Abstract
Aims
Doxycycline (DFD‐09) oral capsules 40 mg are approved for the treatment of inflammatory lesions of rosacea. Unlike the food‐induced lowering of doxycycline's peak plasma concentration (Cmax), its exposure under fed conditions in the skin, the drug's target site for rosacea, is unknown. The present study explored the effect of food on the dermal pharmacokinetics of doxycycline.
Methods
The pharmacokinetics of doxycycline in the dermal interstitial fluid (d‐ISF) and plasma of healthy volunteers were assessed in parallel groups under fed (n = 6) and fasting (n = 6) conditions during a 14‐day once‐daily treatment course with doxycycline oral capsules 40 mg (DFD‐09). Sampling of d‐ISF and plasma was performed on days 1, 10 (fasting group d‐ISF only) and 14.
Results
Twelve subjects were randomized, and 11 analysed. No causally drug‐related adverse events occurred. Dermal doxycycline exposures (Cmax and area under the curve) under the fed state were about 30% lower than under the fasting state at day 1 but were similar at steady state. In analogy to skin, plasma exposure showed no between‐group difference at steady state. Accumulation ratios were higher in the skin than in plasma. Correcting for plasma protein binding (~90%), dermal doxycycline exposure was approximately threefold higher than unbound plasma exposure.
Conclusions
At steady state, doxycycline concentrations in the skin of fed and fasting healthy volunteers were comparable. Doxycycline's efficacy in rosacea is possibly due to considerable dermal accumulation of unbound doxycycline and is independent of the effect of food.
Keywords: antibiotics, dermatology, food/herbal drug interactions, inflammation, pharmacokinetics
What is Already Known about this Subject
Doxycycline 40 mg, indicated for the treatment of inflammatory lesions of rosacea, is recommended for administration under fasting conditions owing to a negative effect of food on plasma exposures.
A ʻfasting‐onlyʼ regimen, based on plasma exposure, restricts patient compliance, and further information on doxycycline's exposure in skin, the drug's target site for rosacea is warranted.
What this Study Adds
This study in healthy adults showed a significantly higher accumulation of free doxycycline in dermal interstitial fluid than in plasma, regardless of food intake, and might explain its clinical efficacy in rosacea.
Comparable dermal steady‐state doxycycline concentrations observed with and without food in the study support doxycycline administration regardless of food intake.
Introduction
http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6464 is approved as a modified‐release formulation at a dose of 40 mg (ORACEA®) for the treatment of inflammatory lesions of rosacea, a disease characterized by transient redness, flushing and inflamed papules/pustules, mostly of facial skin 1. The approved regimen for the treatment of rosacea consists of a once‐daily 40 mg doxycycline capsule to be taken for 16 weeks 1. It was previously shown that, when given with a high‐fat, high‐calorie meal, doxycycline's peak plasma concentration (Cmax) and area under the plasma concentration–time curve (AUC) decreased by 45% and 22%, respectively, compared with fasting conditions 1. As such a lowering of drug exposure with food might compromise therapeutic effectiveness, it is recommended that doxycycline is taken 1 h before or 2 h after a meal. Compliance with this fasting‐only regimen may challenge adherence. Further, the effect of food‐induced lowering of plasma exposures on clinical efficacy is, as yet, unknown. Given that the inflammatory processes which characterize rosacea are localized in the skin, an estimation of doxycycline's availability in dermal compartments becomes imperative in improving knowledge of the food effect associated with this drug.
Doxycycline is postulated to act in rosacea by significantly impairing the secretion of proinflammatory cytokines such as interleukin‐1β and tumour necrosis factor‐α in human monocytes. Moreover, it directly inhibits matrix metalloproteinases (MMPs), which activate cathelicidins, which are antimicrobial peptides responsible for the host response to environmental triggers 2, 3. Doxycycline also indirectly inhibits the catalytic activity of kallikrein‐5 (KLK‐5), a serine protease which activates cathelicidin to its more active form 4. In addition, doxycycline protects capillary wall and connective tissue integrity by inhibiting MMPs, reduces hypersensitivity to vasodilatory stimuli and prevents the leakage of capillaries involved in erythema, all of which may be associated with rosacea 5. In a placebo‐controlled clinical trial, once‐daily administration of doxycycline 40 mg (ORACEA®) for 12 weeks exhibited an approximately 2.5‐fold decrease in epidermal cathelicidins and a numerical decrease in KLK‐5 levels, with no change in MMP and total protease levels in the treatment‐success group compared with the treatment‐failure group 6. The plasma pharmacokinetics (PK) of doxycycline in healthy volunteers (HVs) and different patient populations have been described previously. The most prominent features are improved tissue penetration and long elimination half‐life 7, 8, 9. Absorption of doxycycline was found to be impaired with concomitant food ingestion 9. An improved formulation of doxycycline, DFD‐09, was therefore developed for the same indication, which might ensure sufficient absorption independently of meal times. Bioequivalence of DFD‐09 to the marketed product, ORACEA®, was shown for fasting conditions. Administration of DFD‐09 with a high‐fat, high‐calorie meal resulted in a 30% and 10% reduction in the Cmax and AUC of doxycycline, respectively, compared with dosing under fasting conditions (DFD‐09 clinical study reports: data on file). Doxycycline's exposure in human skin, the target site of action for rosacea, has not been described yet.
The present study explored the effect of food on the dermal PK of doxycycline following the administration of DFD‐09 in HVs. Doxycycline concentrations were measured after once‐daily drug administration at single and repeated doses, to give an overview of the PK of a therapeutic doxycycline regimen in rosacea. In vivo microdialysis was used for dermal interstitial space fluid (d‐ISF) sampling. Microdialysis allows for quantification of drugs in the tissue interstices close to the site of disease manifestation, and consequently the site of drug‐action 10, 11. Plasma doxycycline concentrations were evaluated in parallel for supportive evidence. The results of the study were expected to help in understanding the food effect on dermal exposures to doxycycline, and it was hoped that they would support administration of the drug with or without food.
Methods
All study procedures were performed between July and September 2016 at the Department of Clinical Pharmacology, Medical University of Vienna, Austria, in accordance with International Conference on Harmonization Good Clinical Practice guidelines and the Declaration of Helsinki. The study was registered with the EudraCT number 2016–001622‐34, approved by the Ethics Committee of the Medical University of Vienna (reference number 1530/2016) and authorized by the Austrian Agency for Health and Food Safety.
Eligibility criteria
Within 14 days of the first treatment administration, 12 male HVs who consented for the study underwent a screening visit in which their medical history was taken, they underwent a physical examination, anthropometric data were recorded, blood and urine were collected for routine laboratory and drug‐screening tests, their vital signs were evaluated and a 12‐lead electrocardiogram (ECG) was carried out. Only subjects aged between 18 and 45 years, with a body mass index between 18.5 kg m−2 and 30 kg m−2, who were willing to comply with study requirements were included. Key exclusion criteria were hypersensitivity to doxycycline or tetracyclines, clinically relevant findings in the ECG, and physical and/or laboratory examinations, and skin conditions precluding microdialysis probe insertion.
Study design
This was a randomized, open‐label, two‐cohort, parallel‐design study to evaluate and compare the single‐ and multiple‐dose PK of doxycycline in skin and plasma with once‐daily administration of DFD‐09, both under fed and fasting conditions. Additional objectives were to confirm the achievement of steady‐state doxycycline in the skin of volunteers and estimate the washout duration of doxycycline from the skin after the end of therapy.
Two sets (one main set, one backup set) of sealed envelopes with the randomization number and containing information about the assigned group (fed or fasting), were prepared by study staff for each individual subject and kept throughout the study. By opening the envelopes in consecutive order, the investigator assigned (allocation ratio 1:1) study participants to receive a 14‐day treatment course of once‐daily DFD‐09 under fasting conditions (n = 6) or fed conditions (n = 6).
Study protocol and subjects
Being an exploratory study, a sample size of 12 subjects (six in each cohort) was deemed adequate to achieve the study objectives. All of the subjects fasted for 8 h prior to scheduled dosing. Fasting status was confirmed prior to each dosing by means of a rapid glucose test. Subjects in the fed cohort were served a standardized high‐fat, high‐calorie breakfast (consisting of two croissants, 30 g butter, 75 g jam and 200 ml milk) within 30 min prior to each dose. From day 1 to day 14, a single capsule of DFD‐09 (doxycycline) 40 mg, manufactured and provided by Dr. Reddy's Laboratories Limited, India, was administered with 240 ml water to each subject within a margin of ±60 min from the time of dosing on day 1, under the supervision of study staff in the clinic. After dosing, subjects were allowed to leave the clinic, except on PK sampling days, which required confinement. For the duration of the study, subjects had to avoid alcohol, grapefruit juice, any food containing xanthines, dairy products, antacids and calcium supplements, and avoid concomitant medication, except in the case of adverse events (AEs). Within 7 days from the last PK sampling, a final examination, which included recording of AEs and concomitant medication, measurement of vital signs, a physical examination, a 12‐lead ECG and the collection of blood and urine for routine laboratory tests, was performed.
PK sampling
Blood and d‐ISF samples were collected on study days 1 and 14 to assess the single‐ and multiple‐dose PK of doxycycline, respectively. For plasma sampling on days 1 and 14, venous blood was collected into dipotassium ethylenediamine tetraacetic acid vacuum tubes before and up to 24 h after DFD‐09 administration.
After DFD‐09 administration on study days 1, 10 (only fasting cohort) and 14, d‐ISF samples were collected before dosing and at specified 1‐h intervals over a period of 24 h. These intervals were chosen to ensure continuous d‐ISF sampling in the first 9 h after dosing. Afterwards, collection of d‐ISF was performed only for three distinct 1‐h intervals (11–12 h, 15–16 h and 23–24 h postdose; on study days 1 and 10, the last sampling period was from 21 h to 22 h postdose in order to avoid interference of the subsequent study drug intake with microdialysis probe calibration, which was performed after collection of the last d‐ISF sample).
On study day 28, additional microdialysis samples were collected for intervals 0–1 h, 1–2 h and 2–3 h in the fasting cohort, to determine the levels of any residual doxycycline, to estimate washout duration in d‐ISF. Depending on the study day, six (days 1, 10 and 14, respectively) or three (day 28) linear microdialysis catheters (66 Linear Microdialysis Catheter, μ Dialysis, Stockholm, Sweden) with a polycarbonate luer lock connection, polyurethane inlet and outlet tubing, and a polyarylethersulphone dialysis membrane (membrane length, 30 mm and molecular weight cut‐off, 20 kDa) were inserted into the dermis of one thigh of each HV, with the help of a 21 G, 50 mm introducer needle. Insertion sites were switched between study days (i.e. the same thigh was never used for 2 consecutive study days). Throughout the study days, all microdialysis probes were continuously perfused with 0.9% w/v saline solution by means of precision pumps, at a flow rate of 2 μl min−1. After an equilibration period of 60 min, including 30 min of baseline sampling, the study drug was administered.
The concentrations quantified in the collected microdialysate samples were only a fraction of the actual concentration in the investigated tissue (Cskin > Cdialysate). The factor by which the concentrations in d‐ISF and the perfusion medium are interrelated is termed relative recovery (RR). The RR is determined during the calibration of the microdialysis probes by retrodialysis, and describes the extent of diffusion across the semipermeable membrane 10. The RR as a percentage was calculated as the relative loss of drug from the probes (linear microdialysis catheters) to the d‐ISF according to the following equation:
All microdialysis probes were calibrated individually at the end of each PK sampling period on study days 1, 10, 14 and 28 by perfusing them with 0.5 μg ml−1 doxycycline hyclate (Vibravenös 100 mg 5 ml−1 Ampullen, Pfizer, Austria) at a flow rate of 2 μl min−1. After a run‐in phase of 30 min, two microdialysis calibration samples (0–30 and 30–60 min) and one aliquot of the calibration solution were collected per probe. After calibration, μD probes were removed. Drug concentrations in skin interstitium were calculated using the following equation:
Sample handling and analysis
Blood samples were centrifuged (2000 × g, 10 min, at 4°C) to extract plasma, which was snap frozen at −20°C within 60 min of the respective times of collection. Microdialysis samples were snap frozen at −20°C within 15 min of their respective times of collection. Blood and microdialysis samples were subsequently stored at −80°C until analysis. Doxycycline concentrations in microdialysate and plasma samples were analysed using a validated liquid chromatography–tandem mass spectrometry method, consisting of a Symbiosis™ ALIAS chromatographic system (Spark, The Netherlands) and a Qtrap 5500 system (Sciex, Framingham, MA, USA). Gradient elution was performed at a flow rate of 0.4 ml min−1. The mass spectrometer was operated in positive electrospray ionization mode. The analytical method was validated according to the European Medicines Agency guideline on bioanalytical method validation 12. Quantification was performed by multiple reaction monitoring, with chlortetracycline (Fluka 46 133, Sigma‐Aldrich, St. Louis, MO, USA) used as internal standard. Two calibration ranges were applied for microdialysate samples – a lower range at 1–100 ng ml−1, and a higher range at 50–600 ng ml−1. For plasma, the calibration range used was 100–750 ng ml−1.
PK and statistical analysis
The PK parameters of interest for both plasma and skin under single‐ and multiple‐dose conditions were Cmax, AUC from zero to 24 h (AUC0–24), time to peak concentration (Tmax) and trough concentration at steady state (Cmin). The PK parameters were calculated using Phoenix® WinNonlin® Enterprise Version 7.0 (Certara Inc., Princeton, NJ, USA). The accumulation ratio (AR) was calculated using the AUC method (AUC0–24,day14/AUC0–24,day1). As d‐ISF and plasma samples were collected up to 24 h, terminal elimination half‐lives could not be estimated.
Statistical analysis was performed on the PK data using SAS®, Version 9.4 (SAS Institute Inc., Cary, NC, USA). Descriptive statistics for plasma and dermal PK parameters were computed for doxycycline. Exposure data (such as Cmax, AUC0–24, Cmin) were ln‐transformed and then analysed using appropriate analysis of variance models to determine the mean treatment difference (fed and fasting). A P‐value <0.05 was considered statistically significant. Treatment difference was presented in terms of geometric least square mean ratios with 90% confidence intervals (CIs) for single and multiple doses. The achievement of steady state was verified from four samples collected at predose and at 24 h postdose (trough concentration) on days 10 and 14. Steady‐state analysis was performed for subjects in the fasting cohort by using a linear regression method 13. Achievement of steady state was confirmed if the individual P‐values of the slope were statistically nonsignificant (P > 0.05). The lack of day 10 samples in the fed cohort resulted in the availability of only two concentrations at day 14 (at predose and 24 h postdose), rendering linear regression impossible.
Descriptive statistics were used to summarize AEs, safety results and demographic variables.
Nomenclature of targets and ligands
Key ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 14.
Results
Eleven subjects completed the study. One subject in the fasting cohort discontinued prematurely owing to noncompliance after receiving three doses of the study drug, and was not considered for PK analysis on days 10, 14 and 28. A study flow chart is shown in Figure 1. The demographic characteristics of the study participants are displayed in Table 1. Doxycycline was well tolerated. In total, 48 AEs occurred during the course of the study, three of which were of moderate and 45 of mild severity. More than half (26) of all reported AEs comprised haematoma at the site of microdialysis probe insertion. Of these, 16 occurred in subjects in the fasting group (who had one additional microdialysis sampling day) and 10 in the fed group. No haematoma appeared on the day of microdialysis probe insertion but with a median (range) delay of 2 (1–10) days. The median (range) duration of documented haematomata was 7 (4–32) days in the fasting group and 10 (5–14) days in the fed group. In only one case, probe insertion occurred at a site with a haematoma still visible from a previous study day. In this case, the new probes were placed at a safe distance from the area concerned, and no perturbation of microdialysis probe function or d‐ISF concentrations compared with other measurements was noticed. No AE was judged to be caused by study drug administration.
Figure 1.
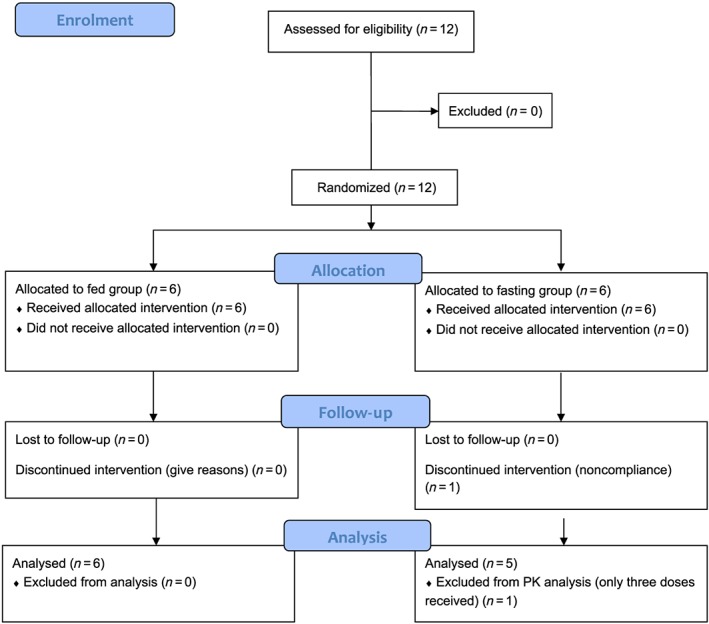
Study flow chart. PK, pharmacokinetic
Table 1.
Demographic data of enrolled subjects (mean ± standard deviation)
| Cohorts | Group A (fasting) | Group B (fed) |
|---|---|---|
| Sample size | n = 6 | n = 6 |
| Ethnicity | Caucasian | Caucasian |
| Age (years) | 28.00 ± 4.20 | 28.83 ± 1.47 |
| Height (cm) | 180.50 ± 6.25 | 184.67 ± 10.46 |
| Weight (kg) | 81.82 ± 14.32 | 87.53 ± 14.23 |
| BMI (kg m −2 ) | 24.98 ± 2.74 | 25.65 ± 3.29 |
BMI, body mass index
Single dose
Concentration–time profiles of doxycycline in the plasma and skin of HVs after administration of a single oral dose of DFD‐09 under fasting and fed conditions are displayed in Figure 2. The median time to Cmax was 4.8 h under fed conditions and 2.5 h under fasting conditions. The single‐dose PK of doxycycline are outlined in Table 2. After dosing with food, the mean Cmax and AUC0–24 were 11% and 14% lower, respectively, in plasma than under fasting conditions (Table 3). Analogous to plasma, dermal exposure to doxycycline was reduced by food intake. In the skin, the median Tmax under fed conditions was 7.0 h, compared with 5.0 h in fasting subjects. With concomitant food administration, the dermal Cmax and AUC0–24 of doxycycline decreased by 27% and 32%, respectively, compared with the fasting state (Table 3).
Figure 2.
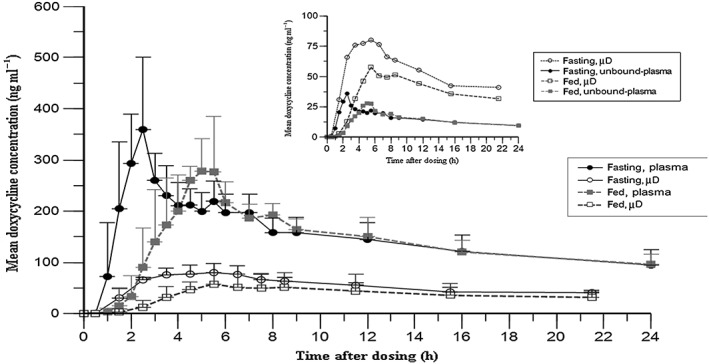
Single‐dose (day 1) doxycycline concentration–time profiles (mean ± standard deviation) in plasma and skin (measured using microdialysis (μD)). Inset: mean unbound doxycycline concentration–time profiles in plasma (calculated assuming 90% protein binding 21) and skin (measured using microdialysis (μD))
Table 2.
Doxycycline pharmacokinetic parameters (mean ± standard deviation) in healthy adults
| Day 1 | Day 14 | |||||||
|---|---|---|---|---|---|---|---|---|
| Tmax (h)b | Cmax (ng ml−1) | AUC0–24 (ng.h ml−1) | Tmax (h)b | Cmax (ng ml−1) | Cmin (ng ml−1) | AUC0–24 (ng.h ml−1) | Accumulation ratioa | |
| Plasma | ||||||||
| Fasting | 2.5 (1.5, 4.5) | 376 ± 127 | 3890 ± 1030 | 2.5 (2.0,2.5) | 483 ± 123 | 138 ± 31.6 | 5593 ± 871 | 1.59 ± 0.14 |
| Fed | 4.8 (3.0, 5.5) | 326 ± 80.4 | 3249 ± 370 | 4.3 (1.5,7.0) | 476 ± 142 | 156 ± 41.7 | 5673 ± 1545 | 1.72 ± 0.35 |
| Skin | ||||||||
| Fasting | 5.0 (3.5, 6.5) | 80.5 ± 18.7 | 1149 ± 196 | 3.5 (2.5,5.5) | 136 ± 34.6 | 64.7 ± 14.0 | 2182 ± 403 | 1.79 ± 0.28 |
| Fed | 7.0 (4.5, 8.5) | 62.4 ± 25.0 | 854 ± 402 | 5.5 (2.5,8.5) | 141 ± 52.4 | 66.8 ± 25.4 | 2172 ± 791 | 2.69 ± 0.69 |
AUC0–24, area under the curve from zero to 24 h; Cmax, peak concentration; Cmin, trough concentration; Tmax, time to peak concentration
Accumulation ratio = AUC0–24, day14/AUC0–24, day1
Median (minimum, maximum)
Table 3.
Comparative bioavailability statistics for doxycycline
| Plasma | |||||
|---|---|---|---|---|---|
| Parameters | Day 1 | Day 14 | |||
| Cmax | AUC0–24 | Cmax | Cmin | AUC0–24 | |
| Geo LSM fed/fasting (90% CI) | 0.89 (0.65, 1.23) | 0.86 (0.69, 1.06) | 0.97 (0.68, 1.37) | 1.10 (0.80, 1.53) | 0.98 (0.73, 1.33) |
| Skin | |||||
| Geo LSM fed/fasting (90% CI) | 0.73 (0.49, 1.08) | 0.68 (0.46, 1.01) | 1.01 (0.70, 1.46) | 0.97 (0.62, 1.50) | 0.94 (0.65, 1.38) |
AUC0–24, area under the curve from zero to 24 h; CI, confidence interval; Cmax, peak concentration; Cmin, trough concentration; Geo LSM, geometric least square means
The relative recovery of doxycycline, as determined by retrodialysis in fasting individuals, was between 35% and 39% across the days 1, 10, 14 and 28. Recovery values in fed subjects were 30% and 33% on days 1 and 14, respectively.
Repeated dose
Concentration–time profiles of doxycycline in the plasma and skin after 14 days of therapy under fasting and fed conditions are displayed in Figure 3. Here, the between‐cohort differences in plasma exposure observed after a single dose were no longer present. Mean plasma Cmax, AUC0–24 and Cmin were almost identical between the cohorts. In both the skin and plasma, the Tmax of doxycycline was delayed by about 2 h when administered with a high‐fat meal, compared with under fasting conditions (Table 2). Doxycycline concentrations in the skin of HVs at day 14 reflected the findings in the plasma, with Cmax, Cmin and AUC0–24 being comparable between the two cohorts (Table 3).
Figure 3.
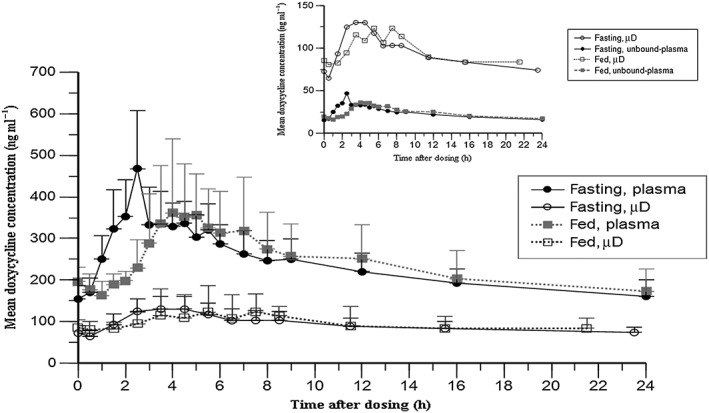
Steady‐state (day 14) doxycycline concentration‐time profiles (mean ± standard deviation) in plasma and skin (measured using microdialysis (μD)). Inset: mean unbound doxycycline concentration–time profiles in plasma (calculated assuming 90% protein binding 21) and skin (measured using microdialysis (μD))
Linear regression of predose and trough dialysate concentrations on days 10 and 14 from four of five subjects had slopes with individual P‐values >0.05, confirming the achievement of steady‐state levels of doxycycline in the skin of fasting subjects at day 14. In the absence of day 10 measurements, no such conclusion could be reached for the fed cohort.
The AR (calculated as AR = AUC0–24,day14/AUC0–24,day1) in the skin was 1.79 ± 0.28 and 2.69 ± 0.69 for fasting and fed cohorts, respectively. In the plasma, the AR was 1.59 ± 0.14 and 1.72 ± 0.35 for fasting and fed cohorts, respectively (Table 2). After single‐dose administration, the AUC0–24 was about 3–4‐fold higher in the plasma than for skin exposure, and this difference waned over time, with an observed 2.5‐fold higher plasma than dermal exposure at day 14. This was because of higher drug accumulation in the skin compared to plasma at day 14 (see Table 2).
There were no detectable doxycycline concentrations in plasma and microdialysate samples collected on day 28, indicating complete washout from plasma and healthy skin after 14 days of stopping the therapy.
Discussion
Dermal microdialysis has emerged as a vital tool to evaluate comparative bioavailability and for establishing PK–pharmacodynamic relationships for dermal drugs. Regulatory authorities have specifically recognized dermal microdialysis as one of the methods suitable for providing relevant information during drug development when blood concentrations are inappropriate 15, 16, 17. This was the first study assessing the influence of food on dermal exposures of doxycycline indicated for rosacea. Use of microdialysis for quantification of doxycycline from ORACEA® in healthy human skin has been reported before in a 7‐day multiple‐dose study 18. However, the latter work did not elucidate all elements of the PK of doxycycline. In that study, doxycycline showed higher accumulation in the skin (AR = 3) than the plasma at day 7. Although the study was not designed to achieve steady‐state concentrations, the dermal elimination half‐life of doxycycline estimated from the AR (AR = 1/(1–e–λ.τ, where λ is the elimination rate constant and τ is the dosing interval) was 43 h, or about 2 days 18. This elimination half‐life was considered for the determination of steady‐state sampling in the present study, in spite of the knowledge that this might not be the true terminal elimination half‐life, being derived from the AR of a drug (doxycycline) which follows biexponential decay 19, 20.
Assuming that steady state is achieved after five half‐lives, microdialysate sampling at day 10, and additionally at day 14, was considered adequate for the estimation of dermal steady state. Conventionally, steady state is estimated through comparison of trough concentrations from consecutive dosing intervals 13. However, sampling on consecutive days from 10 to 14 in the present study was not considered practical owing to the risk of probe insertions at multiple times for a parameter which was completely unknown. The basis of linear regression of predose and trough concentrations at days 10 and 14 was to present statistically the ʻflatnessʼ of the trough concentrations, which reflects approaching steady‐state levels. With similar predose (72.5 ± 17.7 ng ml−1) and trough (74.1 ± 11.5 ng ml−1) concentrations, steady‐state levels of doxycycline in the skin were confirmed at day 14. Given that the plasma elimination half‐life of doxycycline ranges between 15 and 25 h, steady‐state levels in the plasma might have been achieved much earlier than day 14 21.
We observed relatively higher dermal accumulation in the fed group than the fasting group, which might have been due to higher individual variabilities in the fed group, associated with the AR. The comparatively higher drug accumulation in the skin than plasma, irrespective of food intake status, might have been due to a complex process of drug disposition involving tissue diffusion, binding to collagen and tissue proteins, and lymphatic drainage 22, 23.
As unbound doxycycline is believed to be responsible for tissue penetration and action in rosacea, the unbound plasma concentrations of this agent have been estimated theoretically after correcting for plasma protein binding (about 90%) from both fed and unfed groups 21. Regardless of food intake status, doxycycline Cmax in the dermal microdialysate were found in the present study to be almost double the theoretical unbound plasma Cmax at day 1, and triple at day 14, demonstrating the considerable skin‐specific accumulation of doxycycline. However, protein binding in the plasma was not measured in the present study, limiting individual comparison of the unbound fraction in different compartments.
In the current study, after a single drug dose, food decreased Cmax by 11% and AUC by 14% in the plasma, compared with a reduction of 30% in Cmax and 10% in AUC found in the previous single‐dose crossover study with DFD‐09 10. The lower food effect in the present study might be attributed to the parallel study design, which might have prevented the true effect of food consumption on dermal and plasma exposures after a single dose to be observed. This study design was selected based on the low intersubject variability in doxycycline exposure (about 26%) found in the previous DFD‐09 study, and also because the dermal elimination half‐life of doxycycline, which determines the washout duration, was unknown (DFD‐09 clinical study reports: data on file) 18. Inclusion of steady‐state determination, as well as estimating the food effect on dermal exposures, in a crossover design could potentially have involved more blood loss, a higher frequency of visits to the clinic, multiple dermal probe insertions and a longer study duration. With the proposed study objectives, conducting the trial in a crossover design would have been challenging, particularly due to the unknown dermal elimination half‐life of doxycycline.
A previous dose–response analysis for doxycycline in rosacea demonstrated that clinical efficacy, in terms of reducing primary lesion counts, did not correlate with the dose, whereas plasma exposure (in terms of AUC) correlated significantly with the dose 24. This implies that leaner patients receiving a higher doxycycline dose (in terms of mg kg−1 bodyweight) could result in higher drug exposure but confer similar efficacy to that in heavier patients receiving a lower dose (in mg kg−1). Counterintuitively, these data indicate that lower doxycycline concentrations in the plasma or skin resulting from high‐fat food intake should not compromise the clinical efficacy. The data from the present study might help to explain this lack of correlation between plasma exposure and dose–response relationship. Although the therapeutic concentration of unbound dermal doxycycline responsible for clinical efficacy in rosacea is unknown, we were able to demonstrate that, at the target site (the skin), steady‐state doxycycline concentrations are comparable in fed and fasting conditions. This re‐emphasizes the need for target site determination of the PK of drugs, in order to understand their pharmacodynamic behaviour. The present exploratory study showed that free doxycycline accumulates to a considerable extent in the dermal interstitial fluid draining the skin, the actual site of drug action for rosacea, and is independent of the food effect. It also demonstrated that dermal interstitial fluid acts as a potential repository of doxycycline for drug action in the skin. The efficacy of this drug in rosacea may be due to the considerable accumulation of unbound doxycycline in the skin, demonstrated by higher exposure and a longer elimination half‐life, and was found to be independent of food intake.
Competing Interests
For the conduct of this study, the Medical University of Vienna received funding from Dr. Reddy's Laboratories Ltd.
The authors would like to thank research nurse Edith Lackner for her invaluable work in the preparation and conduct of this study. Martin Bauer, Stephan Carrera, Sabine Eberl, Alina Nussbaumer‐Pröll and Maria Weber (in alphabetical order) assisted in study drug administration. Daka Krishna Reddy is acknowledged for statistical support.
Contributors
A.P., P.M., A.G. and M.Z. planned the study. P.M., Z.O., B.W. and M.Z. performed the study. B.R. and T.S. performed the analysis. A.P., P.M., A.G. and M.Z. interpreted the data and drafted the manuscript. All authors were involved in critical revision of the manuscript, approved its final version and confirm the accuracy and integrity of the work.
Pal, A. , Matzneller, P. , Gautam, A. , Österreicher, Z. , Wulkersdorfer, B. , Reiter, B. , Stimpfl, T. , and Zeitlinger, M. (2018) Target site pharmacokinetics of doxycycline for rosacea in healthy volunteers is independent of the food effect. Br J Clin Pharmacol, 84: 2625–2633. 10.1111/bcp.13721.
References
- 1. ORACEA® . Oracea package insert. 2017. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/050805s002lbl.pdf (last accessed 13 September 2017).
- 2. Bender A, Zapolanski T, Watkins S, Khosraviani A, Seiffert K, Ding W, et al Tetracycline suppresses ATP gamma S‐induced CXCL8 and CXCL1 production by the human dermal microvascular endothelial cell‐1 (HMEC‐1) cell line and primary human dermal microvascular endothelial cells. Exp Dermatol 2008; 17: 752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kanada KN, Nakatsuji T, Gallo RL. Doxycycline indirectly inhibits proteolytic activation of tryptic kallikrein‐related peptidases and activation of cathelicidin. J Invest Dermatol 2012; 132: 1435–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berman B, Zell D. Subantimicrobial dose doxycycline: a unique treatment for rosacea. Cutis 2005; 75 (4 Suppl): 19–24. [PubMed] [Google Scholar]
- 5. Golub LM, McNamara TF, D'Angelo G, Greenwald RA, Ramamurthy NS. A non‐antibacterial chemically‐modified tetracycline inhibits mammalian collagenase activity. J Dent Res 1987; 66: 1310–1314. [DOI] [PubMed] [Google Scholar]
- 6. Evaluation of rosacea‐related inflammatory biochemical markers in adult skin when treated with Oracea® vs. placebo (NCT01308619). 2017. Available at https://clinicaltrials.gov/ct2/show/NCT01308619 (last accessed 7 August 2018).
- 7. Beringer PM, Owens H, Nguyen A, Benitez D, Rao A, D'Argenio DZ. Pharmacokinetics of doxycycline in adults with cystic fibrosis. Antimicrob Agents Chemother 2012; 56: 70–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Newton PN, Chaulet JF, Brockman A, Chierakul W, Dondorp A, Ruangveerayuth R, et al Pharmacokinetics of oral doxycycline during combination treatment of severe falciparum malaria. Antimicrob Agents Chemother 2005; 49: 1622–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Welling PG, Koch PA, Lau CC, Craig WA. Bioavailability of tetracycline and doxycycline in fasted and nonfasted subjects. Antimicrob Agents Chemother 1977; 11: 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chaurasia CS, Muller M, Bashaw ED, Benfeldt E, Bolinder J, Bullock R, et al AAPS‐FDA workshop white paper: microdialysis principles, application and regulatory perspectives. Pharm Res 2007; 24: 1014–1025. [DOI] [PubMed] [Google Scholar]
- 11. Muller M. Science, medicine, and the future: microdialysis. BMJ 2002; 324: 588–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. European Medicines Agency, Committee for Medicinal Products for Human Use . Guideline on bioanalytical method validation: EMEA/CHMP/EWP/192217/2009 rev. 1 Corr. 2. 2009. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/08/WC500109686.pdf (last accessed 7 August 2018).
- 13. Maganti L, Panebianco DL, Maes AL. Evaluation of methods for estimating time to steady state with examples from phase 1 studies. AAPS J 2008; 10: 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al The IUPHAR/BPS guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 2018; 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brunner M, Derendorf H. Clinical microdialysis: current applications and potential use in drug development. Trends Anal Chem 2006; 25: 674–680. [Google Scholar]
- 16. Holmgaard R, Nielsen JB, Benfeldt E. Microdialysis sampling for investigations of bioavailability and bioequivalence of topically administered drugs: current state and future perspectives. Skin Pharmacol Physiol 2010; 23: 225–243. [DOI] [PubMed] [Google Scholar]
- 17. Lionberger RA. FDA critical path initiatives: opportunities for generic drug development. AAPS J 2008; 10: 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matzneller P., Lackner E, Zeitlinger M. Intradermal accumulation of doxycycline could explain its anti‐inflammatory action on rosacea. Poster Presentation, Interscience Conference on Antimicrobial Agents and Chemotherapy, Boston, MA, 2016. Washington, DC, USA: American Society for Microbiology.
- 19. Hopkins AM, Wojciechowski J, Abuhelwa AY, Mudge S, Upton RN, Foster DJ. Population pharmacokinetic model of doxycycline plasma concentrations using pooled study data. Antimicrob Agents Chemother 2017; 61: e02401–e0240116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Toutain PL, Bousquet‐Melou A. Plasma terminal half‐life. J Vet Pharmacol Ther 2004; 27: 427–439. [DOI] [PubMed] [Google Scholar]
- 21. Saivin S, Houin G. Clinical pharmacokinetics of doxycycline and minocycline. Clin Pharmacokinet 1988; 15: 355–366. [DOI] [PubMed] [Google Scholar]
- 22. Dancik Y, Anissimov YG, Jepps OG, Roberts MS. Convective transport of highly plasma protein bound drugs facilitates direct penetration into deep tissues after topical application. Br J Clin Pharmacol 2012; 73: 564–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wiig H, Swartz MA. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol Rev 2012; 92: 1005–1060. [DOI] [PubMed] [Google Scholar]
- 24. Theobald K, Bradshaw M, Leyden J. Anti‐inflammatory dose doxycycline (40 mg controlled‐release) confers maximum anti‐inflammatory efficacy in rosacea. Skinmed 2007; 6: 221–226. [DOI] [PubMed] [Google Scholar]


