron can be taken up into bladder sympathetic nerve terminals to increase noradrenaline release has not been reported or determined.
Because the concentration–response curves to mirabegron in both human and rodent detrusor muscle are typically shallow (Hill slopes less than 1) as compared to isoprenaline‐mediated responses, this may indicate heterogeneity of binding sites or negative cooperativity, which may indicate that mirabegron can act at more than 1 site in detrusor muscle. Whether this occurs from mirabegron's actions at other targets besides the β3‐adrenoceptor, or via action at multiple β3‐adrenoceptor locations in various other cells/tissues in the urinary system (such as pre‐junctional β3‐adrenoceptors in detrusor muscle; Silva et al., 2017), has yet to be determined. This could also occur through oligomerization of the β2‐adrenoceptor with the β3‐adrenoceptor, which has been reported in recombinant HEK293 cells, resulting in a β2/3‐adrenoceptor hetero‐oligomer that displays distinct functional properties compared to the β2‐adrenoceptor or β3‐adrenoceptor alone (Breit et al., 2004). However, the probability of a functional β2/3‐adrenoceptor hetero‐oligomer in detrusor muscle appears remote, due to the lack of β2‐adrenoceptors in detrusor muscle (Igawa et al., 1999; Takeda et al., 1999; Yamaguchi, 2002; Nomiya and Yamaguchi, 2003).
Does mirabegron have a potential role for the treatment of metabolic disorders?
The β3‐adrenoceptor is the predominant β‐adrenoceptor in rodent white and brown adipocytes, where its activation results in increased lipolysis in white adipocytes and increased thermogenesis in brown adipocytes. The effects of β3‐adrenoceptors in adipose tissue has been extensively reviewed previously (Arch and Kaumann, 1993; Cannon and Nedergaard, 2004). Recently, the role of these adrenoceptors in brown adipose tissue (BAT) has regained attention, due to the identification of BAT in adult humans using the glucose derivative [18F]fluoro‐2‐deoxyglucose in positron emission tomography (PET; Nedergaard et al., 2007). The extent of BAT activity is inversely correlated with age, BMI and glycaemic status (Cypess et al., 2009; van Marken Lichtenbelt et al., 2009; Vijgen et al., 2011). This is particularly important as studies in rodents show that increased BAT activity prevents the development and severity of obesity (Arch et al., 1984) and Type 2 diabetes (Kim et al., 2006), while BAT‐deficient mice are obesity‐prone (Lowell et al., 1993). These effects are mainly due to the presence and activation of the BAT‐specific mitochondrial protein http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=207#1066) (Cannon and Nedergaard, 2004). UCP1 generates heat by uncoupling electron transport from ATP production, having the unique ability to dissipate chemical energy stored in triglycerides as heat. To date, no β3‐adrenoceptor agonist has passed beyond Phase II clinical trials for the treatment of metabolic disorders.
There are very few reports of mirabegron acting at metabolically active tissues. One study showed that acute mirabegron administration (0.1 mg·kg−1) to mice increases glucose uptake into BAT, but not into skeletal muscle, perigonadal or inguinal WAT (Roberts‐Toler et al., 2015). In the Astellas report to the Australian TGA, mirabegron was reported to increase lipolysis in rat adipocytes, increase thermogenesis (body temperature) in obese kk/Ay diabetic mice and improve glucose tolerance in Zucker rats. In the same report, Astellas reported that these effects could not be replicated in dogs, monkeys or humans (Department of Health Therapeutic Goods Administration: Australian Public Assessment Report for Mirabegron, 2014) However, these studies have not been published to date.
One recent study (Cypess et al., 2015) using healthy young lean males with detectable BAT activity prior to the trial demonstrated that oral administration of mirabegron (200 mg) increased glucose uptake into BAT and increased the resting metabolic rate in healthy male subjects. Glucose uptake, as measured through [18F]fluoro‐2‐deoxyglucose uptake detected by PET scanning, showed wide distribution of uptake into fat depots in the cervical–supraclavicular–axillary and also paraspinal, periaortic, perihepatic, perirenal and perisplenic regions, suggesting that not only classical BAT was stimulated but also “brite” adipocytes (see below). One main criticism of this study (Cypess et al., 2015) is the dose used (one dose of 200 mg was used which is four times the recommended maximal dose). This dose resulted in adverse cardiac side effects in that cohort of subjects, including a significant increase in heart rate and systolic blood pressure, most likely due to actions at cardiac β‐adrenoceptors. While this study was a proof‐of‐principle study to demonstrate that mirabegron can have metabolic effects in humans, several further studies are now warranted. These include lower dosages of mirabegron that are used clinically and minimize cardiovascular side effects, performing studies in diabetic individuals as opposed to healthy lean individuals and performing studies in diabetic individuals that have lower BAT activity (Orava et al., 2013).
Longer‐term studies using mirabegron in the context of adipose tissue activation are required. A current clinical trial measuring the effectiveness of mirabegron in the prevention of heart failure in individuals with cardiac remodelling with/without heart failure symptoms (clinical http://trials.gov: NCT02599480; completion date early 2020) has a secondary end point in assessing the effects of mirabegron (50 mg daily for 12 months) on various metabolic parameters (fasting glucose, HOMA, HbA1c, serum lipids, maximal exercise capacity and activation of brown/”brite” adipose tissue through PET scanning). Another trial (NCT03049462; completion date mid 2018) is assessing the effect of 200 mg daily (males) or 100 mg daily (females) in healthy individuals for 4 weeks to assess BAT activity (PET scanning) and alterations in body weight, fat mass, glucose tolerance and liver function.
A recent discovery in the context of adipocyte biology is the discovery of “brite” (brown within white) adipocytes (see Merlin et al. (2016) for a review on brite adipocytes). Brite adipocytes are defined as brown‐like adipocytes located predominately in white adipose tissue depots. These cells differ from conventional brown adipocytes, as they develop from a white adipocyte precursor cell and not a brown adipocyte precursor cell. Despite deriving from different precursor cells, both brite and brown adipocytes express UCP1. Importantly, brite adipocytes can be activated by environmental stimuli such as cold exposure and a range of pharmaceutical targets that include the β3‐adrenoceptor (see Merlin et al., 2016). Brite adipocytes are found in adult humans in supraclavicular fat depots surrounded by white adipose tissues (Lidell et al., 2013), and their presence in humans is correlated with a metabolically healthy phenotype (Cypess et al., 2009), which makes them an attractive target for obesity‐related diseases. One caveat is that white adipocytes must firstly be converted to a brite adipocyte before β3‐adrenoceptors can activate them. Typically brite adipocytes are generated in vitro and in vivo from white adipocytes by the addition or administration of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=595 ligands such as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1056 (see Merlin et al., 2016). This allows increased UCP1 mRNA/protein that then can be activated by β3‐adrenoceptor ligands (Li et al., 2017). Hence, the idea that there is a dual therapy for activation of brite adipocytes is gaining attention. In line with this theory, a clinical study is underway in individuals with a BMI of 29–45 treated with either the PPARγ activator http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2694 (30 mg·day−1), mirabegron (50 mg·day−1) or a combination of both drugs for 10 weeks (NCT02919176) before BAT activity (PET scanning), insulin sensitivity, glucose tolerance and biopsies taken to confirm the presence of beige/brite adipocytes are performed. This trial (due to be completed in late 2020) will provide much valuable information as to whether a dual combination of a PPARγ agonist and mirabegron improves metabolic health and the appearance of brite/beige adipocytes in a clinically relevant population.
Use of mirabegron in the treatment of heart failure
The β1‐adrenoceptor is the predominant β‐adrenoceptor subtype in the heart that regulates cardiac function (both the force and rate of myocardial contraction and relaxation). However, in heart failure, there is sustained activation of β1/2‐adrenoceptors due to increased catecholamine release from the sympathetic nerves and adrenal glands. This has adverse effects on cardiac function, leading to desensitization and internalization of β1/2‐adrenoceptors, loss of contractile function, cardiac remodelling, calcium overload and cardiomyocyte loss. As such, β‐adrenoceptor blockers (such as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=551, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7129 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=553) are used to treat heart failure, as they can slow the heart rate, allowing the left ventricle to fill up more completely. The role and function of β3‐adrenoceptors in the heart has been long debated by many researchers. This has primarily arisen due to the differences in species variation, with little or low expression in mouse heart, compared to higher expression in other species including rats, rabbits, dogs and humans (Gauthier et al., 1999). In addition, interpretation of data has been confusing due to the use of β3‐adrenoceptor agonists and antagonists that also have effects on other β‐adrenoceptors, as commented on previously (Michel et al., 2011). Nevertheless, the expression of β3‐adrenoceptors increases in rodents with prolonged stimulation of catecholamines (Germack and Dickenson, 2006), and increases in human myocardium during cardiac disease (Moniotte et al., 2001), which may be a protective mechanism under conditions of increased myocardial stress. In mice with cardiac overexpression of the human β3‐adrenoceptor, while there are no alterations in cardiac function or morphology under normal physiological conditions, activation of β3‐adrenoceptors attenuates the hypertrophic response to constant/repeated isoprenaline or http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2504 stimuli, through a nitric oxide‐mediated pathway (Belge et al., 2014). Furthermore, targeting the β3‐adrenoceptors in heart failure may be advantageous since these receptors are not desensitized following prolonged/repeated agonist exposure, compared with the β1/2‐adrenoceptors.
While the β3‐adrenoceptors have been postulated as a target for the treatment of heart failure (see Michel and Balligand, 2017), a recent clinical trial (the BEAT‐HF trial) of mirabegron in 70 patients with chronic heart failure, failed to reach its primary end point of increases in left ventricular ejection fraction (LVEF) (Bundgaard et al., 2017). These patients were treated with 150 mg mirabegron twice daily for 6 months, with all patients also administered with a β‐blocker to minimize effects of mirabegron on cardiac β1‐adrenoceptors. However, in a subset of patients where starting LVEF was <40%, mirabegron significantly increased LVEF as compared to patients given placebo (Bundgaard et al., 2017). This may suggest that mirabegron may be beneficial in the treatment of heart failure in patients with reduced LVEF. A second clinical trial treating patients with a daily dose of 50 mg of mirabegron daily for 12 months is currently underway (NCT02599480) to assess the efficacy of mirabegron in the prevention of heart failure. This study is due to be completed in early 2020.
Off‐target effects of mirabegron
While mirabegron has been defined as a β3‐adrenoceptor agonist, off‐target effects at other receptors and transporters have been reported. In the Australian Public Assessment Report for mirabegron to the Therapeutic Goods Administration (Department of Health Therapeutic Goods Administration: Australian Public Assessment Report for Mirabegron, 2014), mirabegron was reported to bind weakly (Ki 1–11 μM) to http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=4, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=926&familyId=176&familyType=TRANSPORTER, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=927&familyId=176&familyType=TRANSPORTER, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=14&familyId=2&familyType=GPCR and the sodium channel site 2. These Ki values are more than 30 times the unbound plasma Cmax at the maximal therapeutic dose. However, some of the metabolites of mirabegron (in particular M5 and M16) were reported to inhibit dopamine transporters, noradrenaline transporters and opiate receptors. These off‐target effects of mirabegron were reported by Astellas to the Australian TGA; however, it is hard for the scientific community to gain access to these results which have not been published. An overall of the reported off‐target effects of mirabegron is highlighted in Table 1. The potential uses for mirabegron and its off‐target effects are shown in Figure 3.
Table 1.
Reported off‐target effects of mirabegron
| Target | Effect | Reference |
|---|---|---|
| α1A‐Adrenoceptors | Relaxes mouse urethra smooth muscle | 1 |
| Antagonizes α1‐adrenoceptor mediated human prostate smooth muscle contraction | 2 | |
| Binds to human α1A‐adrenoceptors (pKi 6.36) | 1 | |
| α1D‐Adrenoceptors | Antagonizes noradrenaline‐mediated responses in the rat aorta | 1 |
| β1‐Adrenoceptors | Cardiostimulant | 3 |
| CYP2D6, CYP3A4 | Inhibitor | 4, 5 |
| Dopamine transporter | Binds to dopamine transporters | 6 |
| Muscarinic M2 receptor | Binds to M2 receptors | 6 |
| Noradrenaline transporters | Increase noradrenaline release in the heart; cardiostimulant | 3 |
| Binds to noradrenaline transporters | 6 | |
| Organic cation transporters | Inhibitor | 6, 7 |
| P‐glycoprotein | Weak inhibitor | 6, 7 |
| Sodium channel site 2 | Binds to sodium channels | 6 |
Figure 3.
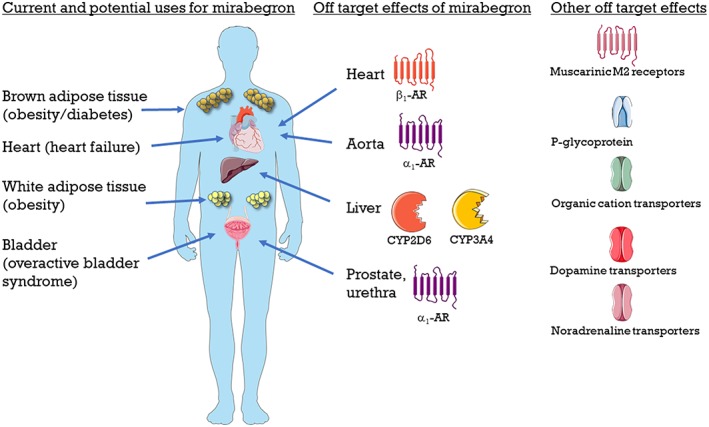
Overview of potential and current uses for mirabegron, and off‐target effects of mirabegron.
Cardiac β‐adrenoceptors
The cardiovascular effects of mirabegron were first noted in several clinical trials (Krauwinkel et al., 2012; Malik et al., 2012). In healthy volunteers, high doses (100–200 mg) of mirabegron increased heart rate and blood pressure, whereas lower therapeutic doses (50 mg) in OAB patients resulted in minor changes in pulse rate (1 beat per minute) and diastolic/systolic blood pressure (1 mm Hg or less) (Chapple et al., 2013; Nitti et al., 2013). A subsequent recent clinical study (van Gelderen et al., 2017) in healthy volunteers showed that a supra‐therapeutic dose of mirabegron (200 mg) increased heart rate and systolic (but not diastolic) blood pressure via activation of β1‐adrenoceptors, as effects were attenuated by co‐administration of either http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=564 or bisoprolol.
To determine a potential mechanism of off‐target effects of mirabegron in cardiac tissue, a recent study was performed studies in human right atrial appendages (Mo et al., 2017). Mirabegron (1–10 μM) increased contractile force, albeit at only 10% of the maximal effect elicited by isoprenaline. This effect was unaffected by the β3‐adrenoceptor antagonist http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3932, showing that mirabegron increases contractile function via a non β3‐adrenoceptor‐mediated mechanism. In the presence of the β1‐adrenoceptor antagonist http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=541, or the neuronal blockers http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2399 or http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7268, mirabegron reduced contractile force substantially. The authors postulated a dual action of mirabegron in cardiac tissue by which it acts, indirectly, as a cardiostimulant by being taken up into sympathetic nerve terminals by noradrenaline transporters that then cause http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=484 release which then activates β1‐adrenoceptors; it can also act as a cardiodepressant through unknown mechanisms that do not involve β3‐adrenoceptors. The authors postulated that, as mirabegron has a phenylethanolamine structure, this may explain why mirabegron increases blood pressure via an indirect sympathomimetic effect. For example, the phenylethanolamine ephedrine also increases β‐adrenoceptor‐mediated cardiostimulant effects by being taken up into sympathetic nerve terminals, resulting in increased noradrenaline release (Waldeck and Widmark, 1985). This is in comparison to noradrenaline which has meta and para hydroxyl groups in its phenyl group, which directly activates β‐adrenoceptors, without effects on sympathetic nerves. Clearly, further work is warranted in further clarifying the role and mechanism of action of mirabegron in the heart.
As mirabegron can increase blood pressure in patients, it is recommended that periodic blood pressure measurements are taken regularly in any hypertensive patients. Mirabegron is also not recommended in patients with severe uncontrolled hypertension (systolic blood pressure ≥ 180 mm Hg and/or diastolic blood pressure ≥ 110 mm Hg). This can have significant implications in the use of mirabegron in individuals with cardiovascular disease or increased cardiovascular risk factors, which are common in elderly patients with OAB.
Urinary α1‐adrenoceptors
Since the urethra smooth muscle contributes to bladder outlet resistance, Alexandre et al., (2016) investigated the effects of mirabegron on mouse urethral smooth muscle relaxation. Mirabegron relaxed smooth muscle precontracted with http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=485 (α‐adrenoceptor agonist) in a biphasic manner: the first high potency part was antagonized by the β3‐adrenoceptor antagonist L748337 (albeit with low pA2 value of 5.82), while the second lower potency component was unaffected by L748337, suggesting a non‐β3‐adrenoceptor‐mediated mechanism. Mirabegron‐mediated relaxations were only observed when tissues were precontracted with phenylephrine and not with other constrictors (including KCl, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2168 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=989). They subsequently showed that mirabegron acted as a competitive antagonist at α1‐adrenoceptors in mouse urethral smooth muscle, as well as in other http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=22&familyId=4&familyType=GPCR rich tissues including the rat vas deferens and rat prostate (Alexandre et al., 2016). In rabbit prostate smooth muscle, mirabegron relaxes smooth muscle contraction mediated by phenylephrine (Calmasini et al., 2015), and in human prostate smooth muscle, 1 and 10 μM mirabegron reduce the maximal contractile response to phenylephrine, with 10 μM mirabegron also causing a sixfold shift in the phenylephrine concentration–response curve (Calmasini et al., 2015), suggesting that at high concentrations, mirabegron appears to act as a α1‐adrenoceptor antagonist in human prostate smooth muscle. One clinical study has evaluated the safety and efficacy of adding on mirabegron (50 mg·day−1) for overactive bladder in men with benign prostatic hyperplasia treated with http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=488 (an α1‐adrenoceptor antagonist; 0.2 mg·day−1) for 8 weeks. They concluded that combination therapy was more effective for improving symptoms of both benign prostatic hyperplasia and OAB, compared with tamsulosin therapy alone (Ichihara et al., 2015).
Mirabegron was also effective in antagonizing noradrenaline‐mediated responses in the rat aorta (an http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=24&familyId=4&familyType=GPCR preparation) but ineffective in antagonizing http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=23&familyId=4&familyType=GPCR responses in the rat spleen (Alexandre et al., 2016). Consistent with the reported effects (Department of Health Therapeutic Goods Administration: Australian Public Assessment Report for Mirabegron, 2014) of mirabegron binding weakly to the α1‐adrenoceptor, Alexandre et al. (2016) showed that mirabegron could bind to the recombinant human α1‐adrenoceptor subtypes in HEK293 cells with pKi values of 6.36 (α1A‐adrenoceptor), 5.74 (α1B‐adrenoceptor) and 4.59 (Δ1–79α1D‐adrenoceptor). Taken together, these results of Alexandre et al., (2016) showed that in the mouse urethral smooth muscle, mirabegron has dual actions: actions at the β3‐adrenoceptor to relax the smooth muscle and the second as a competitive antagonist at α1A‐adrenoceptors.
The clinical relevance of α1‐adrenoceptor blockade exerted by mirabegron is still a matter of debate (Alexandre et al., 2016; Andersson, 2016, 2017; Michel, 2016). Andersson (2016) argued that if mirabegron antagonism of α1‐adrenoceptors had any clinical significance, then (a) a reduction of blood pressure, (b) an increase in stress incontinence (particularly in females), and (c) an effect on bladder outlet resistance and urethral pressure should be observed. However, clinical doses of mirabegron cause negligible increases (<1 mmHg) in blood pressure (Chapple et al., 2013; Nitti et al., 2013), do not cause stress incontinence in women with OAB (Matsukawa et al., 2015) and do not alter maximum urinary flow and detrusor pressure even after 12 weeks of treatment in men with bladder outlet obstruction and lower urinary tract symptoms (Nitti et al., 2013). Overall, α1A‐ and α1D‐adrenoceptor antagonism mediated by mirabegron appears to be an in vitro pharmacological observation in rodent urinary tract tissues, without any clinical evidence indicating that this off‐target effect could contribute to its efficacy in the treatment of OAB. Nevertheless, further studies are necessary to investigate whether antagonism of the α1‐adrenoceptor by mirabegron could be relevant in the treatment of human lower urinary syndromes.
Muscarinic receptors
There are five types of http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=207 (M1–5), of which the M2 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=15&familyId=2&familyType=GPCR subtype are highly expressed in the human bladder detrusor muscle. The M3 receptor is primarily responsible for cholinergic contraction of the detrusor muscle in both normal physiology and under pathophysiological conditions such as OAB/benign prostatic hyperplasia (BPH) (Yamanishi et al., 2015), with the role of M2 receptors unclear, although it may have a role at least in pig bladder contraction under conditions where the M3 receptor is inactivated (Yamanishi et al., 2002). Given that mirabegron has been reported to bind to M2 muscarinic receptors (Department of Health Therapeutic Goods Administration: Australian Public Assessment Report for Mirabegron, 2014), it is surprising that no data exist on any potential interaction of mirabegron with the M3 receptor. Most studies investigating the ability of mirabegron to relax human and rodent detrusor muscle have been performed in detrusor muscle precontracted with carbachol, with EC50 values for mirabegron typically in the high nM to low μM concertation range, with typically very shallow concentration–response curves for mirabegron as well (e.g. Takasu et al., 2007; Svalo et al., 2013; Cernecka et al., 2015).
Unlike studies performed in mouse urethral smooth muscle (Alexandre et al., 2016), mirabegron‐mediated relaxations are observed in rat detrusor muscle when precontracted with either http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=298 or KCl (Cernecka et al., 2015). However, mirabegron responses appear more pronounced (greater efficacy and potency) when tissues are precontracted with carbachol (Cernecka et al., 2015). While speculative, this may suggest that the efficacy of mirabegron to relax detrusor muscle through agonism only at β3‐adrenoceptors may be overestimated and may result from mirabegron also antagonizing M3 receptors. This clearly needs further investigation.
Recent studies (D'Agostino et al., 2015; Silva et al., 2017) show that mirabegron decreases electrically evoked http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=294 release in human detrusor muscle strips, where β3‐adrenoceptors potentially have an additional mechanism to inhibit parasympathetic activity. In Silva et al. (2017), the authors showed that β3‐adrenoceptor activation (albeit mainly using isoprenaline) increases http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2844 release from detrusor muscle via equilibrative nucleoside transporters, leading to retrograde activation of prejunctional http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=18, which inhibit acetylcholine release from cholinergic nerves in both rat and human urinary bladders. The potential significance and relevance of these results has recently been reviewed (Okeke et al., 2017).
P‐glycoprotein and organic cation transporters
Mirabegron is a weak inhibitor of http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=152#768 (ABCB1; an ATP‐dependent efflux transporter responsible for limiting the cellular uptake and distribution of xenobiotics and toxic substances) (Department of Health Therapeutic Goods Administration: Australian Public Assessment Report for Mirabegron, 2014) (unpublished studies cited in Groen‐Wijnberg et al., 2017). When mirabegron is co‐administered with http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4726 (a cardiac glycoside that is a known substrate for P‐glycoprotein), this results in a significant increase in digoxin levels in humans (Groen‐Wijnberg et al., 2017). The authors concluded that titration of digoxin should be considered for patients prescribed digoxin and mirabegron, due to the narrow therapeutic dose window for digoxin.
Mirabegron is also an inhibitor of organic cation transporters (http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=196s; primarily hepatic uptake transporters involved in the uptake of positively and neutrally charged compounds) (Department of Health Therapeutic Goods Administration: Australian Public Assessment Report for Mirabegron, 2014). This may be relevant when patients are also prescribed other medications that are substrates for OCT, such as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4779 (an anti‐diabetic medication). Co‐administration of mirabegron (160 mg) does not affect the pharmacokinetic profile of metformin but plasma mirabegron levels were reduced with co‐administration of metformin (Groen‐Wijnberg et al., 2017). These changes were considered by the authors not to be clinically relevant, and hence, no changes in dosage should be required when mirabegron is co‐administered with metformin.
Cytochrome P450 enzymes
Studies examining the metabolism of mirabegron revealed that it is an (quasi‐) irreversible, metabolism‐dependent inhibitor of cytochrome P450 2D6 (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1329) and to a lesser degree, an inhibitor of http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1337 (Takusagawa et al., 2012a; Takusagawa et al., 2012b). Mirabegron directly inhibits CYP2D6, with an IC50 of 0.67 μM for the recombinant enzyme and 13 μM in human liver microsomes. It inhibits CYP3A4 with an IC50 of 42.5 μM forthe recombinant enzyme, but not in human liver microsomes (Takusagawa et al., 2012a). Mirabegron failed to affect a range of other CYP450 enzymes (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1319, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1324, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1326&familyId=262&familyType=ENZYME, CYP2C19 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1330&familyId=262&familyType=ENZYME). This raises the possibility of mirabegron affecting the metabolism of several drugs that are metabolized by CYP2D6 or CYP3A4. In patient studies, mirabegron (100 or 160 mg·day−1; a dose that will saturate CYP2D6 from in vitro studies) affected the metabolism of two well‐known drugs metabolized by CYP2D: metoprolol, a β1‐adrenoceptor antagonist and desipramine, a tricylic anti‐depressant. This results in an increase in the exposure patients had to metoprolol or desipramine, and mirabegron was suggested to be a moderate CYP2D6 inhibitor (Krauwinkel et al., 2014). However, there were no alterations in the pharmacokinetic profile of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6853, an oral anticoagulant that is metabolized by CYP3A4 (Groen‐Wijnberg et al., 2017). Therefore, further studies into drug–drug interactions between mirabegron and other drugs metabolized by CYP2D6 or CYP3A4 should be performed.
Conclusions
The development of β3‐adrenoceptor agonists has been fraught with significant challenges. Initially a target for the treatment of obesity and diabetes, early β3‐adrenoceptor agonists failed in clinical trials primarily due to their lack of efficacy at the human receptor. The development of β3‐adrenoceptor agonists effective at the human receptor has led to the first β3‐adrenoceptor agonist to be approved for the use of OAB in several countries or regions and re‐focused attention on β3‐adrenoceptors for the potential treatment of other diseases such as heart failure and metabolic disease, of which several clinical trials are currently being performed.
However, mirabegron has reported side effects against several other related receptors, several transporters and liver enzymes. The clinical relevance of these off‐target effects are relatively unknown, with perhaps the exception of its actions at cardiac β1‐adrenoceptors in the heart, which may impose limitations on its use in patients with heart conditions, and its effects with CYP3A4 and CYP2A6 which could interfere with the metabolism of other drugs. Several of the off targets effects of mirabegron (or its metabolites) may be relevant in the treatment of OAB. For example, α1‐ adrenoceptor antagonists (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=493 and tamsulosin) are used clinically to treat lower urinary tract symptoms exaggerated by increased α1‐adrenoceptor‐mediated smooth muscle contraction, particularly in patients with BPH. An additional α1‐adrenoceptor antagonist role of mirabegron may therefore further improve symptoms in patients with OAB and BPH, as suggested by one study (Ichihara et al., 2015). The postulated action of mirabegron at muscarinic receptors needs further investigation before its relevance to OAB can be known but is highly significant, given that anti‐cholinergics are the recommended first‐line treatment for OAB patients.
In conclusion, mirabegron and other β3‐adrenoceptor agonists have the potential to be used in the treatment of not only OAB but also other diseases such as heart failure and metabolic diseases. Further studies should address the off‐target (and indirect signalling) effects of mirabegron and determine whether its off‐target actions have any clinical relevance.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to Pharmacology (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a,b,c,d).
Conflict of interest
T.B. own stocks in the following pharmaceutical companies, Sigrid Therapeutics AB, Atrogi AB and Glucox Biotechnology AB; D.S.H. owns stocks in Glucox Biotechnology AB and is a scientific advisor for Atrogi AB.
Acknowledgements
The artwork contained in this manuscript was obtained from Servier Medical Art (http://smart.servier.com/). The amino acid topological model of the human β3‐adrenoceptor was obtained from http://www.gpcrdb.org/protein/adrb3_human.
Dehvari, N. , da Silva Junior, E. D. , Bengtsson, T. , and Hutchinson, D. S. (2018) Mirabegron: potential off target effects and uses beyond the bladder. British Journal of Pharmacology, 175: 4072–4082. 10.1111/bph.14121.
References
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Cidlowski JA, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017c). The Concise Guide to PHARMACOLOGY 2017/18: Nuclear hormone receptors. Br J Pharmacol 174: S208–S224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017d). The Concise Guide to PHARMACOLOGY 2017/18: Transporters. Br J Pharmacol 174: S360–S446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandre EC, Kiguti LR, Calmasini FB, Silva FH, da Silva KP, Ferreira R et al (2016). Mirabegron relaxes urethral smooth muscle by a dual mechanism involving β3‐adrenoceptor activation and α1‐adrenoceptor blockade. Br J Pharmacol 173: 415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson KE (2016). Pharmacology: on the mode of action of mirabegron. Nat Rev Urol 13: 131–132. [DOI] [PubMed] [Google Scholar]
- Andersson KE (2017). On the site and mechanism of action of β3‐adrenoceptor agonists in the bladder. Int Neurourol J 21: 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arch JR, Ainsworth AT, Cawthorne MA, Piercy V, Sennitt MV, Thody VE et al (1984). Atypical β‐adrenoceptor on brown adipocytes as target for anti‐obesity drugs. Nature 309: 163–165. [DOI] [PubMed] [Google Scholar]
- Arch JR, Kaumann AJ (1993). β3 and atypical β‐adrenoceptors. Med Res Rev 13: 663–729. [DOI] [PubMed] [Google Scholar]
- Belge C, Hammond J, Dubois‐Deruy E, Manoury B, Hamelet J, Beauloye C et al (2014). Enhanced expression of β3‐adrenoceptors in cardiac myocytes attenuates neurohormone‐induced hypertrophic remodeling through nitric oxide synthase. Circulation 129: 451–462. [DOI] [PubMed] [Google Scholar]
- Breit A, Lagace M, Bouvier M (2004). Hetero‐oligomerization between β2‐ and β3‐adrenergic receptors generates a β‐adrenergic signaling unit with distinct functional properties. J Biol Chem 279: 28756–28765. [DOI] [PubMed] [Google Scholar]
- Bundgaard H, Axelsson A, Hartvig Thomsen J, Sorgaard M, Kofoed KF, Hasselbalch R et al (2017). The first‐in‐man randomized trial of a β3 adrenoceptor agonist in chronic heart failure: the BEAT‐HF trial. Eur J Heart Fail 19: 566–575. [DOI] [PubMed] [Google Scholar]
- Calmasini FB, Candido TZ, Alexandre EC, D'Ancona CA, Silva D, de Oliveira MA et al (2015). The β3 adrenoceptor agonist, mirabegron relaxes isolated prostate from human and rabbit: new therapeutic indication? Prostate 75: 440–447. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J (2004). Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359. [DOI] [PubMed] [Google Scholar]
- Cernecka H, Kersten K, Maarsingh H, Elzinga CR, de Jong IJ, Korstanje C et al (2015). β3‐Adrenoceptor‐mediated relaxation of rat and human urinary bladder: roles of BKCa channels and Rho kinase. Naunyn Schmiedebergs Arch Pharmacol 388: 749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapple CR, Kaplan SA, Mitcheson D, Klecka J, Cummings J, Drogendijk T et al (2013). Randomized double‐blind, active‐controlled phase 3 study to assess 12‐month safety and efficacy of mirabegron, a β3‐adrenoceptor agonist, in overactive bladder. Eur Urol 63: 296–305. [DOI] [PubMed] [Google Scholar]
- Cui Y, Zong H, Yang C, Yan H, Zhang Y (2014). The efficacy and safety of mirabegron in treating OAB: a systematic review and meta‐analysis of phase III trials. Int Urol Nephrol 46: 275–284. [DOI] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB et al (2009). Identification and importance of brown adipose tissue in adult humans. N Engl J Med 360: 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Weiner LS, Roberts‐Toler C, Elia EF, Kessler SH, Kahn PA et al (2015). Activation of human brown adipose tissue by a β3‐adrenergic receptor agonist. Cell Metab 21: 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino G, Maria Condino A, Calvi P (2015). Involvement of β3‐adrenoceptors in the inhibitory control of cholinergic activity in human bladder: direct evidence by [3H]‐acetylcholine release experiments in the isolated detrusor. Eur J Pharmacol 758: 115–122. [DOI] [PubMed] [Google Scholar]
- Department of Health Therapeutic Goods Administration: Australian Public Assessment Report for Mirabegron . (2014). [Online] Available at: https://www.tga.gov.au/auspar/auspar-mirabegron (accessed 12/07/2017).
- Evans BA, Papaioannou M, Hamilton S, Summers RJ (1999). Alternative splicing generates two isoforms of the β3‐adrenoceptor which are differentially expressed in mouse tissues. Br J Pharmacol 127: 1525–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura T, Tamura K, Tsutsumi T, Yamamoto T, Nakamura K, Koibuchi Y et al (1999). Expression and possible functional role of the β3‐adrenoceptor in human and rat detrusor muscle. J Urol 161: 680–685. [PubMed] [Google Scholar]
- Gauthier C, Tavernier G, Trochu JN, Leblais V, Laurent K, Langin D et al (1999). Interspecies differences in the cardiac negative inotropic effects of β3‐adrenoceptor agonists. J Pharmacol Exp Ther 290: 687–693. [PubMed] [Google Scholar]
- van Gelderen M, Stolzel M, Meijer J, Kerbusch V, Collins C, Korstanje C (2017). An exploratory study in healthy male subjects of the mechanism of mirabegron‐induced cardiovascular effects. J Clin Pharmacol . 10.1002/jcph.952. [DOI] [PubMed] [Google Scholar]
- Germack R, Dickenson JM (2006). Induction of β3‐adrenergic receptor functional expression following chronic stimulation with noradrenaline in neonatal rat cardiomyocytes. J Pharmacol Exp Ther 316: 392–402. [DOI] [PubMed] [Google Scholar]
- Groen‐Wijnberg M, van Dijk J, Krauwinkel W, Kerbusch V, Meijer J, Tretter R et al (2017). Pharmacokinetic interactions between mirabegron and metformin, warfarin, digoxin or combined oral contraceptives. Eur J Drug Metab Pharmacokinet 42: 417–429. [DOI] [PubMed] [Google Scholar]
- Hatanaka T, Ukai M, Watanabe M, Someya A, Ohtake A, Suzuki M et al (2013a). Pharmacological profile of the selective β3‐adrenoceptor agonist mirabegron in cynomolgus monkeys. Naunyn Schmiedebergs Arch Pharmacol 386: 1001–1008. [DOI] [PubMed] [Google Scholar]
- Hatanaka T, Ukai M, Watanabe M, Someya A, Ohtake A, Suzuki M et al (2013b). In vitro and in vivo pharmacological profile of the selective β3‐adrenoceptor agonist mirabegron in rats. Naunyn Schmiedebergs Arch Pharmacol 386: 247–253. [DOI] [PubMed] [Google Scholar]
- Hutchinson DS, Bengtsson T, Evans BA, Summers RJ (2002). Mouse β3a‐ and β3b‐adrenoceptors expressed in Chinese hamster ovary cells display identical pharmacology but utilize distinct signalling pathways. Br J Pharmacol 135: 1903–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara K, Masumori N, Fukuta F, Tsukamoto T, Iwasawa A, Tanaka Y (2015). A randomized controlled study of the efficacy of tamsulosin monotherapy and its combination with mirabegron for overactive bladder induced by benign prostatic obstruction. J Urol 193: 921–926. [DOI] [PubMed] [Google Scholar]
- Igawa Y, Yamazaki Y, Takeda H, Hayakawa K, Akahane M, Ajisawa Y et al (1999). Functional and molecular biological evidence for a possible β3‐adrenoceptor in the human detrusor muscle. Br J Pharmacol 126: 819–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Pennisi PA, Gavrilova O, Pack S, Jou W, Setser‐Portas J et al (2006). Effect of adipocyte β3‐adrenergic receptor activation on the type 2 diabetic MKR mice. Am J Physiol Endocrinol Metab 290: E1227–E1236. [DOI] [PubMed] [Google Scholar]
- Krauwinkel W, Dickinson J, Schaddelee M, Meijer J, Tretter R, van de Wetering J et al (2014). The effect of mirabegron, a potent and selective β3‐adrenoceptor agonist, on the pharmacokinetics of CYP2D6 substrates desipramine and metoprolol. Eur J Drug Metab Pharmacokinet 39: 43–52. [DOI] [PubMed] [Google Scholar]
- Krauwinkel W, van Dijk J, Schaddelee M, Eltink C, Meijer J, Strabach G et al (2012). Pharmacokinetic properties of mirabegron, a β3‐adrenoceptor agonist: results from two phase I, randomized, multiple‐dose studies in healthy young and elderly men and women. Clin Ther 34: 2144–2160. [DOI] [PubMed] [Google Scholar]
- Li YL, Li X, Jiang TT, Fan JM, Zheng XL, Shi XE et al (2017). An additive effect of promoting thermogenic gene expression in mice adipose‐derived stromal vascular cells by combination of rosiglitazone and CL316,243. Int J Mol Sci 18 (5) pii: E1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidell ME, Betz MJ, Dahlqvist Leinhard O, Heglind M, Elander L, Slawik M et al (2013). Evidence for two types of brown adipose tissue in humans. Nat Med 19: 631–634. [DOI] [PubMed] [Google Scholar]
- Lowell BB, S‐Susulic V, Hamann A, Lawitts JA, Himms‐Hagen J, Boyer BB et al (1993). Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 366: 740–742. [DOI] [PubMed] [Google Scholar]
- Malik M, van Gelderen EM, Lee JH, Kowalski DL, Yen M, Goldwater R et al (2012). Proarrhythmic safety of repeat doses of mirabegron in healthy subjects: a randomized, double‐blind, placebo‐, and active‐controlled thorough QT study. Clin Pharmacol Ther 92: 696–706. [DOI] [PubMed] [Google Scholar]
- Maman K, Aballea S, Nazir J, Desroziers K, Neine ME, Siddiqui E et al (2014). Comparative efficacy and safety of medical treatments for the management of overactive bladder: a systematic literature review and mixed treatment comparison. Eur Urol 65: 755–765. [DOI] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND et al (2009). Cold‐activated brown adipose tissue in healthy men. N Engl J Med 360: 1500–1508. [DOI] [PubMed] [Google Scholar]
- Matsukawa Y, Takai S, Funahashi Y, Yamamoto T, Gotoh M (2015). Urodynamic evaluation of the efficacy of mirabegron on storage and voiding functions in women with overactive bladder. Urology 85: 786–790. [DOI] [PubMed] [Google Scholar]
- Merlin J, Evans BA, Dehvari N, Sato M, Bengtsson T, Hutchinson DS (2016). Could burning fat start with a brite spark? Pharmacological and nutritional ways to promote thermogenesis. Mol Nutr Food Res 60: 18–42. [DOI] [PubMed] [Google Scholar]
- Michel LYM, Balligand JL (2017). New and emerging therapies and targets: β3 agonists. Handb Exp Pharmacol 243: 205–223. [DOI] [PubMed] [Google Scholar]
- Michel MC (2016). How β3‐adrenoceptor‐selective is mirabegron? Br J Pharmacol 173: 429–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel MC, Harding SE, Bond RA (2011). Are there functional β3‐adrenoceptors in the human heart? Br J Pharmacol 162: 817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel MC, Korstanje C (2016). β3‐Adrenoceptor agonists for overactive bladder syndrome: Role of translational pharmacology in a repositioning clinical drug development project. Pharmacol Ther 159: 66–82. [DOI] [PubMed] [Google Scholar]
- Mo W, Michel MC, Lee XW, Kaumann AJ, Molenaar P (2017). The β3‐adrenoceptor agonist mirabegron increases human atrial force through β1‐adrenoceptors: an indirect mechanism? Br J Pharmacol 174: 2706–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniotte S, Kobzik L, Feron O, Trochu JN, Gauthier C, Balligand JL (2001). Upregulation of β3‐adrenoceptors and altered contractile response to inotropic amines in human failing myocardium. Circulation 103: 1649–1655. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Bengtsson T, Cannon B (2007). Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab 293: E444–E452. [DOI] [PubMed] [Google Scholar]
- Nitti VW, Auerbach S, Martin N, Calhoun A, Lee M, Herschorn S (2013). Results of a randomized phase III trial of mirabegron in patients with overactive bladder. J Urol 189: 1388–1395. [DOI] [PubMed] [Google Scholar]
- Nomiya M, Yamaguchi O (2003). A quantitative analysis of mRNA expression of α1 and β‐adrenoceptor subtypes and their functional roles in human normal and obstructed bladders. J Urol 170: 649–653. [DOI] [PubMed] [Google Scholar]
- Okeke K, Gravas S, Michel MC (2017). Do β3‐adrenoceptor agonists cause urinary bladder smooth muscle relaxation by inhibiting acetylcholine release? Am J Physiol Renal Physiol . 10.1152/ajprenal.00215.2017. [DOI] [PubMed] [Google Scholar]
- Orava J, Nuutila P, Noponen T, Parkkola R, Viljanen T, Enerback S et al (2013). Blunted metabolic responses to cold and insulin stimulation in brown adipose tissue of obese humans. Obesity (Silver Spring) 21: 2279–2287. [DOI] [PubMed] [Google Scholar]
- Roberts‐Toler C, O'Neill BT, Cypess AM (2015). Diet‐induced obesity causes insulin resistance in mouse brown adipose tissue. Obesity (Silver Spring) 23: 1765–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva I, Costa AF, Moreira S, Ferreirinha F, Magalhaes‐Cardoso MT, Calejo I et al (2017). Inhibition of cholinergic neurotransmission by β3‐adrenoceptors depends on adenosine release and A1‐receptor activation in human and rat urinary bladders. Am J Physiol Renal Physiol 313: F388–F403. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svalo J, Nordling J, Bouchelouche K, Andersson KE, Korstanje C, Bouchelouche P (2013). The novel β3‐adrenoceptor agonist mirabegron reduces carbachol‐induced contractile activity in detrusor tissue from patients with bladder outflow obstruction with or without detrusor overactivity. Eur J Pharmacol 699: 101–105. [DOI] [PubMed] [Google Scholar]
- Takasu T, Ukai M, Sato S, Matsui T, Nagase I, Maruyama T et al (2007). Effect of (R)‐2‐(2‐aminothiazol‐4‐yl)‐4′‐{2‐[(2‐hydroxy‐2‐phenylethyl)amino]ethyl} acetanilide (YM178), a novel selective β3‐adrenoceptor agonist, on bladder function. J Pharmacol Exp Ther 321: 642–647. [DOI] [PubMed] [Google Scholar]
- Takeda M, Obara K, Mizusawa T, Tomita Y, Arai K, Tsutsui T et al (1999). Evidence for β3‐adrenoceptor subtypes in relaxation of the human urinary bladder detrusor: analysis by molecular biological and pharmacological methods. J Pharmacol Exp Ther 288: 1367–1373. [PubMed] [Google Scholar]
- Takusagawa S, Miyashita A, Iwatsubo T, Usui T (2012a). In vitro inhibition and induction of human cytochrome P450 enzymes by mirabegron, a potent and selective β3‐adrenoceptor agonist. Xenobiotica 42: 1187–1196. [DOI] [PubMed] [Google Scholar]
- Takusagawa S, Yajima K, Miyashita A, Uehara S, Iwatsubo T, Usui T (2012b). Identification of human cytochrome P450 isoforms and esterases involved in the metabolism of mirabegron, a potent and selective β3‐adrenoceptor agonist. Xenobiotica 42: 957–967. [DOI] [PubMed] [Google Scholar]
- Vijgen GH, Bouvy ND, Teule GJ, Brans B, Schrauwen P, van Marken Lichtenbelt WD (2011). Brown adipose tissue in morbidly obese subjects. PLoS ONE 6: e17247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldeck B, Widmark E (1985). The interaction of ephedrine with β‐adrenoceptors in tracheal, cardiac and skeletal muscles. Clin Exp Pharmacol Physiol 12: 439–442. [DOI] [PubMed] [Google Scholar]
- Warren K, Burden H, Abrams P (2016). Mirabegron in overactive bladder patients: efficacy review and update on drug safety. Ther Adv Drug Saf 7: 204–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S, Chambers JK, Park JE, Ladurner A, Cronk DW, Chapman CG et al (1996). Agonist potency at the cloned human β3 adrenoceptor depends on receptor expression level and nature of assay. J Pharmacol Exp Ther 279: 214–221. [PubMed] [Google Scholar]
- Yamaguchi O (2002). β3‐Adrenoceptors in human detrusor muscle. Urology 59: 25–29. [DOI] [PubMed] [Google Scholar]
- Yamaguchi O, Chapple CR (2007). β3‐Adrenoceptors in urinary bladder. NeurourolUrodyn 26: 752–756. [DOI] [PubMed] [Google Scholar]
- Yamanishi T, Chapple CR, Yasuda K, Chess‐Williams R (2002). The role of M2 muscarinic receptor subtypes in mediating contraction of the pig bladder base after cyclic adenosine monophosphate elevation and/or selective M3 inactivation. J Urol 167: 397–401. [PubMed] [Google Scholar]
- Yamanishi T, Kaga K, Fuse M, Shibata C, Kamai T, Uchiyama T (2015). The role of muscarinic receptor subtypes on carbachol‐induced contraction of normal human detrusor and overactive detrusor associated with benign prostatic hyperplasia. J Pharmacol Sci 128: 65–70. [DOI] [PubMed] [Google Scholar]


