Key Points
Control of posttransplant AIHA refractory to conventional treatments with anti-CD38 antibodies.
Abstract
New-onset autoimmune hemolytic anemia (AIHA) occurs in 2% to 6% of pediatric patients post–hematopoietic stem cell transplantation (HSCT) and is a significant complication. Incomplete immune recovery following HSCT may predispose to immune dysregulation including autoimmune cytopenias. We describe an innovative therapy for AIHA refractory to proteasome inhibition. In potentially life-threatening AIHA in the context of HSCT, daratumumab may be an effective rescue therapy.
Visual Abstract
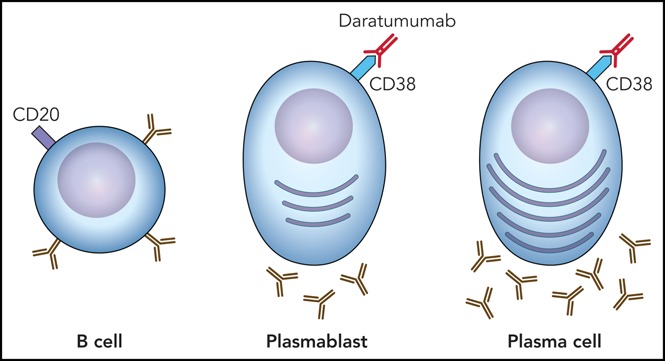
Professional illustration by Patrick Lane, ScEYEnce Studios.
Introduction
New-onset autoimmune hemolytic anemia (AIHA) occurs in 2% to 6%1,2 of pediatric patients post–hematopoietic stem cell transplantation (HSCT). It is potentially life-threatening and causes significant morbidity and mortality.3 Incomplete immune recovery may predispose to immune dysregulation following HSCT including autoimmune cytopenias. Although prednisolone or other immunosuppressive agents control most episodes, up to 60% of patients respond incompletely to first- or second-line therapies including rituximab.3 We describe an innovative therapy for AIHA post-HSCT refractory to established first- and second-line options.
Methods
We retrospectively evaluated data from 3 patients treated with daratumumab (Darzalex) for posttransplant AIHA. Written informed consent of patients and families for compassionate use of the medication according to the Declaration of Helsinki and regulations of local review boards was obtained.
Results and discussion
Patient clinical features and course post-HSCT
A 19-year-old woman with pre–B-cell acute lymphoblastic leukemia (B ALL) with positive minimal residual disease (MRD) underwent myeloablative HSCT from a matched unrelated donor (MUD). Four months after HSCT, and 1 month following a second donor lymphocyte infusion (DLI) because of persistently positive MRD and declining donor chimerism, the patient developed Coombs-positive AIHA (hemoglobin [Hb], 4.7 g/dL). Response was partial following treatment with high-dose methylprednisolone (MPN), rituximab, and bortezomib. Because of persistent MRD and AIHA, the patient was given a stem cell boost after treatment with high-dose cyclophosphamide and antithymocyte globulin (ATG). MRD became negative; however, during the following 14 months, persistent AIHA prompted therapy with MPN, rituximab, alemtuzumab, bortezomib, mycophenolate mofetil (MMF), sirolimus, and ibrutinib. Her Coombs test remained positive and she required >2 packed red blood cell (PRBC) transfusions per week. Daratumumab (16 mg/kg per week IV) was administered for 6 weeks during which time she required only 1 additional PRBC 5 days following the first infusion. Her hemoglobin increased without further transfusions and lactate dehydrogenase (LDH) normalized. At 4 weeks after her first dose of daratumumab, antibody titers in her Coombs test decreased, but haptoglobin remained undetectable. She required monthly PRBC transfusions over a period of 8 months after starting daratumumab before recurrence of AIHA. She died of refractory AIHA 2 months later despite reinitiation of prior immunosuppressants and having been treated with plasmapheresis and splenic irradiation (see Table 1 for more details).
Table 1.
Characteristics of patients, HSCT, and posttransplant AIHA
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Diagnosis | Pre–B-ALL | XLT/WAS | DNA-ligase IV deficiency |
| Age at HSCT | 19 y | 25 mo | 20 mo |
| Donor | MUD (11/12, HLA-DP locus) | MUD (9/10, B locus) | MFD |
| Conditioning regimen | Bu (targeted cAUC 90); Flu (40 mg/m2); Clo (120 mg/m2); alemtuzumab (0.6 mg/kg) | Treo (36 g/m2); Flu (150 mg/m2); Thio (10 mg/kg); alemtuzumab (0.7 mg/kg) | Flu (150 mg/m2); Cy (20 mg/kg); alemtuzumab (1 mg/kg) |
| Blood groups, recipient/donor | A+/A+ | 0+/0+ | 0+/0+ |
| Onset of post-HSCT AIHA, days post-HSCT | 116 | 138 | 286 |
| Chimerism at diagnosis, % donor cells | 92 | 100 | 95 |
| Isotype/specific antibodies | IgG/anti Rh-E-antigen | IgG/warm autoantibody | IgG/anti-RH5 antibody |
| Activation of complement | Yes | No | Yes |
| Immunological phenotyping at onset of AIHA | ND | CD4+ 48/μL, CD19+ 108/μL, CD56+/CD16+ 192/μL | CD4+ 792/μL, CD19+ 528/μL, CD56+/CD16+ 44/μL |
| PRBC transfusions prior to daratumumab | ∼200 (total) | 20 | 4 |
| Cumulative days of steroid treatment* prior to daratumumab | 503 | 54 | 379 |
| Initial treatment | MPN, rituximab | PDN, rituximab, plasmapheresis | MPN/PDN, rituximab |
| Plasmapheresis sessions | No | 36 | No |
| Cumulative number of rituximab doses, ×375 mg/m2, prior to daratumumab | 11 | 8 | 7 |
| Cumulative number of bortezomib courses, 4 × 1.3 mg/m2, prior to daratumumab | 3 | 2 | 3 |
| Ongoing second- or third-line treatments at start of daratumumab treatment | Bortezomib, sirolimus, MMF, ibrutinib, Cy, ATG | MMF, plasmapheresis | MMF, sirolimus, eculizumab |
| Day of start daratumumab after onset of AIHA (post-HSCT day+) | 503 (619) | 265 (403) | 333 (619) |
| Total doses of daratumumab, ×16 mg/kg, given once weekly | 11 | 4 | 7 |
| Duration of daratumumab treatment, d | 49 | 17 | 43 |
| Response to daratumumab | Partial/transient (8 mo) | Yes | Yes |
| PRBC following daratumumab | Until death | None | 1 |
| Outcome (follow-up time in months after daratumumab/rituximab) | Died | a/w (16/12) | a/w (13/19) |
| CD4+ T cells, CD45RA % | NA | 1898/μL, 75% | 1565/μL, 44% |
| Time to B-cell recovery, mo | NA | 7.5 | 6.5 |
| CD19+CD20+ B-cell recovery at last follow-up | NA | 164/μL | 656/μL |
| IgM | NA | 0.44 g/L | 0.42 g/L |
| CD19+CD38++ plasmablasts | NA | Present | Present |
| On IVIG | Until death | No | Yes |
a/w, alive and well; B ALL, B-cell acute lymphocytic leukemia; Bu, busulfan; Clo, clofarabine; cAUC, cumulative area under the curve; Cy, cyclophosphamide; HLA-DP, human leucocyte antigen DP; Flu, fludarabine; MFD, matched family donor; NA, not applicable; ND, not done; PDN, prednisolone; Thio, thiotepa; Treo, treosulfan; WAS, Wiskott-Aldrich syndrome; XLT, X-linked thrombocytopenia.
PDN ≥ 1 mg/kg.
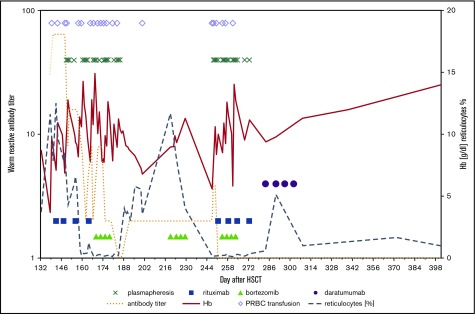
A 25-month-old boy with Wiskott-Aldrich syndrome underwent HSCT from a MUD. Five months later, following an upper airway infection, he developed Coombs-positive AIHA (Hb, 3 g/dL). He showed insufficient response to prednisolone and rituximab requiring frequent transfusions (Figure 1). Even though warm-reactive antibody titers dropped and remained low after the second dose of rituximab, and plasmapheresis was able to stabilize hemolysis (Figure 1), AIHA recurred and was merely temporarily controlled with 3 cycles of bortezomib started 30 days after onset of hemolysis. With the fourth recurrence of active AIHA, daratumumab was administered (Table 1; Figure 1). No further PRBC transfusions have been necessary since. The patient has been stable for 16 months after daratumumab treatment and has been off of immunoglobulin substitution for 2 months. CD19+ B-cell numbers are below the normal range (164/µL); immunoglobulin M (IgM) levels (0.44 g/L) are increasing. The detection of switched memory B cells (0.3% of CD19+ cells are IgG+/CD20+/CD27+) and plasmablasts (0.5% of CD19+ cells are CD38high/CD20−) in peripheral blood indicates recovery of B-cell maturation (Table 1). The child is still on deferasirox for iron overload.
Figure 1.
Representation of laboratory parameters and treatment strategies in patient 2.
A 20-month-old girl with DNA-ligase IV deficiency received a transplant from her HLA-identical father. Nine months after HSCT, she developed Coombs-positive AIHA (Hb, 5.9 g/dL). She had only partial response to steroids, MMF, rituximab, and bortezomib. Treatment with eculizumab given twice weekly 10.5 months after onset of AIHA was ineffective. Seven weekly infusions of daratumumab led to persistent remission of AIHA with reconstitution of donor B cells as early as 3 months after the last daratumumab dose and presence of IgM 2 months after completed treatment with daratumumab. The patient is still being substituted with immunoglobulins, but vaccinations have been initiated. B-cell numbers are in the normal range (656/µL), with presence of switched memory B cells (0.3% of CD19+ cells are IgD−/CD27+) and CD19+/CD38high/CD20− plasmablasts.
In the patients described, AIHA occurred between 4 and 9 months following HSCT. Donors and recipients were ABO-compatible; donor chimerism was between 92% and 100% (Table 1). An infectious trigger was reported for the second patient. All patients had incomplete immune reconstitution when AIHA occurred. First- and second-line treatment consisted of steroids, MMF, sirolimus, and rituximab (the latter was used for all patients). Plasmapheresis was applied in the second patient with transient success only. Even though antibody titers dropped with rituximab, hemolysis recurred or persisted after complete depletion of circulating CD20+ B cells. Other treatments with transient response included cyclophosphamide, bortezomib, eculizumab, and ibrutinib. Persistence of plasma cells was suspected despite treatment with bortezomib. With the risk of peripheral neuropathy as a limiting side effect we were reluctant to escalate cumulative doses of bortezomib. Patients 2 and 3 were treated according to the positive response reported for the patient 1 (Table 1).
Daratumumab was approved for treatment of multiple myeloma in 2015 as a specific antibody targeting CD38 on the surface of malignant plasma cells.4 As CD38 is also expressed on nonmalignant plasma cells, there is a rationale for daratumumab to target autoantibody-producing plasma cells after the failure of established therapies. Described side effects include fever, fatigue, nausea, and cough. Of note, CD38 is weakly expressed on red blood cells. Daratumumab mimics clinically important antibodies for up to 5 months, interfering, for example, with blood bank compatibility testing (false-positive indirect Coombs test), but has no interference with ABO/RhD antigens.4,5 Therefore, any patient should be typed prior to treatment with daratumumab and the blood bank should be informed. As CD38 is also expressed on various other hematopoietic cells, some patients may develop lymphopenia, neutropenia, anemia, and thrombocytopenia.4 With depletion of B and plasma cells by rituximab and daratumumab, immunoglobulin substitution was necessary. The probability and time for complete recovery of humoral immunity after this regimen is unknown. In the 2 survivors, B cells reappeared in the periphery 12 and 2.5 months following treatment with daratumumab (8 and 7 months following the last rituximab infusion). Other long-term side effects so far have not been documented.
In the reported 3 patients with life-threatening posttransplant AIHA, daratumumab was curative in 2, with the third showing a transient response before relapse of AIHA 8 months after this therapy. The survivors are currently free of any signs of hemolysis with a follow-up of 18 and 13 months after administration of daratumumab. Clinical effects on hemolysis and independency of transfusions were observed within a few days after the first dose for patient 2 (Figure 1). Reported side effects of daratumumab were minor, namely bronchial hyperreactivity, nausea, and diarrhea. There are currently no preclinical or clinical data available to prove that a plasma cell–directed therapy with daratumumab has a role in treatment of autoimmune disease by reducing autoantibody-dependent effector mechanisms.5 The mechanisms explaining the rapid remission of AIHA following treatment with daratumumab are yet to be defined, and may differ from those postulated for the antitumor activity in multiple myeloma.5,6 With limited clinical experience and no studies available for this indication, daratumumab is a potentially effective therapeutic agent in AIHA, but its use should currently remain confined to patients with life-threatening disease unresponsive to established therapies.
Acknowledgment
The authors are very grateful to the patients and nursing staff.
Footnotes
C.S. and M.H. share first authorship.
A.S.S. and C.C.D. share last authorship.
Authorship
Contribution: C.S., M.H., D.M., A.S.S., and C.C.D. designed the research and collected data; C.S., M.H., and C.C.D. wrote the report; D.M. and A.S.S. contributed to the study design and revised the manuscript of the report; and C.S., M.H., A.S.S., D.M., M.C., M.B., C.C.D., K.S., V.T., and C.W. contributed to patients’ clinical care and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Catharina Schuetz, Department of Pediatrics and Adolescent Medicine, University Medical Center Ulm, Eythstr 24, 89075 Ulm, Germany; e-mail: catharinaschuetz@gmx.de; and Christopher C. Dvorak, Division of Pediatric Allergy, Immunology & Bone Marrow Transplantation, Benioff Children's Hospital, University of California San Francisco, 550 16th Street, 4th Floor, Box 0434, San Francisco, CA; e-mail: christopher.dvorak@ucsf.edu.
References
- 1.Ahmed I, Teruya J, Murray-Krezan C, Krance R. The incidence of autoimmune hemolytic anemia in pediatric hematopoietic stem cell recipients post-first and post-second hematopoietic stem cell transplant. Pediatr Transplant. 2015;19(4):391-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watz E, Remberger M, Ringden O, et al. . Analysis of donor and recipient ABO incompatibility and antibody-associated complications after allogeneic stem cell transplantation with reduced-intensity conditioning. Biol Blood Marrow Transplant. 2014;20(2):264-271. [DOI] [PubMed] [Google Scholar]
- 3.Faraci M, Zecca M, Pillon M, et al. ; Italian Association of Paediatric Haematology and Oncology. Autoimmune hematological diseases after allogeneic hematopoietic stem cell transplantation in children: an Italian multicenter experience. Biol Blood Marrow Transplant. 2014;20(2):272-278. [DOI] [PubMed] [Google Scholar]
- 4.Costello C. An update on the role of daratumumab in the treatment of multiple myeloma. Ther Adv Hematol. 2017;8(1):28-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van de Donk NW, Janmaat ML, Mutis T, et al. . Monoclonal antibodies targeting CD38 in hematological malignancies and beyond. Immunol Rev. 2016;270(1):95-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shallis RM, Terry CM, Lim SH. The multi-faceted potential of CD38 antibody targeting in multiple myeloma. Cancer Immunol Immunother. 2017;66(6):697-703. [DOI] [PMC free article] [PubMed] [Google Scholar]