Abstract
Contrast-enhanced ultrasound (CEUS) is widely used to evaluate tumor microcirculation, which is useful in the differential diagnosis between benignity and malignancy. In the last 10 years, the applicability of CEUS to thyroid nodules has greatly improved due to technological refinements and the development of second-generation contrast agents. In this review, we summarize the applications of CEUS for thyroid nodules, focusing on the imaging findings of malignant and benign nodules in the existing literature and the use of those findings to predict malignancies, with an additional brief description of the utilization of CEUS for other thyroid-related diseases.
Keywords: Contrast media, Ultrasonography, Thyroid nodule, Elasticity imaging techniques, Diagnosis
Introduction
Contrast-enhanced ultrasound (CEUS) is considered to be an effective technique to evaluate microvascularization, which is important because angiogenesis is the basis for neoplastic growth [1]. CEUS parameters aid in characterizing lesions and evaluating tumor angiogenesis [2]. CEUS was first used in the diagnostic work-up of focal liver lesions, and international ultrasonography guidelines recommend CEUS as the method of choice for focal liver lesions if B-mode and Doppler ultrasonography (US) are inconclusive [3]. As time passed, CEUS was additionally applied to the study of the kidneys [4], prostate, breasts [5], and testes [6].
Thyroid nodules are a common finding in the general population, and their detection rate is increasing with the widespread use of US. Most thyroid nodules are benign, and fewer than 5% are malignant [7]. In the last decade, the applicability of CEUS to thyroid-related disease greatly improved due to the development of more advanced US equipment and the introduction of second-generation contrast agents (SonoVue, Bracco Imaging, Milan, Italy) for the characterization of malignant thyroid nodules. SonoVue consists of sulfur hexafluoride microbubbles (2-10 μm); after configuration, it is injected intravenously as a small bolus when used for CEUS. The microbubbles stay in the circulation for a period of time [8], during which the ultrasound system is focused on the region of interest (ROI). When microbubbles in the blood flow past the imaging window, they reflect a unique echo that stands in contrast to the surrounding tissue due to the mismatch of multiple orders of magnitude between the echogenicity of the microbubbles and the tissue. The ultrasound device converts the patterns of strong echogenicity into a contrast-enhanced image of the ROI. In this way, the echo of the bloodstream is enhanced, permitting the dynamic detection of microvessels.
The use of SonoVue in the thyroid gland was first reported by Bartolitta, who stated that malignant thyroid nodules measuring less than 1 cm in diameter mostly did not show vascularization, those between 1 and 2 cm in diameter showed faint dotted contrast enhancement, and nodules with a diameter larger than 2 cm presented diffuse contrast enhancement [9]. This pivotal finding demonstrated that malignant thyroid nodules had an enhancement pattern related to the size of the tumor. Since that report was published, CEUS has been widely performed for the differential diagnosis between benign and malignant thyroid nodules.
CEUS Examination of Thyroid Nodules
Most studies have performed CEUS for thyroid nodules according to the following criteria: (1) histopathologically confirmed (surgical or US-guided biopsy) thyroid nodules, (2) solid or almost solid (<25% cystic) nodules on conventional US, (3) thyroid nodules with malignant tendencies (e.g., Thyroid Imaging Reporting and Data System [TI-RADS] category 4 on conventional US), and (4) modules larger than 0.5 cm in diameter.
Common exclusion criteria are (1) nodules with coarse calcifications, (2) predominantly cystic nodules, (3) a nodule diameter <5 mm, and (4) pregnancy.
The general process of CEUS for thyroid nodules is as follows. The largest view of the nodule is chosen before switching to the CEUS mode from conventional US. The focus zone is always placed at the bottom level of the nodule, and CEUS is performed using a low mechanical index (MI) (<0.10). SonoVue is injected intravenously as a bolus at a dose from 1.2 to 4.8 mL, followed by a 5-mL saline flush. The timer on the US machine is started during the CEUS process, and the images obtained during the next 2-3 minutes are digitally stored as raw data.
The diagnosis of thyroid nodules includes both a qualitative analysis and a quantitative analysis, mainly depending on the CEUS enhancement patterns of thyroid nodules relative to the surrounding parenchyma, including the following parameters. The enhancement degree (i.e., hyperenhancement, isoenhancement, and hypoenhancement) of the thyroid nodule is defined as a peak intensity higher than, equal to, or lower than the perinodular tissue, respectively. The enhancement pattern is defined as either centripetal enhancement, if enhancement moves from the periphery to the center, or entire enhancement, if the peripheral and central areas synchronously show enhancement. Homogeneous lesions are defined as those in which the entire lesion shows full enhancement, regardless of the enhancement degree, while heterogeneous lesions are enhanced lesions that contain areas without any enhancement. Wash-in and wash-out refer to enhancement that appears or disappears earlier, at the same time as, or later than the perinodular tissue, respectively. Ring enhancement is defined as an enhanced rim of peritumoral tissue that appears in the early phase and becomes more distinct in the late phase [10].
During the post-processing quantitative analysis of thyroid nodules, the operator manually draws an ROI covering the tissue to be studied, and a color map is automatically generated by the built-in analysis package or by using quantification software. Then, further and smaller ROIs of a similar size are hand-drawn on the color map within the tumor, and the following quantitative parameters are automatically calculated: peak intensity, the maximum intensity of the time-intensity curve, time to peak, the time needed to reach peak intensity, the mean transit time, the time for which intensity is higher than the mean value, and area under the time-intensity curve, proportional to the total volume of blood in the ROI and the sum of the areas of wash-in and wash-out [11].
Complications and Disadvantages of CEUS
CEUS suffers from the following limitations that have been described in the literature. First, microbubbles last only 5-10 minutes in circulation because they are taken up either by immune system cells or by the liver or spleen. Second, ultrasound produces more heat as the frequency increases, so the ultrasonic frequency must be carefully adjusted. Third, microbubbles burst at low ultrasound frequencies and at high MIs, which is a measure of the negative acoustic pressure of the ultrasound imaging system. Increasing the MI increases image quality, but there are tradeoffs with microbubble destruction. Microbubble destruction can cause local microvasculature ruptures and hemolysis [12].
Rare reports of fatal events have been reported with CEUS. The overall rates of adverse reactions from the use of SonoVue was reported to be 0.0086%, which appears to be lower than the comparable rates for contrast agents used in computed tomography or magnetic resonance imaging [13].
CEUS Patterns Characteristic of Malignant Thyroid Nodules
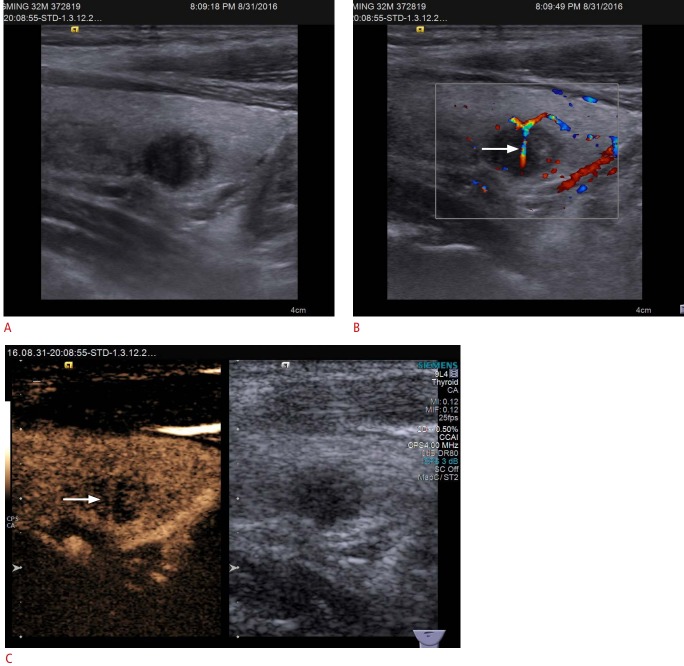
Hypoenhancement is considered to be a major CEUS pattern characteristic of malignant thyroid nodules (Fig. 1) [14-17], especially for thyroid tumors 10 mm or less in diameter [18]. This finding is counterintuitive, because malignant tumors in other organs are well supplied by blood vessels. Hypoenhancement in malignant thyroid nodules has also been termed an "absent" pattern in some studies [19]. The main reason that thyroid malignant tumors show a lack of blood supply is that the tumors grows with complex neovascularization inside it; once the growth outweighs neovascularization, necrosis and embolus formation happens within the tumor, ultimately leading to hypoenhancement on CEUS. Moreover, Zhou et al. [20] found that instead of hypoenhancement, the nodule-to-perinodule peak intensity ratio showed the best diagnostic efficiency, with an optimal cutoff value of 0.9.
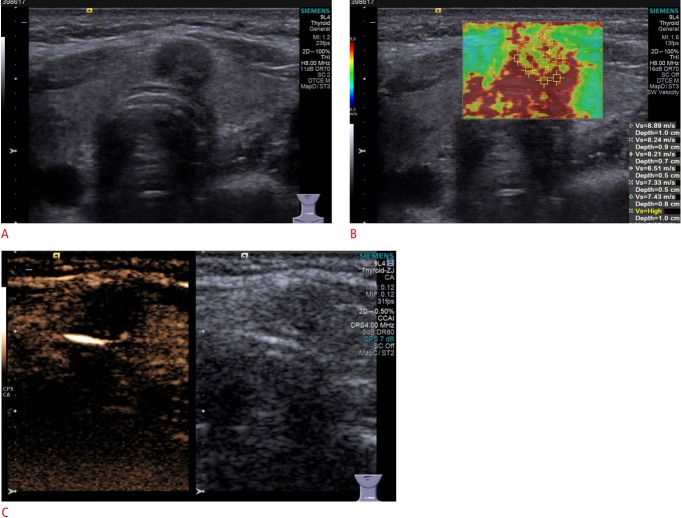
Fig. 1. Conventional ultrasonography (US) and contrast-enhanced ultrasound (CEUS) imaging of papillary thyroid carcinoma in a 32-year-old man.
A. Gray-scale US shows a hypoechoic solid nodule in the right thyroid lobe with a poorly defined margin, assessed as Thyroid Imaging Reporting and Data System category 4C. B. Color Doppler US shows branching vessels (arrow) within the mass. C. CEUS shows hypoenhancement with penetrating vessels (arrow) in the mass.
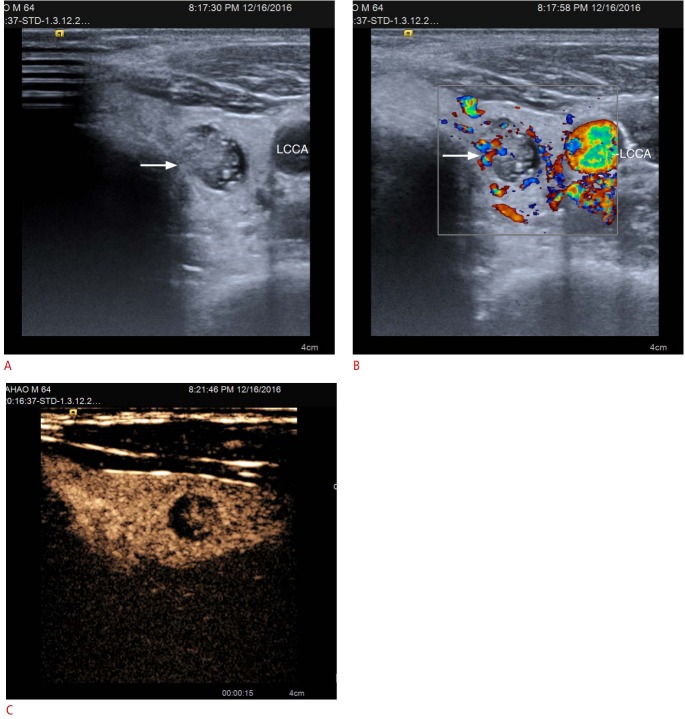
Heterogeneous enhancement is another important CEUS pattern characteristic of malignant thyroid nodules (Fig. 2) [21-24]. Ma et al. [21] observed that the blood vessels of malignant nodules are typically aberrant and tortuous. Most malignant nodules contain areas of fibrosis, calcification, or focal necrosis, which may explain the trend for heterogeneous enhancement. Moreover, heterogeneous enhancement was found to be the best indicator for predicting malignancy in multivariate logistic regression analysis.
Fig. 2. Conventional ultrasonography (US) and contrast-enhanced ultrasound (CEUS) imaging of papillary thyroid carcinoma in a 64-year-old man.
A. Gray-scale US shows a mixed-texture nodule (arrow) in the left thyroid lobe with calcification, assessed as Thyroid Imaging Reporting and Data System category 4B. LCCA, left common carotid artery. B. Color Doppler US shows peripheral and interior blood flow. C. CEUS shows heterogeneous isoenhancement in the mass.
Heterogeneity has generally been considered to be a subjective, qualitative CEUS characteristic. However, Jin et al. [25] used an algorithm to quantify the heterogeneity of enhancement of solid thyroid nodules on CEUS. The heterogeneity value (HV) was calculated as standard deviation/mean intensity×100 (using Adobe Photoshop). The heterogeneity ratio (HR) was calculated as the ratio of the HV of the nodule to that of the surrounding parenchyma. HV and HR were significantly higher in malignant nodules than in benign nodules. This algorithm used for the assessment of image heterogeneity on CEUS examinations may be a useful adjunct to conventional US for the differential diagnosis of solid thyroid nodules.
As well as quantifying enhancement heterogeneity, CEUS can also be used to assess thyroid nodules using other indicators, most notably arrival time and time to peak. Some published studies have shown that earlier wash-in, wash-out, and time to peak were associated with malignancy [18,26-30]. This could be due to the reduced microvascularization in carcinomas as a result of necrosis. Conversely, Yuan et al. [31] and Wu et al. [32] recently reported that the majority of thyroid carcinomas were enhanced later than the surrounding thyroid gland. Furthermore, in some studies, quantitative evaluations did not prove useful for distinguishing between benign and malignant nodules, as the time parameters were different between malignant and benign lesions, but not significantly so [33].
Nemec et al. [29] found that relative enhancement during the wash-out curve (ratio of enhancement at 20 seconds after peak enhancement to baseline intensity) was lower in malignant nodules than in benign nodules, and 2.35 was the optimal threshold of this parameter for differentiating benign and malignant thyroid nodules.
CEUS Patterns Characteristic of Benign Thyroid Nodules
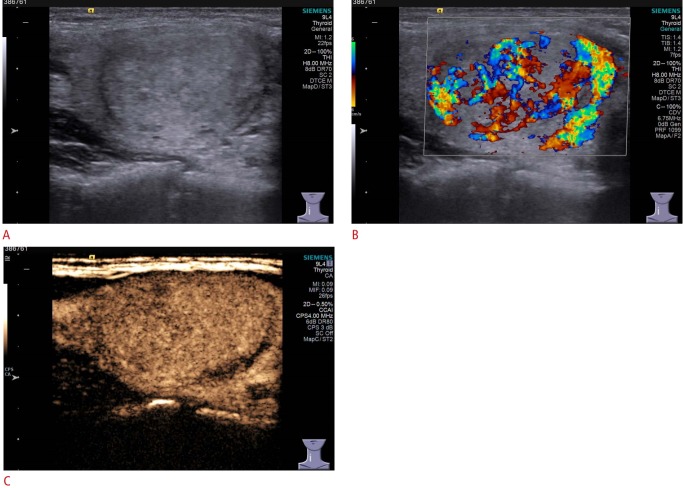
In contrast to the various CEUS patterns characteristic of malignant nodules, the consensus on diagnostic criteria for benign nodules is that homogeneity and ring enhancement are the most two valuable CEUS indicators for predicting benignity (Fig. 3), and these parameters have frequently been evaluated in studies [14,15,22- 24,34].
Fig. 3. Conventional ultrasonography (US) and contrast-enhanced US (CEUS) imaging of thyroid adenoma in a 35-year-old woman.
A. Gray-scale US shows an isoechoic nodule in the left thyroid lobe with a well-defined margin, assessed as Thyroid Imaging Reporting and Data System category 3. B. Color Doppler US shows mainly interior blood flow, with absent peripheral blood flow. C. CEUS shows homogeneity and ring enhancement in the mass.
To summarize, CEUS is a promising noninvasive technique for the differential diagnosis of benign and malignant thyroid nodules (Table 1). According to meta-analyses, its sensitivity is 85% to 88%, and its specificity is 88% to 90% [35,36]. However, some studies [17,21,23,24,28,33] have found that the contrast enhancement pattern might overlap between malignant and benign thyroid nodules. Some nodules with typically benign contrast enhancement patterns exhibited malignant behavior; for instance, some benign nodules had non-homogeneous enhancement due to necrosis. Therefore, many scholars combined CEUS with other US methods to improve diagnostic accuracy.
Table 1.
Criteria and diagnostic value for CEUS in the differential diagnosis between benign and malignant thyroid nodules
| Study | Year | Diagnostic criteria for malignancy | Sensitivity | Specificity | Accuracy |
|---|---|---|---|---|---|
| Schleder et al. [14] | 2015 | No wash-out or wash-out in late phase | 21/26 | 69/75 | 90/101 |
| Jiang et al. [15] | 2015 | Non-homogeneous, hypoenhancement | 44/49 | 67/73 | 111/122 |
| Ma et al. [21] | 2014 | Heterogeneous enhancement | 85/94 | 71/78 | 156/172 |
| Zhang et al. [22] | 2010 | Heterogeneous enhancement | 45/51 | 49/53 | 94/104 |
| Zhang et al. [24] | 2016 | Low enhancement or a peripheral irregular ring | 82/82 | 49/75 | 121/157 |
| Cantisani et al. [26] | 2013 | Heterogeneous, absent wash-in | 15/19 | 31/34 | 46/53 |
| Wendl et al. [28] | 2016 | Accelerated TTP at the center of the lesion | 16/20 | 19/30 | 35/50 |
| Nemec et al. [29] | 2012 | Relative enhancement of 2.35 as the optimal threshold | 10/13 | 22/33 | 32/46 |
| Yuan et al. [31] | 2015 | Irregular shape of enhanced lesions | 35/37 | 36/41 | 71/78 |
| Zhang et al. [37] | 2017 | Heterogeneous enhancement | 58/75 | 230/244 | 288/319 |
| Li et al. [42] | 2015 | Non-homogeneous and low enhancement | 44/50 | 24/30 | 68/80 |
| Giusti et al. [43] | 2014 | TTP index >0.98 | 7/13 | 46/50 | 53/63 |
| Deng et al. [45] | 2014 | Hypoenhancement | 46/56 | 101/119 | 147/175 |
CEUS, contrast-enhanced ultrasound; TTP, time to peak.
CEUS Combined with Conventional US
Zhang et al. [37] combined CEUS with an assessment of TI-RADS categories based on conventional US in the differential diagnosis of thyroid nodules, and found that the combination significantly improved the diagnostic accuracy (96% vs. 90%), especially for TI-RADS class 4 thyroid nodules. In another study, Kwak et al. [38] used TI-RADS and hypo-enhancement by CEUS criteria to predict malignancy. Furthermore, Zhao et al. [16], Li and Luo [17], and Zhang et al. [39] similarly concluded that combining CEUS with conventional US could improve diagnostic accuracy.
CEUS Combined with Real-Time Elastography and Conventional US
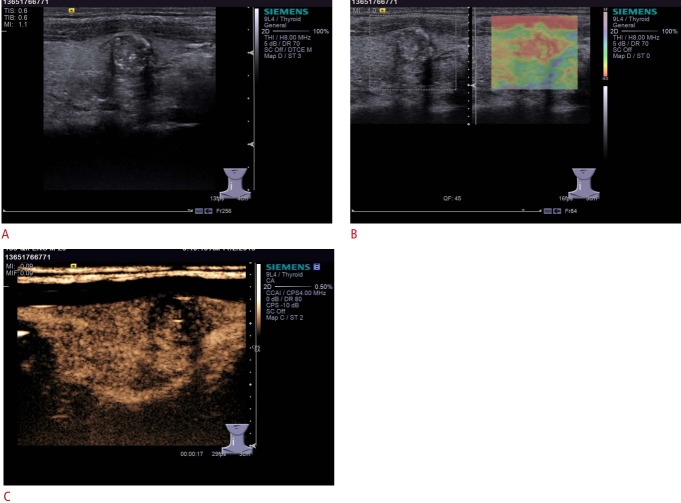
Real-time elastography (RTE) is a reproducible sonographic assessment of tissue consistency using external compression or carotid artery vibrations [40]. Similar to palpation, the rationale of RTE is that cancerous nodules are stiffer, with less elastic deformation than the surrounding thyroid tissue or benign thyroid nodules (Fig. 4).
Fig. 4. Conventional ultrasonography (US), real-time elastography (RTE), and contrast-enhanced US (CEUS) imaging of thyroid adenoma in a 35-year-old man.
A. Gray-scale US shows a hypoechoic solid nodule in the right thyroid lobe with calcification assessed as Thyroid Imaging Reporting and Data System category 4C. B. RTE is mainly red and slightly yellow, which means that the nodule is probably hard. C. CEUS shows heterogeneous isoenhancement in the mass.
A previous study [41] reported that the combination of US, RTE, and CEUS could increase both the sensitivity and specificity of all three methods. With regard to comparisons between individual methods, most studies [20,42,43] showed that RTE was the most valuable tool. Giusti et al. [44] found that RTE and CEUS showed no advantages over US, and in a further study, demonstrated that the information added by CEUS was less sensitive than that provided by US and RTE.
CEUS Combined with Acoustic Radiation Force Impulse Imaging
Acoustic radiation force impulse imaging (ARFI) is a novel US-based elastography method enabling the quantitative measurement of tissue stiffness derived from qualitative RTE. Deng et al. [45] reported that the combination of conventional US, ARFI, and CEUS significantly improved diagnostic accuracy compared with any of these modalities singly or a combination of conventional US and ARFI (Fig. 5).
Fig. 5. Conventional ultrasonography (US), acoustic radiation force impulse imaging (ARFI), and contrastenhanced US (CEUS) imaging of thyroid adenoma in a 35-year-old man.
A. Gray-scale US shows a hypoechoic solid nodule in the isthmus with capsule contact assessed as Thyroid Imaging Reporting and Data System category 4C. B. ARFI is mainly red and slightly yellow, and the median shear wave velocity was 7.82 m/sec, which means that the nodule was very hard. C. CEUS shows hypoenhancement in the mass.
Zhan et al. [46] used ARFI as a preliminary screening tool for the differential diagnosis of thyroid nodules, while CEUS was used as a supplement for nodules that were difficult to distinguish by ARFI. CEUS can be combined with a processing technique to represent 3-dimensional (3D) internal flow.
Molinari et al. [47] developed an image processing technique for characterizing the intranodular vascularization of thyroid lesions by 3D CEUS imaging. The results showed that malignant nodules had higher values for the number of vascular trees, vascular density, the number of branching nodes, 2D and 3D tortuosity, and the inflection count metric. This technique enables quantitative 3D CEUS and skeletonization to be used in the differential diagnosis of thyroid lesions.
In addition to the differential diagnosis of benign and malignant thyroid nodules, CEUS has been applied to assess other thyroid-related diseases.
CEUS Evaluation of US-Guided Laser Ablation for Benign Thyroid Nodules
Ma et al. [48] evaluated the single-session complete ablation rate of US-guided percutaneous laser ablations for benign thyroid nodules, and found that all nodule volumes significantly decreased from the original size at 1 day after ablation.
CEUS is regarded as the main method for evaluating laser ablation treatment. During the procedure, if CEUS shows nodules with a small amount of residual tissue at the edge, the patient requires further ablation treatment until the remnants of the lesion disappear completely.
CEUS Detection of Extrathyroid Extension or Neck Lymph Node Metastasis in Papillary Thyroid Carcinoma
In a previous study, CEUS and tumor size were compared between extrathyroid extension (ETE) and non-ETE groups [49]. Multivariate stepwise logistic regression analysis demonstrated that the time from peak to one-half tumor size and wash-in slope were significantly different between the ETE and non-ETE groups.
Xiang et al. [50] evaluated the value of CEUS as a non-invasive tool for detecting neck lymph node metastasis and the enhancement patterns of malignant lymph nodes for papillary thyroid carcinoma. Their results showed that heterogeneous enhancement, perfusion defects, microcalcification, and centripetal/hybrid enhancement were specific criteria for malignant lymph nodes in a univariate analysis. Furthermore, Hong et al. [51] reported a similar conclusion.
CEUS Used to Evaluate Papillary Thyroid Carcinoma Patients with Breast Cancer
Wei et al. [52] found that the mean and peak intensities were higher in thyroid cancers in patients with breast cancer than in those without breast cancer, and these parameters were significantly associated with the microvessel density count and vascular endothelial growth factor expression.
Conclusion
The present review showed that no isolated CEUS feature is capable of predicting malignancy in thyroid nodules with acceptable diagnostic accuracy. However, the presence of some CEUS features such as hypoenhancement and heterogeneity can be used to identify nodules with an elevated risk for malignancy; in addition, homogeneity and ring enhancement indicate benignity.
Moreover, with further research, CEUS could potentially play a more important role in the detection of extranodular extension of malignant nodules and in evaluating treatment response. Nevertheless, more studies are required to standardize the technique and to confirm its usefulness.
Acknowledgments
This work was supported by grant No. 14411970400 from the Medical Guide Project of the Shanghai Science and Technology Commission.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Sidhu PS, Cantisani V, Dietrich CF, Gilja OH, Saftoiu A, Bartels E, et al. The EFSUMB guidelines and recommendations for the Clinical Practice of Contrast-Enhanced Ultrasound (CEUS) in non-hepatic applications: update 2017 (long version) Ultraschall Med. 2018;39:e2–e44. doi: 10.1055/a-0586-1107. [DOI] [PubMed] [Google Scholar]
- 2.Bertolotto M, Catalano O. Contrast-enhanced ultrasound: past, present, and future. Ultrasound Clin. 2009;4:339–367. [Google Scholar]
- 3.Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsoe CP, et al. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver: update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol. 2013;39:187–210. doi: 10.1016/j.ultrasmedbio.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Gulati M, King KG, Gill IS, Pham V, Grant E, Duddalwar VA. Contrast-enhanced ultrasound (CEUS) of cystic and solid renal lesions: a review. Abdom Imaging. 2015;40:1982–1996. doi: 10.1007/s00261-015-0348-5. [DOI] [PubMed] [Google Scholar]
- 5.Drudi FM, Cantisani V, Gnecchi M, Malpassini F, Di Leo N, de Felice C. Contrast-enhanced ultrasound examination of the breast: a literature review. Ultraschall Med. 2012;33:E1–E7. doi: 10.1055/s-0031-1299408. [DOI] [PubMed] [Google Scholar]
- 6.Drudi FM, Maghella F, Martino G, Messineo D, Ciccariello M, Cantisani V, et al. Detection of small testicular masses in monorchid patients using US, CPDUS, CEUS and US-guided biopsy. J Ultrasound. 2016;19:25–28. doi: 10.1007/s40477-015-0158-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Remonti LR, Kramer CK, Leitao CB, Pinto LC, Gross JL. Thyroid ultrasound features and risk of carcinoma: a systematic review and meta-analysis of observational studies. Thyroid. 2015;25:538–550. doi: 10.1089/thy.2014.0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferraioli G, Meloni MF. Contrast-enhanced ultrasonography of the liver using SonoVue. Ultrasonography. 2018;37:25–35. doi: 10.14366/usg.17037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartolotta TV, Midiri M, Galia M, Runza G, Attard M, Savoia G, et al. Qualitative and quantitative evaluation of solitary thyroid nodules with contrast-enhanced ultrasound: initial results. Eur Radiol. 2006;16:2234–2241. doi: 10.1007/s00330-006-0229-y. [DOI] [PubMed] [Google Scholar]
- 10.Lu Q, Li CX, Huang BJ, Xue LY, Wang WP. Triphasic and epithelioid minimal fat renal angiomyolipoma and clear cell renal cell carcinoma: qualitative and quantitative CEUS characteristics and distinguishing features. Abdom Imaging. 2015;40:333–342. doi: 10.1007/s00261-014-0221-y. [DOI] [PubMed] [Google Scholar]
- 11.Park AY, Seo BK. Up-to-date Doppler techniques for breast tumor vascularity: superb microvascular imaging and contrast-enhanced ultrasound. Ultrasonography. 2018;37:98–106. doi: 10.14366/usg.17043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantisani V, Bertolotto M, Weskott HP, Romanini L, Grazhdani H, Passamonti M, et al. Growing indications for CEUS: the kidney, testis, lymph nodes, thyroid, prostate, and small bowel. Eur J Radiol. 2015;84:1675–1684. doi: 10.1016/j.ejrad.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Piscaglia F, Bolondi L, Italian Society for Ultrasound in Medicine and Biology (SIUMB) Study Group on Ultrasound Contrast Agents The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol. 2006;32:1369–1375. doi: 10.1016/j.ultrasmedbio.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 14.Schleder S, Janke M, Agha A, Schacherer D, Hornung M, Schlitt HJ, et al. Preoperative differentiation of thyroid adenomas and thyroid carcinomas using high resolution contrast-enhanced ultrasound (CEUS) Clin Hemorheol Microcirc. 2015;61:13–22. doi: 10.3233/CH-141848. [DOI] [PubMed] [Google Scholar]
- 15.Jiang J, Shang X, Wang H, Xu YB, Gao Y, Zhou Q. Diagnostic value of contrast-enhanced ultrasound in thyroid nodules with calcification. Kaohsiung J Med Sci. 2015;31:138–144. doi: 10.1016/j.kjms.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Zhao RN, Zhang B, Yang X, Jiang YX, Lai XJ, Zhang XY. Logistic regression analysis of contrast-enhanced ultrasound and conventional ultrasound characteristics of sub-centimeter thyroid nodules. Ultrasound Med Biol. 2015;41:3102–3108. doi: 10.1016/j.ultrasmedbio.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 17.Li F, Luo H. Comparative study of thyroid puncture biopsy guided by contrast-enhanced ultrasonography and conventional ultrasound. Exp Ther Med. 2013;5:1381–1384. doi: 10.3892/etm.2013.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen HY, Liu WY, Zhu H, Jiang DW, Wang DH, Chen Y, et al. Diagnostic value of contrast-enhanced ultrasound in papillary thyroid microcarcinoma. Exp Ther Med. 2016;11:1555–1562. doi: 10.3892/etm.2016.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Q, Li Y, Wang Y. Diagnostic value of "absent" pattern in contrast-enhanced ultrasound for the differentiation of thyroid nodules. Clin Hemorheol Microcirc. 2016;63:325–334. doi: 10.3233/CH-152020. [DOI] [PubMed] [Google Scholar]
- 20.Zhou X, Zhou P, Hu Z, Tian SM, Zhao Y, Liu W, et al. Diagnostic efficiency of quantitative contrast-enhanced ultrasound indicators for discriminating benign from malignant solid thyroid nodules. J Ultrasound Med. 2018;37:425–437. doi: 10.1002/jum.14347. [DOI] [PubMed] [Google Scholar]
- 21.Ma JJ, Ding H, Xu BH, Xu C, Song LJ, Huang BJ, et al. Diagnostic performances of various gray-scale, color Doppler, and contrast-enhanced ultrasonography findings in predicting malignant thyroid nodules. Thyroid. 2014;24:355–363. doi: 10.1089/thy.2013.0150. [DOI] [PubMed] [Google Scholar]
- 22.Zhang B, Jiang YX, Liu JB, Yang M, Dai Q, Zhu QL, et al. Utility of contrast-enhanced ultrasound for evaluation of thyroid nodules. Thyroid. 2010;20:51–57. doi: 10.1089/thy.2009.0045. [DOI] [PubMed] [Google Scholar]
- 23.Yuan Z, Quan J, Yunxiao Z, Jian C, Zhu H. Contrast-enhanced ultrasound in the diagnosis of solitary thyroid nodules. J Cancer Res Ther. 2015;11:41–45. doi: 10.4103/0973-1482.147382. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Luo YK, Zhang MB, Li J, Li J, Tang J. Diagnostic accuracy of contrast-enhanced ultrasound enhancement patterns for thyroid nodules. Med Sci Monit. 2016;22:4755–4764. doi: 10.12659/MSM.899834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin L, Xu C, Xie X, Li F, Lv X, Du L. An Algorithm of image heterogeneity with contrast-enhanced ultrasound in differential diagnosis of solid thyroid nodules. Ultrasound Med Biol. 2017;43:104–110. doi: 10.1016/j.ultrasmedbio.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Cantisani V, Consorti F, Guerrisi A, Guerrisi I, Ricci P, Di Segni M, et al. Prospective comparative evaluation of quantitative-elastosonography (Q-elastography) and contrast-enhanced ultrasound for the evaluation of thyroid nodules: preliminary experience. Eur J Radiol. 2013;82:1892–1898. doi: 10.1016/j.ejrad.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Hornung M, Jung EM, Georgieva M, Schlitt HJ, Stroszczynski C, Agha A. Detection of microvascularization of thyroid carcinomas using linear high resolution contrast-enhanced ultrasonography (CEUS) Clin Hemorheol Microcirc. 2012;52:197–203. doi: 10.3233/CH-2012-1597. [DOI] [PubMed] [Google Scholar]
- 28.Wendl CM, Janke M, Jung W, Stroszczysnski C, Jung EM. Contrast-enhanced ultrasound with perfusion analysis for the identification of malignant and benign tumours of the thyroid gland. Clin Hemorheol Microcirc. 2015;63:113–121. doi: 10.3233/CH-151966. [DOI] [PubMed] [Google Scholar]
- 29.Nemec U, Nemec SF, Novotny C, Weber M, Czerny C, Krestan CR. Quantitative evaluation of contrast-enhanced ultrasound after intravenous administration of a microbubble contrast agent for differentiation of benign and malignant thyroid nodules: assessment of diagnostic accuracy. Eur Radiol. 2012;22:1357–1365. doi: 10.1007/s00330-012-2385-6. [DOI] [PubMed] [Google Scholar]
- 30.Appetecchia M, Bacaro D, Brigida R, Milardi D, Bianchi A, Solivetti F. Second generation ultrasonographic contrast agents in the diagnosis of neoplastic thyroid nodules. J Exp Clin Cancer Res. 2006;25:325–330. [PubMed] [Google Scholar]
- 31.Yuan Z, Quan J, Yunxiao Z, Jian C, Zhu HE. Association between real-time contrast-enhanced ultrasound characteristics and thyroid carcinoma size. Mol Clin Oncol. 2015;3:743–746. doi: 10.3892/mco.2015.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu Q, Wang Y, Li Y, Hu B, He ZY. Diagnostic value of contrast-enhanced ultrasound in solid thyroid nodules with and without enhancement. Endocrine. 2016;53:480–488. doi: 10.1007/s12020-015-0850-0. [DOI] [PubMed] [Google Scholar]
- 33.Wiesinger I, Kroiss E, Zausig N, Hornung M, Zeman F, Stroszczynski C, et al. Analysis of arterial dynamic micro-vascularization with contrast-enhanced ultrasound (CEUS) in thyroid lesions using external perfusion software: first results. Clin Hemorheol Microcirc. 2016;64:747–755. doi: 10.3233/CH-168044. [DOI] [PubMed] [Google Scholar]
- 34.Agha A, Jung EM, Janke M, Hornung M, Georgieva M, Schlitt HJ, et al. Preoperative diagnosis of thyroid adenomas using high resolution contrast-enhanced ultrasound (CEUS) Clin Hemorheol Microcirc. 2013;55:403–409. doi: 10.3233/CH-131777. [DOI] [PubMed] [Google Scholar]
- 35.Sun B, Lang L, Zhu X, Jiang F, Hong Y, He L. Accuracy of contrast-enhanced ultrasound in the identification of thyroid nodules: a meta-analysis. Int J Clin Exp Med. 2015;8:12882–12889. [PMC free article] [PubMed] [Google Scholar]
- 36.Yu D, Han Y, Chen T. Contrast-enhanced ultrasound for differentiation of benign and malignant thyroid lesions: meta-analysis. Otolaryngol Head Neck Surg. 2014;151:909–915. doi: 10.1177/0194599814555838. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Y, Zhou P, Tian SM, Zhao YF, Li JL, Li L. Usefulness of combined use of contrast-enhanced ultrasound and TI-RADS classification for the differentiation of benign from malignant lesions of thyroid nodules. Eur Radiol. 2017;27:1527–1536. doi: 10.1007/s00330-016-4508-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kwak JY, Han KH, Yoon JH, Moon HJ, Son EJ, Park SH, et al. Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology. 2011;260:892–899. doi: 10.1148/radiol.11110206. [DOI] [PubMed] [Google Scholar]
- 39.Zhang YZ, Xu T, Gong HY, Li CY, Ye XH, Lin HJ, et al. Application of high-resolution ultrasound, real-time elastography, and contrast-enhanced ultrasound in differentiating solid thyroid nodules. Medicine (Baltimore) 2016;95:e5329. doi: 10.1097/MD.0000000000005329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Itoh A, Ueno E, Tohno E, Kamma H, Takahashi H, Shiina T, et al. Breast disease: clinical application of US elastography for diagnosis. Radiology. 2006;239:341–350. doi: 10.1148/radiol.2391041676. [DOI] [PubMed] [Google Scholar]
- 41.Friedrich-Rust M, Sperber A, Holzer K, Diener J, Grunwald F, Badenhoop K, et al. Real-time elastography and contrast-enhanced ultrasound for the assessment of thyroid nodules. Exp Clin Endocrinol Diabetes. 2010;118:602–609. doi: 10.1055/s-0029-1237701. [DOI] [PubMed] [Google Scholar]
- 42.Li F, Zhang J, Wang Y, Liu L. Clinical value of elasticity imaging and contrast-enhanced ultrasound in the diagnosis of papillary thyroid microcarcinoma. Oncol Lett. 2015;10:1371–1377. doi: 10.3892/ol.2015.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giusti M, Orlandi D, Melle G, Massa B, Silvestri E, Minuto F, et al. Is there a real diagnostic impact of elastosonography and contrast-enhanced ultrasonography in the management of thyroid nodules? J Zhejiang Univ Sci B. 2013;14:195–206. doi: 10.1631/jzus.B1200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giusti M, Campomenosi C, Gay S, Massa B, Silvestri E, Monti E, et al. The use of semi-quantitative ultrasound elastosonography in combination with conventional ultrasonography and contrast-enhanced ultrasonography in the assessment of malignancy risk of thyroid nodules with indeterminate cytology. Thyroid Res. 2014;7:9. doi: 10.1186/s13044-014-0009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deng J, Zhou P, Tian SM, Zhang L, Li JL, Qian Y. Comparison of diagnostic efficacy of contrast-enhanced ultrasound, acoustic radiation force impulse imaging, and their combined use in differentiating focal solid thyroid nodules. PLoS One. 2014;9:e90674. doi: 10.1371/journal.pone.0090674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhan J, Diao XH, Chen L, Jin JM, Chen Y. Role of contrast-enhanced ultrasound in diagnosis of thyroid nodules in acoustic radiation force impulse "gray zone". Ultrasound Med Biol. 2017;43:1179–1186. doi: 10.1016/j.ultrasmedbio.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Molinari F, Mantovani A, Deandrea M, Limone P, Garberoglio R, Suri JS. Characterization of single thyroid nodules by contrast-enhanced 3-D ultrasound. Ultrasound Med Biol. 2010;36:1616–1625. doi: 10.1016/j.ultrasmedbio.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 48.Ma S, Zhou P, Wu X, Tian S, Zhao Y. Detection of the single-session complete ablation rate by contrast-enhanced ultrasound during ultrasound-guided laser ablation for benign thyroid nodules: a prospective study. Biomed Res Int. 2016;2016:9565364. doi: 10.1155/2016/9565364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y, Liu H, Qian CL, Lin MS, Li FH. Utility of quantitative contrast-enhanced ultrasound for the prediction of extracapsular extension in papillary thyroid carcinoma. Sci Rep. 2017;7:1472. doi: 10.1038/s41598-017-01650-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiang D, Hong Y, Zhang B, Huang P, Li G, Wang P, et al. Contrast-enhanced ultrasound (CEUS) facilitated US in detecting lateral neck lymph node metastasis of thyroid cancer patients: diagnosis value and enhancement patterns of malignant lymph nodes. Eur Radiol. 2014;24:2513–2519. doi: 10.1007/s00330-014-3288-5. [DOI] [PubMed] [Google Scholar]
- 51.Hong YR, Luo ZY, Mo GQ, Wang P, Ye Q, Huang PT. Role of contrast-enhanced ultrasound in the pre-operative diagnosis of cervical lymph node metastasis in patients with papillary thyroid carcinoma. Ultrasound Med Biol. 2017;43:2567–2575. doi: 10.1016/j.ultrasmedbio.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 52.Wei X, Li Y, Zhang S, Ming G. Evaluation of thyroid cancer in Chinese females with breast cancer by vascular endothelial growth factor (VEGF), microvessel density, and contrast-enhanced ultrasound (CEUS) Tumour Biol. 2014;35:6521–6529. doi: 10.1007/s13277-014-1868-2. [DOI] [PubMed] [Google Scholar]