Abstract
An 87‐year‐old woman with a long‐standing history of hypertension, hypothyroidism and diabetes presented to us with scaly and pruritic vesicles of an erythematous base and crusted surface of 2‐month duration. They first appeared on her abdomen and gradually spread to her lower back, thighs, before spreading to her upper and lower limbs. Her lesions were non‐painful, aggravated by sun exposure only, and sparing mucous membranes. Nikolsky sign was positive with no discernible fluid‐filled bullae. History was remarkable only for a doubling of her Lisinopril dosage 2 months prior to the appearance of her lesions, with no other potential environmental and/or drug triggers recognizable on history taking. In light of the appearance of her lesions after her Lisinopril dose escalation, in the absence of any other discernible triggers, an adverse drug reaction (ADR) was entertained, yielding a corresponding Naranjo ADR probability score of 7. Particularly, drug‐induced pemphigus foliaceus was initially suspected given her clinical presentation and the morphology and distribution of her lesions. However, her skin biopsy altered our diagnosis to drug‐induced bullous pemphigoid (BP) instead, making this the second case reported to date on Lisinopril‐induced BP, and the first to report a dose–response variant of this adverse reaction.
Keywords: angiotensin II receptor, angiotensin‐converting enzyme (ACE), autoimmune, blisters, drug‐induced, pharmacovigilance
Introduction
Bullous pemphigoid (BP) is an autoimmune blistering skin disorder characterized by diffuse, erythematous and pruritic skin lesions that often begin as papular and/or urticarial, before transforming into deeper and tense vesico‐bullous eruptions 1, 2. The exact pathophysiology underlying the disorder remains incompletely understood. However, anti‐BP180 (BPAg2) and/or anti‐BP230 (BPAg1) autoantibody deposition within the dermal–epidermal junction constitutes hallmark histological and biochemical findings 1, 3. This immunoglobulin G (IgG)‐mediated disruption of the hemidesmosomes at the dermal–epidermal interface is what accounts for the tense and bullous nature of BP 1, 3. BP primarily affects elderly patients beyond 70 years of age, with a median age of onset of 80 years and an arguably higher incidence in women compared to men 1, 2.
BP is generally classified based on its etiology into idiopathic versus drug induced 4, 5, with loop diuretics such as furosemide being classical culprit drugs of the latter form of the disease 1, 5. Likewise, non‐steroidal anti‐inflammatory drugs (NSAIDs), antipsychotics and a few http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1613 inhibitors (ACEIs), primarily Captopril, Ramipril and Enalapril, have also been reported to induce BP 1, 5, 6, 7, 8, 9. Alternatively, various infectious and/or environmental factors such as HIV infection and radiation exposure respectively have also been associated with BP development 3.
However, to our best knowledge, there has been only one report to date associating Lisinopril use with BP development 10.
Thus, the following case is the second reported to date on Lisinopril‐associated BP 10, with the exception that, unlike the previously reported case where the patient was Lisinopril‐naïve and developed BP after newly starting Lisinopril, our patient was already maintained on 20 mg of daily Lisinopril for several years, and only developed her BP lesions when her dose was escalated to 40 mg. Therefore, her case represents a dose–response variant of the previously described all‐or‐none nature of Lisinopril‐associated BP.
Case presentation
An 87‐year‐old Lebanese woman with a long‐standing history of hypertension, hypothyroidism and type 2 diabetes mellitus presented to us with new‐onset skin lesions of 2‐month duration. The lesions appeared first on her trunk and gradually spread to her lower back, and posteromedial thighs, before spreading to her shoulders and dorsal aspects of her upper and lower extremities (Figure 1). Her lesions were non‐painful, but severely itchy and aggravated by sun exposure.
Figure 1.
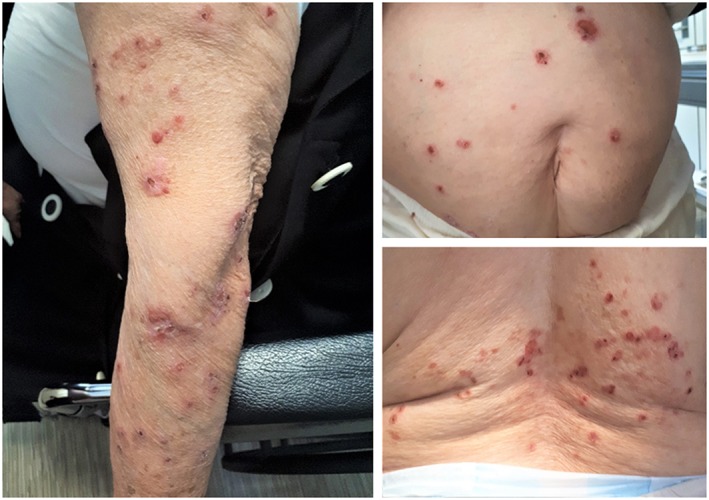
Scaly and eroded vesicles with superficial crusts and an erythematous base on the patient's trunk (top right), lower back (bottom right), and upper extremity (left)
On presentation, she was afebrile, in no apparent distress, and weighed 82 kg. Her physical examination showed disseminated eroded vesicles of 4–6 mm diameter, with a few discrete and sharply demarcated non‐blanching reddish‐to‐purple plaques of 2–3 cm diameter. Her vesicles were non‐tender to palpation and spared mucous membranes. Nikolsky sign was positive with no bullae or blisters discernible at the time. Patient history was negative for any allergies, previous drug reactions, NSAID or antibiotic use for the past 6 months, history of radiation exposure, immunodeficient status or any recent travel or sick contacts.
Patient has been managed only by thyroid‐replacement therapy with 100 ug of levothyroxine daily for her hypothyroidism ever since diagnosis. She was also taking 50 mg of vildagliptin with a 2.5 mg/400 mg glyburide‐metformin combination for her diabetes, and a 12.5 mg/20 mg hydrochlorothiazide‐Lisinopril combination for her hypertension. She had been well maintained on all those medications for over 7 years at her time of presentation to us, with no prior adverse reactions to them.
However, recent medical history was remarkable for a hospital admission due to syncope 2 months prior to presenting to us, during which she reported having no skin lesions whatsoever while being maintained on the above medications only. On discharge from that admission however, her admitting physician added a 20 mg Lisinopril tablet to her medication regimen for better blood pressure control without changing any of her other medications. The patient's son who is her primary caregiver confirms his mother's compliance with all prescribed medications, including her recently escalated dose of Lisinopril.
Thus, given the temporal association between the patient's isolated Lisinopril dose escalation and her skin eruptions 2 months later, a drug‐induced adverse reaction was entertained. Particularly, drug‐induced pemphigus foliaceus (PF), another autoimmune blistering skin disorder, was highly suspected, given its previous reporting twice within the literature in association with Lisinopril use 11, 12, along with the flaccid, eroded and crusted nature of our patient's skin lesions and their mucous‐sparing distribution 13.
Complete blood count (CBC), electrolytes, liver enzymes, thyroid‐stimulating hormone (TSH) level and erythrocyte sedimentation rate (ESR) were all ordered and found to be normal (i.e. within reference range). We therefore took a skin biopsy for definitive diagnosis, which showed diffuse IgG and C3 deposition at the dermo‐epidermal junction upon direct immunofluorescence, with superficial perivascular and interstitial lympho‐eosinophilic infiltrates on histology. Such a finding is actually consistent with a diagnosis of drug‐induced BP, rather than PF as we initially suspected. Thus, we then used the Naranjo adverse drug reaction (ADR) probability scale 14 to assess the likelihood of our patient's BP as being an ADR to her increased Lisinopril dosage, only to attain a total score of 7 which is suggestive of a ‘probable ADR’ (Figure 2).
Figure 2.
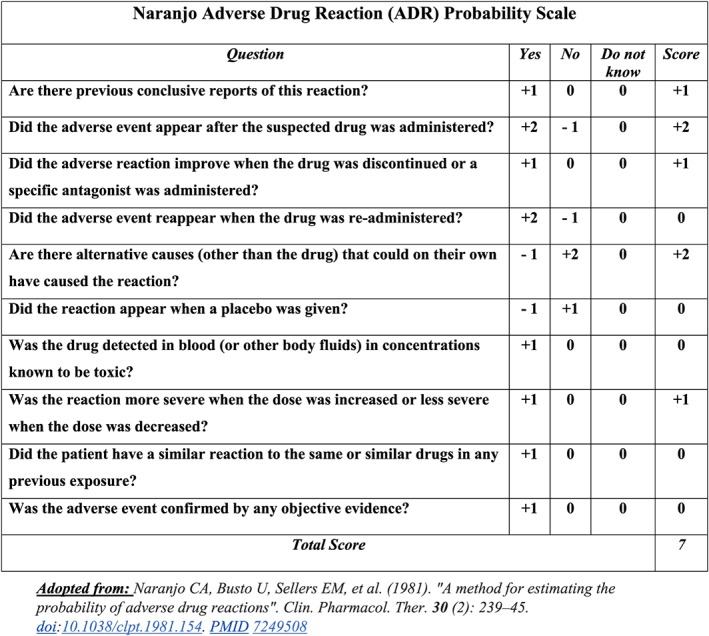
Naranjo ADR probability assessment score for the likelihood of our patient's case being an ADR to Lisinopril
Consequently, we scheduled a follow‐up visit for our patient to communicate our findings to her, only to discover that she now has intra‐oral involvement of her lesions, a finding more consistent with BP compared to PF, given the latter's notable mucosal membrane‐sparing characteristic 15. We thus discontinued her two Lisinopril‐containing antihypertensive medications, replacing them with a calcium channel blocker (CCB) instead, and prescribed a high‐dose topical steroid for application twice daily on her lesions. Two weeks later during her scheduled follow‐up, we find that most of her lesions have crusted and coalesced, indicating that they started to resolve with no new lesions being noted elsewhere. The patient was thus maintained on the same steroid regimen for another 6 weeks, after which most of her lesions had completely healed without scarring. We then sought her written informed consent to have her case reported.
Discussion
The uniqueness of our case lies in the dose‐dependent nature of our patient's Lisinopril‐induced BP in contrast to its all‐or‐none nature in the previously reported case 10.
Despite the rarity of this ADR, Patsatsi et al. highlighted an association between Lisinopril use and BP development in their case series as well, when they discovered that one of their patients diagnosed with BP had also been taking Lisinopril in retrospect 16. Interestingly, BP has also been twice reported as a possible adverse reaction to the functionally‐related class of medications, i.e., http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=6 blockers, namely, Losartan and Valsartan 17, 18.
Two mechanisms have been suggested to date on how ACEIs may induce BP: first, through activating and/or potentiating the pro‐inflammatory kinin system via inhibiting the system's inactivating angiotensin‐converting enzyme (ACE) 19, 20 and second, through their hapten‐like properties of binding to and modifying lamina lucida proteins, thereby triggering the production of autoantibodies against those ‘neo‐antigens’ 16. However, the latter mechanism has been previously often more accredited, owing to Lisinopril's intrinsic amide group which has been shown to exhibit acantholysis‐triggering properties in vitro 11. This may potentiate BP development in already predisposed individuals such as elderly patients, who undergo a dose escalation of their Lisinopril, such as the case of our patient. This however, remains only a hypothetical model and a subject of future research.
Finally, while pemphigus disorders such as PF are listed as possible adverse reactions to Lisinopril within the medication's leaflet, pemphigoid disorders such as BP are not 21. Similarly, an international database reviewing all FDA reports on ADRs reported a 0.02% incidence of pemphigus disorders in patients taking Lisinopril (as of October 2017) 22, compared to not providing any estimates for pemphigoid disorders in those patients (as of November 2017), due to a ‘lack of reports’ 23. This therefore highlights the need to enlist pemphigoid disorders, such as BP, as rare yet possible ADRs of Lisinopril, as previously suggested 10.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 24, and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 25, 26.
Competing Interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Contributors
R.B. was the fourth‐year medical student interviewing the patient with U.M. during her first presentation. R.B. conducted a thorough physical examination of the patient, which was subsequently replicated by U.M. for validation of preliminary findings. J.K. advised R.B. and U.M. on how to proceed with the dermatological workup of the patient and obtained a skin biopsy from her. R.B. searched the medical literature for similar reports as our patient's manifestations and reviewed the literature on blistering disorders, relaying his findings to U.M. and J.K. R.B. and U.M. saw the patient during her second visit and followed up with her after 6 weeks. R.B. sought the patient's written informed consent and drafted the preliminary draft of the entire manuscript which was subsequently revised and validated by U.M. and J.K. All authors reviewed and approved the final draft.
Ballout, R. A. , Musharrafieh, U. , and Khattar, J. (2018) Lisinopril‐associated bullous pemphigoid in an elderly woman: a case report of a rare adverse drug reaction. Br J Clin Pharmacol, 84: 2678–2682. 10.1111/bcp.13737.
References
- 1. Bernard P, Antonicelli F. Bullous pemphigoid: a review of its diagnosis, associations and treatment. Am J Clin Dermatol 2017; 18: 513–528. [DOI] [PubMed] [Google Scholar]
- 2. Langan SM, Smeeth L, Hubbard R, Fleming KM, Smith CJ, West J. Bullous pemphigoid and pemphigus vulgaris – incidence and mortality in the UK: population based cohort study. BMJ 2008; 337: a180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lo Schiavo A, Ruocco E, Brancaccio G, Caccavale S, Ruocco V, Wolf R. Bullous pemphigoid: etiology, pathogenesis, and inducing factors: facts and controversies. Clin Dermatol 2013; 31: 391–399. [DOI] [PubMed] [Google Scholar]
- 4. Brenner S, Goldberg I. Drug‐induced pemphigus. Clin Dermatol 2011; 29: 455–457. [DOI] [PubMed] [Google Scholar]
- 5. Stavropoulos PG, Soura E, Antoniou C. Drug‐induced pemphigoid: a review of the literature. J Eur Acad Dermatol Venereol 2014; 28: 1133–1140. [DOI] [PubMed] [Google Scholar]
- 6. Mallet L, Cooper JW, Thomas J. Bullous pemphigoid associated with captopril. DICP 1989; 23: 63. [DOI] [PubMed] [Google Scholar]
- 7. Smith EP, Taylor TB, Meyer LJ, Zone JJ. Antigen identification in drug‐induced bullous pemphigoid. J Am Acad Dermatol 1993; 29 (5 Pt 2): 879–882. [DOI] [PubMed] [Google Scholar]
- 8. Zaballos P, Morales AL, Rodero J, Lafuente F, Grasa MP, Martin J, et al Bullous pemphigoid associated with captopril. Med Cutan Ibero Lat Am 2002; 30: 100–102. [Google Scholar]
- 9. Agrawal SS P, Yadav SC, Singh AK. Ramipril‐induced bullous drug eruptions. J Indian Acad Clin Med 2013; 14: 181–183. [Google Scholar]
- 10. Kalinska‐Bienias A, Rogozinski TT, Wozniak K, Kowalewski C. Can pemphigoid be provoked by lisinopril? Br J Dermatol 2006; 155: 854–855. [DOI] [PubMed] [Google Scholar]
- 11. Dobrosavljević Vukojević D, Stojković Filipović J, Sjerobabin M, Vuković J, Vesić S. Lisinopril‐induced pemphigus foliaceus in a patient with diabetes mellitus and Kaposi‐Juliusberg varicelliform eruption. Serb J Derm Venereol 2012; 4: 153–162. [Google Scholar]
- 12. Patterson CR, Davies MG. Pemphigus foliaceus: an adverse reaction to lisinopril. J Dermatolog Treat 2004; 15: 60–62. [DOI] [PubMed] [Google Scholar]
- 13. James KA, Culton DA, Diaz LA. Diagnosis and clinical features of pemphigus foliaceus. Dermatol Clin 2011; 29: 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30: 239–245. [DOI] [PubMed] [Google Scholar]
- 15. Fonseca LAF, Alves C, Aprahamian I, Pinto CAL. Pemphigus foliaceus as a differential diagnosis in vesicobullous lesions. Einstein (Sao Paulo) 2017; 15: 220–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patsatsi A, Vyzantiadis TA, Chrysomallis F, Devliotou‐Panagiotidou D, Sotiriadis D. Medication history of a series of patients with bullous pemphigoid from northern Greece – observations and discussion. Int J Dermatol 2009; 48: 132–135. [DOI] [PubMed] [Google Scholar]
- 17. Femiano F. Mucocutaneous bullous pemphigoid induced by valsartan. A clinical case. Minerva Stomatol 2003; 52: 187–190. [PubMed] [Google Scholar]
- 18. Saraceno R, Citarella L, Spallone G, Chimenti S. A biological approach in a patient with psoriasis and bullous pemphigoid associated with losartan therapy. Clin Exp Dermatol 2008; 33: 154–155. [DOI] [PubMed] [Google Scholar]
- 19. Campbell DJ. Angiotensin converting enzyme (ACE) inhibitors and kinin metabolism: evidence that ACE inhibitors may inhibit a kininase other than ACE. Clin Exp Pharmacol Physiol 1995; 22: 903–911. [DOI] [PubMed] [Google Scholar]
- 20. Ruocco V, Satriano RA, Guerrera V. “Two‐step” pemphigus induction by ace‐inhibitors. Int J Dermatol 1992; 31: 33–36. [DOI] [PubMed] [Google Scholar]
- 21. ZESTRIL® (lisinopril) Tablets In: FDA, editor.: AstraZeneca Pharmaceuticals LP Wilmington, DE 19850.
- 22. Information ePH. Lisinopril and Pemphigus – from FDA reports. In: FDA, editor. eHeathMe; 2017. Available at https://www.ehealthme.com/ds/lisinopril/pemphigus/ (last accessed 13 August 2018).
- 23. Information ePH. Lisinopril and Bullous pemphigoid – from FDA reports. In: FDA, editor. eHeathMe; 2017. Available at https://www.ehealthme.com/ds/lisinopril/bullous-pemphigoid/ (last accessed 13 August 2018).
- 24. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 2018; 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E, et al The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 2017; 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA, et al The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 2017; 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]


