Abstract
Bruton’s tyrosine kinase (Btk) is a crucial regulator of B cell signaling and is a therapeutic target for lymphoma and autoimmune disease. BTK-deficient patients suffer from humoral immunodeficiency, as their B cells fail to progress beyond the bone marrow. However, the role of Btk in fully developed, mature peripheral B cells is not well understood. Analysis using BTK-inhibitors is complicated by suboptimal inhibition, off-target effects, or failure to eliminate BTK’s adaptor function. Therefore a Btkflox/Cre-ERT2 mouse model was developed and used to excise Btk after B cell populations were established. Mice lacking Btk from birth are known to have reduced follicular (FO) compartments, with expanded transitional populations, suggesting a block in development. In adult Btkflox/Cre-ERT2 mice, Btk-excision did not reduce FO B cells, which persisted for weeks. Autoimmune-prone B1 cells also survived conditional Btk-excision, contrasting their near-absence in global Btk-deficient mice. Therefore Btk supports BCR-signaling during selection into the follicular and B1 compartments, but is not needed to maintain these cell populations. B1-related natural IgM levels remained normal, contrasting global Btk-deficiency, but B cell proliferation and T-independent type II immunization responses were blunted. Thus, B cells have nuanced signaling responses that are differentially regulated by Btk for development, survival, and function. These findings raise the possibility that Btk may also be expendable for survival of mature human B cells, therefore requiring prolonged dosing to be effective, and that success of BTK-inhibitors may depend in part on off-target effects.
Bruton’s tyrosine kinase (Btk) is a Tec-family kinase expressed in B lymphocytes and in innate immune cells. Btk plays a role in signaling through the B cell receptor (BCR), as well as through innate receptors such as the Fcγ receptor (FcγR) and various toll like receptors (TLRs)(1–6). The role of Btk has been mostly studied in B lymphocytes. In BTK-deficient human patients, B cells fail to progress beyond the bone marrow resulting in fewer than 1% normal circulating B cells, together with severe hypogammaglobulinemia. However, the role of BTK in established peripheral B cells populations is unknown. The recent emergence of small molecular BTK-inhibitors, and interest in their use for treatment of autoimmune disease, highlights the importance of understanding the actions of Btk in mature B cell populations, which will already have developed beyond the bone marrow when drug dosing begins.
In mice, Btk is not required for maturation in the bone marrow and B cells are able to move into the periphery. However, there is a ~50% decrease in the total number of peripheral B cells in Btk-deficient B6 mice (4). This seems to be due to a developmental block, with increased proportions of late transitional (T2) B cells and a concurrent loss of mature naïve follicular B cells (4, 7–9). The effects of Btk-deficiency on autoreactive B cells are more severe. Innate-like B1 cells, the anergic autoreactive An1 subset, and transgenic anti-insulin and anti-DNA B cells are all absent or impaired in Btk-deficient models (4, 10, 11). B1 cells are initially generated in fetal liver and found primarily in peritoneal and pleural cavities (12). They exhibit slow turnover, are self-renewing, and produce polyreactive natural IgM that is germline-configured to recognize bacterial antigens but can also cross-react with autoantigens (13). B1 cells quickly respond to antigen and are therefore well-suited for early, T-independent responses to infection (14, 15). Signaling through the B cell receptor (BCR) is regulated differently in B1 cells as compared to their B2 counterparts. B1 cells do not mobilize calcium or proliferate in response to BCR crosslinking, but have higher basal levels of cytoplasmic free calcium (16, 17), characteristics they share with the anergic, autoreactive An1 B cell subset (18). Both cell subsets also exhibit constitutive ERK (extracellular signal-related kinase) phosphorylation (19, 20). Btk-deficient mice lack B1 B cells and natural IgM (21) and are unable to respond to T-independent immunization due to the loss of this B cell population (22–24). Humans are reported to have a similar subset of polyreactive IgM-producing B cells, found in umbilical cord and adult peripheral blood, which are characterized by expression of CD20, CD27, and CD43 (25–27).
The reliance of autoreactive-prone subsets such as An1 and B1 cells upon Btk for development has been well studied, but the lack of an inducible knockout model has rendered Btk’s role in their survival and function unclear. One study used an inhibitor to study Btk’s role in mature B cell subsets (28); however, this inhibitor also inhibits TEC and BMX and has a half-life of only five hours. Discrete study of the specific role of Btk therefore requires a genetic knock-down model. The studies reported here use a newly developed Btkflox/Cre-ERT2 model and show that Btk is not required for the survival of mature naïve follicular B cells, the B1 subset or the production of natural IgM, but is required for B cell responses to a T-independent polysaccharide antigen. These data show for the first time the differential contribution of Btk to the development, survival, and function of B lymphocytes.
Materials and Methods
Mice and Cre-ERT2 induction
Btkflox mice were developed at the University of Miami Transgenic Mouse facility. Mice with the potential for conditional deletion of the Btk gene were generated from C57BL/6 embryonic stem cells from EUCOMM (clone HEPD0522_1_A11) carrying LoxP-flanked Btk exons 6 and 7 (Btkflox) and neor gene and a lacZ reporter genes flanked by FRT sites. To generate conditional-ready Btk mice, the neor and lacZ reporter genes were deleted by crossing the mice to C57Bl/6 Flp deleter mice (Taconic). For inducible deletion of Btk, Btkflox mice were paired with a mouse expressing a tamoxifen (Tam)-inducible estrogen receptor. Cre-ERT2 mice were purchased from the Jackson Laboratory (B6.Cg-Tg(UBC-cre/ERT2)1Ejb/1J). Btknull B6 mice were generated as previously described (4). Mice were bred and maintained under specific pathogen free conditions. To induce Cre activation, mice were injected intraperitoneally (I.P.) on days −2, −1, and 0 with 3mg of tamoxifen-free base (Sigma) in 200μL of safflower oil, or vehicle alone. Experiments used age-matched male and female mice, unless otherwise stated, and no difference in efficacy of Btk deletion between genders was observed. Btkflox and Btkflox/Cre-ERT2 animals were cohoused littermates, while Cre-ERT2 and Btknull mice came from separate lines. All studies have been approved by the Vanderbilt University Institutional Animal Care and Use Committee.
Flow cytometry and antibodies
Single-cell suspensions of spleen, bone marrow, and peritoneal cavity were obtained as previously described (1) and stained using fluorochrome or biotin-conjugated antibodies against B220 (RA3-6B2), IgM (μ-chain, Life Technology), IgMb (AF6-78), IgD (11-26c.2a), CD5 (53–7.3), CD11b (M1/70), CD11c (HL3), F4/80 (BM8, eBioscience), Ly6G (IA8), CD19 (ID3), CD21 (7G6), CD23 (B3B4), CD93 (AA4.1), CD86 (GL1), CD44 (IM7), CD43 (S7), CD9 (KMC8) and/or CD138 (281-2). Unless otherwise stated, antibodies were procured from BD Biosciences. Biotin-conjugated antibodies were secondarily stained with streptavidin-conjugated fluorochromes and dead cells were excluded using fixable viability dye 455UV or eFluor 450 (eBioscience) or Alexa Fluor 700-conjugated succinimidyl ester (Life Technologies). For intracellular staining, cells were fixed using 1.6% paraformaldehyde (Electron Microscopy Sciences), then permeabilized with a solution of 0.05% Triton-X-100 (SigmaUltra) and stained with rabbit anti-mouse BTK (D3H5, Cell Signaling), followed by a fluorochrome-conjugated anti-Rabbit IgG (F′ab2) secondary (Cell Signaling). Samples were collected on an LSRII flow cytometer (BD Biosciences) and data analyzed using FlowJo software (TreeStar).
B cell proliferation
Splenocytes were stained with CFSE (Life Technologies) or CellTrace Violet (Life Technologies) according to manufacturer’s instructions, and then cultured at 1x106 cells/mL for three days in cRPMI alone, stimulated with 5μg/mL goat anti-mouse IgM (μ-chain specific, Jackson Immunoresearch) or stimulated with 1μg/mL lipopolysaccharide (LPS, Dibco). Following incubation, cells were harvested and analyzed by flow cytometry.
Immunization studies
Five days after tamoxifen injections, mice were immunized I.P. with 50μg of TNP37-Ficoll (Biosearch Technologies) diluted in 200μL sterile PBS or mock-immunized with PBS alone. Blood for serum Ab analysis was collected one day pre- and five days post-immunization. For TNP-Ficoll specific B cell analysis, cells were isolated from spleen or peritoneal lavage five days post-immunization and incubated with 20μg/mL of TNP65-Ficoll-Fluorescein in PBS containing 2% fetal calf serum, then subsequently stained for analysis by flow cytometry.
ELISAs
Serum anti-phosphoryl-choline (PC) IgM, anti-TNP-Ficoll IgM, and anti-TNP-Ficoll IgG were measured. 96-well flat-bottom NUNC plates were coated with 1μg/mL of PC-BSA (Biosearch Technologies) or TNP37-Ficoll (Biosearch Technologies) in borate-buffered saline or carbonate buffer overnight at 4ºC. Plates were blocked with 1% BSA in PBS+0.05% Tween-20 (PBST). For anti-PC IgM ELISA, samples were serially diluted starting at 1:10. For anti-TNP IgM and IgG, samples were diluted at 1:5000. IgM and IgG antibodies were detected using goat anti-mouse IgM or IgG conjugated to alkaline phosphatase (AP) (Southern Biotech). p-Nitorphenyl phosphate (PNPP) was added and the plate read on a Microplate Autoreader (Bio-Tek Instruments) at O.D. 405nm. Plates were washed in between steps using PBST.
Statistical Analysis
Statistics were performed using GraphPad Prism version 6.00 for Windows, (GraphPad Software, La Jolla California USA). p-values were calculated using one-way or two-way ANOVAs, or Kruskal-Wallice with Dunn’s multiple comparison test, as appropriate.
Results
Cre activation in mature Btkflox/Cre-ERT2 mice depletes Btk at all stages of B cell development
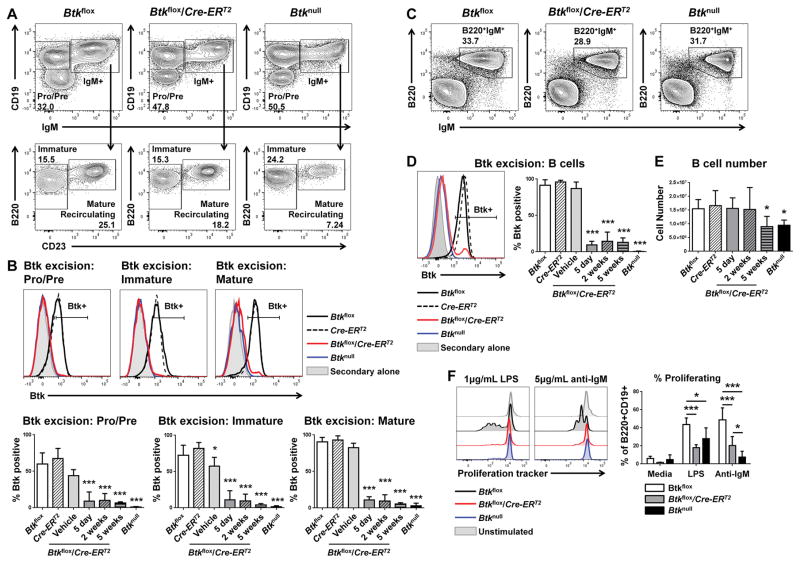
To determine the role of Btk in mature cells, we employed a novel mouse model from C57BL/6 embryonic stem cells carrying LoxP-flanked Btk exons 6 and 7 (Btkflox, Figure 1). For inducible deletion of Btk, Btkflox mice were paired with a mouse expressing a tamoxifen (Tam)-inducible estrogen receptor (Cre-ERT2). Induction of the Cre-ERT2 by administration of tamoxifen in Btkflox/Cre-ERT2 mice resulted in successful knockdown of Btk within five days (Figure 2). Analysis of bone marrow showed successful protein deletion begins at the earliest stages of B cell development (Figure 2A, 2B). Btk was successfully knocked down in pre- and pro- (91.03%±12.68%, p<0.001) and immature B cells (88.79%±12.38%, p<0.001), as well as in mature recirculating B cells (89.20%±4.66%, p<0.001), and all bone marrow B cell subsets remained largely Btk-negative even up to five weeks after injection (Figure 2B). Knockdown was equally successful in splenic B cells five days after injection (90.39%±4.9% Btk-negative, p<0.001, Figure 2D). This knockdown was stable, as B cells from tamoxifen-treated Btkflox/Cre-ERT2 mice remained Btk-negative five weeks later (86.82%±5.96% Btk-negative B cells, p<0.001, Figure 2D). As expected, treated Btkflox/Cre-ERT2 mice also exhibited stable knockdown in macrophages and conventional dendritic cells in the spleen (Supplemental Figure 1). These data demonstrate the efficacy and stability of inducible Btk knockdown. Of note, one out of four vehicle treated female Btkflox/Cre-ERT2 control mice did exhibit a Btk-negative B cell population in the bone marrow, resulting in the appearance of a slight, but significant, loss of Btk in immature B cells (42.75%±12.36% Btk negative) (p=0.049). This mouse also exhibited a slight loss of Btk in pro- and pre- B cells, but the trend was less evident in the spleen. This confirms previous findings of others that endogenous estrogen can occasionally induce some degree of nonspecific activation in the Cre-ERT2 system. However, no differences were seen in Btk knockdown between male and female Btkflox/Cre-ERT2 mice treated with tamoxifen.
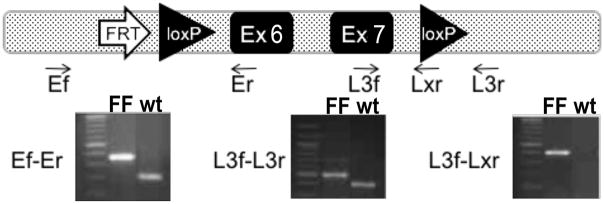
Figure 1. Conditional Btk allele and genotyping strategy for conditional deletion of Btk gene.
Conditional Btk allele following deletion of LacZ and Neor through through breeding with FLP1 transgenic mice. One remaining Flippase recombination enzyme-recognition target (FRT), and Btk Exons 6 and 7 flanked by loxP recombination sites are shown (top). Several primers were used to genotype the mice for homozygocity. Results from PCR reactions from tail snips of the three genotyping reactions of homozygous Btk flox/flox and wt mice. Mw marker = 100bp. Left) Ef-Er showing the presence of the 5′ FRT and loxP sites. Middle) L3f-L3r showing the presence of the 3′ loxP and intronic space. Right) L3f-Lxr showing the presence of the 3′ loxp in the FF mouse, which is absent in the wt mouse.
Figure 2. Inducible knockdown of Btk in Btkflox/Cre-ERT2 is stably achieved in splenic and bone marrow B cells.
(A and C) Representative flow plots for Btkflox (left), Btkflox/Cre-ERT2 (middle) and Btknull (right) showing bone marrow B cells (A) or splenic B cells (C), five days after tamoxifen injections. (B and D) Representative histograms of B cell Btk expression five days after treatment: Btkflox (black, solid), Cre-ERT2 (black, dashed), Btkflox/Cre-ERT2 (red), Btknull (blue), and isotype control (gray). Bar charts show the percent of B cells that are Btk positive in each condition. (E) Total B cell numbers five days, two weeks, or five weeks after Btk knockdown. For B–E: Btkflox (solid white, n=16–24), Cre-ERT2 (diagonal pattern, n=9–13), vehicle control (light gray, n=4–5), Btkflox/Cre-ERT2 after five days (solid gray, n=8–13), two weeks (diagonal pattern, gray, n=5), or five weeks (horizontal pattern, gray, n=3–10), Btknull (black, n=11–15). (F) Five days post tamoxifen treatment, splenocytes from Btkflox (n=4), Btkflox/Cre-ERT2 (n=6), and Btknull (n=4) animals were harvested and cultured for three days in media alone, 1μg/mL LPS or 5μg/mL of anti-IgM. B cell proliferation was measured by dye dilution (histograms, left) and quantified by percent proliferating of CD19+B220+ (right). *p<0.05, ***p<0.001 as calculated by one-way or two-way ANOVA compared to Btkflox.
Splenic B cells survive after Btk loss, but have blunted proliferative responses
Conventional Btknull B6 mice exhibit a loss of total B cell numbers. However, there was no immediate B cell depletion after Btk was deleted using the Btkflox/Cre-ERT2 system. Btkflox/Cre-ERT2 spleens contained equivalent numbers of B cells five days (1.57e7±4.54e6) and two weeks (1.53e7±6.45e6) after Btk loss, as compared to Btkflox controls (1.55e7±7.51e6) (p=0.999). Five weeks after Btk knockdown, Btkflox/Cre-ERT2 mice did exhibit B cell loss (9.05e6±5.12e6, p=0.041). Therefore, B cells in the spleen do not require Btk for their survival, but are depleted after B cell turnover during development (Figure 2E). To determine if loss of Btk results in a defective B cell response to stimuli, we harvested spleens from Btkflox, Btkflox/Cre-ERT2, and Btknull five days after tamoxifen injection and stimulated B cells with anti-IgM or LPS (Figure 2F). Btkflox/Cre-ERT2 B cells showed blunted proliferation after Btk deletion compared with Btkflox control B cells in response to LPS (17.95±3.39% proliferation vs. 43.68%±7.28% proliferation, p<0.001) or anti-IgM (20.49%±9.82% proliferation vs. 48.78%±13.34% proliferation, p<0.001). In fact, Btk knockdown was functionally equivalent to Btk-deficiency in Btknull B cells in response to LPS (27.95%±11.98% proliferation, p=0.14), though slightly increased compared to Btknull B cells in response to anti-IgM (7.77%±6.34% proliferation, p=0.04). These data confirm that Btk deletion after cellular maturation results in a functional defect in proliferation response to LPS and anti-IgM that is similar to lifelong Btk-deficiency.
Btk knockdown results in immediate increase in the late transitional (T2) stage, but requires weeks to reduce the follicular compartment
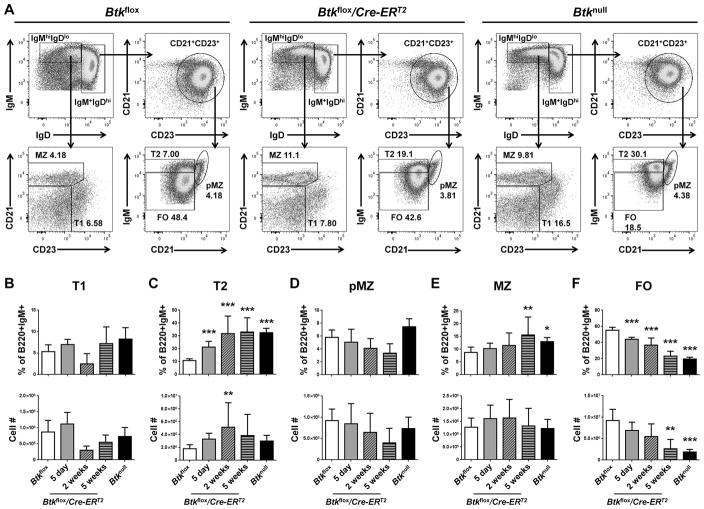
Conventional Btknull genetic mouse models show a loss of total B cell numbers, and exhibit an increased percentage of B cells at the transitional 2 (T2) stage of development with a concurrent loss of follicular (FO) B cells (4, 29). This phenotype is interpreted to mean that B cells are developmentally blocked at T2. To determine if mature FO B cells require Btk for their survival, B cell subsets were assessed five days, two weeks, and five weeks after Btk knockdown. Transitional 1 (T1), T2, pre-marginal zone (pMZ), marginal zone (MZ) and FO B cells were determined by expression of IgM, IgD, CD21, and CD23, as shown in Figure 3A. The most immediate effect of Btk knockdown was increased surface IgM expression in Btkflox/Cre-ERT2 five days post injection, leading to a significant increase in the percentage of T2 B cells (21.35%±5.25%) as compared to Btkflox controls (10.71%±2.78%) (p=0.001). This was accompanied by a reciprocal trend toward decreased FO B cell proportions, but did not reduce their numbers. The T2 developmental block with concurrent loss of follicular B cells continued to emerge over the next five weeks, finally resulting in significantly decreased FO B cell numbers (2.64e6±2.07e6) compared to Btkflox controls (9.20e6±5.13e6) (p<0.001). As in the Btknull B6 mouse, there were no changes in cell numbers of T1 (Figure 3B), pMZ (Figure 3D) or MZ B cells (Figure 3E). Interestingly, this model shows no immediate loss of FO B cells at the time of Btk knockdown. Rather, follicular B cell similarity to Btknull models emerges five weeks later, after B cell turnover has occurred. This shows for the first time that the phenotype seen in Btk-deficient B cells is due to developmental factors, and supports the idea that murine B cells require Btk to mature through the transitional stages, but is not required for survival of mature FO B cells.
Figure 3. Follicular B cell compartment survives initial Btk knockdown, decreasing over 5 weeks to match global Btk-deficiency.
(A) Representative flow plots for Btkflox (left), Btkflox/Cre-ERT2 (middle) and Btknull (right) splenic B cells, pre-gated as B220+IgM+ single live lymphocytes, five days after tamoxifen injection. Transitional 1 (T1), transitional 2 (T2), pre-marginal zone (pMZ), marginal zone (MZ) and follicular (FO) B cell subsets are determined by expression of IgM, IgD, CD21 and CD23. (B–F) B cell subsets are quantified by percent of B220+IgM+ (top) and total cell number (bottom) for Btkflox (white, n=17), Btkflox/Cre-ERT2 5 days (gray, n=8), 2 weeks (diagonal pattern, n=5), or 5 weeks (horizontal pattern, n=6) post tamoxifen injection, or Btknull (black, n=12). *p<0.05, **p<0.01, ***p<0.001 as calculated by one-way ANOVA compared to Btkflox.
An1 B cells are depleted following Btk knockdown
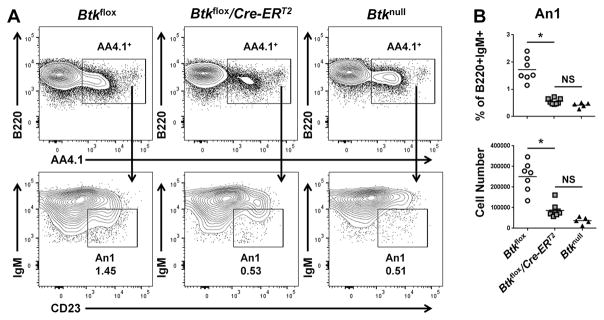
An1 B cells are an anergic, autoreactive B cell subset (30) that we and others have found to be strikingly reduced in Btk-deficient mice (8, 10). Btkflox, Btkflox/Cre-ERT2, and Btknull An1 B cells were assessed in the spleen five days after tamoxifen treatment, by expression of B220, AA4.1, IgM, and CD23 (Figure 4A). As shown in Figure 4B, An1 B cells in Btkflox/Cre-ERT2 animals were significantly decreased in both percentage (0.56%±0.10%) and number (8.67e4 ±3.29e4) as compared to Btkflox controls’ percentage (1.72%±0.437%, p=0.012) and number (2.5e5±7.2e4, p=0.036). An1 B cells are known to have a short life cycle (8). Therefore, it is unclear if this loss of cell numbers is due to a block in development or a reliance on Btk for An1 B cell survival. Regardless, these data show that An1 B cells are rapidly depleted following Btk knockdown.
Figure 4. Autoreactive-prone anergic An1 B cell subset is depleted in Btkflox/Cre-ERT2 mice five days after tamoxifen treatment.
(A) Representative flow plots for Btkflox (left), Btkflox/Cre-ERT2 (middle) and Btknull (right) spleen B cells, pre-gated as B220+IgM+ single live lymphocytes, five days after tamoxifen treatment. Anergic An1 B cells are determined by expression of AA4.1, IgM, and CD23. (B) An1 B cells are quantified by % of B220+IgM+ (top) and total cell number (bottom) for Btkflox (circles, n=7), Btkflox/Cre-ERT2 5 days after tamoxifen injection (squares, n=8), or Btknull (triangles, n=5). *p<0.05 as calculated by Kruskal-Wallace with Dunn’s multiple comparison test.
B1 cells do not require Btk for survival
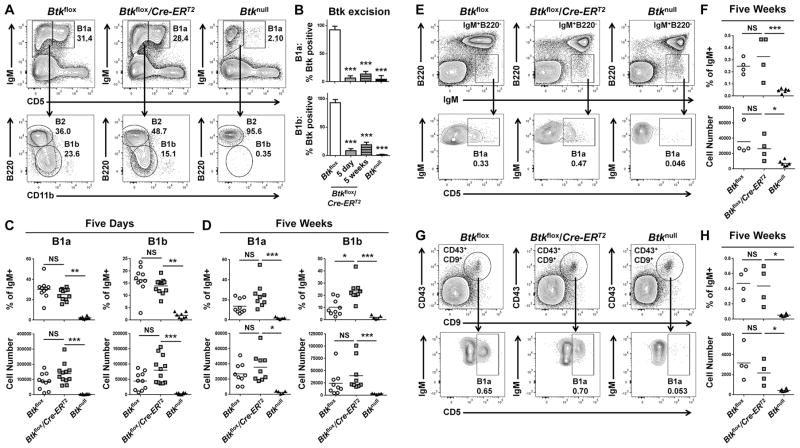
The innate-like, autoreactive-prone B1 cell subset is known to be important for the production of natural IgM (13, 31) and response to polysaccharide antigens (14, 15, 32) and is absent in Btknull models (21). To determine if Btk is required for development or survival of the B1 cell subset, we induced Btk knockdown and assessed B1a and B1b cells in the peritoneal lavage by expression of IgM, CD5, B220, and CD11b (Figure 5A). Knockdown was successful and stable up to five weeks after injections in both subsets (Figure 5B). Five days after tamoxifen treatment, B1a cell percentages were not significantly changed, forming 25.13%±5.68% of total IgM+ cells in treated Btkflox/Cre-ERT2, and 30.27%±9.58% in Btkflox controls (p=0.38). B1b cell percentages also remained unchanged, forming 13.91%±3.03% of IgM+ cells in Btkflox/Cre-ERT2, and 16.38%±5.61% in Btkflox controls (p=0.33) (Figure 5C). To determine if this effect persisted over time, we assessed B1a and B1b cells in the peritoneal lavage five weeks after injection (Figure 5D). Even at this later timepoint, Btkflox/Cre-ERT2 animals retained similar B1a cell numbers (3.57e4±1.87e4) in comparison to Btkflox controls (2.69e4±1.35e4) (p>0.999). B1b numbers were also maintained, as Btkflox/Cre-ERT2 lavages contained 4.01e4±3.14e4 B1b cells and Btkflox control lavages contained 2.45e4±2.51e4 (p>0.397). Five weeks after tamoxifen injection, both B1a and B1b cell numbers were decreased compared to their numbers five days after tamoxifen injection. This decrease in B1a and B1b cell numbers occurred in both the Btkflox and Btkflox/Cre-ERT2 peritoneal lavages, and was therefore not an effect of Btk deletion. Rather, this finding may reflect side effects of the injection itself, such as cellular perturbance secondary to the presence of an oil emulsion in the peritoneal cavity at either time point.
Figure 5. The survival of B1a and B1b cells does not depend upon Btk.
(A, E, G) Representative flow plots for Btkflox (left), Btkflox/Cre-ERT2 (middle) and Btknull (right) peritoneal (A), splenic (E), or bone marrow (G) cells. Cells are pre-gated as single live lymphocytes, peritoneal cells as Ly6G−. (A) Peritoneal B1a, B1b, and B2 cells are determined by expression of IgM, CD5, B220, and CD11b. (B) Btk knockdown is reported as % Btk positive for B1a (top) and B1b (bottom) cells of genotypes Btkflox (white), Btkflox/Cre-ERT2 5 days (gray) or 5 weeks (horizontal pattern) after tamoxifen injection, or Btknull (black). (C–D) B1a (left) and B1b (right) cells of Btkflox (circles, n=9–10), Btkflox/Cre-ERT2 (squares, n=9–12) and Btknull (triangles, n=5–8), five days (C) or five weeks (D) post injection are quantified by % of IgM+ (top) and total cell number (bottom). Splenic B1a (E) are identified by expression of IgM and CD5, and by low B220 expression. Bone marrow B1a (G) are pre-gated as IgM+CD19+, then further identified by expression of CD43, CD9, and CD5. (F, H) B1a cells of Btkflox (n=4), Btkflox/Cre-ERT2 (n=4) and Btknull (n=6) are quantified by percent of IgM+ (top) or total cell number (bottom) in the spleen (F) and bone marrow (H) five weeks after tamoxifen injection. *p<0.05, **p<0.01, ***p<0.001 as calculated by Kruskal-Wallace with Dunn’s multiple comparison test.
Though B1s are the major B cell subset present in the peritoneal cavity, B1a cells are also found in the spleen and bone marrow, and it is these B1 cells that produce large amounts of natural IgM (31, 33, 34). Therefore, we assessed splenic and bone marrow B1a cells at five weeks after Btk knockdown to determine if these crucial subsets were preserved after Btk loss. B1a cells in the spleen were identified by high expression of IgM, low expression of B220, and as CD5-positive (Figure 5F). B1a cells were not significantly reduced in number in Btkflox/Cre-ERT2 (2.64e4±1.54e4) as compared to Btkflox controls (3.56e4±1.92e4) (p>0.999). B1a cells were also maintained in the bone marrow, where they were identified as IgM+CD19+, then by expression of CD43, CD9, and CD5, all reported to be markers of B1a cells in the bone marrow niche (Figure 5G) (31). Btkflox/Cre-ERT2 maintained 2.17e3±1.17e3 B1a cells, as compared to Btkflox controls at 3.26e3±1.54e3 (p=0.788). These data show that B1a and B1b cells do not depend on the presence of Btk for survival, despite its crucial developmental contributions.
The production of anti-phosphoryl-choline antibody is not decreased by loss of Btk
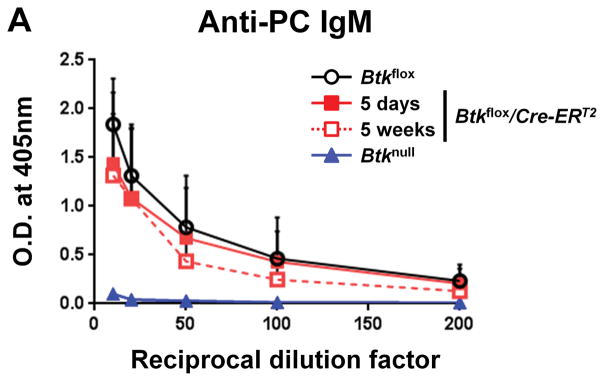
Though the above data showed that B1a cells in the spleen and bone marrow do not require Btk to survive, it was unclear if Btk is required to support B1 function. Early studies found that B1 cells are responsible for up to 80% of natural IgM (13). Recent work by Reynolds et al. and Savage et al. has shown that natural IgM is more specifically produced by two B cell subsets, IgM+CD138− B1a cells and IgM+CD138+ cells that are plasma cell-like and of B1 origin (34, 35). The natural IgM repertoire contains anti-phosphoryl choline antibody (anti-PC), which is germline-encoded and present in serum even in germ-free conditions (36–38). To determine whether Btk is required to maintain natural IgM production, ELISA was used to measure anti-PC antibodies in serum after Btk knockdown. There was no significant difference between anti-PC antibody levels in Btkflox serum (1.840±0.469) and Btkflox/Cre-ERT2 five days (1.436±0.732) or five weeks (1.315±0.631) after injections (p=0.4041, p=0.5257) (Figure 6A). As expected anti-PC antibody levels in Btknull animals were nearly undetectable (0.102±0.038) due to their lack of B1 cells. As the half-life of IgM in serum is estimated at two days (39), the continued level of anti-PC IgM shows that Btk is not required to maintain natural IgM production.
Figure 6. Production of natural IgM is independent of Btk.
(A) Anti-phosphocholine (PC) IgM is measured by ELISA for Btkflox (black, n-7), Btkflox/Cre-ERT2 five days after treatment (red, solid line, n=4), Btkflox/Cre-ERT2 five weeks after treatment (red, dashed line, n=3) and Btknull (blue, n=4). Statistics were performed using a 2-way ANOVA and listed in Supplemental Table 1.
Mice have reduced responses to T-independent type II immunization after Btk deletion
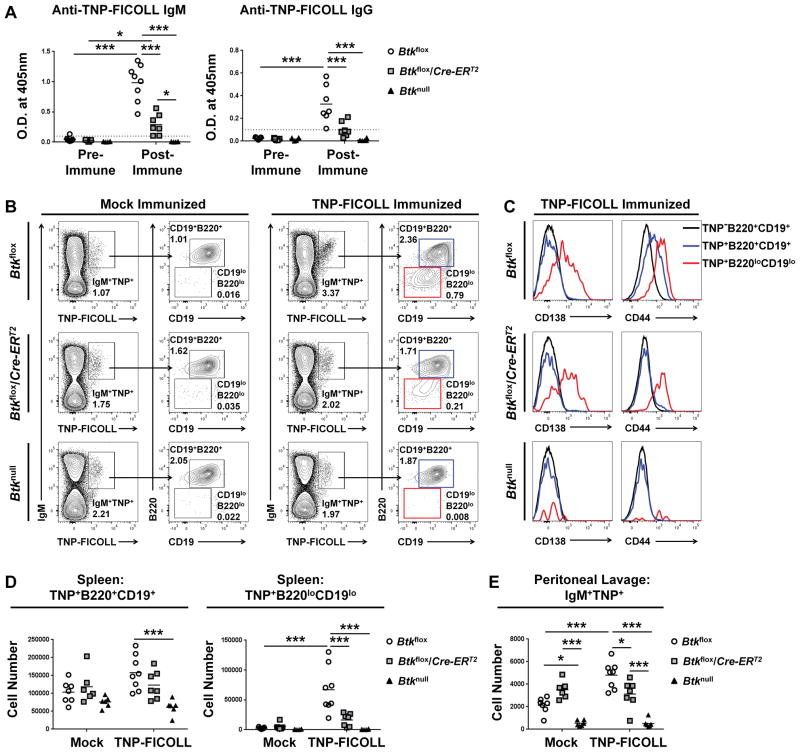
Early studies of Btknull mice established that Btk is necessary for T-independent type II (TI-II) immunization responses (22–24). In part, this deficiency is due to a lack of B1 cells, known to be critical for TI-II responses (14, 32). In addition, the increased frequency of immature T2 and loss of FO B cells in the spleen could play a role in the response of Btknull animals to T-independent immunization. Therefore, we injected Btkflox, Btkflox/Cre-ERT2, and Btknull mice five days after Btk knockdown with a mock injection of PBS or a TNP-Ficoll immunization. Figure 7A shows anti-TNP IgM (left) and IgG (right) from serum before and after immunization. Though immunized Btkflox/Cre-ERT2 mice did exhibit a significant IgM response to TNP-Ficoll immunization, with an O.D. of 0.292±0.172 after immunization compared to an O.D. of 0.028±0.014 before immunization (p=0.037), this post-immunization response was significantly decreased compared to the response of Btkflox control mice, which reached an anti-TNP-Ficoll IgM O.D. of 0.986±0.304 (p<0.001). In addition, the Btkflox/Cre-ERT2 mice did not produce anti-TNP-ficoll IgG (0.104±0.066) after immunization, as compared to pre-immune control sera (0.017±0.008) (p=0.4134). B1 cells are important contributors in the response to TNP-Ficoll immunization (32), and MZ B cells also contribute to TI-II antibody responses (40). Though B1 cells survive after Btk loss, and MZ B cells are retained in both Btkflox/Cre-ERT2 and Btknull models, Btkflox/Cre-ERT2 mice have significantly reduced ability to produce anti-TNP-Ficoll IgM as compared to Btk-sufficient controls, and cannot produce significant anti-TNP-Ficoll IgG. These data point to a loss of function in B1 and MZ B cells after Btk loss.
Figure 7. T-independent type II immune response depends on Btk.
Five days post tamoxifen treatment, Btkflox, Btkflox/Cre-ERT2, and Btknull animals were immunized with 50μg of TNP37-Ficoll in 200μL of PBS or mock immunized with PBS alone. (A) Anti-TNP IgM (left) and IgG (right) is measured one day pre-immunization and five days post-immunization by ELISA for Btkflox (circles, n=7–8), Btkflox/Cre-ERT2 (squares, n=7) and Btknull (triangles, n=6) animals. (B) Representative flow plots of anti-TNP B cells, gated by expression of IgM and binding to TNP-Ficoll-FITC, then by expression of B220 and CD19. Cells are pre-gated as single live lymphocytes. Mock (left) and TNP-Ficoll (right) from Btkflox (top), Btkflox/Cre-ERT2 (middle) and Btknull (bottom) mice. (C) Representative expression of CD138 (left) and CD44 (right) on TNP−B220+CD19+ (black), TNP+B220+CD19+ (blue), and TNP+B220−CD19−(red) from TNP-ficoll immunized Btkflox (top), Btkflox/Cre-ERT2 (middle) and Btknull (bottom) mice. Cells are pre-gated as IgM+. (D) Total splenic cell numbers of TNP+B220+CD19+ (left), and TNP+B220−CD19− (right) from mock or TNP-Ficoll immunized Btkflox (circles, n=7–8), Btkflox/Cre-ERT2 (squares, n=6–7) and Btknull (triangles, n=6). (E) Total cell numbers of IgM+TNP+ cells in the peritoneal lavage of mock and TNP-Ficoll immunized mice. *p<0.05, **p<0.01, ***p<0.001 as calculated by two-way ANOVA.
To further characterize the TI-II immunization response, we used FITC conjugated TNP-Ficoll to track antigen specific B cells in the spleen and peritoneal lavage. Figure 7B shows representative Btkflox (top), Btkflox/Cre-ERT2 (middle), and Btknull (bottom) splenic anti-TNP-Ficoll B cells in mock-immunized (left) or TNP-Ficoll immunized (right) animals. In immunized Btkflox controls, we observed two TNP-Ficoll-specific IgM+ populations, one of which was CD19+B220+ and the other CD19loB220lo. The IgM+TNP-Ficoll+CD19loB220lo also exhibited higher levels of CD138 and CD44 (Figure 7C), leading to the conclusion that this population is most likely expanding plasmablasts. These TNP-Ficoll-specific plasmablasts were significantly increased in number in the spleens of immunized Btkflox mice (6.71e4±3.84e4) compared to mock-immunized controls (2.61e3±1.38e3) (p<0.001). This contrasts Btkflox/Cre-ERT2 mice after Btk deletion, in which the number of TNP-Ficoll-specific plasmablasts was not significantly increased in TNP-Ficoll immunized mice (1.64e4±1.08e4) compared to mock-immunized controls (5.33e3±5.67e3) (p=0.9925). Furthermore, these numbers were significantly reduced compared to immunized Btkflox control mice (p<0.001), indicating that Btk contributes to development of antigen-specific plasmablasts. Non-plasmablast anti-TNP-Ficoll B cells (IgM+TNP-Ficoll+CD19+B220+) (Figure 7D, left) were not different in mock-immunized Btkflox (1.04e5±3.08e4), Btkflox/Cre-ERT2 (1.19e5±4.55e4), and Btknull (7.59e5±1.50e4), and none of the genotypes showed significantly increased numbers of this subset after TNP-Ficoll immunization.
We also assessed IgM+TNP-Ficoll+ cells in the peritoneal lavage to evaluate contributions by B1 cells there (Figure 7E). After TNP-ficoll immunization, Btkflox controls had significantly higher numbers of IgM+TNP-Ficoll+ cells (4.80e3±1.17e3) than Btkflox/Cre-ERT2 (3.12e3±1.25e3) (p=0.011). IgM+TNP-Ficoll+ cell number in TNP-immunized Btkflox/Cre-ERT2 (3.12e3±1.25e3) was not different from that of mock-immunized Btkflox/Cre-ERT2 (3.513e3±8.038e2) (p=0.9998). Due to the significantly decreased IgM response, a lack of IgG responses, a loss of plasmablasts in the spleen, and a failure to increase numbers of TNP-specific B cells in the peritoneal lavage, we conclude that though B1 cells do not require Btk for their survival (Figure 5), Btk is required for TI-II responses by both B1 and B2 cells.
Discussion
The phenotype of Btknull mice has been extensively reported, by our own work and the work of others. Btknull mice lack B cell subsets such as anergic An1s and B1s, and fail to respond to T-independent antigens (4, 10, 11, 22–24). However, the lack of an inducible knockout has resulted in a gap in our understanding of how mature B cell subsets rely on Btk. As BTK inhibitors are now approved and under consideration to treat a growing number of human diseases, understanding the role of BTK in mature B cell survival and function is vital. Previously, in the absence of an inducible knockout, inhibitors were sometimes used to try to characterize the role of Btk in mature cells. However, inhibitors are incomplete substitutes given the varied off-target effects of different drugs and the failure to eliminate the adaptor role of the protein. In this report, we detail the first use of Btkflox/Cre-ERT2 inducible knockdown to study the effect of loss of Btk on the survival and function of fully developed B cell subsets.
First, we established the efficacy of the Btkflox/Cre-ERT2 system (Figure 2, Supplemental Figure 1). Treatment of Btkflox/Cre-ERT2 with tamoxifen resulted in efficient knockdown in B cells at all subsets and developmental stages, as well as in splenic macrophages and dendritic cells. This knockdown was stable up to five weeks after injection. We then analyzed splenic B cell subsets to assess the effect of Btk knockdown on mature B cells (Figure 3). Btknull mice exhibit a block in transition from T2 to mature FO B cells (4, 41). T2 and FO B cells both express IgD, CD21, and CD23, and are differentiated by expression of IgM, which is high on T2 and lower on FO B cells. Because IgM surface expression is generally higher in the absence of Btk, it was possible that FO B cells would shift to a more T2-like appearance immediately. However, though there was an immediate increase in IgM after Btk loss, the treated Btkflox/Cre-ERT2 did not fully mimic the phenotype of Btknull mice until five weeks after knockdown, when cell turnover would essentially recapitulate a Btknull B cell repertoire. This contrasts with previously published work showing that rapid B2 cell loss occurs when the BCR, Igα, or Syk are removed (42–44), suggesting that tonic signaling through the BCR is required for survival of B2 cells in the periphery. The data reported here show that this type of low-level BCR signaling does not rely on Btk. This contrasts signaling responses induced by IgM crosslinking or LPS, as Btkflox/Cre-ERT2 B cells proliferated significantly less to these stimuli within days of tamoxifen treatment (Figure 2C). This model reveals the split role of Btk in survival versus function in B cells, and shows that the impaired proliferation in B cells lacking Btk is not simply due to maturational defect. Further study is required to elucidate how specific signaling pathways are affected when Btk is excised from mature cells, and how they may differ in response to tonic versus active signaling. It is important to note that these nuanced Btk contributions may or may not apply in the same way to human B cells, which are much more sensitive to its loss at very early developmental stages. Since B cells in XLA patients fail to develop at all beyond the bone marrow, it is possible that they also have increased requirements for this protein in the periphery. Study of normal human B cell outcomes in response to highly specific Btk-inhibitors, currently under development, would be helpful to better understand whether human B cells have similarly divergent reliance on BTK.
The lack of B1s in Btknull models correlates with a loss of natural IgM and deficient responses to T-independent antigens. However, the lack of a genetic knockdown model has prevented the study of Btk’s role in the survival and function of this important B cell subset. The data show that Btk is unnecessary for cell survival, as normal numbers of Btk-deficient B1a and B1b cells are present in the peritoneal lavage, spleen, and bone marrow of Btkflox/Cre-ERT2 mice even five weeks after Btk knockdown (Figure 5). Previously published work showed that short term dosing using a BTK inhibitor (PF-303) was equally unable to eliminate these cells (28). However, it was possible that the drug inadequately targeted Btk. Furthermore, kinase inhibitors do not affect the adaptor domain of Btk, which is known to have independent function (45, 46). In addition, inhibitors may have off-target effects that could eliminate counterbalancing negative signals. The Btkflox/Cre-ERT2 model completes this picture by genetic verification that the failure of the drug to reduce B1’s was not due to poor kinase inhibition. Further, these data indicate that the adaptor function is also expendable for B1 cellular survival. This model is therefore the first to show definitively that B1a and B1b cells do not depend on Btk for survival, but instead require it for development. These data may also be used as comparators for future-inhibitor studies.
The exact mechanism by which Btk contributes to the development of B1 cells remains unclear. The data in this report contribute to gathering evidence that B1 cells require a positive selective step mediated by the BCR during development. B1 cells rely on the classical NF-κB pathway for development (47), and Btk is known to link BCR signaling to NF-κB (5, 6, 48). In addition, the normal B1 compartment contains autoreactive anti-Thy-1 B cells that require BCR binding to develop. Mice lacking Thy-1 do not develop anti-Thy-1 B1 cells, demonstrating positive selection. (49). These combined data suggest that antigen-mediated BCR-stimulation drives Btk-mediated signaling to select B cells into this compartment via activation of NF-κB, contrasting constitutive BCR-signaling that may mediate B1 survival without the need for Btk.
Natural IgM is produced both by IgM+CD138+ B1 cells and IgM+CD138+ antibody secreting cells of B1 origin (31, 33–35), and serves several important roles. Natural antibodies serve as a first defense against many pathogens, such as Streptococcus pneumoniae (14), Listeria monocytogenes (50), influenza virus (51, 52) and others (53, 54) In addition, natural IgM contributes to tissue homeostasis through the binding of self-antigens (55, 56) and have been shown to be atheroprotective (57, 58). It is known that Btknull mice lack natural IgM; however, this lack cannot be separated from their lack of B1 cells. Also, little is known about what factors maintain natural IgM production after B1a and B1a-like antibody producing cells are already formed. In Figure 6, we have shown that the natural antibody anti-phosphoryl-choline IgM remains present in Btkflox/Cre-ERT2 serum five days and even five weeks after Btk knockdown. The half-life of IgM in vivo is estimated at two days (39). Therefore, natural anti-PC antibody production continues even after the loss of Btk, contrasting its absence in Btknull controls. Thus, the production of natural IgM is independent of continued signaling through Btk.
Another role of B1 cells is in the initial response to infection. B1b cells are known to rapidly produce protective IgM in response to pathogens such as Borrelia hermsii (15, 59) and Streptococcus pneumoniae (14). B1b, as well as B1a and marginal zone B2 cells are known to be the main contributors to T-independent (TI) antibody production (40, 60–62). We immunized Btkflox/Cre-ERT2 mice with TNP-Ficoll after Btk knockdown, to determine if the surviving B1 and marginal zone B cells could respond to a model TI-type II (TI-II) antigen (Figure 7). Both B1 cells in the peritoneal lavage and B2 cells in the spleen were unable to respond to antigen in vivo. Btkflox/Cre-ERT2 animals exhibited significantly reduced anti-TNP IgM and very little anti-TNP IgG post immunization, compared to Btkflox controls. This finding shows that though mature B1 cells survive the loss of Btk, and retain their ability to produce natural IgM, they are unable to respond to TI-II immunization. These data could also have implications for the use of BTK inhibitors in human disease, particularly in long term use, as BTK inhibitors may decrease the efficacy of some immunizations, such as unconjugated pneumococcal vaccines.
As for T-dependent immunizations, early studies of xid and Btknull mice showed they could respond to booster doses of vaccines (4, 63, 64). Although their IgG responses were somewhat blunted, they were able to achieve adequate protection against infection (65). On the other hand, impaired germinal center formation and memory cell “burst” have been reported during BTK-inhibitor treatment, suggesting a more powerful role than may have been previously appreciated (28), unless off-target effects were responsible for those findings. Further study using the Btkflox/Cre-ERT2 model is therefore needed to better assess how Btk contributes to germinal center responses, including cellular functions that support selection and affinity maturation.
Systemic autoimmune disorders like RA are mediated by autoantibody production by autoreactive B cell subsets. An1 B cells are an endogenous autoreactive B cell subset (30). A similar subset is present in humans and is increased in autoimmunity (66, 67). Our lab and others have found that the development of An1 B cells is dependent upon Btk. Therefore, we assessed the An1 subset in Btkflox/Cre-ERT2 animals five days after Btk knockdown. An1 cells were swiftly depleted, and were significantly reduced after Btk loss (Figure 4). However, it remains unclear as to whether this is due to a survival defect or a loss at development, as An1 B cells are known to have a life cycle of only five days (8). Nevertheless, the swift depletion of this subset that we show by Btk knockdown and that Benson et al showed using Btk inhibition (28) implies that short courses of Btk inhibition may impact similar autoreactive anergic populations in humans, without greatly impacting non-autoreactive B2 subsets. This provides hope that some autoimmune patients could benefit from short-term, intermittent, courses of BTK-inhibitors to eliminate this autoimmune-prone subset.
In summary, this work is the first to use an inducible genetic knockdown of Btk to rigorously study its role in the survival and function of mature B cells. These studies reveal that despite the developmental blocks in follicular and B1 B cells seen in Btknull models, mature B cells that have already passed selective checkpoints into these subsets do not require Btk for survival. In addition, the production of natural IgM is intact following the loss of Btk. However, the loss of Btk greatly impacts An1 B cells, which are swiftly depleted. This decrease in autoreactive-prone An1 B cells may have implications for BTK-inhibition as short-term therapy for autoimmune disorders. B cells are functionally impacted by loss of Btk, with splenic B cells showing an inability to proliferate to LPS or anti-IgM, and the B1 compartment showing reduced responses to T-independent immunization. As investigations of BTK inhibitors for cancer and autoimmunity increase, these data may serve as a resource to further inform drug discovery and to aid in designing dosing parameters and expected outcomes for clinical trials.
Supplementary Material
Acknowledgments
This work was supported by the Department of Veterans’ Affairs (PLK) and by National Institutes of Health Grants: National Institute of Diabetes and Digestive and Kidney Diseases R01 DK084246 (PLK), R01 AI060729 (WNK), P30 A1073961 (ESC) and T32HL069765 (LEN), as well as by Juvenile Diabetes Research Foundation Grant 3-2013-121(RHB), and by the Jeffrey Modell Foundation (WNK). Flow Cytometry experiments were performed in the VMC Flow Cytometry Shared Resource. The VMC Flow Cytometry Shared Resource is supported by the Vanderbilt Ingram Cancer Center (P30 CA68485) and the Vanderbilt Digestive Disease Research Center (DK058404). Btkflox mice were generated at the transgenic core, Miller School of Medicine, University of Miami, Miami, FL 33136.
References
- 1.Kendall PL, Moore DJ, Hulbert C, Hoek KL, Khan WN, Thomas JW. Reduced diabetes in btk-deficient nonobese diabetic mice and restoration of diabetes with provision of an anti-insulin IgH chain transgene. J Immunol. 2009;183:6403–6412. doi: 10.4049/jimmunol.0900367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antony P, Petro JB, Carlesso G, Shinners NP, Lowe J, Khan WN. B cell receptor directs the activation of NFAT and NF-kappaB via distinct molecular mechanisms. Experimental cell research. 2003;291:11–24. doi: 10.1016/s0014-4827(03)00338-0. [DOI] [PubMed] [Google Scholar]
- 3.Khan WN. Regulation of B lymphocyte development and activation by Bruton’s tyrosine kinase. Immunologic research. 2001;23:147–156. doi: 10.1385/IR:23:2-3:147. [DOI] [PubMed] [Google Scholar]
- 4.Khan WN, Alt FW, Gerstein RM, Malynn BA, Larsson I, Rathbun G, Davidson L, Muller S, Kantor AB, Herzenberg LA, et al. Defective B cell development and function in Btk-deficient mice. Immunity. 1995;3:283–299. doi: 10.1016/1074-7613(95)90114-0. [DOI] [PubMed] [Google Scholar]
- 5.Petro JB, Rahman SM, Ballard DW, Khan WN. Bruton’s tyrosine kinase is required for activation of IkappaB kinase and nuclear factor kappaB in response to B cell receptor engagement. The Journal of experimental medicine. 2000;191:1745–1754. doi: 10.1084/jem.191.10.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petro JB, Khan WN. Phospholipase C-gamma 2 couples Bruton’s tyrosine kinase to the NF-kappaB signaling pathway in B lymphocytes. The Journal of biological chemistry. 2001;276:1715–1719. doi: 10.1074/jbc.M009137200. [DOI] [PubMed] [Google Scholar]
- 7.Hardy RR, Hayakawa K, Parks DR, Herzenberg LA. Demonstration of B-cell maturation in X-linked immunodeficient mice by simultaneous three-colour immunofluorescence. Nature. 1983;306:270–272. doi: 10.1038/306270a0. [DOI] [PubMed] [Google Scholar]
- 8.Allman D, Lindsley RC, DeMuth W, Rudd K, Shinton SA, Hardy RR. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J Immunol. 2001;167:6834–6840. doi: 10.4049/jimmunol.167.12.6834. [DOI] [PubMed] [Google Scholar]
- 9.Su TT, Rawlings DJ. Transitional B lymphocyte subsets operate as distinct checkpoints in murine splenic B cell development. J Immunol. 2002;168:2101–2110. doi: 10.4049/jimmunol.168.5.2101. [DOI] [PubMed] [Google Scholar]
- 10.Bonami RH, Sullivan AM, Case JB, Steinberg HE, Hoek KL, Khan WN, Kendall PL. Bruton’s tyrosine kinase promotes persistence of mature anti-insulin B cells. J Immunol. 2014;192:1459–1470. doi: 10.4049/jimmunol.1300125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halcomb KE, Musuka S, Gutierrez T, Wright HL, Satterthwaite AB. Btk regulates localization, in vivo activation, and class switching of anti-DNA B cells. Mol Immunol. 2008;46:233–241. doi: 10.1016/j.molimm.2008.08.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayakawa K, Hardy RR, Herzenberg LA. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. The Journal of experimental medicine. 1985;161:1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baumgarth N, Herman OC, Jager GC, Brown L, Herzenberg LA. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:2250–2255. doi: 10.1073/pnas.96.5.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–390. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 16.Chumley MJ, Dal Porto JM, Cambier JC. The unique antigen receptor signaling phenotype of B-1 cells is influenced by locale but induced by antigen. J Immunol. 2002;169:1735–1743. doi: 10.4049/jimmunol.169.4.1735. [DOI] [PubMed] [Google Scholar]
- 17.Morris DL, Rothstein TL. Abnormal transcription factor induction through the surface immunoglobulin M receptor of B-1 lymphocytes. The Journal of experimental medicine. 1993;177:857–861. doi: 10.1084/jem.177.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Healy JI, Dolmetsch RE, Timmerman LA, Cyster JG, Thomas ML, Crabtree GR, Lewis RS, Goodnow CC. Different nuclear signals are activated by the B cell receptor during positive versus negative signaling. Immunity. 1997;6:419–428. doi: 10.1016/s1074-7613(00)80285-x. [DOI] [PubMed] [Google Scholar]
- 19.Holodick NE, Tumang JR, Rothstein TL. Continual signaling is responsible for constitutive ERK phosphorylation in B-1a cells. Molecular immunology. 2009;46:3029–3036. doi: 10.1016/j.molimm.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong SC, Chew WK, Tan JE, Melendez AJ, Francis F, Lam KP. Peritoneal CD5+ B-1 cells have signaling properties similar to tolerant B cells. The Journal of biological chemistry. 2002;277:30707–30715. doi: 10.1074/jbc.M202460200. [DOI] [PubMed] [Google Scholar]
- 21.Reid RR, Prodeus AP, Khan W, Hsu T, Rosen FS, Carroll MC. Endotoxin shock in antibody-deficient mice: unraveling the role of natural antibody and complement in the clearance of lipopolysaccharide. J Immunol. 1997;159:970–975. [PubMed] [Google Scholar]
- 22.Amsbaugh DF, Hansen CT, Prescott B, Stashak PW, Barthold DR, Baker PJ. Genetic control of the antibody response to type 3 pneumococcal polysaccharide in mice. I. Evidence that an X-linked gene plays a decisive role in determining responsiveness. The Journal of experimental medicine. 1972;136:931–949. doi: 10.1084/jem.136.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scher I, Ahmed A, Strong DM, Steinberg AD, Paul WE. X-linked B-lymphocyte immune defect in CBA/HN mice. I. Studies of the function and composition of spleen cells. The Journal of experimental medicine. 1975;141:788–803. [PMC free article] [PubMed] [Google Scholar]
- 24.Boswell HS, Nerenberg MI, Scher I, Singer A. Role of accessory cells in B cell activation. III. Cellular analysis of primary immune response deficits in CBA/N mice: presence of an accessory cell-B cell interaction defect. The Journal of experimental medicine. 1980;152:1194–1309. doi: 10.1084/jem.152.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. The Journal of experimental medicine. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Griffin DO, Rothstein TL. Human b1 cell frequency: isolation and analysis of human b1 cells. Frontiers in immunology. 2012;3:122. doi: 10.3389/fimmu.2012.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rothstein TL, Quach TD. The human counterpart of mouse B-1 cells. Annals of the New York Academy of Sciences. 2015;1362:143–152. doi: 10.1111/nyas.12790. [DOI] [PubMed] [Google Scholar]
- 28.Benson MJ, Rodriguez V, von Schack D, Keegan S, Cook TA, Edmonds J, Benoit S, Seth N, Du S, Messing D, Nickerson-Nutter CL, Dunussi-Joannopoulos K, Rankin AL, Ruzek M, Schnute ME, Douhan J., 3rd Modeling the clinical phenotype of BTK inhibition in the mature murine immune system. J Immunol. 2014;193:185–197. doi: 10.4049/jimmunol.1302570. [DOI] [PubMed] [Google Scholar]
- 29.Khan WN, Sideras P, Rosen FS, Alt FW. The role of Bruton’s tyrosine kinase in B-cell development and function in mice and man. Ann NY Acad Sci. 1995;764:27–38. doi: 10.1111/j.1749-6632.1995.tb55802.x. [DOI] [PubMed] [Google Scholar]
- 30.Merrell KT, Benschop RJ, Gauld SB, Aviszus K, Decote-Ricardo D, Wysocki LJ, Cambier JC. Identification of anergic B cells within a wild-type repertoire. Immunity. 2006;25:953–962. doi: 10.1016/j.immuni.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 31.Choi YS, Dieter JA, Rothaeusler K, Luo Z, Baumgarth N. B-1 cells in the bone marrow are a significant source of natural IgM. European journal of immunology. 2012;42:120–129. doi: 10.1002/eji.201141890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haas KM. Programmed cell death 1 suppresses B-1b cell expansion and long-lived IgG production in response to T cell-independent type 2 antigens. J Immunol. 2011;187:5183–5195. doi: 10.4049/jimmunol.1101990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holodick NE, Vizconde T, Rothstein TL. Splenic B-1a Cells Expressing CD138 Spontaneously Secrete Large Amounts of Immunoglobulin in Naive Mice. Frontiers in immunology. 2014;5:129. doi: 10.3389/fimmu.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Savage HP, Yenson VM, Sawhney SS, Mousseau BJ, Lund FE, Baumgarth N. Blimp-1-dependent and -independent natural antibody production by B-1 and B-1-derived plasma cells. The Journal of experimental medicine. 2017;214:2777–2794. doi: 10.1084/jem.20161122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reynolds AE, Kuraoka M, Kelsoe G. Natural IgM is produced by CD5- plasma cells that occupy a distinct survival niche in bone marrow. J Immunol. 2015;194:231–242. doi: 10.4049/jimmunol.1401203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hooijkaas H, Benner R, Pleasants JR, Wostmann BS. Isotypes and specificities of immunoglobulins produced by germ-free mice fed chemically defined ultrafiltered “antigen-free” diet. European journal of immunology. 1984;14:1127–1130. doi: 10.1002/eji.1830141212. [DOI] [PubMed] [Google Scholar]
- 37.Bos NA, Kimura H, Meeuwsen CG, De Visser H, Hazenberg MP, Wostmann BS, Pleasants JR, Benner R, Marcus DM. Serum immunoglobulin levels and naturally occurring antibodies against carbohydrate antigens in germ-free BALB/c mice fed chemically defined ultrafiltered diet. European journal of immunology. 1989;19:2335–2339. doi: 10.1002/eji.1830191223. [DOI] [PubMed] [Google Scholar]
- 38.Haury M, Sundblad A, Grandien A, Barreau C, Coutinho A, Nobrega A. The repertoire of serum IgM in normal mice is largely independent of external antigenic contact. European journal of immunology. 1997;27:1557–1563. doi: 10.1002/eji.1830270635. [DOI] [PubMed] [Google Scholar]
- 39.Vieira P, Rajewsky K. The half-lives of serum immunoglobulins in adult mice. European journal of immunology. 1988;18:313–316. doi: 10.1002/eji.1830180221. [DOI] [PubMed] [Google Scholar]
- 40.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 41.Loder F, Mutschler B, Ray RJ, Paige CJ, Sideras P, Torres R, Lamers MC, Carsetti R. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals. The Journal of experimental medicine. 1999;190:75–89. doi: 10.1084/jem.190.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 43.Kraus M, Alimzhanov MB, Rajewsky N, Rajewsky K. Survival of resting mature B lymphocytes depends on BCR signaling via the Igalpha/beta heterodimer. Cell. 2004;117:787–800. doi: 10.1016/j.cell.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 44.Schweighoffer E, Vanes L, Nys J, Cantrell D, McCleary S, Smithers N, Tybulewicz VL. The BAFF receptor transduces survival signals by co-opting the B cell receptor signaling pathway. Immunity. 2013;38:475–488. doi: 10.1016/j.immuni.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Middendorp S, Dingjan GM, Maas A, Dahlenborg K, Hendriks RW. Function of Bruton’s tyrosine kinase during B cell development is partially independent of its catalytic activity. J Immunol. 2003;171:5988–5996. doi: 10.4049/jimmunol.171.11.5988. [DOI] [PubMed] [Google Scholar]
- 46.Middendorp S, Zijlstra AJ, Kersseboom R, Dingjan GM, Jumaa H, Hendriks RW. Tumor suppressor function of Bruton tyrosine kinase is independent of its catalytic activity. Blood. 2005;105:259–265. doi: 10.1182/blood-2004-07-2708. [DOI] [PubMed] [Google Scholar]
- 47.Pedersen GK, Adori M, Karlsson Hedestam GB. NF-kappaB signaling in B-1 cell development. Annals of the New York Academy of Sciences. 2015;1362:39–47. doi: 10.1111/nyas.12800. [DOI] [PubMed] [Google Scholar]
- 48.Petro JB, Castro I, Lowe J, Khan WN. Bruton’s tyrosine kinase targets NF-kappaB to the bcl-x promoter via a mechanism involving phospholipase C-gamma2 following B cell antigen receptor engagement. FEBS letters. 2002;532:57–60. doi: 10.1016/s0014-5793(02)03623-2. [DOI] [PubMed] [Google Scholar]
- 49.Hayakawa K, Asano M, Shinton SA, Gui M, Allman D, Stewart CL, Silver J, Hardy RR. Positive selection of natural autoreactive B cells. Science. 1999;285:113–116. doi: 10.1126/science.285.5424.113. [DOI] [PubMed] [Google Scholar]
- 50.Ochsenbein AF, Fehr T, Lutz C, Suter M, Brombacher F, Hengartner H, Zinkernagel RM. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 1999;286:2156–2159. doi: 10.1126/science.286.5447.2156. [DOI] [PubMed] [Google Scholar]
- 51.Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, Chen J. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. The Journal of experimental medicine. 2000;192:271–280. doi: 10.1084/jem.192.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jayasekera JP, Moseman EA, Carroll MC. Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. Journal of virology. 2007;81:3487–3494. doi: 10.1128/JVI.02128-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boes M, Prodeus AP, Schmidt T, Carroll MC, Chen J. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. The Journal of experimental medicine. 1998;188:2381–2386. doi: 10.1084/jem.188.12.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou ZH, Zhang Y, Hu YF, Wahl LM, Cisar JO, Notkins AL. The broad antibacterial activity of the natural antibody repertoire is due to polyreactive antibodies. Cell host & microbe. 2007;1:51–61. doi: 10.1016/j.chom.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ehrenstein MR, Notley CA. The importance of natural IgM: scavenger, protector and regulator. Nature reviews. Immunology. 2010;10:778–786. doi: 10.1038/nri2849. [DOI] [PubMed] [Google Scholar]
- 56.Silverman GJ, Gronwall C, Vas J, Chen Y. Natural autoantibodies to apoptotic cell membranes regulate fundamental innate immune functions and suppress inflammation. Discovery medicine. 2009;8:151–156. [PubMed] [Google Scholar]
- 57.Rosenfeld SM, Perry HM, Gonen A, Prohaska TA, Srikakulapu P, Grewal S, Das D, McSkimming C, Taylor AM, Tsimikas S, Bender TP, Witztum JL, McNamara CA. B-1b Cells Secrete Atheroprotective IgM and Attenuate Atherosclerosis. Circulation research. 2015;117:e28–39. doi: 10.1161/CIRCRESAHA.117.306044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shaw PX, Horkko S, Chang MK, Curtiss LK, Palinski W, Silverman GJ, Witztum JL. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. The Journal of clinical investigation. 2000;105:1731–1740. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Colombo MJ, Alugupalli KR. Complement factor H-binding protein, a putative virulence determinant of Borrelia hermsii, is an antigenic target for protective B1b lymphocytes. J Immunol. 2008;180:4858–4864. doi: 10.4049/jimmunol.180.7.4858. [DOI] [PubMed] [Google Scholar]
- 60.Foote JB, Kearney JF. Generation of B cell memory to the bacterial polysaccharide alpha-1,3 dextran. J Immunol. 2009;183:6359–6368. doi: 10.4049/jimmunol.0902473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cole LE, Yang Y, Elkins KL, Fernandez ET, Qureshi N, Shlomchik MJ, Herzenberg LA, Vogel SN. Antigen-specific B-1a antibodies induced by Francisella tularensis LPS provide long-term protection against F. tularensis LVS challenge. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:4343–4348. doi: 10.1073/pnas.0813411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alugupalli KR. A distinct role for B1b lymphocytes in T cell-independent immunity. Current topics in microbiology and immunology. 2008;319:105–130. doi: 10.1007/978-3-540-73900-5_5. [DOI] [PubMed] [Google Scholar]
- 63.Janeway CA, Jr, Barthold DR. An analysis of the defective response of CBA/N mice to T-dependent antigens. J Immunol. 1975;115:898–900. [PubMed] [Google Scholar]
- 64.Scher I, Berning AK, Asofsky R. X-linked B lymphocyte defect in CBA/N mice. IV. Cellular and environmental influences on the thymus dependent IgG anti-sheep red blood cell response. J Immunol. 1979;123:477–486. [PubMed] [Google Scholar]
- 65.McDaniel LS, Scott G, Kearney JF, Briles DE. Monoclonal antibodies against protease-sensitive pneumococcal antigens can protect mice from fatal infection with Streptococcus pneumoniae. The Journal of experimental medicine. 1984;160:386–397. doi: 10.1084/jem.160.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Duty JA, Szodoray P, Zheng NY, Koelsch KA, Zhang Q, Swiatkowski M, Mathias M, Garman L, Helms C, Nakken B, Smith K, Farris AD, Wilson PC. Functional anergy in a subpopulation of naive B cells from healthy humans that express autoreactive immunoglobulin receptors. The Journal of experimental medicine. 2009;206:139–151. doi: 10.1084/jem.20080611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Quach TD, Manjarrez-Orduno N, Adlowitz DG, Silver L, Yang H, Wei C, Milner EC, Sanz I. Anergic responses characterize a large fraction of human autoreactive naive B cells expressing low levels of surface IgM. J Immunol. 2011;186:4640–4648. doi: 10.4049/jimmunol.1001946. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.