Abstract
Background and Purpose
Strontium ranelate, a drug approved and until recently used for the treatment of osteoporosis, mediates its effects on bone at least in part via the calcium‐sensing (CaS) receptor. However, it is not known whether bone‐targeted CaS receptor positive allosteric modulators (PAMs; calcimimetics) represent an alternative (or adjunctive) therapy to strontium (Sr2+ o).
Experimental Approach
We assessed three structurally distinct calcimimetics [cinacalcet, AC‐265347 and a benzothiazole tri‐substituted urea (BTU‐compound 13)], alone and in combination with extracellular calcium (Ca2+ o) or Sr2+ o, in G protein‐dependent signalling assays and trafficking experiments in HEK293 cells and their effects on cell differentiation, tartrate‐resistant acid phosphatase (TRAP) activity and hydroxyapatite resorption assays in human blood‐derived osteoclasts.
Key Results
Sr2+ o activated CaS receptor‐dependent signalling in HEK293 cells in a similar manner to Ca2+ o, and inhibited the maturation, TRAP expression and hydroxyapatite resorption capacity of human osteoclasts. Calcimimetics potentiated Ca2+ o‐ and Sr2+ o‐mediated CaS receptor signalling in HEK293 cells with distinct biased profiles, and only cinacalcet chaperoned an endoplasmic reticulum‐retained CaS mutant receptor to the cell surface in HEK293 cells, indicative of a conformational state different from that engendered by AC‐265347 and BTU‐compound 13. Intriguingly, only cinacalcet modulated human osteoclast function, reducing TRAP activity and profoundly inhibiting resorption.
Conclusion and Implications
Although AC‐265347 and BTU‐compound 13 potentiated Ca2+ o‐ and Sr2+ o‐induced CaS receptor activation, they neither replicated nor potentiated the ability of Sr2+ o to inhibit human osteoclast function. In contrast, the FDA‐approved calcimimetic, cinacalcet, inhibited osteoclast TRAP activity and hydroxyapatite resorption, which may contribute to its clinical effects on bone mineral density
Linked Articles
This article is part of a themed section on Molecular Pharmacology of GPCRs. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v175.21/issuetoc
Abbreviations
- BMD
bone mineral density
- CaS receptor
calcium‐sensing receptor
- Ca2+o
extracellular calcium
- M‐CSF
macrophage colony‐stimulating factor
- PBMC
peripheral blood mononuclear cell
- PAM
positive allosteric modulator
- PTH
parathyroid hormone
- RANK‐L
receptor activator of NF‐κB ligand
- Sr2+o
strontium
- TRAP
tartrate‐resistant acid phosphatase
Introduction
Bone remodelling is essential for bone integrity and primarily involves the interaction between bone‐resorbing osteoclasts and bone‐forming osteoblasts. This process is tightly regulated at many levels, including by osteocyte‐, osteoblast‐ and osteoclast‐derived factors, mediators released by the bone matrix upon resorption by osteoclasts and other local and systemic hormones (Sims and Martin, 2014; Sims and Martin, 2015). Osteoporosis reflects an imbalance of bone remodelling, favouring resorption over formation, and is typified by low bone mineral density (BMD) and increased fracture risk (Watts et al., 2013). Many osteoporosis therapeutics are based on reducing osteoclast‐mediated bone resorption (Marcus, 2013). Strontium (Sr2+o) ranelate has been used clinically for the treatment of osteoporosis and reduces morphometric vertebral fractures in postmenopausal women (Meunier et al., 2004; Reginster et al., 2005). Unfortunately, it is also associated with an increased incidence of cardiac disorders and thromboembolic events, leading to its reclassification for restricted use by the European Medicines Agency in 2014 (EMA, 2014). Currently, it is prescribed only for the treatment of severe osteoporosis in postmenopausal women and adult men at high risk of fracture. One advantage of strontium ranelate is its proposed dual action as an anti‐resorptive and anabolic agent, further aided by its high incorporation into bone (Dahl et al., 2001). This remained a driver for continued use in Europe (EMA, 2014), although the restrictions in place have led to cessation of its marketing in 2017 for commercial reasons (Servier, 2017).
Sr2+ o inhibits the maturation and activity of osteoclast cultures (Bonnelye et al., 2008; Caudrillier et al., 2010), induces apoptosis (Hurtel‐Lemaire et al., 2009) and promotes osteoblast differentiation and recruitment (Chattopadhyay et al., 2007; Brennan et al., 2009). However, the anabolic activity of Sr2+ o has been questioned; improved bone strength associated with incorporation of Sr2+ o into the bone matrix and the anti‐resorptive actions on osteoclasts may have been sufficient to produce the increase in BMD observed in clinical trials (Bain et al., 2009; Chavassieux et al., 2014). Whilst the exact mechanism of action of Sr2+ o remains unclear, the calcium‐sensing receptor (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=54) may be partly responsible (Chattopadhyay et al., 2007; Fromigué et al., 2009; Hurtel‐Lemaire et al., 2009; Caudrillier et al., 2010). Thus, a combination therapy, comprising Sr2+ o and a CaS receptor positive allosteric modulator (PAM), in a bone‐targeted formulation, could reduce the required dose of Sr2+ o and therefore the risk of adverse effects.
The CaS receptor is a Class C GPCR that pleiotropically couples to G proteins and is expressed in many tissues that regulate extracellular calcium (Ca2+ o) levels, including the parathyroid gland [where it inhibits http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1785 (PTH) release], osteoblasts/osteoclasts (where it regulates bone turnover), thyroid parafollicular C cells (where it stimulates http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=685 release) and the kidney (where it regulates renal Ca2+ o re‐absorption) (Chang et al., 1999; Kantham et al., 2009; Thomsen et al., 2012; Goltzman and Hendy, 2015; Riccardi and Valenti, 2016). However, the CaS receptor is also expressed in many tissues not directly involved in Ca2+ o homeostasis, including cardiovascular cells (where calcimimetics can display hypertensive effects) (Bonomini et al., 2012) and mesenchymal stem cells (where it contributes to osteogenic differentiation) (Pipino et al., 2014), amongst others. The CaS receptor is responsive to Ca2+ o and other cations (including Sr2+ o) that can preferentially activate distinct signalling pathways; in HEK293 cells expressing the bovine CaS receptor, Sr2+ o is less potent than Ca2+ o in Ca2+ i mobilization and IP1 accumulation assays but is equipotent to Ca2+ o in ERK1/2 phosphorylation assays (Chattopadhyay et al., 2007). Intriguingly, Sr2+ o is more potent than Ca2+ o at stimulating calcitonin release from rat medullary thyroid carcinoma 6–23 cells, highlighting the context‐dependence of agonist potency at the CaS receptor (Thomsen et al., 2012).
Small molecule PAMs have been identified for the CaS receptor (Jian‐Nong et al., 2011; Deprez et al., 2013), most notably http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3308, which is used clinically for the treatment of secondary hyperparathyroidism (Cunningham et al., 2005; Davey et al., 2012; Cook et al., 2015). Cinacalcet has been extensively characterized for its ability to modulate CaS receptor‐mediated signalling (Leach et al., 2013; Leach et al., 2016). The structurally distinct calcimimetic, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3947, which reduces serum PTH levels in rats, has also been relatively well characterized as an allosteric modulator (Smajilovic et al., 2011;Cook et al., 2015 ; Leach et al., 2016). These studies reveal that, like positively charged cations, cinacalcet and AC‐265347 stabilize receptor conformations that differentially regulate CaS receptor‐signalling pathways, a phenomenon termed biased modulation (Cook et al., 2015; Leach et al., 2016). This bias extends to actions as pharmacochaperones, where cinacalcet, but not AC‐265347, traffics a naturally occurring endoplasmic reticulum‐retained mutant CaS receptor (Cook et al., 2015). In contrast to cinacalcet and AC‐265347, relatively little is known about the molecular pharmacology of other calcimimetics, such as the structurally distinct benzothiazole tri‐substituted urea series (Deprez et al., 2013).
In the present study, we have extended our previous observations of PAM‐mediated bias at the CaS receptor to include effects on agonist‐mediated luciferase transcription under the control of serum response factor response element (SRF‐RE)‐Luc transcription and a surrogate of RhoA‐mediated signalling. We also profiled the structurally distinct PAM, BTU‐compound 13 (Deprez et al., 2013) (Figure 1) and, to test the propensity for ‘probe‐dependence’ (Valant et al., 2012), characterized the ability of each calcimimetic to modulate both Ca2+ o‐ and Sr2+ o‐activity in a potentially differential manner. Furthermore, to establish whether Sr2+ o and calcimimetic combination treatment could be considered for the treatment of osteoporosis, we evaluated how calcimimetic pharmacology translates into human CD14+ monocyte‐derived osteoclasts. Using a quantitative, high throughput, multi‐staining method (Diepenhorst et al., 2017), we assessed osteoclast tartrate‐resistant acid phosphatase (TRAP) activity and nucleation state. Coupled with hydroxyapatite resorption assays, we show that cinacalcet, but not AC‐265347 or BTU‐compound 13, inhibits osteoclast‐mediated resorption without impacting osteoclast differentiation and with only a small reduction in TRAP activity, in contrast to Sr2+ o, which robustly reduces TRAP activity, osteoclast maturation and resorption.
Figure 1.

Structures of the calcimimetics used in this study.
Methods
Recombinant cell culture
Flp‐In HEK293‐TREx‐c‐myc‐CASR cells containing the human CaS receptor gene (CASR) were used in all recombinant cell assays and were maintained in DMEM and 10% FBS (Invitrogen as previously described; Davey et al., 2012). For ERK1/2 phosphorylation and Ca2+ i mobilization assays, cells were seeded at 80 000 cells per well into Corning 96‐well plates coated with 2.5 μg per well poly‐D lysine. CASR expression was induced by incubation with 100 ng·mL−1 tetracycline overnight at 37°C in an atmosphere of 5% CO2.
Ca2+ i mobilization assay
Ca2+ i mobilization assays were performed as previously described (Davey et al., 2012). Seeded, tetracycline‐induced cells were washed twice in assay buffer (150 mM NaCl, 2.6 mM KCl, 0.1 mM CaCl2, 1.18 mM MgCl2, 10 mM D‐glucose, 10 mM HEPES, 4 mM probenecid, 0.5% w·v−1 BSA, pH 7.4) and loaded with 0.9 μM Fluo‐4‐AM for 1 h at 37°C. Cells were washed twice with 100 μL per well assay buffer before co‐addition (in duplicate) of the calcimimetics and Ca2+ o or Sr2+ o. Intracellular calcium mobilization was determined by the peak change in fluorescence using a Flexstation‐1 microplate reader (Molecular Devices, Sunnyvale, CA, USA) using 485 nm excitation and 525 nm emission filters.
IP1 accumulation assay
Induced Flp‐In HEK293‐TREx‐c‐myc‐CASR cells were harvested and resuspended in assay buffer (150 mM NaCl, 2.6 mM KCl, 0.1 mM CaCl2, 1.18 mM MgCl2, 10 mM D‐glucose, 10 mM HEPES, 50 mM LiCl2, pH 7.4); 1 × 104 cells in 7 μL were added to wells of a 384‐well white Proxiplate (PerkinElmer) in addition to 7 μL agonist (Ca2+ o or Sr2+ o) with or without calcimimetic (in duplicate). Plates were briefly centrifuged and allowed to incubate at 37°C for 45 min. The reaction was stopped and cells lysed by the addition of 6 μL per well lysis buffer (CisBio Bioassays). Lysates were analysed for IP1 accumulation using the IP‐One Tb™ assay kit (CisBio Bioassays) according to the manufacturer's protocol as previously described (Cook et al., 2015).
ERK1/2 phosphorylation assay
ERK1/2 phosphorylation assays were performed on 4 h serum‐starved cells in 0.1 mM Ca2+‐containing serum‐free DMEM, as described above. Concentration responses to Ca2+ o or Sr2+ o in the presence and absence of calcimimetic were performed (in duplicate) at 37°C and were normalized to the maximal Ca2+ o‐ or Sr2+ o‐responses. Cells were co‐stimulated for 2.5 min with calcimimetics and Ca2+ o or Sr2+ o. The medium was removed and the cells lysed with the 100 μL SureFire lysis buffer (TGR Biosciences). Lysates were frozen prior to analysis with the SureFire™ ERK1/2 (Thr201/Tyr204) phosphorylation Assay Kit according to the manufacturer's protocol (PerkinElmer) using an EnVision multilabel plate reader (PerkinElmer) for detection.
Serum response factor response element (SRF‐RE)‐Luc reporter gene assay
As a surrogate for CaS receptor coupling to RhoA activity, a reporter gene assay was used to measure luciferase transcription under the control of SRF‐RE‐Luc. Briefly, Flp‐In HEK293‐TREx‐c‐myc‐CASR, at 80 000 cells per well, were transfected with 0.1 μg per well pGL4.34[luc2P/SRF‐RE/Hygro] (Promega) DNA using a ratio of 1:3 DNA:PEI, in Corning 96‐well white plates coated with 2.5 μg per well poly‐D lysine. CaS receptor expression was simultaneously induced with 100 ng·mL−1 tetracycline. After incubation at 37°C in an atmosphere of 5% CO2 for 5 h, the media were exchanged for 100 μL per well serum‐free, low Ca2+ o (0.1 mM) DMEM with 100 ng·mL−1 tetracycline, and cells were incubated overnight at 37°C in an atmosphere of 5% CO2. The following day, DMEM was aspirated and cells were washed once with PBS, and 100 μL assay buffer (150 mM NaCl, 2.6 mM KCl, 0.1 mM CaCl2, 1.18 mM MgCl2, 10 mM D‐glucose, 10 mM HEPES, pH 7.4) was added to each well. Cells were stimulated with agonist with or without calcimimetic (in duplicate) for 6 h at 37°C. The plate was equilibrated to room temperature prior to the addition of Bright‐Glo™ (Promega) reagent at a final dilution of 1:2. Following incubation at room temperature for 2 min, luminescence was measured (EnVision multilabel plate reader, PerkinElmer). Data were normalized to the maximal response stimulated by Ca2+ o or Sr2+ o in the absence of any calcimimetic.
Source of human blood
Collection and use of human blood samples (buffy coat and whole blood) was conducted according to the guidelines and approval of Monash University (Clayton, Australia) and the Monash University Research Ethics Committee (Clayton, Australia). Blood (100 mL) was obtained from human volunteers from the Victorian Blood Donor Registry or from buffy coat preparations from the Australian Red Cross blood service (Melbourne, Australia, under the Monash University Research Ethics Committee approval; HREC CF14/999‐2014000425).
Osteoclast differentiation and culture
Osteoclasts were differentiated from CD14+ monocytes isolated from human blood as previously described (Nicholson et al., 2000). Briefly, buffy coat or whole blood was diluted 1:1 in 1 × PBS; 30 mL of diluted blood was layered over 15 mL Ficoll (GE Healthcare) in a Leucosep tube and centrifuged at 800× g for 20 min, at room temperature (21°C) with slow deceleration. The peripheral blood mononuclear cells (PBMC) layer was aspirated and washed three times in PBS by centrifugation at 300× g for 10 min at room temperature. CD14+ PBMCs were isolated using human CD14 MACS MicroBeads (Miltenyl Biotec) as per the manufacturer's protocol.
Isolated CD14+ PBMCs were seeded at 200 000 cells per well in 96‐well plates in α‐MEM (Life Technologies, 1.8 mM CaCl2) supplemented with 10% heat‐inactivated FBS (Life Technologies) and cultured overnight at 37°C in 5% CO2. Cells were differentiated with the addition of 20 ng·mL−1 macrophage colony‐stimulating factor (M‐CSF) (Sigma‐Aldrich) 24 h after isolation and 20 ng·mL−1 M‐CSF and 20 ng·mL−1 receptor activator of NF‐κB ligand (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5066) (Merck) 72 h later. Cells were cultured for up to 2 weeks at 37°C, 5% CO2 with media refreshed every 3 days.
Reverse transcriptase PCR
CD14+ monocytes were seeded at 2 × 106 cells per well in a six‐well plate and were incubated with 20 ng·mL−1 M‐CSF for 3 days. Cells were then treated with 20 ng·mL−1 M‐CSF or 20 ng·mL−1 M‐SCF and 20 ng·mL−1 RANK‐L for a further 7 days. Cells were lysed using 350 μL lysis buffer with 3.5 μL β‐mercaptoethanol, and RNA was extracted using the Isolate II RNA Mini Kit (BioLine) according to the manufacturer's protocol. RNA yields were determined using a NanoDrop 2000 and A260/280 and A260/230 ratios recorded for quality assessment. Immediately, 2 μg of RNA was converted to cDNA using Tetro cDNA Synthesis Kit (BioLine) according to the manufacturer's protocol using the random hexamer primer mix, in duplicate. Subsequent cDNA was diluted 1:5 in nuclease‐free water and samples stored at −20°C. RT‐PCR reactions were carried out using 2 μL cDNA, 2 μL forward and reverse primers at 5 μM (Supporting Information Table S1) and SybrGreen (Roche) as per the manufacturer's protocol and reactions were run on a LightCycler ® 480 Instrument II (Roche). Primer efficiency was calculated using the standard curve method. Data were analysed using the Pfaffl method (Pfaffl, 2001), expressed as ΔΔCt normalized to a calibrator sample and to both reference genes, plotted as mean ± SEM and represent five independent donors with samples run in duplicate.
Western blot for human CaS receptor
CD14+ human monocytes were seeded on six‐well plates and incubated for the indicated time in the presence or absence of 20 ng·mL−1 RANK‐L. Cells were then washed with PBS, 50 μL of SDS lysis buffer + DTT + β‐mercaptoethanol + protein inhibitor added to each well and cells recovered using a scraper. Samples were sonicated at 40% amplitude for 30 s and loaded into a 6% polyacrylamide separating gel. After running samples for 1 h at 100 V, gel was transferred overnight at 30 V onto a nitrocellulose membrane using a wet transfer system at 4°C. Membrane was then blocked using PBS‐T 1% milk for 1 h and incubated with 1 μg·mL−1 of CaS receptor antibody (5C10, ADD clone, ThermoFisher) overnight at 4°C. Membrane was then washed three times with PBS‐T 1% milk and incubated with anti‐mouse HRP (1:1000) secondary antibody for 1 h at room temperature. Following three washes with PBS‐T, the membrane was imaged using the Chemidoc Touch imager (Bio‐rad) and developed with Luminata™ Forte Western HRP Substrate (Merck Millipore) according to the manufacturer's instructions.
Tartrate‐resistant acid phosphatase (TRAP) activity assay
TRAP activity was quantified as previously described (Diepenhorst et al., 2017). Briefly, CD14+ PBMCs were isolated and seeded at 200 000 cells per well into 96‐well plates and were incubated with 20 ng·mL−1 M‐CSF for 3 days followed by differentiation with 20 ng·mL−1 M‐CSF and 20 ng·mL−1 RANK‐L in the presence or absence of 5, 10 or 20 mM SrCl2 and/or 3 μM calcimimetic for 8 days. Cells were fixed, stained and imaged as previously described (Diepenhorst et al., 2017) with additional staining for the calcitonin receptor. Briefly, staining for the calcitonin receptor involved blocking with 0.5% BSA/PBS for 1 h at room temperature, overnight incubation with 1:500 mouse anti‐human calcitonin receptor antibody (Welcome receptor) in PBS at 4°C and incubation with anti‐mouse Alexa‐594 (1:1000, Life Technologies) prior to imaging cells on the In Cell 2000 (GE Healthcare), four fields of view per well in duplicate wells using a 10× objective. TRAP activity is presented as the mean number of TRAP foci per cell ± SEM for 10 individual experiments (each with four fields of view analysed), binned by osteoclast maturation state (number of nuclei; Diepenhorst et al., 2017).
Resorption assay
CD14+ PBMCs were isolated and seeded as previously described into Corning® Osteo Assay Surface 96‐well plates and incubated with 20 ng·mL−1 M‐CSF for 3 days. Cells were then differentiated into osteoclasts in the presence or absence of 5, 10, 20 mM SrCl2 or 3 μM calcimimetic (in duplicate) with 20 ng·mL−1 M‐CSF, 20 ng·mL−1 RANK‐L for a further 8 days. The area of the Osteo Assay surface resorbed was determined using Von Kossa staining at the end of 8 days of differentiation. Briefly, cells were removed by incubation with 10% bleach solution at 22°C for 5 min. Wells were washed twice with water before allowing to air‐dry overnight. Wells were then stained with 5% (w.v−1) aqueous silver nitrate at 22°C in the dark for 30 min prior to a 5 min wash in water. Wells were then incubated with 5% (w·v−1) sodium carbonate in formalin for 4 min. Wells were imaged using an In Cell 2000 (GE Healthcare) and images analysed in Fiji to determine the proportion of hydroxyapatite Osteo Assay surface resorbed. Data were collated from 10 donors, expressed as mean percentage area resorbed ± SEM.
Flow cytometry for the detection of cell‐surface receptor expression
Flow cytometry was used to measure changes in the cell surface expression of either WT CaS receptor or the G670E mutant CaS receptor as previously described (Cook et al., 2015). Briefly, Flp‐In HEK293‐TREx‐c‐myc‐CASR and CaS receptor‐G670E cells were seeded at 80 000 cells per well into a 96‐well plate and expression was induced with 100 ng·mL−1 tetracycline in the presence of vehicle or 3 μM calcimimetic overnight at 37°C. The following day, cells were harvested, washed in 1 × PBS with 0.1% BSA and 2 mM EDTA (wash buffer) by centrifugation (350× g, 4°C for 3 min) before resuspension and 30 min incubation in 100 μL blocking buffer (1 × PBS, 5% BSA and 2 mM EDTA); 1 μg·mL−1 AF647‐conjugated 9E10 made in‐house as previously described was then added and incubated for 1 h. Cells were washed as previously described and resuspended in wash buffer containing Sytox blue stain (Invitrogen). Fluorescence was measured using a FACS Canto (Becton Dickinson). Data are mean ± SEM of five independent experiments.
Data analysis and statistics
All non‐linear regression analysis was performed in GraphPad Prism 7. Interaction experiments between Ca2+ o or Sr2+ o and cinacalcet, AC‐265347 or BTU‐compound 13 were fitted globally to the following operational model of allosterism and agonism (Leach et al., 2007; Aurelio et al., 2009) where the transducer slope was constrained to be less than four:
EC50 is the agonist concentration that elicits a half maximal response; τB denotes the efficacy of the allosteric ligand; α and β denote allosteric effects on orthosteric ligand binding affinity and efficacy, respectively; K B represents the functional affinity of the allosteric ligand; [A] and [B] represent orthosteric and allosteric ligand concentrations; E m denotes the maximal possible response of the system; and n is the slope of the transducer function linking agonist receptor occupancy to response. Affinity, cooperativity and efficacy parameters were estimated as logarithms (Christopoulos, 1998).
To determine whether the calcimimetics engendered biased modulation of CaS receptor signalling, the statistical differences between either pKB or log αβ values across different pathways for a given PAM were determined by one‐way ANOVA with Tukey's multiple comparisons test for effects in combination with either Ca2+ o or Sr2+ o. In order to determine any probe‐dependent effects, variations in calcimimetic log αβ determined from interaction with either Ca2+ o or Sr2+ o at a given signalling pathway were compared by two‐way ANOVA (with orthosteric probe and signalling endpoints as factors) with Sidak's multiple comparisons post test. TRAP expression data were analysed by repeated measures two‐way ANOVA (with Sr2+ o treatment and osteoclast size as variables) followed by Dunnett's multiple comparisons test.
Due to the inherent variability in the resorptive capacity of human donor‐derived osteoclasts, hydroxyapatite resorption data were subject to Grubb's test for outliers (α = 0.05). For the Sr2+ o dataset, two data points (out of a total of 44; n = 11, four treatment conditions) were excluded; the data were analysed by one‐way ANOVA with Dunnett's multiple comparisons compared to vehicle. For the calcimimetics ± Sr2+ o, the data from one donor were excluded (n = 10 remain); the data were analysed by repeated measures two‐way ANOVA with calcimimetic and Sr2+ o treatment as variables and Dunnett's multiple comparisons compared to vehicle. Flow cytometry data were analysed by two‐way ANOVA with drug treatment and genotype as variables and Dunnett's multiple comparisons compared to vehicle. The data and statistical analyses comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). The data and statistical analyses were performed in GraphPad Prism 7. Group sizes and number of independent experiments appropriately reflect the variability in the datasets and magnitude of the signal size. A difference was considered significant when P < 0.01 and was marked on figures accordingly.
Compounds
Cinacalcet was synthesized in‐house as described previously (Davey et al., 2012); AC‐265347 was purchased from Sigma‐Aldrich; BTU‐compound 13 was synthesized by Institut de Recherches Servier using published methods (Deprez et al., 2013).
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017).
Results
Calcimimetic effects on Ca2+ o‐mediated CaS receptor signalling
The interaction between cinacalcet or AC‐265347 and Ca2+ o in Ca2+ i‐mobilization, IP1 accumulation and ERK1/2 phosphorylation assays has been previously described (Cook et al., 2015; Leach et al., 2016) and suggests that cinacalcet is biased towards Ca2+ i mobilization and away from ERK1/2 phosphorylation, as evidenced by a significantly lower cooperativity with Ca2+ o in ERK1/2 phosphorylation versus Ca2+ i mobilization assays. Using SRF‐RE‐Luc transcription as a surrogate for RhoA activity, we now show that cinacalcet also exhibits bias towards RhoA versus ERK1/2 phosphorylation, as evidenced by a significantly greater pKB for the receptor conformation that couples to this pathway (Table 1; Figure 2; Supporting Information Figure S1). AC‐265347 has been shown to exhibit the greatest positive modulation of Ca2+ o in ERK1/2 phosphorylation assays versus Ca2+ i mobilization assays and also binds with higher affinity to the receptor conformation that couples to IP1 accumulation (Table 1; Figure 2). Accordingly, we now reveal that AC‐265347, like cinacalcet, is a strong positive modulator of Ca2+ o‐mediated RhoA activity in comparison to Ca2+ i mobilization (Table 1; Figure 2; Supporting Information Figure S2).
Table 1.
Affinity (pKB) and cooperativity (log αβ) estimates from operational model analysis of cinacalcet, AC‐265347 and BTU‐compound 13‐mediated allosteric modulation of Ca2+ o‐ and Sr2+ o‐ stimulated Ca2+ i mobilization, IP1 accumulation, ERK1/2 phosphorylation and the SRF‐RE‐Luc transcription in HEK293 cells induced to express CaS receptors
| Cinacalcet | AC‐265347 | BTU‐compound 13 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca2+ o | Sr2+ o | Ca2+ o | Sr2+ o | Ca2+ o | Sr2+ o | ||||||||
| n | n | n | n | n | |||||||||
| Ca2+ i | pKB | 6.29 ± 0.04 | 29 | 6.12 ± 0.08 | 5 | 6.21 ± 0.07 | 13 | 6.11 ± 0.17 | 5 | 6.66 ± 0.14 | 5 | 6.76 ± 0.11 | 5 |
| log αβ (αβ) | 0.42 ± 0.03 (2.6) | 0.67 ± 0.07 (4.6) | 0.44 ± 0.08 (2.5) | 0.92 ± 0.23 (8.3) | 0.21 ± 0.15 (3.2) | 0.20 ± 0.11 (1.6) | |||||||
| IP1 | pKB | 6.09 ± 0.10 | 5 | 6.17 ± 0.06 | 5 | 7.34 ± 0.08 | 5 | 7.38 ± 0.14 | 5 | 7.15 ± 0.11 | 5 | 7.24 ± 0.08 | 7 |
| log αβ (αβ) | 0.41 ± 0.15 (2.6) | 0.40 ± 0.09 (2.5) | 0.67 ± 0.10 (4.7) | 0.45 ± 0.23 (2.8) | 0.46 ± 0.16 (2.9) | 0.23 ± 0.13 (1.7) | |||||||
| ERK1/2 | pKB | 6.52 ± 0.07 | 5 | 6.42 ± 0.18 | 5 | 6.06 ± 0.11 | 10 | 6.00 ± 0.20 | 5 | 6.18 ± 0.18 | 5 | 5.96 ± 0.18 | 5 |
| log αβ (αβ) | 0.12 ± 0.05 (1.3) | 0.41 ± 0.05 (2.5) | 1.07 ± 0.11 (10) | 1.12 ± 0.23 (13) | 0.08 ±0.19 (1.2) | 0.18 ± 0.11 (1.5) | |||||||
| SRF‐RE | pKB | 7.12 ± 0.19 | 5 | 6.53 ± 0.17 | 5 | 6.19 ± 0.13 | 5 | 5.97 ± 0.23 | 5 | 6.47 ± 0.21 | 5 | 5.69 ± 0.37 | 5 |
| log αβ (αβ) | 0.65 ± 0.11 (4.5) | 0.82 ± 0.16 (6.6) | 1.13 ± 0.15 (13) | 1.28 ± 0.26 (19) | 1.23 ± 0.23 (17) | 1.74 ± 0.41 (55) | |||||||
All data are mean ± SEM of the stated number of independent experiments.
Figure 2.
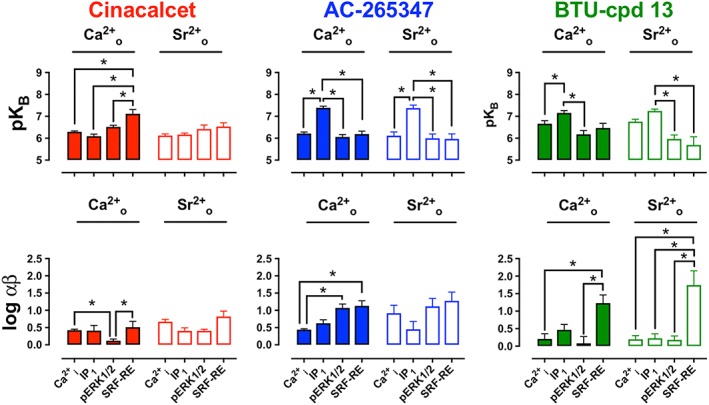
Cooperativity (log αβ) and affinity (pKB) values for the modulation by cinacalcet, AC‐265347 and BTU‐compound 13 of Ca2+ o‐ and Sr2+ o‐mediated activation of multiple signalling endpoints in Flp‐In HEK293‐TREx‐c‐myc‐CASR cells (graphed by calcimimetic). Affinity and cooperativity estimates were derived from analysis of grouped datasets using an operational model of allosterism and agonism (see Methods). Data are mean ± SEM for 5–29 individual experiments performed in duplicate (individual ‘n’ numbers are shown in Table 1). *P < 0.01, one‐way ANOVA with Tukey's multiple comparisons test for differences between pathways.
Since there has been no quantitative evaluation of the pharmacology of BTU‐compound 13 as a calcimimetic (Deprez et al., 2013), we assessed its ability to modulate Ca2+ o‐mediated Ca2+ i mobilization, IP1 accumulation, ERK1/2 phosphorylation and SRF‐RE‐Luc transcription in Flp‐In HEK293‐TREx‐c‐myc‐CASR cells. BTU‐compound 13 potentiated Ca2+ o‐mediated signalling responses in all assays, confirming its classification as a calcimimetic (Table 1; Figure 2; Supporting Information Figure S3). Like cinacalcet, BTU‐compound 13 was biased towards modulation of SRF‐RE‐Luc transcription assay and away from ERK1/2 phosphorylation (Table 1; Figure 2), but unlike cinacalcet, BTU‐compound 13 was also biased away from Ca2+ i mobilization, exhibiting relatively weak potentiation in this assay. Similar to AC‐265347, BTU‐compound 13 bound with the highest affinity to the receptor conformation that couples to IP1 accumulation.
Collectively, interaction studies with calcimimetics and Ca2+ o suggest that each calcimimetic engenders its own profile of biased modulation, providing evidence for different conformational states favoured by structurally distinct calcimimetics.
Calcimimetics potentiate Sr2+ o‐mediated CaS receptor signalling
Having established that all three calcimimetics potentiated Ca2+ o‐mediated CaS receptor signalling, we sought to determine whether they displayed similar pharmacology when using Sr2+ o as the orthosteric agonist probe. Sr2+ o stimulates CaS receptor‐mediated Ca2+ i mobilization, IP1 accumulation, activation of non‐selective cation channels and ERK1/2 activity in HEK293 cells transiently transfected with bovine CaS receptor (Chattopadhyay et al., 2007) in addition to calcitonin release, IP1 accumulation, Ca2+ i mobilization, inhibition of cAMP and ERK1/2 phosphorylation in rat medullary thyroid carcinoma 6–23 cells (Thomsen et al., 2012). Here, we demonstrate that Sr2+ o robustly stimulates Ca2+ i‐mobilization, IP1 accumulation, ERK1/2 phosphorylation and SRF‐RE‐Luc transcription in Flp‐In HEK293‐TREx‐c‐myc‐CASR cells (Figure 2; Supporting Information Figures S1–S3). Consequently, the same panel of signalling assays were used to evaluate calcimimetic‐mediated modulation of Sr2+ o‐stimulated CaS receptor signalling (Supporting Information Figures S1–S3). Broadly speaking, all calcimimetics potentiated Sr2+ o‐mediated signalling across all pathways. Analogous to observations using Ca2+ o as the agonist, both AC‐265347 and BTU‐compound 13 bound with highest affinity to the receptor conformation that coupled to Sr2+ o‐mediated IP1 accumulation, and BTU‐compound 13 was strongly biased towards Sr2+ o‐mediated SRF‐RE‐Luc transcription. However, in contrast to the Ca2+ o dataset, cinacalcet showed no bias when Sr2+ o was the agonist, and AC‐265347 was not significantly biased towards Sr2+ o‐mediated ERK1/2 phosphorylation or SRF‐RE‐Luc transcription (Figure 2; Table 1). Interestingly, although cinacalcet bound with higher affinity to the receptor conformation that coupled to Ca2+ o‐mediated SRF‐RE‐Luc transcription, it showed no preference for the conformation that coupled to Sr2+ o‐mediated SRF‐RE‐Luc transcription.
Collectively, these data suggest that all CaS receptor calcimimetics potentiate Ca2+ o and Sr2+ o‐mediated signalling, though there is evidence for both biased modulation and probe‐dependence.
Cinacalcet, but not AC‐265347 or BTU‐compound 13, pharmacochaperones an ER‐retained CaS receptor variant
In addition to biased modulation, calcimimetics differentially pharmacochaperone a naturally occurring loss‐of‐expression CASR‐G670E variant (Cook et al., 2015). Thus, whereas cinacalcet rescued G670E cell surface expression, AC‐265347 had no pharmacochaperone effect (despite retaining affinity for the mutated receptor; Cook et al., 2015). The divergent pharmacochaperone abilities of cinacalcet versus AC‐265347 is either further evidence that these two calcimimetics preferentially stabilize different receptor conformations, or it suggests that AC‐265347 is unable to access intracellular receptors, a form of ‘location bias’ (Irannejad et al., 2017). Therefore, as the three calcimimetics in CaS receptor signalling assays displayed subtle differences in their biased profile, we next explored their potential to differentially regulate CaS receptor trafficking. As wild‐type CaS receptor is robustly cell‐surface expressed in HEK293 cells (and hence, it is not possible to measure chaperoning; Figure 3), we once again exploited the naturally occurring CaS receptor‐G670E variant, that is, predominantly retained in the endoplasmic reticulum and only has low cell surface expression (White et al., 2009; Leach et al., 2012). Cinacalcet (3 μM), but not AC‐265347 or BTU‐compound 13, significantly enhanced CaS receptor‐G670E cell‐surface expression as measured by flow cytometry. This suggests that cinacalcet can access and stabilize a conformation of the CaS receptor‐G670E variant that is trafficked to the cell surface, but AC‐265347 and BTU‐compound 13 cannot (Figure 3). All three compounds are lipophilic and are predicted to have high cell permeability (Supporting Information Table S2; Broccatelli et al., 2016; Pires et al., 2015), suggesting that the effects could be due to distinct receptor conformations stabilized by the calcimimetics. However, we cannot rule out that divergent active transport or permeability of the drugs leads to differential access to the intracellular CaS receptor‐G670E variant.
Figure 3.
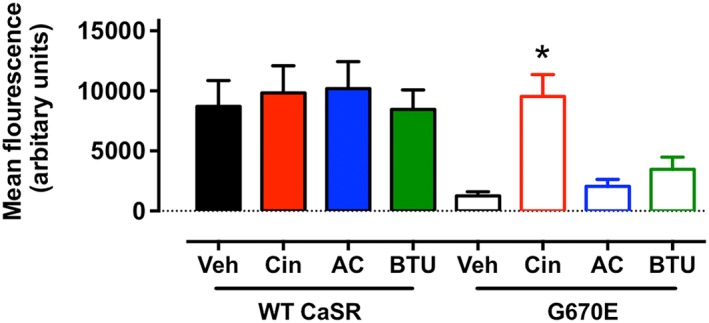
Effect of calcimimetics (3 μM) on the cell surface expression of either c‐myc‐tagged wild‐type or mutant (G670E) CaS receptors expressed in HEK293 cells. Cinacalcet, but not AC‐256347 or BTU‐compound 13, was able to chaperone the intracellular‐retained G670E mutant to the cell surface as detected by flow cytometry (*P < 0.01 vs. respective vehicle control; two‐way ANOVA with Dunnett's multiple comparisons test, for five individual experiments).
Characterization of human CD14+ monocyte derived osteoclasts
Since cinacalcet, AC‐265347 and BTU‐compound 13 all differentially potentiate Ca2+ o and Sr2+ o signalling and have distinct pharmacochaperone activity, we evaluated the behaviour of all three calcimimetics, alone and in combination with Sr2+ o, in phenotypic assays of human osteoclast function.
Osteoclasts are large, multinucleated cells responsible for mediating bone resorption. They are sensitive to Sr2+ o in vivo, resulting in decreased differentiation, reduced resorption activity and increased apoptosis (Baron and Tsouderos, 2002; Takahashi et al., 2003;Bonnelye et al., 2008 ; Caudrillier et al., 2010). Osteoclasts were differentiated from human CD14+ monocytes isolated from the blood of 10 donors by incubation with M‐CSF and RANK‐L and used to assess the phenotypic and functional effects of Sr2+ o and calcimimetics. These cultures comprise a heterogeneous population, from mono‐nucleated cells through to osteoclasts containing 20+ nuclei, consistent with previous studies (Diepenhorst et al., 2017).
Our osteoclast cultures were characterized based on morphology and the expression of key markers, determined by qPCR and imaging. Osteoclast cultures expressed mRNA for osteoclastic genes, including TRAP (ACAP5), RANK (the receptor for RANK‐L), TNF receptor‐associated factor 6 (TRAF6; involved in RANK‐L signalling), calcitonin receptor (CALCR), cathepsin K (CTSK) and integrin subunit alphaV (ITGAV) (Figure 4A), the latter being a commonly used marker for osteoclasts owing to its role in the formation of the sealing zone. Importantly, these cultures also expressed CASR mRNA (Figure 4A). Western blotting studies confirmed CaS receptor protein expression in osteoclast cultures (Figure 4B), with a similar glycosylation state to the CaS receptor protein expressed in the HEK293‐TREx‐c‐myc‐CASR cells (Figure 4C). Protein expressions of calcitonin receptor and TRAP were confirmed by immunofluorescence and ELF‐97® staining, respectively (Figure 4D, E). A large number of cells were multi‐nucleated with the distinct actin ring staining of osteoclasts (which facilitates formation of the sealing zone for bone resorption; Figure 4F). Figure 4G, H shows Von Kossa staining of hydroxyapatite surface with cells cultured in the absence (G) and presence (H) of 20 ng·mL−1 RANK‐L, demonstrating its key role in the differentiation of functional osteoclasts.
Figure 4.

Characterization of human osteoclast cultures. (A) mRNA expression of markers of mature osteoclasts including the CaS receptor (CASR), TRAP (ACAP5), the receptor for RANK‐L (RANK), TNF receptor associated factor‐6 (TRAF6), calcitonin receptor (CALCR), cathepsin K (CTSK) and integrin subunit α V (ITGAV). (B) Expression of CaS receptor protein as detected by Western blotting in osteoclasts with varying days of culture with RANK‐L and (C) comparison with human CaS receptors expressed in HEK293 cells. Human osteoclasts are characteristically multinucleated (Hoechst, blue) and express calcitonin receptors (mouse‐anti human calcitonin receptor 1o with anti‐mouse IgG‐Alexa 594 2o, yellow; D) and TRAP (ELF‐97®, green; E) and have distinct actin ring morphology typical of active, resorbing osteoclasts (Phaloidin‐647, red; F). Von Kossa staining of osteoclast‐mediated hydroxyapatite surface resorption after culture in the absence (G) and presence (H) of RANK‐L.
Sr2+ o inhibits osteoclast differentiation and resorptive activity
Given the mixed cell populations obtained in cultures of differentiated osteoclasts, ranging from mono‐nucleated cells to osteoclasts containing 20+ nuclei, a method for single cell analysis of osteoclast activity and maturation was developed (Diepenhorst et al., 2017), taking into account the nucleation state of an osteoclast, which is indicative of its activity and maturation (Piper et al., 1992; Boissy et al., 2002). This method uses TRAP as a marker of osteoclast activity based on its central role in bone matrix resorption and use as a diagnostic measure of bone turnover (Halleen et al., 2006). Originally, this approach was developed to monitor the effect of RANK‐L on a differentiating osteoclast population (Diepenhorst et al., 2017). Here, we quantitatively assess the effects of Sr2+ o and calcimimetics on osteoclast differentiation and activity.
Previous studies show that Sr2+ o inhibits osteoclast differentiation and resorptive activity (Bonnelye et al., 2008; Caudrillier et al., 2010). Here, we determine the extent of Sr2+ o‐mediated inhibition of osteoclast maturation and activity (Sr2+ o was added to the cells upon initiation of differentiation with RANK‐L, 8 days prior to assay). Sr2+ o did not significantly change osteoclast maturation (the proportion of multinucleated cells in RANK‐L‐differentiated osteoclast cultures; Figure 5A). However, Sr2+ o treatment caused a concentration‐dependent and significant reduction in osteoclast activity (TRAP activity) in higher‐order osteoclasts (10+ nuclei; TRAP foci per cell reduced from 125.4 ± 12.5 in vehicle‐treated cells to 79.5 ± 18.2 with 20 mM Sr2+ o; Figure 5B).
Figure 5.
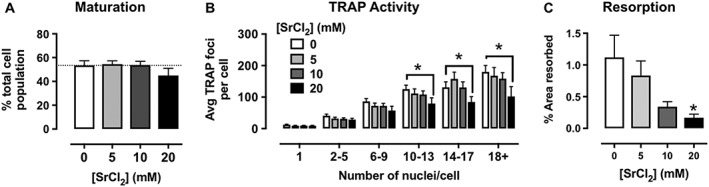
Sr2+ o (20 mM) inhibits osteoclast differentiation and activity. (A) Sr2+ o had no significant effect on the proportion of multi‐nucleated cells within the population and (B) significantly decreased TRAP activity (fewer TRAP foci per cell) in higher‐order osteoclasts (bins of cells containing greater than 10 nuclei; *P < 0.01 vs. vehicle; repeated measures two‐way ANOVA with Dunnett's multiple comparisons test). (C) Unsurprisingly, osteoclast‐mediated hydroxyapatite surface resorption was impaired in the presence of increasing concentrations of Sr2+ o (*P < 0.01 vs. vehicle, one‐way ANOVA with Dunnett's multiple comparisons test). Data are mean ± SEM from 11 individual experiments with analysis of four fields of view per experiment per treatment.
The Sr2+ o‐mediated reduction in TRAP activity in higher‐order osteoclasts was associated with a significant impairment in the resorptive capacity of osteoclasts; Sr2+ o (20 mM) inhibited osteoclast‐mediated resorption of hydroxyapatite artificial bone matrix in a concentration‐dependent manner (Figure 5C). These results correlate well with previous studies of Sr2+ o on bone cell function (Bonnelye et al., 2008; Caudrillier et al., 2010) and provide a framework to investigate the effects of calcimimetics on osteoclasts and, by extension, their therapeutic potential for the treatment of osteoporosis.
Cinacalcet, but not AC‐265347 or BTU‐compound 13, potentiates Sr2+ o‐mediated inhibition of TRAP activity
To determine the effects of each calcimimetic or in combination with a sub‐effective concentration of Sr2+ o, CD14+ monocytes from 11 donors were differentiated to osteoclasts in the presence of 3 μM cinacalcet, AC‐265347 or BTU‐compound 13 in the absence or presence of 5 mM Sr2+ o. After 8 days, osteoclast cultures were analysed for differentiation and TRAP activity as previously described (Diepenhorst et al., 2017).
None of the calcimimetics adversely affected the appearance of osteoclast cultures (data not shown). Despite robust effects on Sr2+ o‐mediated signalling, none of the calcimimetics changed the proportion of multinucleated cells in culture (either alone or in the presence of 5 mM Sr2+ o; Figure 6). However, a combination of cinacalcet plus Sr2+ o significantly inhibited TRAP activity in the most active (18+ nuclei) osteoclasts (Figure 7A), whereas changes in TRAP activity were absent in cultures containing AC‐2654347 or BTU‐compound 13 (alone or in the presence of 5 mM Sr2+ o; Figure 7B, C). Although BTU‐compound 13 appeared to enhance TRAP activity in osteoclasts with 18+ nuclei, this effect was absent in the presence of 5 mM Sr2+ o (Figure 7C).
Figure 6.

Calcimimetics do not modulate osteoclast differentiation, either alone (3 μM) or in combination with a sub‐effective (5 mM) concentration of Sr2+ o. Data are mean ± SEM from 10 individual experiments with four fields of view analysed per experiment per treatment.
Figure 7.
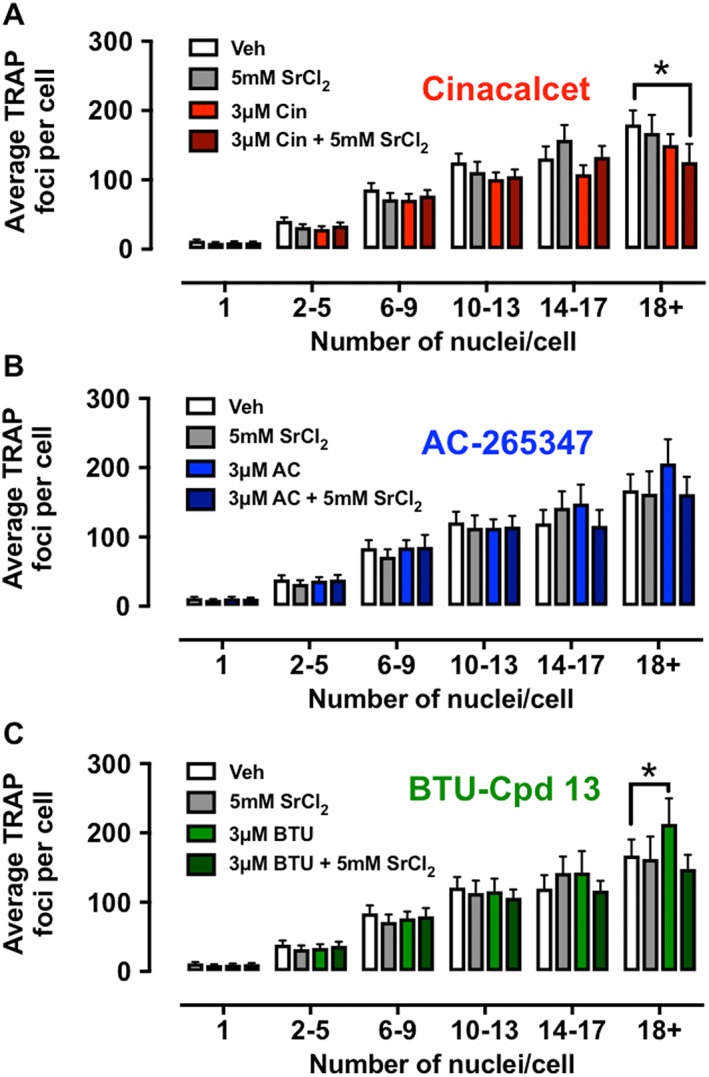
Quantitative analysis of TRAP activity (average number of TRAP foci per cell) in osteoclasts treated with calcimimetics alone (3 μM) or in the presence of a sub‐effective concentration of Sr2+ (5 mM), binned by nuclei number. Cinacalcet (A; in the presence of Sr2+ o) significantly reduced TRAP activity in the highest‐order osteoclasts (18+ nuclei), whereas AC‐265347 (B) was without effect. BTU‐compound 13 (C) paradoxically appeared to increase TRAP activity alone in osteoclasts with 18+ nuclei, although there was no effect when combined with Sr2+. Data are mean ± SEM from 10 individual experiments with four fields of view analysed (*P < 0.01 vs. vehicle; repeated measures two‐way ANOVA with Dunnett's multiple comparisons test).
Cinacalcet robustly inhibits osteoclast‐mediated hydroxyapatite resorption
Given that cinacalcet inhibited TRAP activity in the mature 18+ nuclei‐containing cells, the effect of calcimimetics on osteoclast‐mediated resorption was assessed. CD14+ monocytes from 10 donors were differentiated to osteoclasts on hydroxyapatite artificial bone matrix in the presence of calcimimetic (3 μM) in the absence or presence of 5 mM Sr2+ o. After 8 days, the resorbed area was visualized by Von Kossa staining and quantified in Fiji. Remarkably, cinacalcet profoundly inhibited osteoclast‐mediated resorption of the hydroxyapatite surface, both alone and in the presence of Sr2+ o (Figure 8). Although BTU‐compound 13 marginally reduced resorption when applied alone, no effect was observed in the presence of 5 mM Sr2+ o, and AC‐2654347 was without effect.
Figure 8.

Osteoclasts were differentiated with calcimimetics alone (3 μM) or in the presence of a sub‐effective concentration of Sr2+ o (5 mM) for 8 days. Cinacalcet alone (and in combination with Sr2+ o) significantly inhibited osteoclast‐mediated hydroxyapatite surface resorption; BTU‐compound 13 had a small inhibitory effect alone (which was not seen in combination with Sr2+ o) and AC‐256347 was without significant effect. Data are mean ± SEM from 10 individual experiments performed in duplicate (*P < 0.01 vs. vehicle; repeated measures two‐way ANOVA with Dunnett's multiple comparisons test).
Discussion
In this study, we showed that three structurally distinct calcimimetics, including the clinically used agent cinacalcet, positively modulate Sr2+ o‐mediated CaS receptor signalling in Flp‐In HEK293‐TREx‐c‐myc‐CASR cells. Across a broad range of G protein‐dependent signalling events, cinacalcet‐mediated positive modulation of Sr2+ o was unbiased, in contrast to its modulation of Ca2+ o, where it was biased away from ERK1/2 phosphorylation and displayed the highest affinity for the receptor conformation that couples to a signalling readout downstream of RhoA activity (SRF‐RE‐Luc transcription). These latter data are consistent with and extend previous findings (Davey et al., 2012; Leach et al., 2013; Cook et al., 2015; Leach et al., 2016). AC‐265347 and BTU‐compound 13 also engender distinct biased modulation of CaS receptor signalling pathways depending on which orthosteric agonist is used as the probe (at the level of both affinity and efficacy). Notably, AC‐265347 and BTU‐compound 13 are biased towards ERK1/2 phosphorylation and/or SRF‐RE‐Luc transcription when Ca2+ o is the probe, but only BTU‐compound 13 shows bias in the presence of Sr2+ o. Nonetheless, both compounds preferentially bind to the receptor conformation that couples to IP1 accumulation.
Intriguingly, cinacalcet, but not AC‐265347 or BTU‐compound 13, pharmacochaperoned the endoplasmic reticulum‐retained CaS receptor‐G670E mutant to the cell surface. Although we cannot rule out that these compounds differentially access intracellular compartments, they are all predicted to be cell permeable (pkCSM predictor; Broccatelli et al., 2016; Pires et al., 2015), Collectively, these data indicate that the different calcimimetics preferentially stabilize different conformational states of the CaS receptor and thus engender biased modulation and/or exhibit location bias (Irannejad et al., 2017) through access to different receptor pools.
In agreement with previous findings, we show that Sr2+ o reduces human osteoclast maturation, TRAP activity and osteoclast‐mediated artificial bone matrix resorption with the most profound effects on the highest‐order (and most active) osteoclasts (Figure 5). However, due to its clinical side‐effect profile (Compston, 2014; Reginster, 2014), the use of Sr2+o ranelate as a therapeutic was limited to treatment of severe osteoporosis in postmenopausal women and adult men at high risk for fracture (it was withdrawn from the market for commercial reasons in August 2017; Servier, 2017). In order to verify whether bone‐targeted calcimimetics alone, or in combination with low‐dose Sr2+o ranelate, might represent an alternative therapeutic approach in osteoporosis, we evaluated their effect on osteoclast maturation and activity. In broad terms, none of the calcimimetics, either alone or in combination with a sub‐effective concentration of Sr2+ o, reduced osteoclast maturation or TRAP activity, although cinacalcet (when combined with 5 mM Sr2+ o) did significantly reduce TRAP activity in the highest‐order osteoclasts. Whereas cinacalcet profoundly inhibited osteoclast‐mediated artificial bone matrix resorption (even without Sr2+ o), BTU‐compound 13 had a small inhibitory effect (that was absent in the presence of Sr2+ o) and AC‐265347 was without effect. This suggests that a cinacalcet/Sr2+ o combination might represent a useful approach in targeting osteoclasts; however, as neither AC‐265347 nor BTU‐compound 13 shared the same profile on TRAP activity and hydroxyapatite resorption, it appears that this effect may be specific to cinacalcet, rather than calcimimetics per se.
One previous study showed that cinacalcet (1 μM) did not alter the differentiation, TRAP activity or bone resorption of human osteoclasts (Shalhoub et al., 2003) or potentiate the effects of Ca2+ o (1.6–6.1 mM). However, this lack of efficacy may reflect an absence of CASR mRNA in the human monocyte‐derived osteoclast cultures, in contrast to our findings. Nonetheless, the differences between the effect of cinacalcet and AC‐265347 or BTU‐compound 13 in our osteoclast cultures are perhaps surprising, although they could be explained by the differences in biased modulatory profile of the calcimimetics.
The expression and trafficking of the CaS receptor is highly regulated at many levels, including within the endoplasmic reticulum, where mutated and misfolded receptors can be ubiquitinated and targeted for degradation (Huang et al., 2006). Calcimimetics and calcilytics have been shown to act as pharmacological chaperones for CaS receptor mutants (Huang and Breitwieser, 2007; Peacock et al., 2009; White et al., 2009; Leach et al., 2013; Cook et al., 2015); it is possible that the calcimimetics herein may differentially regulate cell‐surface receptor expression. By examining the trafficking of the loss‐of‐expression, naturally occurring, G670E point mutation CaS receptor (Leach et al., 2013; Cook et al., 2015), we showed that cinacalcet, but not AC‐265347 or BTU‐compound 13, rescues cell‐surface receptor expression. Whilst these data are from studies in recombinant cells (and using a polymorphic variant), they suggest that the different modulators stabilize distinct conformational states to permit (or not) receptor trafficking. Thus, these different conformations could contribute to phenotypic differences between the calcimimetics in regulating osteoclast function. This observation requires further investigation in a physiologically relevant context; unfortunately, due to the lack of well‐validated antibodies, it was not possible to determine whether cinacalcet was able to differentially traffic the endogenous CaS receptor in human osteoclasts.
We cannot discount that the differences between Sr2+ o, cinacalcet, AC‐265347 and BTU‐compound 13 in osteoclasts are due to off‐target effects of Sr2+ o or cinacalcet acting beyond the CaS receptor. Sr2+ o can interact with other molecular targets of Ca2+ o, including gap‐junction hemi‐channels (Hofer and Brown, 2003), a ryanodine receptor‐like protein that is expressed in osteoclasts (Zaidi et al., 1995) and GPRC6, a close relative of the CaS receptor that also plays an important role in bone physiology (Pi et al., 2000; Pi et al., 2005); indeed, Sr2+ o‐mediated activation of the GPRC6 receptor is reported as partially responsible for its effects on osteoblasts (Pi et al., 2005; Rybchyn et al., 2009). Collectively, it is likely that both CaS receptor and non‐CaS receptor mechanisms mediate the Sr2+ o effects; therefore, it might be useful to examine these effects on osteoclasts derived from patients with naturally occurring CaS receptor mutations. Off‐target effects of cinacalcet have not been well‐defined, though results from a pan‐target screening panel of various neuronal GPCRs, ion channels and transporters (coupled with the relatively high lipophilicity of the compound) suggest the propensity for a number of additional interactions, though most of the targets identified in this study (e.g. monoamine transporters) are not involved in osteoclast physiology (Wu‐Wong et al., 2013). Furthermore, these data would need to be coupled with knowledge of the broader pharmacology of AC‐265347 or BTU‐compound 13 to identify a causative factor in the effects of cinacalcet.
The broad lack of interaction between Sr2+ o and calcimimetics at the level of osteoclast function indicates that a bone‐targeted combination therapy approach for osteoporosis may not be viable; nonetheless, the effect of cinacalcet alone on osteoclast function (irrespective of aetiology) is intriguing and potentially important from a clinical perspective. There are mixed reports on the impact of cinacalcet on BMD in the clinic (Peacock et al., 2005; Malluche et al., 2008; Peacock et al., 2009; Shigematsu et al., 2009; Tsuruta et al., 2013), most likely reflecting modulation of the CaS receptor in multiple cell types and organs (e.g. parathyroid, osteoclasts and osteoblasts). Nevertheless, these data suggest that an inhibitory effect on osteoclasts may contribute to this overall profile.
In summary, we showed that three structurally diverse calcimimetics potentiate Sr2+ o‐mediated activation of the CaS receptor in HEK293 cells in a similar manner to Ca2+ o; however, for AC‐265347 and BTU‐compound 13, these effects do not generally translate to potentiation of Sr2+ o to inhibit human osteoclast maturation and function, suggesting that Sr2+o ranelate may mediate its effects via both CaS receptor‐ and non‐CaS receptor‐dependent pathways. However, the clinically approved agent, cinacalcet, did inhibit osteoclast function (both TRAP activity and resorption), although it is not clear to what degree these effects are related to its allosteric modulation of the CaS receptor or an unappreciated off‐target activity (or a combination thereof). Nonetheless, an evaluation of direct effects on bone cell function may well be warranted for the development of any future calcimimetic or calcilytic agents.
Author contributions
N.A.D., K.L., A.N.K., P.R., A.E.C., T.L.P. performed all experimental work. N.A.D., K.L., C.N. and C.J.L. analyzed data. N.A.D., K.L. and C.J.L. wrote the manuscript. P.P., M.S., P.R., K.L., R.J.S., W.N.C., P.M.S., A.C. and C.J.L. conceived the studies and reviewed the manuscript draft(s).
Conflict of interest
P.P. and M.S. are employed by Servier, which until 2017 marketed strontrium ranelate for the treatment of osteoporosis. N.A.D., K.L., A.N.K., P.R., A.E.C., T.L.P., C.N., R.J.S., W.N.C., P.M.S., A.C. and C.J.L. are (or were) employed by Monash University and receive financial support from Servier for G protein‐coupled receptor drug discovery.
Supporting information
Figure S1 Concentration response curves for Ca2+ o‐ and Sr2+ o‐ mediated CaS receptor signalling across multiple signalling pathways in the presence of increasing concentrations of cinacalcet in Flp‐In HEK293‐TREx‐c‐myc‐CASR cells. Data are grouped from 5–29 individual experiments performed in duplicate; the curves represent model fits to the data according to the operational model of allosterism and agonism (see Methods).
Figure S2 Concentration response curves for Ca2+ o‐ and Sr2+ o‐ mediated CaS receptor signalling across multiple signalling pathways in the presence of increasing concentrations of AC‐265347 in Flp‐In HEK293‐TREx‐c‐myc‐CASR cells. Data are grouped from 5–13 individual experiments performed in duplicate; the curves represent model fits to the data according to the operational model of allosterism and agonism (see Methods).
Figure S3 Concentration response curves for Ca2+ o‐ and Sr2+ o‐ mediated CaS receptor signalling across multiple signalling pathways in the presence of increasing concentrations of BTU compound 13 in Flp‐In HEK293‐TREx‐c‐myc‐CASR cells. Data are grouped from five individual experiments performed in duplicate; the curves represent model fits to the data according to the operational model of allosterism and agonism (see Methods).
Table S1 Primers for the characterization of mature osteoclast cultures using RT‐PCR.
Table S2 Calculated physicochemical and predicted cell permeability parameters for calcimimetics.
Acknowledgements
P.M.S. is a Principal Research Fellow, and A.C. is a Senior Principal Research Fellow of the National Health and Medical Research Council of Australia. K.L. is an Australian Research Council Future Fellow. This work was partly funded by Servier.
Diepenhorst, N. A. , Leach, K. , Keller, A. N. , Rueda, P. , Cook, A. E. , Pierce, T. L. , Nowell, C. , Pastoureau, P. , Sabatini, M. , Summers, R. J. , Charman, W. N. , Sexton, P. M. , Christopoulos, A. , and Langmead, C. J. (2018) Divergent effects of strontium and calcium‐sensing receptor positive allosteric modulators (calcimimetics) on human osteoclast activity. British Journal of Pharmacology, 175: 4095–4108. 10.1111/bph.14344.
References
- Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA et al (2017). The Concise Guide To PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurelio L, Valant C, Flynn BL, Sexton PM, Christopoulos A, Scammells PJ (2009). Allosteric modulators of the adenosine A1 receptor: synthesis and pharmacological evaluation of 4‐substituted 2‐amino‐3‐benzoylthiophenes. J Med Chem 52: 4543–4547. [DOI] [PubMed] [Google Scholar]
- Bain S, Jerome C, Shen V, Dupin‐Roger I, Ammann P (2009). Strontium ranelate improves bone strength in ovariectomized rat by positively influencing bone resistance determinants. Osteoporos Int 20: 1417–1428. [DOI] [PubMed] [Google Scholar]
- Baron R, Tsouderos Y (2002). In vitro effects of S12911‐2 on osteoclast function and bone marrow macrophage differentiation. Eur J Pharmacol 450: 11–17. [DOI] [PubMed] [Google Scholar]
- Boissy P, Saltel F, Bouniol C, Jurdic P, Machuca‐Gayet I (2002). Transcriptional activity of nuclei in multinucleated osteoclasts and its modulation by calcitonin. Endocrinology 143: 1913–1921. [DOI] [PubMed] [Google Scholar]
- Bonnelye E, Chabadel A, Saltel F, Jurdic P (2008). Dual effect of strontium ranelate: stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro . Bone 42: 129–138. [DOI] [PubMed] [Google Scholar]
- Bonomini M, Giardnelli A, Morabito C, Di Silvestre S, Di Cesare M, Di Pietro N et al (2012). Calcimimetic R‐568 and Its Enantiomer S‐568 Increase Nitric Oxide Release in Human Endothelial Cells. PLOS One. 10.1371/journal.pone.0030682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan T, Rybchyn M, Green W, Atwa S, Conigrave A, Mason R (2009). Osteoblasts play key roles in the mechanisms of action of strontium ranelate. Br J Pharmacol 157: 1291–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broccatelli F, Salphati L, Plise E, Cheong J, Gobbi A, Lee ML et al (2016). Predicting passive permeability of drug‐like molecules from chemical structure: where are we? Mol Pharm 13: 4199–4208. [DOI] [PubMed] [Google Scholar]
- Caudrillier A, Hurtel‐Lemaire A‐S, Wattel A, Cournarie F, Godin C, Petit L et al (2010). Strontium ranelate decreases receptor activator of nuclear factor‐κB ligand‐induced osteoclastic differentiation in vitro: involvement of the calcium‐sensing receptor. Mol Pharm 78: 569–576. [DOI] [PubMed] [Google Scholar]
- Chang W, Tu C, Chen T‐H, Komuves L, Oda Y, Pratt SA et al (1999). Expression and signal transduction of calcium‐sensing receptors in cartilage and bone 1. Endocrinology 140: 5883–5893. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay N, Quinn SJ, Kifor O, Ye C, Brown EM (2007). The calcium‐sensing receptor (CaR) is involved in strontium ranelate‐induced osteoblast proliferation. Biochem Pharmacol 74: 438–447. [DOI] [PubMed] [Google Scholar]
- Chavassieux P, Meunier PJ, Roux JP, Portero‐Muzy N, Pierre M, Chapurlat R (2014). Bone histomorphometry of transiliac paired bone biopsies after 6 or 12 months of treatment with oral strontium ranelate in 387 osteoporotic women: randomized comparison to alendronate. J Bone Miner Res 29: 618–628. [DOI] [PubMed] [Google Scholar]
- Christopoulos A (1998). Assessing the distribution of parameters in models of ligand–receptor interaction: to log or not to log. Trends Pharmacol Sci 19: 351–357. [DOI] [PubMed] [Google Scholar]
- Compston J (2014). Strontium ranelate lives to fight another day. Maturitas 78: 75–76. [DOI] [PubMed] [Google Scholar]
- Cook A, Mistry S, Gregory K, Furness S, Sexton P, Scammells P et al (2015). Biased allosteric modulation at the CaS receptor engendered by structurally diverse calcimimetics. Br J Pharmacol 172: 185–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham J, Danese M, Olson K, Klassen P, Chertow GM (2005). Effects of the calcimimetic cinacalcet HCl on cardiovascular disease, fracture, and health‐related quality of life in secondary hyperparathyroidism. Kidney Int 68: 1793–1800. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander S, Giembycz MA et al (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl SG, Allain P, Marie PJ, Mauras Y, Boivin G, Ammann P et al (2001). Incorporation and distribution of strontium in bone. Bone 28: 446–453. [DOI] [PubMed] [Google Scholar]
- Davey AE, Leach K, Valant C, Conigrave AD, Sexton PM, Christopoulos A (2012). Positive and negative allosteric modulators promote biased signaling at the calcium‐sensing receptor. Endocrinology 153: 1232–1241. [DOI] [PubMed] [Google Scholar]
- Deprez P, Temal T, Jary H, Auberval M, Lively S, Guédin D et al (2013). New potent calcimimetics: II. Discovery of benzothiazole trisubstituted ureas. Bioorg Med Chem 23: 2455–2459. [DOI] [PubMed] [Google Scholar]
- Diepenhorst NA, Nowell CJ, Rueda P, Henriksen K, Pierce T, Cook AE et al (2017). High throughput, quantitative analysis of human osteoclast differentiation and activity. Anal Biochem 519: 51–56. [DOI] [PubMed] [Google Scholar]
- EMA (2014) European Medicines Agency recommends that Protelos/Oressor remain available but with further restrictions, in EMA/84749/2014 (EMA ed).
- Fromigué O, Haÿ E, Barbara A, Petrel C, Traiffort E, Ruat M et al (2009). Calcium sensing receptor‐dependent and receptor‐independent activation of osteoblast replication and survival by strontium ranelate. J Cell Mol Med 13: 2189–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goltzman D, Hendy GN (2015). The calcium‐sensing receptor in bone mechanistic and therapeutic insights. Nat Rev Endocrinol 11: 298–307. [DOI] [PubMed] [Google Scholar]
- Halleen JM, Tiitinen SL, Ylipahkala H, Fagerlund KM, Vaananen HK (2006). Tartrate‐resistant acid phosphatase 5b (TRACP 5b) as a marker of bone resorption. Clin Lab 52: 499–510. [PubMed] [Google Scholar]
- Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S et al (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer AM, Brown EM (2003). Extracellular calcium sensing and signalling. Nat Rev Mol Cell Biol 4: 530–538. [DOI] [PubMed] [Google Scholar]
- Huang Y, Breitwieser GE (2007). Rescue of calcium‐sensing receptor mutants by allosteric modulators reveals a conformational checkpoint in receptor biogenesis. J Biol Chem 282: 9517–9525. [DOI] [PubMed] [Google Scholar]
- Huang Y, Niwa J‐i, Sobue G, Breitwieser GE (2006). Calcium‐sensing receptor ubiquitination and degradation mediated by the E3 ubiquitin ligase dorfin. J Biol Chem 281: 11610–11617. [DOI] [PubMed] [Google Scholar]
- Hurtel‐Lemaire AS, Mentaverri R, Caudrillier A, Cournarie F, Wattel A, Kamel S et al (2009). The calcium‐sensing receptor is involved in strontium ranelate‐induced osteoclast apoptosis: new insights into the associated signaling pathways. J Biol Chem 284: 575–584. [DOI] [PubMed] [Google Scholar]
- Irannejad R, Pessino V, Mika D, Huang B, Wedegaertner PB, Conti M et al (2017). Functional selectivity of GPCR‐directed drug action through location bias. Nat Chem Biol 13: 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian‐Nong Ma, Michelle Owens, Magnus Gustafsson, Jacob Jensen, Ali Tabatabaei, Kara Schmelzer et al (2011). Characterization of Highly Efficacious Allosteric Agonists of the Human Calcium‐Sensing Receptor. JPET 337: 275–284. [DOI] [PubMed] [Google Scholar]
- Kantham L, Quinn SJ, Egbuna OI, Baxi K, Butters R, Pang JL et al (2009). The calcium‐sensing receptor (CaSR) defends against hypercalcemia independently of its regulation of parathyroid hormone secretion. Am J Physiol Endocrinol Metab 297: E915–E923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach K, Gregory KJ, Kufareva I, Khajehali E, Cook AE, Abagyan R et al (2016). Towards a structural understanding of allosteric drugs at the human calcium‐sensing receptor. Cell Res 26: 574–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach K, Sexton PM, Christopoulos A (2007). Allosteric GPCR modulators: taking advantage of permissive receptor pharmacology. Trends Pharmacol Sci 28: 382–389. [DOI] [PubMed] [Google Scholar]
- Leach K, Wen A, Cook AE, Sexton PM, Conigrave AD, Christopoulos A (2013). Impact of clinically relevant mutations on the pharmacoregulation and signaling bias of the calcium‐sensing receptor by positive and negative allosteric modulators. Endocrinology 154: 1105–1116. [DOI] [PubMed] [Google Scholar]
- Leach K, Wen A, Davey AE, Sexton PM, Conigrave AD, Christopoulos A (2012). Identification of molecular phenotypes and biased signaling induced by naturally occurring mutations of the human calcium‐sensing receptor. Endocrinology 153: 4304–4316. [DOI] [PubMed] [Google Scholar]
- Malluche H, Monier‐Faugere M, Wang G, Frazã OJ, Charytan C, Coburn J et al (2008). An assessment of cinacalcet HCl effects on bone histology in dialysis patients with secondary hyperparathyroidism. Clin Nephrol 69: 269–278. [DOI] [PubMed] [Google Scholar]
- Marcus R (2013). Current Therapies for Osteoporosis. Rheumatology 40. [Google Scholar]
- Meunier PJ, Roux C, Seeman E, Ortolani S, Badurski JE, Spector TD et al (2004). The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med 350: 459–468. [DOI] [PubMed] [Google Scholar]
- Nicholson GC, Malakellis M, Collier FM, Cameron PU, Holloway WR, Gough TJ et al (2000). Induction of osteoclasts from CD14‐positive human peripheral blood mononuclear cells by receptor activator of nuclear factor kappaB ligand (RANKL). Clin Sci 99: 133–140. [PubMed] [Google Scholar]
- Peacock M, Bilezikian JP, Klassen PS, Guo MD, Turner SA, Shoback D (2005). Cinacalcet hydrochloride maintains long‐term normocalcemia in patients with primary hyperparathyroidism. J Clin Endocrinol Metab 90: 135–141. [DOI] [PubMed] [Google Scholar]
- Peacock M, Bolognese MA, Borofsky M, Scumpia S, Sterling LR, Cheng S et al (2009). Cinacalcet treatment of primary hyperparathyroidism: biochemical and bone densitometric outcomes in a five‐year study. J Clin Endocrinol Metab 94: 4860–4867. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW (2001) A new mathematical model for relative quantification in real‐time RT‐PCR. Nucleic Acids Res 29: e45, 45e, 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi M, Faber P, Ekema G, Jackson PD, Ting A, Wang N et al (2005). Identification of a novel extracellular cation‐sensing G‐protein‐coupled receptor. J Biol Chem 280: 40201–40209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi M, Garner SC, Flannery P, Spurney RF, Quarles LD (2000). Sensing of extracellular cations in CasR‐deficient osteoblasts: evidence for a novel cation‐sensing mechanism. J Biol Chem 275: 3256–3263. [DOI] [PubMed] [Google Scholar]
- Piper K, Boyde A, Jones SJ (1992). The relationship between the number of nuclei of an osteoclast and its resorptive capability in vitro. Anat Embryol 186: 291–299. [DOI] [PubMed] [Google Scholar]
- Pipino C, Tomo P, Mandatori D, Cianci E, Lanuti P, Cutrona MB et al (2014). Calcium Sensing Receptor Activation by Calcimimetic R‐568 in Human Amniotic Fluid Mesenchymal Stem Cells: Correlation with Osteogenic Differentiation. Stem Cells and Development 23: 2959–2971. [DOI] [PubMed] [Google Scholar]
- Pires DEV, Blundell TL, Ascher DB (2015). pkCSM: predicting small‐molecule pharmacokinetic and toxicity properties using graph‐based signatures. J Med Chem 58: 4066–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reginster J‐Y (2014). Cardiac concerns associated with strontium ranelate. Expert Opin Drug Saf 13: 1209–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reginster J‐Y, Seeman E, De Vernejoul M, Adami S, Compston J, Phenekos C et al (2005). Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) study. J Clin Endocrinol Metab 90: 2816–2822. [DOI] [PubMed] [Google Scholar]
- Riccardi D, Valenti G (2016). Localization and function of the renal calcium‐sensing receptor. Nat Rev Nephrol 12: 414–425. [DOI] [PubMed] [Google Scholar]
- Rybchyn M, Green W, Conigrave A, Mason R (2009). Involvement of both GPRC6A and the calcium‐sensing receptor in strontium ranelate‐induced osteoclastogenic signal expression and replication in primary human osteoblasts. Bone 44: S317. [Google Scholar]
- Servier LL (2017) Cessation of marketing of Protelos/Osseor: extract of the letter sent to European Medicine Agency (EMA) and national European Agencies on 10 February 2017, Les Laboratories Servier, Available at: http://www.servier.co.uk/sites/default/files/media/servier_protelos_medical_industry_pr_final.pdf [Accessed 15 Aug. 2017].
- Shalhoub V, Grisanti M, Padagas J, Scully S, Rattan A, Qi M et al (2003). In vitro studies with the calcimimetic, cinacalcet HCl, on normal human adult osteoblastic and osteoclastic cells. Crit Rev Eukaryot Gene Expr 13: 89–106. [DOI] [PubMed] [Google Scholar]
- Shigematsu T, Akizawa T, Uchida E, Tsukamoto Y, Iwasaki M, Koshikawa S (2009). Long‐term cinacalcet HCl treatment improved bone metabolism in Japanese hemodialysis patients with secondary hyperparathyroidism. Am J Nephrol 29: 230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims NA and Martin TJ (2014) Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. BoneKEy Rep. [DOI] [PMC free article] [PubMed]
- Sims NA, Martin TJ (2015). Coupling signals between the osteoclast and osteoblast: how are messages transmitted between these temporary visitors to the bone surface? Front Endocrinol . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smajilovic S, Yano S, Jabbari R, Tfelt‐Hansen J (2011). The calcium‐sensing receptor and calcimimetics in blood pressure modulation. Br J Pharmacol 164: 884–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Sasaki T, Tsouderos Y, Suda T (2003). S 12911‐2 inhibits osteoclastic bone resorption in vitro. J Bone Miner Res 18: 1082–1087. [DOI] [PubMed] [Google Scholar]
- Thomsen ARB, Worm J, Jacobsen SE, Stahlhut M, Latta M, Bräuner‐Osborne H (2012). Strontium is a biased agonist of the calcium‐sensing receptor in rat medullary thyroid carcinoma 6‐23 cells. J Pharmacol Exp Ther 343: 638–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruta Y, Okano K, Kikuchi K, Tsuruta Y, Akiba T, Nitta K (2013). Effects of cinacalcet on bone mineral density and bone markers in hemodialysis patients with secondary hyperparathyroidism. Clin Exp Nephrol 17: 120–126. [DOI] [PubMed] [Google Scholar]
- Valant C, Felder CC, Sexton PM, Christopoulos A (2012). Probe dependence in the allosteric modulation of a G protein‐coupled receptor: implications for detection and validation of allosteric ligand effects. Mol Pharmacol 81: 41–52. [DOI] [PubMed] [Google Scholar]
- Watts JJ, Abimanyi‐Ochom J and Sanders KM (2013) Osteoporosis costing all Australians: a new burden of disease analysis–2012 to 2022, Osteoprosis Australia; [Google Scholar]
- White E, McKenna J, Cavanaugh A, Breitwieser GE (2009). Pharmacochaperone‐mediated rescue of calcium‐sensing receptor loss‐of‐function mutants. Mol Endocrinol 23: 1115–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu‐Wong JR, Nakane M, Chen Y, Mizobuchi M (2013). Mechanistic analysis for time‐dependent effects of cinacalcet on serum calcium, phosphorus, and parathyroid hormone levels in 5/6 nephrectomized rats. Physiol Rep 1: (Epub 2013, Aug 2022): e00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi M, Shankar V, Re T, Adebanjo O, Mackrill J, Pazianas M et al (1995). A ryanodine receptor‐like molecule expressed in the osteoclast plasma membrane functions in extracellular Ca2+ sensing. J Clin Invest 96: 1582–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Concentration response curves for Ca2+ o‐ and Sr2+ o‐ mediated CaS receptor signalling across multiple signalling pathways in the presence of increasing concentrations of cinacalcet in Flp‐In HEK293‐TREx‐c‐myc‐CASR cells. Data are grouped from 5–29 individual experiments performed in duplicate; the curves represent model fits to the data according to the operational model of allosterism and agonism (see Methods).
Figure S2 Concentration response curves for Ca2+ o‐ and Sr2+ o‐ mediated CaS receptor signalling across multiple signalling pathways in the presence of increasing concentrations of AC‐265347 in Flp‐In HEK293‐TREx‐c‐myc‐CASR cells. Data are grouped from 5–13 individual experiments performed in duplicate; the curves represent model fits to the data according to the operational model of allosterism and agonism (see Methods).
Figure S3 Concentration response curves for Ca2+ o‐ and Sr2+ o‐ mediated CaS receptor signalling across multiple signalling pathways in the presence of increasing concentrations of BTU compound 13 in Flp‐In HEK293‐TREx‐c‐myc‐CASR cells. Data are grouped from five individual experiments performed in duplicate; the curves represent model fits to the data according to the operational model of allosterism and agonism (see Methods).
Table S1 Primers for the characterization of mature osteoclast cultures using RT‐PCR.
Table S2 Calculated physicochemical and predicted cell permeability parameters for calcimimetics.


