Abstract
During the past decade we have been witnessing a rise in medical cannabis use, yet the evidence for the safety and efficacy of the various cannabinoid compounds is scarce. The State of Israel has always been at the forefront of clinical and translational research in support of Evidence Based Medicine. With respect to cannabis Israel has created a medical and regulatory environment that enables clinical studies with cannabis which may lead to improved Evidence Based use of these compounds. This opinion paper discusses selected studies into the safety and effects of cannabis derived products.
During the past decade we have been witnessing a rise in medical cannabis use. Although the evidence for the safety and effectiveness of the different cannabinoid compounds is scarce, claims of the miracle effects are plenty. Supporters cheer cannabis as the universal source for the next generation of medical products capable of treating a wide variety of maladies (cancer, epilepsy, ALS, MS, ADHD, autism, Alzheimer's, etc.). Clinicians trying to express scepticism and asking to accumulate and assess the evidence are considered to be “Big Pharma Collaborators” and opponents of progress. It is safe to say that medical cannabis is currently at the peak of the inflated expectations on the Gartner hype cycle 1 and it is therefore important that the clinical research community determines potential benefits and safety of cannabis‐derived compounds under the Evidence Based Medicine paradigm.
Despite the significant increase in publications on medical cannabis‐related topics (Figure 1), a summary of the current research activities expected to provide appropriate levels of evidence is remarkably short. Based on a PubMed search (see the first paragraph of the Appendix), there was a substantial increase in cannabis clinical trials, from 165 in the years 1990–1999 to 504 in the years 2000–2009 and 734 in the years 2010–2017. However, the majority of the published literature is dedicated to surveys of willing cannabis users.
Figure 1.
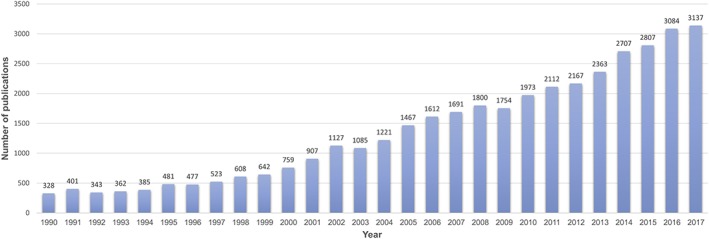
PubMed search results on annual number of articles on medical cannabis
Whiting et al. 2, in a systematic review based on the literature search from inception to April 2015, identified 79 randomized controlled trials of cannabis and cannabinoids, 58 of which were double blind, placebo‐controlled trials. The indications, in decreasing order with respect to number of trials per indication, were: chronic pain, nausea and vomiting due to chemotherapy, spasticity due to multiple sclerosis or paraplegia, HIV/AIDS, sleep disorder, psychosis, Tourette's syndrome, anxiety disorder and glaucoma. A similar search (see the second paragraph of the Appendix) from April 2015 to the end of February 2018 yields an additional 37 clinical trials (22 of them randomized, double blind, placebo‐controlled). Chronic pain studies described the effect of dronabinol and vaporized or smoked cannabis containing different concentrations of THC in patients with neuropathic pain from spinal cord injury of from diabetes and noncardiac chest pain 3, 4, 5, 6, 7, 8. The sample size was between 13 and 215 patients. The pain reduction measured by Cold‐Pressor Test, visual analog scales, McGill Pain Questionnaire, chest pain symptom questionnaire was significant in all studies. Most trials in multiple sclerosis were small scaled (sample size up to 219 patients), all used nabiximols (Sativex®), and only one of them was a double‐blind placebo‐controlled trial showing improvement in neurophysiological measures of multiple sclerosis associated spasticity 9, 10, 11, 12, 13.
Four trials evaluated the effect of different cannabinoids: dronabinol, Δ (9)‐tetrahydrocannabivarin (THCV) or cannabidiol (CBD) on different metabolic parameters, such as glycemic control, lipid profile, insulin sensitivity, markers of inflammation, leptin, incretin, insulin‐like growth factor I (IGF‐I), urinary free cortisol (UFC) and adipokines. CBD and THCV had significant positive effect compared to placebo on reduction of fasting plasma glucose, pancreatic β‐cell function, adiponectin and apolipoprotein A, but dronabinol had no clinically meaningful metabolic effect. The sample size in these studies was relatively small: 13 to 130 subjects 14, 15, 16, 17, 18. The therapeutic effects of cannabinoids, specifically cannabidiol (CBD), on epilepsy, mainly in young adults and children, were investigated in three trials 19, 20, 21. Only one of the trials was placebo‐controlled, with a sample size of 120 patients, and the results demonstrated seizures reduction. These studies led the Food and Drug Administration (FDA) to approve, for the first time, a drug with an active ingredient derived directly from herbal cannabis 22.
The International Clinical Trials Registry Platform of the World Health Organization includes 17 national and international registries. A search of active cannabis trials on 27 June 2018 (see the third paragraph of the Appendix) showed that there are 295 randomized, double blind, placebo controlled clinical trials registered worldwide (after exclusion of duplications) 23. Only 199 of these trials were recruiting during the time of search. Of note, a majority of cannabis‐related clinical trials focus on the various aspects of treating cannabis as an illicit drug and the detrimental effect of cannabis abuse (i.e. substance abuse, mental and psychotic disorders due to cannabis use), rather than investigating the medical effects of cannabis.
Over the last decade, Israel has been emerging as a leading country in cannabis research and development. Detailed, well‐developed regulations 24 together with an advanced agricultural programme and a long scientific tradition of cannabis research started by Prof. Mechoulam provide a fertile ground for a plethora of clinical and basic research programmes. Rather than presenting a systematic review of all cannabis‐related studies in the world or in Israel, we shall describe a selected few of them conducted in Israel which in our opinion can contribute to a better understanding of medical use of cannabis and its derivatives.
Gastrointestinal System
Two small randomized double blind placebo‐controlled trials (RCTs) on 21 and 20 Crohn's disease patients each, showed inconsistent results: one (NCT01040910, 0.5 gram evirecense with 23% THC concentration or placebo twice daily, Tikun‐Olam Ltd provided the cannabis and placebo for the trial), showed a significant reduction in Crohn's disease activity index (CDAI) compared to placebo and mild side effects (nausea, sleepiness, concentration, memory loss, confusion and dizziness) 25, while in the second trial (NCT01037322), administration of the oral CBD (10 mg CBD or placebo twice daily) was not associated with a decrease in CDAI 26. Two additional phase II randomized double‐blind placebo controlled trials are currently in their final stages (supported by Tikun‐Olam Ltd.): the first investigates the safety and efficacy of whole plant oral cannabis oil (15% CBD and 3.7% THC) in 50 patients with Crohn's disease (NCT01826188), and the second trial (NCT01826188) is designed to evaluate the effect of smoking cannabis (20% THC cigarettes or placebo) in 32 ulcerative colitis patients.
Central Nervous System
Three small observational studies in Parkinson's disease patients, suggested improvement in motor symptoms and pain. Two (20 and 22 subjects) found no side effects of medical cannabis consumption 27, 28. The third, a telephone survey of 47 patients with Parkinson disease treated with medical cannabis (supported by Tikun‐Olam Ltd), concluded that the treatment improved PD symptoms 29. Five patients (10.6%) stopped cannabis treatment 3 to 12 months after initiating it. Side effects of the treatment included confusion (reported by eight patients), anxiety (eight patients), hallucinations (eight patients), short‐term amnesia (three patients) and one patient developed psychosis.
In an open label study of 11 Alzheimer's disease patients, THC treatment (2.5 mg) was associated with a reduction in aggressive behavior. Adverse events were reported in three of the patients, with one adjudicated as adverse event related to the treatment (confusion, improved following dose adjustment 30). A currently recruiting phase II, randomized, double‐blind, placebo‐controlled trial (NCT03328676, supported by Tikun‐Olam Ltd), investigates the efficacy and safety of cannabis oil (30% CBD and 1.5% THC) in patients with dementia related agitation. In a pilot study, ten post‐traumatic stress disorder (PTSD) patients showed a reduction in symptoms and improvement of sleep following oral THC (5 mg) administration. Side effects were reported in four cases; dry mouth in two patients, headache in one patient and dizziness in another patient. These effects were mild and continued throughout the 3 weeks of treatment 31.
Graft Versus Host Disease (GVHD)
In a one arm, prospective, phase II trial, the incidence of acute GVHD in 48 patients, scheduled for Allogeneic Hematopoietic Cell Transplantation and receiving CBD (300 mg), was significantly lower than the expected. None of the patients developed GVHD while consuming CBD and no serious adverse effects were experienced by any of the patients 32. The same group is currently conducting an open label interventional study (NCT02392780) aiming to explore the efficacy of 150 mg oral CBD, in the treatment of severe acute GVHD.
Pain
A prospective observational open label study on 176 patients showed pain reduction and a decrease of 44% in opioid consumption. Nine subjects discontinued treatment due to mild to moderate adverse effects: primarily sedation, heaviness, nervousness and difficulty to concentrate. Two additional subjects discontinued treatment due to serious side effects: one subject because of elevated liver transaminases, and one elderly subject was admitted to the emergency room in a confusional state 33. Another observational study compared the incidence of depression and anxiety in patients treated with either medical cannabis (n = 329), opioids (n = 474) or both (n = 77) for chronic pain, found that patients treated with opioids had higher rates of depression and anxiety. Side effects incidence was not reported by authors 34. The subjective nature of pain assessment and the inherent limitations of an open label design put these studies in a significant risk for bias.
Paediatrics
Two trials on spasticity in paediatrics patients suffering from cerebral palsy are in final stages, the first is a double blind, placebo control trial assessing safety and efficacy of Sativex® in 72 patients (NCT01898520, GW Research Ltd.), and the second is a randomized controlled trial comparing two cannabis oil formulations (5% CBD and 0.2% THC) and (5% CBD and 0.8% THC) provided by Tikun Olam Ltd in 40 patients (NCT02470325). In an intermediate analysis on 25 patients, adverse effects were rare and included worsening of seizures in 2 patients, behavioral changes in 2 and somnolence in 1 35. A large double blind, randomized, placebo‐controlled trial with crossover that is now recruiting, is assessing the safety, tolerability and efficacy of whole plant extract versus 99% CBD oil and placebo (provided by BOL pharma ltd) in 120 patients with autism spectrum disorder (NCT02956226).
Prospective Registry
Our group has established a prospective registry of patients treated in the cannabis clinics of Tikun Olam, the largest provider of medical cannabis in Israel. We analyzed safety and efficacy of cannabis formulations in a large unselected group of 7000 patients out of approximately 32 000 receiving treatment in Israel. Our first reports suggest that cannabis treatment is safe, reduces pain and improves quality of life in a population of 2736 patients older than 65 and 2970 cancer patients receiving palliative therapy 36, 37. Moreover, treatment with cannabis seems associated with a decrease in opioids consumption.
Conclusion
There is a serious gap between the public perception of cannabis as being a 21st century panacea and the medical establishment view that cannabis is a 21st century snake oil. While some of the public and mainstream media have already accepted medical cannabis to be effective in a lengthy list of conditions, clinical studies with an appropriate study design are few. Only few studies are prospective and most clinical trials investigating medical cannabis and its derivatives are not placebo controlled, not blinded and have small sample sizes. This situation leads to a division within the medical community: those in favor of using cannabis who also usually see themselves as pioneers in this area and tend to prescribe cannabis even when the evidence is insufficient, while opponents of medical cannabis might perhaps end up denying a patient cannabis even in situations where cannabis has been shown to be beneficial (pain, nausea and sleep disturbance, National Academies of Sciences Engineering and Medicine Report 38). Furthermore, rapidly developing clinical research is rather not focused, and many cannabis research programmes are concentrated around rare diseases, where the regulatory process and thus time to marketing are perceived to be shorter. Yet, we believe that cannabis derived medications should have a significant role in the treatment of chronic pain symptoms and to be a safer substitute for opioids in palliative care.
Israel has developed a regulatory environment where the indications for which cannabis can be prescribed are tightly regulated, and developed based on the accumulation of research and clinical experience data thus far. The approved indications include nausea and vomiting due to chemotherapy treatment, cancer associated pain, inflammatory bowel diseases, neuropathic pain, cachexia in AIDS patients, multiple sclerosis, Parkinson's disease, Tourette syndrome, epilepsy (both adult and pediatric population), and post‐traumatic stress disorder.
It is policies such as these that will lead to an improved evidence‐based medical use of cannabis and its derivatives. Some of the smaller clinical studies conducted within this environment generated promising data and are followed up by larger studies.
Competing Interests
V.N. serves on the scientific advisory board of ‘Tikun Olam Ltd.’ and L.B.‐L.S. is an employee of ‘Tikun Olam Ltd.’.
Search Strategies
-
1
PubMed search for cannabis clinical trials:
“Cannabinoids”[MeSH Terms] OR “Cannabis”[Mesh Terms] OR “Medical Marijuana”[Mesh] OR “Cannabinoids”[tiab] OR “Cannabinoid”[tiab] OR “Cannabinol”[tiab] OR “Cannabidiol”[tiab] OR “Tetrahydrocannabinol”[tiab] OR “THC”[tiab] OR “CBD”[tiab] OR “Marinol”[tiab] OR “Cesamet”[tiab] OR “Sativex”[tiab] OR “Nabilone”[tiab] OR “Dronabinol”[tiab] OR “Delta‐9‐tetrahydrocannabinol”[tiab] OR “Delta‐9‐THC”[tiab] OR “Cannabis”[tiab] OR “Marihuana”[tiab] OR “Marijuana”[tiab] OR “Hashish”[tiab] OR “Epidiolex”[tiab] OR “Syndros”[tiab] NOT (“Cholangiopancreatography Endoscopic Retrograde”[MeSh Terms] OR “Cholecystectomy Laparoscopic”[MeSh Terms] OR “Gallstones”[MeSH Terms]) Filters: Clinical Trial; Publication date from 1990/01/01 to 2017/12/31
-
2
PubMed search for cannabis clinical trials from April 2015 to February 2018:
“Cannabinoids”[MeSH Terms] OR “Cannabis”[Mesh Terms] OR “Medical Marijuana”[Mesh] OR “Cannabinoids”[tiab] OR “Cannabinoid”[tiab] OR “Cannabinol”[tiab] OR “Cannabidiol”[tiab] OR “Tetrahydrocannabinol”[tiab] OR “THC”[tiab] OR “CBD”[tiab] OR “Marinol”[tiab] OR “Cesamet”[tiab] OR “Sativex”[tiab] OR “Nabilone”[tiab] OR “Dronabinol”[tiab] OR “Delta‐9‐tetrahydrocannabinol”[tiab] OR “Delta‐9‐THC”[tiab] OR “Cannabis”[tiab] OR “Marihuana”[tiab] OR “Marijuana”[tiab] OR “Hashish”[tiab] OR “Epidiolex”[tiab] OR “Syndros”[tiab] AND (Clinical Trial[ptyp] AND (“2015/03/01”[PDAT] : “2018/12/31”[PDAT])) NOT (“Cholangiopancreatography, Endoscopic Retrograde”[MeSh Terms] OR “Cholecystectomy, Laparoscopic”[MeSh Terms] OR “Gallstones”[MeSH Terms])
-
3
International Clinical Trials Registry Platform (ICTRP) search for registered cannabis double‐blind placebo‐controlled trials (date performed – June 27th, 2018):
Title: cannabis OR cannabinoids OR Marihuana OR Marijuana OR Sativex OR Nabiximols OR Nabilone OR Cesamet OR Canemes OR Dronabinol OR Marinol OR Syndros OR Epidiolex OR THC OR tetrahydrocannabinol OR delta‐9‐tetrahydrocannabinol OR Cannabidiol OR cannabinol AND Intervention: placebo.
Bar‐Lev Schleider, L. , Abuhasira, R. , and Novack, V. (2018) Medical cannabis: aligning use to evidence‐based medicine approach. Br J Clin Pharmacol, 84: 2458–2462. 10.1111/bcp.13657.
References
- 1. Gartner Inc . Gartner Hype Cycle [online]. Available at https://www.gartner.com/technology/research/methodologies/hype-cycle.jsp (last accessed 20 March 2018).
- 2. Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al Cannabinoids for medical use. JAMA 2015; 313: 2456–2473. [DOI] [PubMed] [Google Scholar]
- 3. Walter C, Oertel BG, Felden L, Kell CA, Nöth U, Vermehren J, et al Brain mapping‐based model of Δ(9)‐Tetrahydrocannabinol effects on connectivity in the pain matrix. Neuropsychopharmacology 2016; 41: 1659–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Malik Z, Bayman L, Valestin J, Rizvi‐Toner A, Hashmi S, Schey R. Dronabinol increases pain threshold in patients with functional chest pain: a pilot double‐blind placebo‐controlled trial. Dis Esophagus 2017; 30: 1–8. [DOI] [PubMed] [Google Scholar]
- 5. Ware MA, Wang T, Shapiro S, Collet J‐P, Boulanger A, Esdaile JM, et al Cannabis for the management of pain: assessment of safety study (COMPASS). J Pain 2015; 16: 1233–1242. [DOI] [PubMed] [Google Scholar]
- 6. Wallace MS, Marcotte TD, Umlauf A, Gouaux B, Atkinson JH. Efficacy of inhaled cannabis on painful diabetic neuropathy. J Pain 2015; 16: 616–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilsey B, Marcotte TD, Deutsch R, Zhao H, Prasad H, Phan A. An exploratory human laboratory experiment evaluating vaporized cannabis in the treatment of neuropathic pain from spinal cord injury and disease. J Pain 2016; 17: 982–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cooper ZD, Haney M. Sex‐dependent effects of cannabis‐induced analgesia. Drug Alcohol Depend 2016; 167: 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haupts M, Vila C, Jonas A, Witte K, Álvarez‐Ossorio L. Influence of previous failed antispasticity therapy on the efficacy and tolerability of THC:CBD Oromucosal spray for multiple sclerosis spasticity. Eur Neurol 2016; 75: 236–243. [DOI] [PubMed] [Google Scholar]
- 10. Carotenuto A, Iodice R, Petracca M, Inglese M, Cerillo I, Cocozza S, et al Upper motor neuron evaluation in multiple sclerosis patients treated with Sativex® . Acta Neurol Scand 2017; 135: 442–448. [DOI] [PubMed] [Google Scholar]
- 11. Russo M, Naro A, Leo A, Sessa E, D’Aleo G, Bramanti P, et al Evaluating Sativex® in neuropathic pain management: a clinical and neurophysiological assessment in multiple sclerosis. Pain Med 2016; 17: pnv080. [DOI] [PubMed] [Google Scholar]
- 12. Leocani L, Nuara A, Houdayer E, Schiavetti I, Del Carro U, Amadio S, et al Sativex® and clinical–neurophysiological measures of spasticity in progressive multiple sclerosis. J Neurol 2015; 262: 2520–2527. [DOI] [PubMed] [Google Scholar]
- 13. Russo M, Dattola V, Logiudice AL, Ciurleo R, Sessa E, De Luca R, et al The role of Sativex in robotic rehabilitation in individuals with multiple sclerosis. Medicine (Baltimore) 2017; 96: e8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pu S, Eck P, Jenkins DJA, Connelly PW, Lamarche B, Kris‐Etherton PM, et al Interactions between dietary oil treatments and genetic variants modulate fatty acid ethanolamides in plasma and body weight composition. Br J Nutr 2016; 115: 1012–1023. [DOI] [PubMed] [Google Scholar]
- 15. Jadoon KA, Ratcliffe SH, Barrett DA, Thomas EL, Stott C, Bell JD, et al Efficacy and safety of Cannabidiol and Tetrahydrocannabivarin on Glycemic and lipid parameters in patients with type 2 diabetes: a randomized, double‐blind, placebo‐controlled, parallel group pilot study. Diabetes Care 2016; 39: 1777–1786. [DOI] [PubMed] [Google Scholar]
- 16. Reichenbach ZW, Sloan J, Rizvi‐Toner A, Bayman L, Valestin J, Schey R. A 4‐week pilot study with the Cannabinoid receptor Agonist Dronabinol and its effect on metabolic parameters in a randomized trial. Clin Ther 2015; 37: 2267–2274. [DOI] [PubMed] [Google Scholar]
- 17. Andries A, Frystyk J, Flyvbjerg A, Støving RK. Changes in IGF‐I, urinary free cortisol and adipokines during dronabinol therapy in anorexia nervosa: results from a randomised, controlled trial. Growth Horm IGF Res 2015; 25: 247–252. [DOI] [PubMed] [Google Scholar]
- 18. Chia CW, Carlson OD, Liu DD, González‐Mariscal I, Santa‐Cruz Calvo S, Egan JM. Incretin secretion in humans is under the influence of cannabinoid receptors. Am J Physiol Metab 2017; 313: E359–E366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, et al Trial of Cannabidiol for drug‐resistant seizures in the Dravet syndrome. N Engl J Med 2017; 376: 2011–2020. [DOI] [PubMed] [Google Scholar]
- 20. Devinsky O, Marsh E, Friedman D, Thiele E, Laux L, Sullivan J, et al Cannabidiol in patients with treatment‐resistant epilepsy: an open‐label interventional trial. Lancet Neurol 2016; 15: 270–278. [DOI] [PubMed] [Google Scholar]
- 21. Szaflarski M, Hansen B, Bebin EM, Szaflarski JP. Social correlates of health status, quality of life, and mood states in patients treated with cannabidiol for epilepsy. Epilepsy Behav 2017; 70: 364–369. [DOI] [PubMed] [Google Scholar]
- 22. The U.S. Food and Drug Administration USD of H and HS . Press Announcements ‐ FDA approves first drug comprised of an active ingredient derived from marijuana to treat rare, severe forms of epilepsy. Office of the Commissioner. Available at https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm611046.htm (last accessed 27 June 2018).
- 23. World Health Organization . International Clinical Trials Registry Platform (ICTRP). Available at http://apps.who.int/trialsearch/default.aspx (last accessed 26 February 2018).
- 24. Abuhasira R, Shbiro L, Landschaft Y. Medical use of cannabis and cannabinoids containing products ‐ regulations in Europe and North America. Eur J Intern Med 2018; 49: 2–6. [DOI] [PubMed] [Google Scholar]
- 25. Naftali T, Bar‐Lev Schleider L, Dotan I, Lansky EP, Sklerovsky Benjaminov F, Konikoff FM. Cannabis induces a clinical response in patients with Crohn's disease: a prospective placebo‐controlled study. Clin Gastroenterol Hepatol 2013; 11: 1276–1280. [DOI] [PubMed] [Google Scholar]
- 26. Naftali T, Mechulam R, Marii A, Gabay G, Stein A, Bronshtain M, et al Low‐dose Cannabidiol is safe but not effective in the treatment for Crohn's disease, a randomized controlled trial. Dig Dis Sci 2017; 62: 1615–1620. [DOI] [PubMed] [Google Scholar]
- 27. Shohet A, Khlebtovsky A, Roizen N, Roditi Y, Djaldetti R. Effect of medical cannabis on thermal quantitative measurements of pain in patients with Parkinson's disease. Eur J Pain 2017; 21: 486–493. [DOI] [PubMed] [Google Scholar]
- 28. Lotan I, Treves TA, Roditi Y, Djaldetti R. Cannabis (medical Marijuana) treatment for motor and non–motor symptoms of Parkinson disease. Clin Neuropharmacol 2014; 37: 41–44. [DOI] [PubMed] [Google Scholar]
- 29. Balash Y, Bar‐Lev Schleider L, Korczyn AD, Shabtai H, Knaani J, Rosenberg A, et al Medical Cannabis in Parkinson disease. Clin Neuropharmacol 2017; 40: 268–272. [DOI] [PubMed] [Google Scholar]
- 30. Shelef A, Barak Y, Berger U, Paleacu D, Tadger S, Plopsky I, et al Safety and efficacy of medical Cannabis oil for behavioral and psychological symptoms of dementia: an-open label, add‐on, pilot study. J Alzheimer's Dis 2016; 51: 15–19. [DOI] [PubMed] [Google Scholar]
- 31. Roitman P, Mechoulam R, Cooper-Kazaz R, Shalev A. Preliminary, open‐label, pilot study of add‐on oral Δ9‐Tetrahydrocannabinol in chronic post‐traumatic stress disorder. Clin Drug Investig 2014; 34: 587–591. [DOI] [PubMed] [Google Scholar]
- 32. Yeshurun M, Shpilberg O, Herscovici C, Shargian L, Dreyer J, Peck A, et al Cannabidiol for the prevention of graft‐versus‐host‐disease after Allogeneic Hematopoietic cell transplantation: results of a phase II study. Biol Blood Marrow Transplant 2015; 21: 1770–1775. [DOI] [PubMed] [Google Scholar]
- 33. Haroutounian S, Ratz Y, Ginosar Y, Furmanov K, Saifi F, Meidan R, et al The effect of medicinal Cannabis on pain and quality‐of‐life outcomes in chronic pain: a prospective open‐label study. Clin J Pain 2016; 32: 1036–1043. [DOI] [PubMed] [Google Scholar]
- 34. Feingold D, Brill S, Goor‐Aryeh I, Delayahu Y, Lev‐Ran S. Depression and anxiety among chronic pain patients receiving prescription opioids and medical marijuana. J Affect Disord 2017; 218: 1–7. [DOI] [PubMed] [Google Scholar]
- 35. Libzon S, Schleider LB‐L, Saban N, Levit L, Tamari Y, Linder I, et al Medical Cannabis for pediatric moderate to severe complex motor disorders. J Child Neurol 2018; 33: 565–571. [DOI] [PubMed] [Google Scholar]
- 36. Abuhasira R, Schleider LB‐L, Mechoulam R, Novack V. Epidemiological characteristics, safety and efficacy of medical cannabis in the elderly. Eur J Intern Med 2018; 49: 44–50. [DOI] [PubMed] [Google Scholar]
- 37. Bar‐Lev Schleider L, Mechoulam R, Lederman V, Hilou M, Lencovsky O, Betzalel O, et al Prospective analysis of safety and efficacy of medical cannabis in large unselected population of patients with cancer. Eur J Intern Med 2018; 49: 37–43. [DOI] [PubMed] [Google Scholar]
- 38. National Academies of Sciences, Engineering, and Medicine . The health effects of Cannabis and Cannabinoids: the current state of evidence and recommendations for research. Washington, DC: The National Academies Press, 2017. [PubMed] [Google Scholar]


