Abstract
Traditionally, signal transduction from GPCRs is thought to emanate from the cell surface where receptor interactions with external stimuli can be transformed into a broad range of cellular responses. However, emergent data show that numerous GPCRs are also associated with various intracellular membranes where they may couple to different signalling systems, display unique desensitization patterns and/or exhibit distinct patterns of subcellular distribution. Although many GPCRs can be activated at the cell surface and subsequently endocytosed and transported to a unique intracellular site, other intracellular GPCRs can be activated in situ either via de novo ligand synthesis, diffusion of permeable ligands or active transport of nonpermeable ligands. Current findings reinforce the notion that intracellular GPCRs play a dynamic role in various biological functions including learning and memory, contractility and angiogenesis. As new intracellular GPCR roles are defined, the need to selectively tailor agonists and/or antagonists to both intracellular and cell surface receptors may lead to the development of more effective therapeutic tools.
Linked Articles
This article is part of a themed section on Molecular Pharmacology of GPCRs. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v175.21/issuetoc
Abbreviations
- Ang II
angiotensin II
- CCR2
chemokine receptor 2
- CXCR4
cysteine (C)‐X‐C receptor 4
- ER
endoplasmic reticulum
- ET‐1
endothelin 1
- ETB
endothelin receptor B
- GnRH
gonadotropin‐releasing hormone
- GRK
GPCR kinase
- IP3
inositol 1,4,5‐trisphosphate
- LPA
lysophosphatidic acid
- NLSs
nuclear localization signals
- NM
nuclear membrane
- OA1
ocular albinism I
- PACAP
pituitary adenylate cyclase activating polypeptide
- PM
plasma membrane
Intracellular GPCRs
From their position on the cell surface, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=694&familyType=GPCR are known to transform external stimuli into a broad range of signalling pathways within the cell. GPCRs have also been found on the endoplasmic reticulum (ER), where they are synthesized, folded, modified and assembled, as well as in sorting vesicles on their way to the cell surface, or on endosomes that have just come off the membrane. Emerging studies, however, indicate that certain intracellular membranes may serve as alternate destinations or even the preferred location for a number of GPCRs where they may couple to different signalling systems and exhibit distinct patterns of subcellular distribution (Irannejad et al., 2013, 2017; Jong et al., 2014; Branco and Allen, 2015; Calebiro et al., 2015; Campden et al., 2015; Joyal et al., 2015). One example of an exclusively intracellular GPCR is that of the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=203 (OA1) GPCR, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=203 (Goshima et al., 2014; De Filippo et al., 2017). GPR143 localize to melanosomes and late endosomes/lysosomes in pigmented and non‐pigmented cells, where they appear to act as ‘sensors’ regulating organelle biogenesis and maturation (Schiaffino et al., 1996; d'Addio et al., 2000; Samaraweera et al., 2001). Other early examples of intracellular GPCRs include http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=342 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=343 that signal from endothelial, brain and/or liver nuclei (Bhattacharya et al., 1999) or metabotropic glutamate receptor, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=293, signalling from neuronal ER or nuclear membranes (NMs) (O'Malley et al., 2003). GPCRs have also been found on vesicles, mitochondria (Benard et al., 2012), NMs (Gobeil et al., 2006; Calebiro et al., 2010; Tadevosyan et al., 2012; Joyal et al., 2014) and even within the nucleoplasm on nuclear bodies and/or nuclear invaginations (Lee et al., 2004; Morinelli et al., 2007; Wright et al., 2012). The diversity of the various intracellular destinations has raised many questions such as (i) what are the signals responsible for trafficking or retaining GPCRs in these unique locations; (ii) how are these intracellular GPCRs activated; (iii) what are the functional consequences of activating an intracellular GPCR; and importantly, (iv) what are the pathophysiological consequences of intracellular GPCRs. Since over 30 GPCRs have been described at the NM, here, we will primarily summarize current findings regarding nuclear trafficking, activation and location‐dependent signalling of this expanding class of intracellular receptors (Irannejad et al., 2013, 2017; Jong et al., 2014; Branco and Allen, 2015; Calebiro et al., 2015; Campden et al., 2015; Joyal et al., 2015). Where sufficient information exists, we have included additional information on GPCRs functioning on other intracellular membranes such as endosomes and mitochondria (Vilardaga et al., 2014; Irannejad et al., 2017).
Trafficking of nuclear GPCRs
There appear to be many mechanisms associated with nuclear GPCR translocation. These range from lateral diffusion through peripheral channels between the nuclear pore complex and the pore membrane to movement through the nuclear pore complex using linkers, carrier proteins and even components of the soluble transport machinery (Lusk et al., 2007; Zuleger et al., 2012; Katta et al., 2014). For example, certain GPCRs such as the http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=7, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=34, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=3 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=42 use canonical nuclear localization signals (NLSs), that is, short stretches of basic amino acids that are subsequently recognized by specific members of the karyopherin superfamily for nuclear import (Lee et al., 2004; Morinelli et al., 2007; Wright et al., 2012; Branco and Allen, 2015). In many instances, mutating various residues within the NLS prevents karyopherin recognition and hence nuclear localization of these GPCRs (Branco and Allen, 2015). Some GPCRs contain multiple NLS motifs including the proteinase‐activated receptor http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=348 [also known as F2R‐like trypsin receptor 1 (F2rl1) and coagulation factor II receptor‐like 1], which has two NLS motifs (Joyal et al., 2014). After ligand‐mediated receptor internalization, these motifs are recognized by importin β1, which allows PAR2 to translocate from the plasma membrane (PM) to the NM via sorting nexin11 and dynein transport along microtubules (Joyal et al., 2014). In a similar fashion, agonist stimulation of the http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=66 leads to its β‐arrestin‐mediated internalization followed by binding of the karyopherin, transportin‐1 to NLS sequences within intracellular and C‐terminal domains followed by transport to the nucleus (Di Benedetto et al., 2014). The chemokine receptors http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=59 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=71 are also trafficked to the nucleus via transportin‐1 (Favre et al., 2008; Don‐Salu‐Hewage et al., 2013). Other GPCRs like the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=334 traffic to the nucleus via a process involving the small http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=897s, Rab11a and importin‐5 (Bhosle et al., 2016; 2017). Thus, there is no one single karyopherin or one preferred pathway that is involved in this process.
Not all nuclear GPCRs contain canonical NLS sequences. Some like the metabotropic glutamate receptor, mGlu5, contain previously unidentified targeting sequences that are critical for the receptor's nuclear localization (Sergin et al., 2017). Although the transport proteins responsible for this movement have not been identified, the sequences themselves are necessary and sufficient for mGlu5 nuclear localization (Sergin et al., 2017). Interestingly, the mGlu5 C‐terminal targeting sequence contains several kinase structural motifs, including motifs for http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=284, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=565, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=286&familyType=ENZYME and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1554 (CaMKII; Mao et al., 2008). Conceivably, phosphorylation of this NM targeting sequence might influence binding of the transport protein (s) responsible for redistributing mGlu5 receptors to the NM.
As described above, many GPCRs are first trafficked to the cell surface, activated, internalized and subsequently transported to the nucleus. Interestingly, many of the receptors trafficked from the cell surface are not associated with NMs but rather appear within the nucleoplasm itself (via unknown mechanisms). These include the apelin, CCR2, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=366&familyId=66&familyType=GPCR, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=275, oxytocin and CXCR4 receptors (Lee et al., 2004; Kinsey et al., 2007; Favre et al., 2008; Estrada et al., 2009; Verzijl et al., 2010; Don‐Salu‐Hewage et al., 2013; Di Benedetto et al., 2014; Sun et al., 2016). Some GPCRs, however, are trafficked directly to the NM. The http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=21, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=6 and mGlu5 receptors all seem to fit this model (Bkaily et al., 2006; Jong et al., 2014; Sergin et al., 2017). In these cases, a simple diffusion–retention model has been proposed since the outer NM is contiguous with the ER (Zuleger et al., 2012; Katta et al., 2014). This model suggests that proteins synthesized in the ER rapidly diffuse along the outer NM before passing through peripheral channels located between the nuclear pore complex and the pore membrane to become tethered on the inner NM via interactions with nuclear lamins or chromatin (Lusk et al., 2007; Hinshaw et al., 1992; Zuleger et al., 2012). For example, the mGlu5 receptor NM motif interacts with chromatin via a basic region (pI > 9.8) that may promote its nuclear retention (Sergin et al., 2017). Because most transmembrane proteins are tagged with N‐linked oligosaccharides as they are translocated through the ER and Golgi, differential glycosylation can be used to monitor protein trafficking. At least one nuclear GPCR appears to be directly transferred to NMs without going through the Golgi, the ETB receptor (Merlen et al., 2013). In contrast, mGlu5 and PAF receptors exhibit glycosylation patterns consistent with a more dynamic diffusion model, one in which mGlu5 at least is cycled back from the Golgi to the ER and then undergoes either lateral diffusion or a facilitated process to reach the inner NM (Sergin et al., 2017). Most recently, it was reported that http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=371, a class B GPCR shared by http://www.guidetopharmacology.org/GRAC/FamilyIntroductionForward?familyId=67 and http://www.guidetopharmacology.org/GRAC/FamilyIntroductionForward?familyId=67, was trafficked to the NM via palmitoylation of its most N‐terminal cysteine (Cys37) in the extracellular domain (Yu et al., 2017). Taken together, there seems to be many different signals and types of processes by which nuclear GPCRs arrive at their destination (Figure 1).
Figure 1.
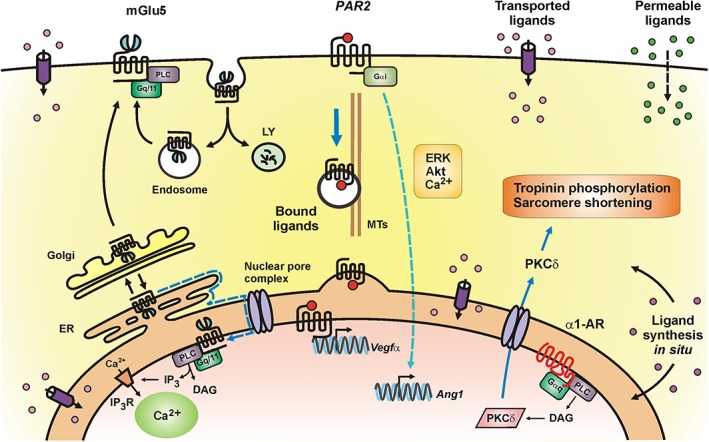
Schematic representation of selected intracellular GPCRs and ways in which they are activated. Left: proposed model of mGlu5 receptor trafficking in neurons in which >90% of mGlu5 receptors traffick through the golgi (Sergin et al., 2017). From there, between 15 and 40% (Hubert et al., 2001; López‐Bendito et al., 2002; O'Malley et al., 2003) go to the cell surface where they undergoe a cycle of constitutive endocytosis and recycling (Trivedi and Bhattacharyya, 2012). Alternatively, 60–85% of mGlu5 receptors are retrogradely trafficked back to the ER and via lateral diffusion (dotted blue line) reach the NM (Vincent et al., 2016; Sergin et al., 2017). Middle: ligand‐bound PAR2 can translocate from the retinal ganglion cell surface to the nucleus via importin‐β, Snx11 and dynein; nuclear PAR2 activates Vegfa expression. In contrast, signalling from cell surface PAR2 results in angiopoietin 1 expression (Joyal et al., 2014). Right: the https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=3&cad=rja&uact=8&ved=0ahUKEwiDsp6cqrvVAhWr64MKHWP9AHIQFgg7MAI&url=https%3A%2F%2Fwww.ncbi.nlm.nih.gov%2Fpmc%2Farticles%2FPMC3930413%2F&usg=AFQjCNEaQB5359zmSlQuF092N7FE4mEbxAceptor (α1‐AR) is localized to the inner NM of adult cardiac myocytes where it is activated by noradrenaline transported via the http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=196#1021 located on the cell surface and ER membranes. The nuclear α1‐adrenoceptor couples to Gαq and PLC, which activate nuclear PKCδ. PKCδ is then transported out of the nucleus through the nuclear pore complex. PKCδ induces phosphorylation of troponin 1 to regulate contractility and ERK to regulate survival signalling (Wu et al., 2014; Wu and O'Connell, 2015). Intracellular GPCRs can be activated via (i) channels, transporters or exchangers recognizing specific ligands such as glutamate in the case of mGlu5 receptors or OCT3 for noradrenaline activation of α1‐adrenoceptors; (ii) alternatively, ligands sufficiently permeable can freely diffuse across cell membranes; (iii) as in the case of PAR2, receptor‐bound ligands can simply be internalized with a given GPCR; and (iv) ligands can be synthesized within the cell and either diffuse or be trafficked to a given cellular compartment.
Ligand activation of nuclear receptors
Peptide receptors are amongst the earliest GPCRs to be described in the nucleus and/or on NMs (Gobeil et al., 2006; Boivin et al., 2008). Frequently, the same receptors are also abundantly expressed on the PM. Although some peptide ligands are known to be destroyed in early endosomes, a process that allows receptors to be resensitized and recycled to the cell surface (Poole and Bunnett, 2016), in other cases, the cognate peptide ligand triggers internalization of the peptide GPCR and its subsequent trafficking to the nucleus (e.g. Figure 1). For example, both PAR2 and the PAF receptor appear to internalize with their ligands bound to the receptor (Joyal et al., 2014; Bhosle et al., 2016). The oxytocin receptor also moves to the NM after ligand binding in osteoblasts, breast cancer cells and primary fibroblasts (Kinsey et al., 2007; Di Benedetto et al., 2014). In the latter two cases, the authors used iodinated oxytocin and fluorescently labelled receptors to unequivocally demonstrate that cells internalize both the receptor and its ligand (Kinsey et al., 2007). In addition, peptide ligands can also activate endogenous peptide GPCRs already present within the nucleus or on NMs. The mechanism underlying this is not clear although ligand application shows radiolabelled co‐localization of nuclear receptors followed by appropriate functional outcomes. Thus, application of radiolabelled http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1162 can be found in the nucleus along with the nuclear GnRH receptor in rat, hamster and human tissue whereupon it triggers histone H3 acetylation and phosphorylation within minutes of stimulation (Re et al., 2010). Re et al. (2010) speculate that GnRH is either transported intracellularly via an active uptake mechanism or that after peptide processing it is somehow transported back to the nucleus where GnRH receptors are activated. Intracrine signalling has also been proposed for CXCR4, which is abundantly expressed in the nuclei of various cancer cells especially metastatic prostate cancer cells. CXCR4 receptors are activated by http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4358 that can be made by the same cells that express CXCR4 on NMs (Don‐Salu‐Hewage et al., 2013). Although the exact mechanisms underlying peptidergic intracrine signalling are not yet known, mounting data suggest that it does indeed occur.
Besides binding ligand at the cell surface and subsequent trafficking to an intracellular site, GPCRs can also be activated at subcellular locations in a variety of ways (Figure 1). Ligands can enter cells via diffusion or be made in situ, endocytosed and/or transported through channels or pores (Boivin et al., 2008; Barlow et al., 2010; Tadevosyan et al., 2012). Since ligand binding sites would be within the vesicle or luminal region of the ER or nucleus, extracellular ligands would have to cross both the PM as well as the intracellular membrane to activate intracellular GPCRs (O'Malley et al., 2003). A highly permeable ligand might freely cross such membranes, whereas a less permeable, charged ligand might require an active transport process. For example, in order to activate mGlu5 receptors, at least two uptake systems are responsible for transporting glutamate into a neuron: the http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=163 and the http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=169#902 (Jong et al., 2005; 2007). Conditions that block either type of transporter reduce agonist uptake in cortical, hippocampal and striatal neurons (Jong et al., 2005; 2007; Purgert et al., 2014). Uptake of radiolabelled ligand can also be observed in isolated nuclei; nuclear ligand uptake can be blocked with sodium or chloride‐free buffers or by inclusion of transporter blockers, such as http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5413 or http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4631. Thus, for nuclear mGlu5 receptors, 90–95% of all ligand‐induced nuclear responses can be accounted for by these transporters (Jong et al., 2005; 2007; Purgert et al., 2014). Direct demonstration of the ‘intracrine’ effects of glutamate can be better achieved using microinjection of caged glutamate into mGlu5‐expressing neurons and then uncaging via restricted photoactivation (Jong and O'Malley, 2017). Only photoactivated neurons expressing caged glutamate exhibit mGlu5‐mediated Ca2+ responses, showcasing the spatial and temporal resolution of intracellular GPCRs (Jong and O'Malley, 2017). Similarly, Tadevosyan et al. (2015) prepared soluble caged Ang II compounds, which upon photoactivation can also stimulate nuclear angiotensin receptors to increase nuclear Ca2+ and, in turn, transcription.
In contrast to ligand transport, ligands might also be made in situ via localized biosynthetic machinery. For example, a large number of GPCRs such as the prostaglandin, platelet‐activating factor and lysophosphatidic acid (http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=36, whose ligands are bioactive lipids derived from membrane hydrolysis, are also located on NMs (Zhu et al., 2006). As ligand‐generating enzymes are present on NMs and because such ligands readily diffuse through lipid bilayers, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1883, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1831 and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2906 can easily activate their cognate receptors. A variation on this paradigm is exemplified by the http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3354 pathway. This pathway is regulated both by induction of its synthetic components and by their translocation and co‐localization at the nucleus. This allows for the effective production of LTC4 followed by its binding to the nuclear receptors, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=269 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=270. In turn, receptor activation leads to the nuclear translocation of NOX4, generation of ROS, subsequent DNA damage and apoptosis (Pedruzzi et al., 2004; Weyemi et al., 2012; Dvash et al., 2015). Alternatively, activation of nuclear‐localized GPCRs may not need ligands. Many GPCRs exhibit constitutive ligand‐independent activity that might allow nuclear receptors to function (Chidiac et al., 1994; Boivin et al., 2008). For example, proteins like Homer1a can lead to agonist‐independent mGlu5 receptor activation (Ango et al., 2001). Agonist‐independent activation of the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=370 also occurs due to a close association with the insulin‐like growth factor 1 receptor and subsequent transactivation by http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2206Delcourt et al., 2007). Collectively, these studies emphasize the notion that nuclear GPCRs are fully functional even when their ligand binding domain is inside the cell and even within an intracellular lumenal domain. Thus, as long as a ligand is either made in situ or transported to the site of action, an intracellular receptor can be activated (Boivin et al., 2008; Vaniotis et al., 2011; Tadevosyan et al., 2012; Figure 1). Whether nuclear GPCR desensitization occurs does not appear to have been specifically addressed to date. Since nuclei are reported to express PKCα, δ and ε (Wu et al., 2014) and striatal nuclei at least express GPCR kinase (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1466 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1469 as well as arrestins 2 and 3 (Bychkov et al., 2012), some desensitization machinery is present in nuclei. However, confirmation or resolution of this issue awaits more definitive studies.
Signalling of intracellular GPCRs
Signalling molecules that have traditionally been considered to be associated with the PM or in the cytosol have also been demonstrated in the nucleus or on NMs. Both monomeric G proteins (e.g. Ras, Rab, Rho and Ran) and heterotrimeric G proteins (Gi, Gs, Gq, G11, G12, G13 and G16) (Campden et al., 2015) have been found in the nucleoplasm along with other effector molecules such as http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=257, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=275, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=274 and http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=276, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=260 (Branco and Allen, 2015), DAG kinase, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=672, β‐arrestin‐1 and http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=283 (Branco and Allen, 2015; Campden et al., 2015). Many other regulatory proteins (PKA, PKC and http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=891&familyType=OTHER proteins) and critical channels [inositol 1,4,5‐trisphosphate (http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=123 and http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=125] are present (Campden et al., 2015). Luminal Ca2+ is refilled at least in part by the nuclear http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=159s (Nicotera et al., 1989; Petersen et al., 1998) located on the outer NM. Thus, although signals originating at the PM may be transmitted to the nucleus (Power and Sah, 2002), the presence of specific signalling machinery on the NM or in the nucleus argues for a nuclear regulatory system independent of the PM. For example, the nuclear mGlu5 receptor couples to Gq/11 and PI‐PLC in striatal nuclei to generate IP3‐mediated release of Ca2+ via IP3 and ryanodine receptors in the nucleus (Kumar et al., 2008). Further, nuclear http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=22 on cardiac myocytes also signal through Gq/11 leading to PKCδ activation. The latter then translocates into the cytoplasm leading to troponin phosphorylation and sarcomere shortening (Wu et al., 2014; Wu and O'Connell, 2015; Figure 1). Mislocalization of either the receptor or PKCδ blocked the effects of these α1A‐adrenoceptors (Wu et al., 2014; Wu and O'Connell, 2015). http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=989 also increases nuclear Ca2+ in isolated cardiac nuclei, cardiomyocytes and whole heart (Branco and Allen, 2015). Similarly, intracellular release of a caged ET‐1 analogue also evokes an increase in Ca2+ that is attenuated by the IP3 receptor blocker, 1,3‐dicyclohexylcarbodiimide (Merlen et al., 2013). Conversely, a caged cell‐permeable endothelin ETB receptor antagonist blocks the ability of intracellular ET‐1 to increase nuclear Ca2+, whereas extracellular ETB receptor antagonists do not (Merlen et al., 2013). Hence, acting as intracrine ligand, ET‐1 is able to trigger an IP3‐mediated release of Ca2+ from perinuclear stores, which is involved in regulating transcription. Therefore, activated nuclear GPCRs can induce nuclear Ca2+ changes independent of cytosolic Ca2+. Moreover, just as cell surface GPCR activation can trigger nuclear changes, activation of nuclear GPCRs can trigger profound cytoplasmic effects as well.
Despite the large number of GPCRs found on NMs, deducing their functional significance remains challenging because of the difficulty in probing the nucleus in situ, and because these same GPCRs are also present at the cell surface. Novel genetic and pharmacological strategies are overcoming some of these hurdles; several GPCRs have now been shown to play unique roles at the NM versus the cell surface – in vivo. For example, activation of the GPCR PAR2 anchored at PMs triggered the expression of the angiogenic gene, angiopoietin 1, whereas nuclear‐activated PAR2 induced an increase in http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5085 in retinal ganglion cells providing in vivo evidence for a physiological function brought about by the receptor's subcellular localization (Joyal et al., 2014). Similarly, ligand‐stimulated, nuclear PARs activate angiogenic genes such as Vegfa and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1249, whereas cell surface PARs induce an up‐regulation of pro‐inflammatory cytokines in vivo (Bhosle et al., 2016). Taken together, these data suggest that nuclear signalling may represent a versatile, independent system by which nuclear function is regulated.
Outside the nuclear membrane
Endosomal GPCRs
Desensitization and endosomal internalization of GPCRs is a well‐known mechanism to regulate receptor number via degradation and/or resensitization. In addition, this process is emerging as a major mechanism by which internalized GPCRs generate unique signalling responses that are different from those initiated at the PM. For example, ligand‐activated GPCRs are phosphorylated by GRKs, which, in turn, promote the recruitment of β‐arrestins. The latter serve as scaffolding proteins that can themselves initiate a programme of G‐protein‐independent signalling (Shenoy and Lefkowitz, 2011). It is thought that while the cell surface GPCR might rapidly desensitize, its internalization as a receptor/G‐protein/β‐arrestin complex leads to a stable complex generating sustained endosomal signals (Bahouth and Nooh, 2017). Although initially arrestin‐mediated signalling focused on the http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=512/http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=514 cascade, many other signalling moieties can interact with the receptor/G‐protein/β‐arrestin complex such as http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=285, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1499, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=518 and activators of transcription, STATs (Reiter et al., 2012). In turn, these proteins mediate downstream functions such as growth, cell survival, apoptosis, contractility, cell migration and cytoskeletal reorganization (Reiter et al., 2012). While recognizing the ability of GPCRs to adopt distinct conformations contributing to the overall outcome of receptor activation, many drug discovery teams are searching for distinct ligands that can modulate these processes (Geppetti et al., 2015; Rankovic et al., 2016).
Besides G‐protein‐independent signalling, internalized endosomal GPCRs can also trigger G‐protein‐dependent signalling. For example, conformation‐specific single‐domain antibodies (nanobodies) were used to directly assess activation of the http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=29. This approach verified bona fide G‐protein signalling from early endosomes (Irannejad et al., 2013, 2017). Using nanobody tools, an activated conformational state of the β2‐adrenoceptor was first detected at the PM seconds after ligand application. Shortly thereafter, a second activation phase was detected on the endosomes, which lasted long after the PM signals had diminished (Tsvetanova and von Zastrow, 2014). Interestingly, although both the PM receptor and the endosomal receptor generated cAMP, the G‐protein‐dependent response of the latter induced unique cAMP‐generated transcriptional responses compared to those generated by the cell surface receptor (Tsvetanova and von Zastrow, 2014). In addition to Gαs endosomal signalling (Vilardaga et al., 2014; Irannejad et al., 2017), Jensen et al. (2017) recently demonstrated that internalized http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=360 contributed to sustained endosomal signalling via Gαq. Endosomal NK1 receptor/Gαq signalling but not cell surface NK1 receptors induced an increase in cytosolic cAMP, PKC and nuclear ERK resulting in neuronal excitation and nociception. As described below, only compounds preventing internalization or blocking the endosomal receptor were effective in blocking pain transmission.
Mitochondrial GPCRs
As with nuclear and endosomal GPCRs, there is a growing list of mitochondrial GPCRs. These include the purine, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=323 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=324 (Belous et al., 2004), http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=9 (Wang et al., 2016), angiotensin (Ang II) types 1 and 2 receptors (Abadir et al., 2011), http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=287 (Gbahou et al., 2017) and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=56 (Benard et al., 2012; Ma et al., 2015: Hebert‐Chatelain et al., 2014a,b, 2016; Xu et al., 2016; Melser et al., 2017). To date, activation and/or inhibition of mitochondrial GPCRs appears to affect processes such as mitochondrial Ca2+ uptake, ATP production, ROS production and even apoptosis. For example, several studies have shown that activated CB1 receptors trigger a cascade of events mediated by intra‐mitochondrial Gαi, which inhibits soluble adenylate cyclase leading to decreased cAMP, decreased PKA phosphorylation of various complex 1 proteins and a subsequent decrease in mitochondrial activity (Benard et al., 2012; Hebert‐Chatelain et al., 2016). Short‐term consequences of mitochondrial CB1 receptor signalling include loss of mitochondrial mobility and synaptic depression; long‐term consequences can include memory loss (Hebert‐Chatelain et al., 2016), metabolic defects and apoptosis (Xu et al., 2016). Tools to determine the contribution of mitochondrial verse cell surface receptors will be critical in developing new drug targets and/or targeted therapeutics going forward.
Pathophysiological consequences of intracellular GPCR signalling
Emerging evidence furthers the notion that GPCR localization also plays an important role in various disease conditions. As described above, several chemokine receptors, such as CXCR4, are found on NMs in cancer cells where they have been shown to be ligand‐responsive. Their activation leads to increased nucleoplasmic Ca2+ levels, which appear to enhance and extend signals initiated in the cytoplasm. Since increased amplitude and/or duration of nuclear Ca2+ is known to differentially activate unique transcription factors, nuclear chemokine receptors may be critical in regulating the process of tumourigenesis in given cell types. If so, impermeable or non‐transported antagonists targeting cell surface receptors may be ineffectual for nuclear receptors. Another intracellular receptor playing a role in a disease process is spinal cord dorsal horn mGlu5. In the spinal cord, the mGlu5 receptor is a key mediator of neuroplasticity underlying persistent pain. Recently, we showed that the expression of mGlu5 receptors is increased on spinal NMs following nerve injury in a model of neuropathic pain (Vincent et al., 2016). Biochemical, pharmacological and ultrastructural studies all support the notion that increased mGlu5 receptor levels are observed on spinal nuclei following nerve injury, whereas decreased numbers of receptors are present on the cell surface or intracellular membranes (Vincent et al., 2016). Amazingly, blockade of spinal nuclear mGlu5 receptors inhibits pain behaviours, whereas blockade of cell surface mGlu5 receptors has little effect. Moreover, inhibition of a glutamate transporter mimics the effects of intracellular mGlu5 antagonism by preventing intracellular uptake of the ligand. The re‐localization of mGlu5 receptors away from the cell surface onto NMs reinforces the importance of considering location in the drug discovery process: drugs designed to antagonize cell surface mGlu5 receptors or unable to pass through the lipid bilayer or be transported into the nuclear lumen would be ineffectual in this chronic pain model. Location‐dependent signalling of receptors redistributed to endosomes also contributes to disease pathology. As described above, Jensen et al. (2017) discovered that substance P/NK1 receptor‐mediated nociception is mediated by internalized, endosomal NK1 receptors, not cell surface receptors. Inhibition of endocytosis (dynamin, clathrin or β‐arrestin inhibitors) prevented pain signalling and promoted antinociception as did targeting NK1 receptor antagonists to the endosomes (Jensen et al., 2017). These findings reinforce the notion that where the receptor is signalling from (location‐dependence) plays a critical role in whether a given ligand can modulate its receptor: conventional NK1 receptor antagonists are clinically ineffective for the treatment of chronic pain because the receptor is no longer on the cell surface but rather is signalling from the early endosome promoting nociception (Jensen et al., 2017). Taken together, GPCR localization can strongly contribute to receptor function and even disease pathology, underscoring the importance of designing drugs to block or enhance a given biological output in the context of where the receptor is signalling from.
New opportunities
Just as biased agonism led to a paradigm shift in GPCR research and drug development, emerging data documenting G‐protein‐dependent signalling from intracellular GPCRs should result in a similar marked change. Although for some GPCRs like the PAR2, receptor activation and/or inhibition may occur at the cell surface; for others, such as the mGlu5 receptor and/or the https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=3&cad=rja&uact=8&ved=0ahUKEwiDsp6cqrvVAhWr64MKHWP9AHIQFgg7MAI&url=https%3A%2F%2Fwww.ncbi.nlm.nih.gov%2Fpmc%2Farticles%2FPMC3930413%2F&usg=AFQjCNEaQB5359zmSlQuF092N7FE4mEbxAceptor, whether a ligand gets across a given cellular membrane may change a its functional response (Figure 1). Thus, drugs with a desirable pharmacokinetic outcome might be further optimized for a desirable cell surface and/or intracellular response. In the latter case, the same key parameters associated with drug development for cell surface receptors such as efficacy, potency and specificity are still essential for intracellular GPCR drug design, with the added requirement that the preferred outcome might necessitate the drug accessing the cell's interior without perturbing its cell surface counterpart.
What are the ways in which an intracellular receptor can be activated or inhibited without blocking cell surface function? Furthermore, can intracellular modulators be targeted to a given subcellular organelle? Nuclei? Mitochondria? Lysosomes? Ways in which to address these issues are emerging as new challenges and opportunities for intracellular drug delivery. The in vitro and/or ex vivo applications have often used so‐called caged ligands in which the biologically active molecule is protected by a functional group, which, upon cellular uptake, can be activated by intracellular enzymes, pH, light and so forth. Indeed, caged compounds have been shown to activate intracellular receptors in vitro (Tadevosyan et al., 2016; Jong and O'Malley, 2017). Although caged compounds are useful for a given set of in vitro applications, in vivo applications would be limited. Fortunately, a wave of new techniques drawn from chemistry, materials science and nanotechnology are creating a plethora of novel delivery strategies that can safely get a drug or a biomolecule into the cell and even to the appropriate organelle in the cytoplasm (Stewart et al., 2016). For example, to prevent NK1 receptor endosomal signalling, Jensen et al. (2017) synthesized tripartite compounds composed of cholestenol to promote membrane insertion, a polyethylene linker for flexibility and a membrane impermeable NK1 receptor antagonist. This strategy successfully delivered NK1 receptor antagonists into the endosome, blocking further NK1 receptor signalling and promoting antinociception (Jensen et al., 2017). Other new techniques include polymer‐based nanocarriers, which can be tailored to display a given charge or combined with other biomolecules such as drugs, antibodies, proteins and oligonucleotides in order to more effectively deliver the desired entity to the particular intracellular location (Cohen and Granek, 2014; Wang et al., 2014; Ye et al., 2016). Collectively, it seems clear that next‐generation technologies will allow an unprecedented ability to deliver therapeutics to every part of the cell.
In summary, current efforts to develop allosteric modulators and biased ligands for GPCRs might be further enhanced by recognizing that intracellular GPCRs are functional. Given the ‘drugability’ of GPCRs, the need to selectively tailor agonists and/or antagonists to both intracellular and cell surface receptors is critical. This would be highly significant for translational applications, since developing the most highly effective and minimally toxic drugs is the long‐term goal for any effective treatment.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015a,b,c,d,e).
Conflict of interest
The authors declare no conflicts of interest.
Jong, Y.‐J. I. , Harmon, S. K. , and O'Malley, K. L. (2018) GPCR signalling from within the cell. British Journal of Pharmacology, 175: 4026–4035. 10.1111/bph.14023.
References
- d'Addio M, Pizzigoni A, Bassi MT, Baschirotto C, Valetti C, Incerti B et al (2000). Defective intracellular transport and processing of OA1 is a major cause of ocular albinism type 1. Hum Mol Genet 9: 3011–3018. [DOI] [PubMed] [Google Scholar]
- Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A et al (2011). Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci U S A 108: 14849–14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Catterall WA, Kelly E, Marrion N, Peters JA, Benson HE et al (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Voltage‐gated ion channels. Br J Pharmacol 172: 5904–5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al (2015b). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al (2015d). The Concise Guide to PHARMACOLOGY 2015/16: Transporters. Br J Pharmacol 172: 6110–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Peters JA, Kelly E, Marrion N, Benson HE, Faccenda E et al (2015e). The Concise Guide to PHARMACOLOGY 2015/16: Ligand‐gated ion channels. Br J Pharmacol 172: 5870–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ango F, Prézeau L, Muller T, Tu JC, Xiao B, Worley PF et al (2001). Agonist‐independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature 411: 962–965. [DOI] [PubMed] [Google Scholar]
- Bahouth SW, Nooh MM (2017). Barcoding of GPCR trafficking and signaling through the various trafficking roadmaps by compartmentalized signaling networks. Cell Signal 36: 42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow CA, Laishram RS, Anderson RA (2010). Nuclear phosphoinositides: a signaling enigma wrapped in a compartmental conundrum. Trends Cell Biol 20: 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belous A, Wakata A, Knox CD, Nicoud IB, Pierce J, Anderson CD et al (2004). Mitochondrial P2Y‐like receptors link cytosolic adenosine nucleotides to mitochondrial calcium uptake. J Cell Biochem 92: 1062–1073. [DOI] [PubMed] [Google Scholar]
- Benard G, Massa F, Puente N, Lourenco J, Bellocchio L, Soria‐Gomez E et al (2012). Mitochondrial CB(1) receptors regulate neuronal energy metabolism. Nat Neurosci 15: 558–564. [DOI] [PubMed] [Google Scholar]
- Bhattacharya M, Peri K, Ribeiro‐da‐Silva A, Almazan G, Shichi H, Hou X et al (1999). Localization of functional prostaglandin E2 receptors EP3 and EP4 in the nuclear envelope. J Biol Chem 274: 15719–15724. [DOI] [PubMed] [Google Scholar]
- Bhosle VK, Rivera JC, Chemtob S (2017). New insights into mechanisms of nuclear translocation of G‐protein coupled receptors. Small GTPases. 10.1080/21541248.2017.1282402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhosle VK, Rivera JC, Zhou TE, Omri S, Sanchez M, Hamel D et al (2016). Nuclear localization of platelet‐activating factor receptor controls retinal neovascularization. Cell Discov 2: 16017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bkaily G, Nader M, Avedanian L, Choufani S, Jacques D, D'Orléans‐Juste P et al (2006). G‐protein‐coupled receptors, channels, and Na+–H+ exchanger in nuclear membranes of heart, hepatic, vascular endothelial, and smooth muscle cells. Can J Physiol Pharmacol 84: 431–441. [DOI] [PubMed] [Google Scholar]
- Boivin B, Vaniotis G, Allen BG, Hébert TE (2008). G protein‐coupled receptors in and on the cell nucleus: a new signaling paradigm? J Recept Signal Transduct Res 28: 15–28. [DOI] [PubMed] [Google Scholar]
- Branco AF, Allen BG (2015). G‐protein‐coupled receptor signaling in cardiac nuclear membranes. J Cardiovasc Pharmacol 65: 101–109. [DOI] [PubMed] [Google Scholar]
- Bychkov E, Zurkovsky L, Garret MB, Ahmed MR, Gurevich EV (2012). Distinct cellular and subcellular distributions of G protein‐coupled receptor kinase and arrestin isoforms in the striatum. PLoS One 7: e48912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calebiro D, Godbole A, Lyga S, Lohse MJ (2015). Trafficking and function of GPCRs in the endosomal compartment. Methods Mol Biol 1234: 97–211. [DOI] [PubMed] [Google Scholar]
- Calebiro D, Nikolaev VO, Persani L, Lohse MJ (2010). Signaling by internalized G protein‐coupled receptors. Trends Pharmacol Sci 31: 221–228. [DOI] [PubMed] [Google Scholar]
- Campden R, Audet N, Hébert TE (2015). Nuclear G protein signaling: new tricks for old dogs. J Cardiovasc Pharmacol 65: 110–122. [DOI] [PubMed] [Google Scholar]
- Chidiac P, Hebert TE, Valiquette M, Dennis M, Bouvier M (1994). Inverse agonist activity of beta‐adrenergic antagonists. Mol Pharmacol 45: 490–499. [PubMed] [Google Scholar]
- Cohen O, Granek R (2014). Nucleus‐targeted drug delivery: theoretical optimization of nanoparticles decoration for enhanced intracellular active transport. Nano Lett 14: 2515–2521. [DOI] [PubMed] [Google Scholar]
- De Filippo E, Schiedel AC, Manga P (2017). Interaction between G protein‐coupled receptor 143 and tyrosinase: implications for understanding ocular albinism type 1. J Invest Dermatol 137: 457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcourt N, Thouvenot E, Chanrion B, Galéotti N, Jouin P, Bockaert J et al (2007). ACAP type I receptor transactivation is essential for IGF‐1 receptor signalling and antiapoptotic activity in neurons. EMBO J 26: 1542–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Benedetto A, Sun L, Zambonin CG, Tamma R, Nico B, Calvano CD et al (2014). Osteoblast regulation via ligand‐activated nuclear trafficking of the oxytocin receptor. Proc Natl Acad Sci U S A 111: 16502–16507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don‐Salu‐Hewage AS, Chan SY, McAndrews KM, Chetram MA, Dawson MR, Bethea DA et al (2013). Cysteine (C)‐x‐C receptor 4 undergoes transportin 1‐dependent nuclear localization and remains functional at the nucleus of metastatic prostate cancer cells. PLoS One 8: e57194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvash E, Har‐Tal M, Barak S, Meir O, Rubinstein M (2015). Leukotriene C4 is the major trigger of stress‐induced oxidative DNA damage. Nat Commun 6: 10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada R, Wang L, Jala VR, Lee JF, Lin CY, Gray RD et al (2009). Ligand‐induced nuclear translocation of S1P(1) receptors mediates Cyr61 and CTGF transcription in endothelial cells. Histochem Cell Biol 131: 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favre N, Camps M, Arod C, Chabert C, Rommel C et al (2008). Chemokine receptor CCR2 undergoes transportin1‐dependent nuclear translocation. Proteomics 8: 4560–4576. [DOI] [PubMed] [Google Scholar]
- Gbahou F, Cecon E, Viault G, Gerbier R, Jean‐Alphonse F, Karamitri A et al (2017). Design and validation of the first cell‐impermeant melatonin receptor agonist. Br J Pharmacol 174: 2409–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppetti P, Veldhuis NA, Lieu T, Bunnett NW (2015). G protein‐coupled receptors: dynamic machines for signaling pain and itch. Neuron 88: 635–649. [DOI] [PubMed] [Google Scholar]
- Gobeil F, Fortier A, Zhu T, Bossolasco M, Leduc M, Grandbois M et al (2006). G‐protein‐coupled receptors signalling at the cell nucleus: an emerging paradigm. Can J Physiol Pharmacol 84: 287–297. [DOI] [PubMed] [Google Scholar]
- Goshima Y, Nakamura F, Masukawa D, Chen S, Koga M (2014). Cardiovascular actions of DOPA mediated by the gene product of ocular albinism 1. J Pharmacol Sci 126: 14–20. [DOI] [PubMed] [Google Scholar]
- Hebert‐Chatelain E, Desprez T, Serrat R, Bellocchio L, Soria‐Gomez E, Busquets‐Garcia A et al (2016). A cannabinoid link between mitochondria and memory. Nature 539: 555–559. [DOI] [PubMed] [Google Scholar]
- Hebert‐Chatelain E, Reguero L, Puente N, Lutz B, Chaouloff F, Rossignol R et al (2014a). Cannabinoid control of brain bioenergetics: exploring the subcellular localization of the CB1. Mol Metab 3: 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert‐Chatelain E, Reguero L, Puente N, Lutz B, Chaouloff F, Rossignol R et al (2014b). Studying mitochondrial CB1 receptors: yes we can. Mol Metab 3: 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw JE, Carragher BO, Milligan RA (1992). Architecture and design of the nuclear pore complex. Cell 69: 1133–1141. [DOI] [PubMed] [Google Scholar]
- Hubert GW, Paquet M, Smith Y (2001). Differential subcellular localization of mGluR1a and mGluR5 in the rat and monkey substantia nigra. J Neurosci 21: 1838–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irannejad R, Pessino V, Mika D, Huang B, Wedegaertner PB, Conti M et al (2017). Functional selectivity of GPCR‐directed drug action through location bias. Nat Chem Biol 13: 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irannejad R, Tomshine JC, Tomshine JR, Chevalier M, Mahoney JP, Steyaert J et al (2013). Conformational biosensors reveal GPCR signalling from endosomes. Nature 495: 534–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen DD, Lieu T, Halls ML, Veldhuis NA, Imlach WL, Mai QN et al (2017). Neurokinin 1 receptor signaling in endosomes mediates sustained nociception and is a viable therapeutic target for prolonged pain relief. Sci Transl Med 9 pii: eaal3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong YI, O'Malley KL (2017). Mechanisms associated with activation of intracellular metabotropic glutamate receptor, mGluR5. Neurochem Res 42: 166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong YJ, Kumar V, Kingston AE, Romano C, O'Malley KL (2005). Functional metabotropic glutamate receptors on nuclei from brain and primary cultured striatal neurons. Role of transporters in delivering ligand. J Biol Chem 280: 30469–30480. [DOI] [PubMed] [Google Scholar]
- Jong YJ, Schwetye KE, O'Malley KL (2007). Nuclear localization of functional metabotropic glutamate receptor mGlu1 in HEK293 cells and cortical neurons: role in nuclear calcium mobilization and development. J Neurochem 101: 458–469. [DOI] [PubMed] [Google Scholar]
- Jong YJ, Sergin I, Purgert CA, O'Malley KL (2014). Location‐dependent signaling of the group 1 metabotropic glutamate receptor mGlu5. Mol Pharmacol 86: 774–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyal JS, Bhosle VK, Chemtob S (2015). Subcellular G‐protein coupled receptor signaling hints at greater therapeutic selectivity. Expert Opin Ther Targets 19: 717–721. [DOI] [PubMed] [Google Scholar]
- Joyal JS, Nim S, Zhu T, Sitaras N, Rivera JC, Shao Z et al (2014). Subcellular localization of coagulation factor II receptor‐like 1 in neurons governs angiogenesis. Nat Med 20: 1165–1173. [DOI] [PubMed] [Google Scholar]
- Katta SS, Smoyer CJ, Jaspersen SL (2014). Destination: inner nuclear membrane. Trends Cell Biol 24: 221–229. [DOI] [PubMed] [Google Scholar]
- Kinsey CG, Bussolati G, Bosco M, Kimura T, Pizzorno MC, Chernin MI et al (2007). Constitutive and ligand‐induced nuclear localization of oxytocin receptor. J Cell Mol Med 11: 96–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Jong YJ, O'Malley KL (2008). Activated nuclear metabotropic glutamate receptor mGlu5 couples to nuclear Gq/11 proteins to generate inositol 1,4,5‐trisphosphate‐mediated nuclear Ca2+ release. J Biol Chem 283: 14072–14083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DK, Lança AJ, Cheng R, Nguyen T, Ji XD, Gobeil F Jr et al (2004). Agonist‐independent nuclear localization of the apelin, angiotensin AT1, and bradykinin B2 receptors. J Biol Chem 279: 7901–7908. [DOI] [PubMed] [Google Scholar]
- López‐Bendito G, Shigemoto R, Fairén A, Luján R (2002). Differential distribution of group I metabotropic glutamate receptors during rat cortical development. Cereb Cortex 12: 625–638. [DOI] [PubMed] [Google Scholar]
- Lusk CP, Blobel G, King MC (2007). Highway to the inner nuclear membrane: rules for the road. Nat Rev Mol Cell Biol 8: 414–420. [DOI] [PubMed] [Google Scholar]
- Ma L, Jia J, Niu W, Jiang T, Zhai Q, Yang L et al (2015). Mitochondrial CB1 receptor is involved in ACEA‐induced protective effects on neurons and mitochondrial functions. Sci Rep 5: 12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao LM, Liu XY, Zhang GC, Chu X, Fibuch EE, Wang LS et al (2008). Phosphorylation of group I metabotropic glutamate receptors (mGluR1/5) in vitro and in vivo. Neuropharmacology 55: 403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melser S, Zottola ACP, Serrat R, Puente N, Grandes P, Marsicano G et al (2017). Functional analysis of mitochondrial CB1 cannabinoid receptors (mtCB1) in the brain. Methods Enzymol 593: 143–174. [DOI] [PubMed] [Google Scholar]
- Merlen C, Farhat N, Luo X, Chatenet D, Tadevosyan A, Villeneuve LR et al (2013). Intracrine endothelin signaling evokes IP3‐dependent increases in nucleoplasmic Ca2+ in adult cardiac myocytes. J Mol Cell Cardiol 62: 189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinelli TA, Raymond JR, Baldys A, Yang Q, Lee MH, Luttrell L et al (2007). Identification of a putative nuclear localization sequence within ANG II AT(1A) receptor associated with nuclear activation. Am J Physiol Cell Physiol 292: C1398–C1408. [DOI] [PubMed] [Google Scholar]
- Nicotera P, McConkey DJ, Jones DP, Orrenius S (1989). ATP stimulates Ca2+ uptake and increases the free Ca2+ concentration in isolated rat liver nuclei. Proc Natl Acad Sci U S A 86: 453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley KL, Jong YJ, Gonchar Y, Burkhalter A, Romano C (2003). Activation of metabotropic glutamate receptor mGlu5 on nuclear membranes mediates intranuclear Ca2+ changes in heterologous cell types and neurons. J Biol Chem 278: 28210–28219. [DOI] [PubMed] [Google Scholar]
- Pedruzzi E, Guichard C, Ollivier V, Driss F, Fay M, Prunet C et al (2004). NAD(P)H oxidase Nox‐4 mediates 7‐ketocholesterol‐induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol Cell Biol 24: 10703–10717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen OH, Gerasimenko OV, Gerasimenko JV, Mogami H, Tepikin AV (1998). The calcium store in the nuclear envelope. Cell Calcium 23: 87–90. [DOI] [PubMed] [Google Scholar]
- Poole DP, Bunnett NW (2016). G protein‐coupled receptor trafficking and signalling in the enteric nervous system: the past, present and future. Adv Exp Med Biol 891: 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JM, Sah P (2002). Nuclear calcium signaling evoked by cholinergic stimulation in hippocampal CA1 pyramidal neurons. J Neurosci 22: 3454–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purgert CA, Izumi Y, Jong YJ, Kumar V, Zorumski CF, O'Malley KL (2014). Intracellular mGlu5 can mediate synaptic plasticity in the hippocampus. J Neurosci 34: 4589–4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankovic Z, Brust TF, Bohn LM (2016). Biased agonism: an emerging paradigm in GPCR drug discovery. Bioorg Med Chem Lett 26: 241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re M, Pampillo M, Savard M, Dubuc C, McArdle CA, Millar RP et al (2010). The human gonadotropin releasing hormone type I receptor is a functional intracellular GPCR expressed on the nuclear membrane. PLoS One 5: e11489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter E, Ahn S, Shukla AK, Lefkowitz RJ (2012). Molecular mechanism of beta‐arrestin‐biased agonism at seven‐transmembrane receptors. Annu Rev Pharmacol Toxicol 52: 179–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaraweera P, Shen B, Newton JM, Barsh GS, Orlow SJ (2001). The mouse ocular albinism 1 gene product is an endolysosomal protein. Exp Eye Res 72: 319–329. [DOI] [PubMed] [Google Scholar]
- Schiaffino MV, Baschirotto C, Pellegrini G, Montalti S, Tacchetti C, De Luca M et al (1996). The ocular albinism type 1 gene product is a membrane glycoprotein localized to melanosomes. Proc Natl Acad Sci U S A 93: 9055–9060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergin I, Jong YI, Harmon SK, Kumar V, O'Malley KL (2017). Sequences within the C terminus of the metabotropic glutamate receptor 5 (mGluR5) are responsible for inner nuclear membrane localization. J Biol Chem 292: 3637–3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ (2011). β‐Arrestin‐mediated receptor trafficking and signal transduction. Trends Pharmacol Sci 32: 521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart MP, Sharei A, Ding X, Sahay G, Langer R, Jensen KF (2016). In vitro and ex vivo strategies for intracellular delivery. Nature 538: 183–192. [DOI] [PubMed] [Google Scholar]
- Sun RL, Huang CX, Bao JL, Jiang JY, Zhang B, Zhou SX et al (2016). CC‐chemokine ligand 2 (CCL2) suppresses high density lipoprotein (HDL) internalization and cholesterol efflux via CC‐chemokine receptor 2 (CCR2) induction and p42/44 mitogen‐activated protein kinase (MAPK) activation in human endothelial cells. J Biol Chem 291: 19532–19544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadevosyan A, Létourneau M, Folch B, Doucet N, Villeneuve LR, Mamarbachi AM et al (2015). Photoreleasable ligands to study intracrine angiotensin II signalling. J Physiol 593: 521–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadevosyan A, Vaniotis G, Allen BG, Hébert TE, Nattel S (2012). G protein‐coupled receptor signalling in the cardiac nuclear membrane: evidence and possible roles in physiological and pathophysiological function. J Physiol 590: 1313–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadevosyan A, Villeneuve LR, Fournier A, Chatenet D, Nattel S, Allen BG (2016). Caged ligands to study the role of intracellular GPCRs. Methods 92: 72–77. [DOI] [PubMed] [Google Scholar]
- Trivedi RR, Bhattacharyya S (2012). Constitutive internalization and recycling of metabotropic glutamate receptor 5 (mGluR5). Biochem Biophys Res Commun 427: 185–190. [DOI] [PubMed] [Google Scholar]
- Tsvetanova NG, von Zastrow M (2014). Spatial encoding of cyclic AMP signaling specificity by GPCR endocytosis. Nat Chem Biol 10: 1061–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaniotis G, Allen BG, Hébert TE (2011). Nuclear GPCRs in cardiomyocytes: an insider's view of β‐adrenergic receptor signaling. Am J Physiol Heart Circ Physiol 301: H1754–H1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzijl D, Peters SL, Alewijnse AE (2010). Sphingosine‐1‐phosphate receptors: zooming in on ligand‐induced intracellular trafficking and its functional implications. Mol Cells 29: 99–104. [DOI] [PubMed] [Google Scholar]
- Vilardaga JP, Jean‐Alphonse FG, Gardella TJ (2014). Endosomal generation of cAMP in GPCR signaling. Nat Chem Biol 10: 700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent K, Cornea VM, Jong YJ, Laferrière A, Kumar N, Mickeviciute A et al (2016). Intracellular mGluR5 plays a critical role in neuropathic pain. Nat Commun 7: 10604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Wang Y, Zhang X, Zhang W, Guo S, Jin F (2014). Recent progress of cell‐penetrating peptides as new carriers for intracellular cargo delivery. J Control Release 174: 126–136. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhang H, Xu H, Guo D, Shi H, Li Y et al (2016). 5‐HTR3 and 5‐HTR4 located on the mitochondrial membrane and functionally regulated mitochondrial functions. Sci Rep 6: 37336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyemi U, Lagente‐Chevallier O, Boufraqech M, Prenois F, Courtin F et al (2012). ROS‐generating NADPH oxidase NOX4 is a critical mediator in oncogenic H‐Ras‐induced DNA damage and subsequent senescence. Oncogene 31: 1117–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CD, Wu SC, Dahl EF, Sazama AJ, O'Connell TD (2012). Nuclear localization drives α1‐adrenergic receptor oligomerization and signaling in cardiac myocytes. Cell Signal 24: 794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SC, Dahl EF, Wright CD, Cypher AL, Healy CL, O'Connell TD (2014). Nuclear localization of a1A‐adrenergic receptors is required for signaling in cardiac myocytes: an “inside‐out” a1‐AR signaling pathway. J Am Heart Assoc 3: e000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SC, OʼConnell TD (2015). Nuclear compartmentalization of α1‐adrenergic receptor signaling in adult cardiac myocytes. J Cardiovasc Pharmacol 65: 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Lv XA, Dai Q, Ge YQ, Xu J (2016). Acute upregulation of neuronal mitochondrial type‐1 cannabinoid receptor and its role in metabolic defects and neuronal apoptosis after TBI. Mol Brain 9: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Liu E, Yu Z, Pei X, Chen S, Zhang P et al (2016). CPP‐assisted intracellular drug delivery, what is next? Int J Mol Sci 17: E1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R, Liu H, Peng X, Cui Y, Song S, Wang L et al (2017). The palmitoylation of the N‐terminal extracellular Cys37 mediates the nuclear translocation of VPAC1 contributing to its anti‐apoptotic activity. Oncotarget https://doi.org/10.18632/oncotarget.17449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T, Gobeil F, Vazquez‐Tello A, Leduc M, Rihakova L, Bossolasco M et al (2006). Intracrine signaling through lipid mediators and their cognate nuclear G‐protein‐coupled receptors: a paradigm based on PGE2, PAF, and LPA1 receptors. Can J Physiol Pharmacol 84: 377–391. [DOI] [PubMed] [Google Scholar]
- Zuleger N, Kerr AR, Schirmer EC (2012). Many mechanisms, one entrance: membrane protein translocation into the nucleus. Cell Mol Life Sci 69: 2205–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]


