Abstract
Aims
Prescribing is a complex skill required of doctors and, increasingly, other healthcare professionals. Use of a personal formulary can help to develop this skill. In 2006–9, we developed a core list of the 100 most commonly prescribed drugs. Our aim in the present study was to update this ‘starter formulary’ to ensure its continued relevance for prescriber training.
Methods
We analysed large contemporary primary and secondary care datasets to identify the most frequently prescribed medicinal products. Items were classified into natural groups, broadly following their British National Formulary classification. The resulting drug groups were included in the core list if they comprised ≥0.1% prescriptions in both settings or ≥0.2–0.3% prescriptions in one setting. Drugs from emergency guidelines that did not qualify by prescribing frequency completed the list.
Results
Over 1 billion primary care items and approximately 1.8 million secondary care prescriptions were analysed. The updated list comprises 81 drug groups commonly prescribed in both settings; six from primary care; seven from secondary care; and six from emergency guidelines. Eighty‐eight per cent of the formulary was unchanged. Notable changes include entry of newer anti‐epileptics and dipeptidyl peptidase‐4 inhibitors and exit of phenytoin and thiazolidinediones.
Conclusions
The relative stability of the core drug list over 9 years and the current update ensure that learning based on this list remains relevant to practice. Trainee prescribers may be encouraged to use this ‘starter formulary’ to develop a sound basis of prescribing knowledge and skills that they can subsequently apply more widely.
Keywords: general medicine, medical education, pharmacoepidemiology
What is Already Known about this Subject
Prescribing is a complex skill, acquisition of which can be facilitated by use of a personal formulary.
In 2006–9, we developed a ‘starter formulary’ of the 100 drugs most commonly prescribed in the UK.
This drug list remained stable over 2 years and was consistent with practice of new prescribers.
What this Study Adds
We used primary and secondary prescribing data from 2015 to update the ‘starter formulary’.
Most drugs in the list remain the same, with 12 differences attributable to changes in practice, disease prevalence and methodology.
The list is intended not to stifle trainees' inquisitiveness, but to provide an evidence‐based starting point from which they can build their prescribing knowledge and skills.
Introduction
In Outcomes for Graduates, the General Medical Council emphasizes the safe, effective and economical prescription of drugs as a core skill for all new UK medical graduates 1. The importance of prescribing skills is further emphasized by the UK Prescribing Safety Assessment, which all new doctors must pass as a requirement of the Foundation Programme 2, 3. Prescribing is a complex, multi‐step process that includes defining the clinical problem and therapeutic objectives; identifying a suitable treatment; starting the treatment; giving appropriate information; and monitoring treatment success 4. The challenge faced by trainee prescribers in acquiring this skill is compounded by the large number of drugs available. For example, in the UK, 1603 drugs and 18 408 preparations are licensed for prescription (personal communication, British National Formulary editorial team, October 2017).
To facilitate development and maintenance of prescribing competence, the World Health Organization (WHO) recommends that prescribers develop a list of ‘P’ drugs – a personal formulary of drugs that they prescribe regularly and can become familiar with 4. This is difficult for undergraduate medical students who are not yet prescribing and who may see diverse practice as they rotate through healthcare settings and specialties. De Vries and colleagues found that provision of any formulary, whether learner or teacher‐led, helped students to improve their prescribing skills 5. In 2011, we therefore developed a ‘starter formulary’ of the 100 drugs most commonly prescribed in the UK from analysis of primary and secondary care prescribing data 6. This helped students to focus their initial learning on drugs they would actually prescribe in practice and supported educators in developing learning resources and assessments 7.
Our original list was developed from analysis of primary and secondary care prescribing data from 2006 to 2009. Over the last 5–10 years, there have been significant therapeutic advances, including the advent of direct oral anticoagulants and dipeptidyl peptidase 4 inhibitors. The aim of this study was to update the starter formulary by identifying the drugs most commonly prescribed in primary and secondary care in 2015, thereby supporting relevant modern‐day learning for new prescribers.
Methods
Overview
NHS prescription cost analysis (PCA) data was used to identify all items dispensed in the community in England in 2015 8. Electronic prescription records were used to identify all items prescribed in the University Hospital Birmingham NHS Foundation Trust in 2015. Medicinal products identified in each healthcare setting were formed into natural groups, guided by their classification in the British National Formulary (BNF) 9. The most commonly prescribed drug groups in both or either setting were combined with drugs identified from emergency guidelines to generate the final core drug list.
Study approvals
This study did not require ethical approval as it was based wholly on aggregate data, with no linkage to patient‐level data.
Data collection
Primary care
NHS PCA data for England 2015 was obtained. This is based on information obtained from prescriptions sent to the Prescription Pricing Division of the NHS Business Services Authority. All prescriptions dispensed in the community are included, the majority of which are written by general practitioners. Analysis was based on the frequency with which each medicinal product was dispensed.
Secondary care
A list of all items prescribed in University Hospital Birmingham NHS Foundation Trust in 2015 was obtained from their electronic prescribing system. Analysis was based on the frequency of medicinal product prescription.
Emergency drugs
A review of hospital guidelines generated a list of all emergency drugs used in hospital emergency settings 10.
Compiling the core list
In accordance with a prospectively defined analysis plan, the PCA dataset was cleaned to remove items that fell outside the definition of a medicinal product 11 (e.g. sunscreens, camouflages, appliances and nutritional supplements). We also removed intravenous fluid preparations and vaccines because, although they fall within the definition of medicinal products, we judged that they represent educationally distinct groups. Finally, we planned to apply clinical–educational judgement to remove drugs used in highly specialized practice that fell outside the scope of a core drug list for trainee prescribers.
The PCA data was used to develop natural drug groups. Medicinal products were first classified by BNF sub‐paragraph. The products within each sub‐paragraph were then classified by chemical name to identify and separate individual drug classes. Where several chemical entities fell naturally into a drug class, this was used as a group for analysis purposes. Conversely, where a chemical entity fell into a class of its own, it was named and analysed as such. For example, the BNF sub‐paragraph ‘Lipid‐regulating drugs’ was separated into statins, fibrates and ezetimibe. In a few cases, e.g. ‘Nicotine replacement and related drugs’, the BNF sub‐paragraph was retained as the basis for the drug group. Where necessary, clinical judgement was applied to ensure groupings were natural and clinically applicable. The drug groups developed from the PCA data were then used to sort drugs in the secondary care data.
Compound products were not included as distinct items if their constituent ingredients were already captured in the top 100 list. Where different members of drug classes were used for more than one indication, the drug class was included only once (e.g. H1 receptor antagonists for nausea and allergy) and the frequencies summed.
Prescribing frequency
For the PCA data, the number of items dispensed for all medicinal products within each drug group was summed and expressed as a percentage of the total number of items dispensed.
For the secondary care data, the number of prescriptions written for all medicinal products within each drug group was summed and expressed as a percentage of the total number of prescriptions.
Generating the top 100 drug list
Prior to the analysis, it was decided that the list would contain 100 drug groups as a number that was educationally attractive, sufficient to cover most prescribing by foundation doctors 6 and limited enough to be considered core.
Drug groups qualified for the top 100 list if they comprised ≥0.1% prescriptions in both primary and secondary care; ≥0.2% prescriptions in primary care but <0.1% prescriptions in secondary care; or ≥0.3% prescriptions in secondary care but <0.1% prescriptions in primary care. These definitions were chosen to optimize inclusion of drugs that were widely prescribed across healthcare systems and to reduce the inclusion of more specialist drugs, e.g. those with high use by a single specialist team in secondary care but not commonly prescribed by non‐specialist doctors. As the number of drug groups meeting these criteria exceeded 100, clinical and educational judgement was used to review the less commonly prescribed drugs on these lists, selecting those considered to be prescribed by generalists over those requiring more specialist expertise. In addition, drugs from emergency guidelines that did not qualify by prescribing frequency but were considered to be clinically important were identified and room was made for them on the list by removing more specialist drugs.
Comparison of methodology between 2006–9 and 2015
Prescription cost analysis data was used to analyse items dispensed in the community in both 2006–9 and 2015 using broadly similar approaches. Minor changes in 2015 included a pre‐planned decision to exclude intravenous fluids and vaccines from the analysis and to exclude combination products (e.g. analgesia, inhalers) from the final list where the constituent drugs were already included.
The main difference between studies was in the methods used to obtain the secondary care data. In 2006–9, a manual audit of paper drug charts of inpatients in two London hospitals was used to identify 7705 individual prescriptions. In 2015, a list of all (2.129 million) items prescribed that year in a single large teaching hospital was obtained from their electronic prescribing system. The 2015 secondary care data gives a much more comprehensive picture of secondary care prescribing, albeit from a single hospital with some distinct tertiary practice.
Results
The PCA 2015 dataset comprised 1.037 billion dispensed items, of which 24.775 million items were ineligible for inclusion (Figure 1). The Birmingham hospital data set comprised 2.129 million prescriptions, of which 360 000 prescriptions were ineligible for inclusion. The primary and secondary care analysis datasets therefore comprised 1.013 billion items dispensed and 1.779 million prescriptions respectively.
Figure 1.
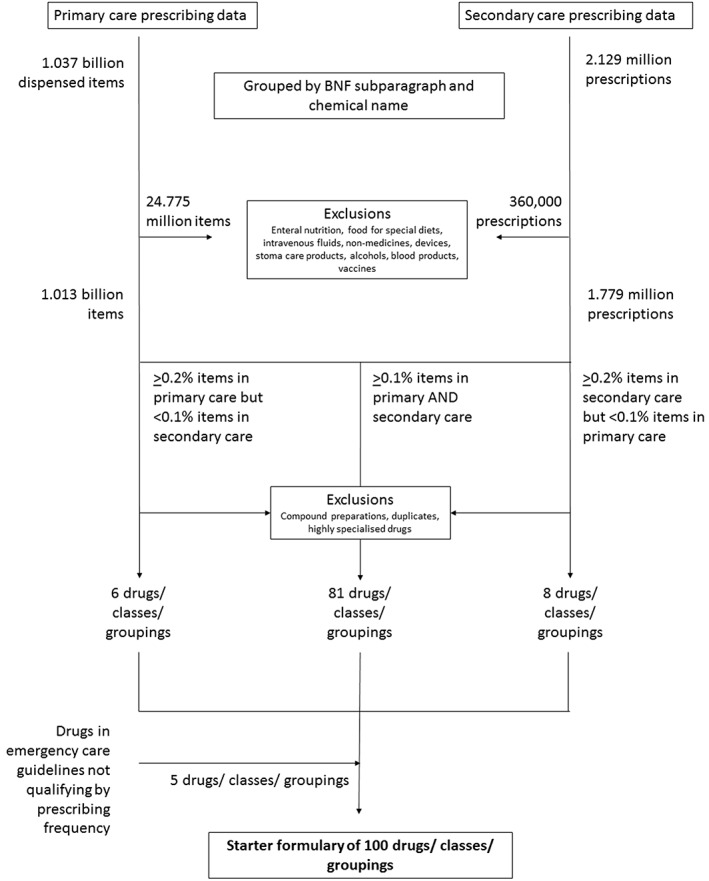
Flow diagram showing acquisition, exclusion and processing of prescribing data from primary and secondary care and emergency guidelines to produce the core drug list
Core drug list
Eighty‐one drug groups that made up ≥0.1% items dispensed in primary care and prescriptions in secondary care comprised the majority of the list (Table 1). Two drugs that met these criteria (nicorandil, 0.1% hospital prescriptions, 0.3% primary care items; hydroxychloroquine 0.1% hospital prescriptions, 0.1% primary care items) were considered more for specialist than generalist use and therefore not included in the final list.
Table 1.
Drugs, classes and BNF groupings comprising ≥0.1% of both primary and hospital prescriptions
| Overall rank | Drug, class or BNF grouping | Most commonly prescribed example(s) | Hosp. rank | PCA rank | Hosp. (%) | PCA (%) |
|---|---|---|---|---|---|---|
| 1 | Proton pump inhibitors | omeprazole, lansoprazole | 3 | 2 | 3.0% | 5.5% |
| 2 | Statins | simvastatin, atorvastatin, pravastatin | 9 | 1 | 2.3% | 6.5% |
| 3 | Paracetamol | 1 | 11 | 6.2% | 2.3% | |
| 4 | Beta‐blockers | bisoprolol, atenolol, propranolol | 17 | 5 | 1.8% | 3.6% |
| 5 | Calcium and vitamin D | 11 | 12 | 2.1% | 2.1% | |
| 6 | Calcium‐channel blockers | amlodipine, felodipine, diltiazem, nifedipine, lercanidipine | 21 | 4 | 1.8% | 3.7% |
| 7 | H1 receptor antagonists | cyclizine, cetirizine, loratadine, fexofenadine, chlorphenamine | 6 | 19 | 2.7% | 1.6% |
| 8 | Aspirin | 18 | 8 | 1.8% | 2.8% | |
| 9 | Opioids: weak/moderate | tramadol, codeine, dihydrocodeine | 5 | 21 | 2.8% | 1.4% |
| 10 | Opioids: strong | morphine | 2 | 27 | 5.2% | 1.2% |
| 11 | Beta2 agonists | salbutamol, salmeterol | 22 | 10 | 1.5% | 2.3% |
| 12 | Angiotensin‐converting enzyme inhibitors | ramipril, lisinopril, perindopril | 30 | 3 | 1.1% | 4.3% |
| 13 | Diuretics, loop | furosemide, bumetanide | 12 | 22 | 2.1% | 1.4% |
| 14 | Vitamin K antagonists | warfarin | 6 | 28 | 2.5% | 1.1% |
| 15 | Vitamins | folic acid, thiamine hydrochloride, vitamin B group | 16 | 20 | 1.8% | 1.5% |
| 16 | Non‐steroidal anti‐inflammatory drugs | naproxen, ibuprofen | 28 | 13 | 1.1% | 2.1% |
| 17 | Penicillins, broad spectrum | amoxicillin, co‐amoxiclav | 19 | 24 | 1.8% | 1.4% |
| 18 | Laxatives ‐ osmotic | macrogol, lactulose | 13 | 33 | 2.1% | 0.9% |
| 19 | Anti‐depressants, selective serotonin re‐uptake inhibitors | citalopram, sertraline, fluoxetine | 42 | 6 | 0.7% | 3.2% |
| 20 | Corticosteroids, systemic | prednisolone | 10 | 38 | 2.1% | 0.8% |
| 21 | Laxatives, stimulant | senna, docusate sodium | 7 | 41 | 2.5% | 0.7% |
| 22 | Corticosteroids, inhaled | beclometasone, fluticasone, budesonide | 39 | 14 | 0.8% | 2.0% |
| 23 | Thyroid hormones | levothyroxine | 50 | 7 | 0.6% | 2.9% |
| 24 | Benzodiazepines | diazepam, temazepam, lorazepam | 26 | 32 | 1.2% | 1.0% |
| 25 | Alpha‐adrenoceptor blocking drugs | doxazosin, tamsulosin | 34 | 25 | 0.8% | 1.3% |
| 26 | Biguanides | metformin | 45 | 15 | 0.7% | 1.9% |
| 27 | Insulin | 24 | 43 | 1.3% | 0.7% | |
| 28 | Angiotensin‐II receptor antagonists | losartan, candesartan, irbesartan | 54 | 16 | 0.5% | 1.8% |
| 29 | Corticosteroids, topical | hydrocortisone | 63 | 9 | 0.4% | 2.4% |
| 30 | Gabapentin and pregabalin | 43 | 29 | 0.7% | 1.0% | |
| 31 | Anti‐depressants, tricyclic and related drugs | amitriptyline | 56 | 19 | 0.4% | 1.6% |
| 32 | Anti‐platelet drugs | clopidogrel | 41 | 34 | 0.7% | 0.9% |
| 33 | Anti‐fungal drugs | clotrimazole, ketononazole | 31 | 45 | 1.0% | 0.6% |
| 34 | Histamine (H2)‐receptor antagonists | ranitidine | 25 | 51 | 1.3% | 0.5% |
| 35 | Diuretics, thiazide and thiazide‐like | bendroflumethiazide, indapamide | 65 | 18 | 0.3% | 1.7% |
| 36 | Emollients | 58 | 31 | 0.4% | 1.0% | |
| 37 | Nitrates | isosorbide mononitrate, glyceryl trinitrate | 48 | 42 | 0.6% | 0.7% |
| 38 | Trimethoprim | 35 | 55 | 0.8% | 0.4% | |
| 39 | Iron | ferrous fumarate, ferrous sulfate | 51 | 40 | 0.6% | 0.7% |
| 40 | Bisphosphonates | alendronic acid | 57 | 36 | 0.4% | 0.8% |
| 41 | Penicillins, penicillinase‐resistant | flucloxacillin | 46 | 54 | 0.6% | 0.4% |
| 42 | Sulfonylureas | gliclazide | 67 | 35 | 0.3% | 0.8% |
| 43 | Macrolides | clarithromycin | 53 | 49 | 0.5% | 0.5% |
| 44 | Gout and hyperuricaemia | allopurinol | 60 | 48 | 0.4% | 0.5% |
| 45 | Alginates and antacids | 59 | 50 | 0.4% | 0.5% | |
| 46 | Anti‐depressant drugs, other | venlafaxine, mirtazapine | 80 | 30 | 0.2% | 1.0% |
| 47 | Z drugs | zopiclone | 66 | 46 | 0.3% | 0.6% |
| 48 | Ocular lubricants (artificial tears) | hypromellose | 75 | 39 | 0.3% | 0.8% |
| 49 | Anti‐emetics, dopamine (D2)‐receptor antagonists | metoclopramide, domperidone | 27 | 88 | 1.2% | 0.2% |
| 50 | Anti‐muscarinics, cardiovascular and gastrointestinal uses | atropine, hyoscine butylbromide | 52 | 64 | 0.1% | 0.5% |
| 51 | Anti‐psychotics: 2nd generation | quetiapine, olanzapine, risperidone | 81 | 37 | 0.2% | 0.8% |
| 52 | Anti‐muscarinics, bronchodilators | tiotropium, ipratropium bromide | 73 | 47 | 0.3% | 0.6% |
| 53 | Cardiac glycosides | digoxin | 61 | 61 | 0.4% | 0.3% |
| 54 | Methotrexate | 44 | 79 | 0.7% | 0.2% | |
| 55 | Anti‐muscarinics, genitourinary uses | solifenacin, tolterodine, oxybutynin | 92 | 44 | 0.2% | 0.6% |
| 56 | Anti‐proliferative immunosuppressants | azathioprine | 32 | 104 | 1.0% | 0.1% |
| 57 | Tetracyclines | doxycycline | 90 | 52 | 0.2% | 0.4% |
| 58 | Aldosterone antagonists | spironolactone | 76 | 66 | 0.3% | 0.3% |
| 59 | Metronidazole | 64 | 81 | 0.4% | 0.2% | |
| 60 | Dipeptidyl peptidase‐4 inhibitors | sitagliptin, linagliptin | 95 | 57 | 0.2% | 0.4% |
| 61 | Anti‐motility drugs | loperamide | 68 | 84 | 0.3% | 0.2% |
| 62 | Quinine sulfate | 97 | 56 | 0.2% | 0.4% | |
| 63 | Dopaminergic drugs used in parkinsonism | co‐careldopa (carbidopa/levodopa) | 99 | 58 | 0.2% | 0.4% |
| 64 | Lamotrigine | 101 | 59 | 0.2% | 0.4% | |
| 65 | Direct oral anticoagulants | rivaroxaban, apixaban, dabigatran | 94 | 69 | 0.2% | 0.3% |
| 66 | Anti‐psychotics: 1st generation | haloperidol | 69 | 94 | 0.3% | 0.1% |
| 67 | Mucolytics | carbocisteine | 81 | 78 | 0.2% | 0.2% |
| 68 | Levetiracetam | 74 | 90 | 0.3% | 0.2% | |
| 69 | Prostaglandin analogues | latanoprost | 112 | 53 | 0.1% | 0.4% |
| 70 | Penicillin | benzylpenicillin, phenoxymethylpenicillin | 93 | 75 | 0.2% | 0.2% |
| 71 | Valproate | 107 | 63 | 0.1% | 0.3% | |
| 72 | 5α‐reductase inhibitors | finasteride | 109 | 62 | 0.1% | 0.3% |
| 73 | Chloramphenicol | 115 | 65 | 0.1% | 0.3% | |
| 74 | Aminosalicylates | mesalazine | 103 | 77 | 0.1% | 0.2% |
| 75 | Nitrofurantoin | 113 | 73 | 0.1% | 0.2% | |
| 76 | Carbamazepine | 117 | 72 | 0.1% | 0.2% | |
| 77 | Antivirals | aciclovir | 84 | 105 | 0.2% | 0.1% |
| 78 | Cephalosporins | ceftriaxone, cefalexin | 85 | 106 | 0.2% | 0.1% |
| 79 | Local anaesthetics | lidocaine | 116 | 92 | 0.1% | 0.1% |
| 80 | Amiodarone | 100 | 108 | 0.2% | 0.1% | |
| 81 | Drugs used in substance dependence | nicotine, methadone | 111 | 100 | 0.1% | 0.1% |
BNF, British national formulary; PCA, prescription cost analysis; Hosp., hospital
For each drug the prescribing frequency in terms of rank and percentage of prescriptions are shown for both primary (PCA) and secondary (hosp.) care. The average rank in both healthcare settings was calculated and determined the overall rank
All five drug groups that made up ≥0.2% items dispensed in primary care alone were included in the core drug list (Table 2). In addition, ‘drugs for breast cancer’, comprising 0.19% items dispensed) was included.
Table 2.
Drugs, classes and BNF groupings comprising ≥0.2% prescriptions in primary care but <0.1% prescriptions in secondary care
| Drug, class or BNF grouping | Most commonly prescribed example(s) | PCA rank | PCA (%) | |
|---|---|---|---|---|
| 1 | Oestrogens and progestogens | combined ethinylestradiol, desogestrel, estradiol | 27 | 1.2% |
| 2 | Phosphodiesterase (type 5) inhibitors | sildenafil | 61 | 0.3% |
| 3 | Acetylcholinesterase inhibitors | donepezil | 72 | 0.2% |
| 4 | Serotonin (5HT1)‐receptor agonists | sumatriptan | 75 | 0.2% |
| 5 | Leukotriene receptor antagonists | montelukast | 79 | 0.2% |
| 6 | Drugs for breast cancer | tamoxifen | 83 | 0.19% |
BNF, British national formulary; PCA, prescription cost analysis
Eleven drug groups made up ≥0.3% prescriptions in secondary care alone and seven of these were included in the final list (Table 3). The four drug groups excluded from the core final list because they were considered to require more specialist than generalist expertise were N‐methyl‐D‐aspartate receptor antagonists (e.g. ketamine), 1.9% prescriptions; immunosuppressants (e.g. tacrolimus, ciclosporin), 1.3% prescriptions; drugs for human immunodeficiency virus (HIV) infection (e.g. ritonavir), 1.1% prescriptions; and carbapenems (e.g. meropenem), 0.5% prescriptions.
Table 3.
Drugs, classes and BNF groupings comprising ≥0.3% prescriptions in secondary care but <0.1% prescriptions in primary care
| Drug, class or BNF grouping | Most commonly prescribed example(s) | Hosp. rank | Hosp. (%) | |
|---|---|---|---|---|
| 1 | Heparins | enoxaparin, heparin | 4 | 2.9% |
| 2 | Serotonin (5HT3)‐receptor antagonists | ondansetron | 8 | 2.4% |
| 3 | Oxygen | 21 | 1.7% | |
| 4 | Quinolones | ciprofloxacin, moxifloxacin | 37 | 0.8% |
| 5 | Penicillins, anti‐pseudomonal | piperacillin sodium/tazobactam sodium | 38 | 0.8% |
| 6 | Vancomycin | 48 | 0.6% | |
| 7 | Aminoglycosides | gentamicin | 72 | 0.3% |
BNF, British national formulary; Hosp., hospital
Six drugs from emergency guidelines that did not qualify by prescribing frequency were considered clinically important and completed the list (Table 4).
Table 4.
Drugs identified from emergency guidelines not qualifying for the core list by prescribing frequency but considered to be core learning for new prescribers
| 1 | Activated charcoal |
| 2 | Adrenaline (epinephrine) |
| 3 | Adenosine |
| 4 | Acetylcysteine |
| 5 | Fibrinolytics, e.g. alteplase |
| 6 | Naloxone |
Drugs from emergency guidelines are in alphabetical order
Changes in core drug list from 2006–2009 to 2015
There were 12 changes to the core list in 2015 from 2006–9 (Table 5). Some of the drugs dropping out of the core drug list did so due to changes in qualification rules set in the prospectively defined analysis plan. Compound products were not included as distinct items if their constituent ingredients were already captured in the top 100 list (compound beta 2 agonist/corticosteroid inhalers; opioids, compound preparations). Where different members of drug classes were used for more than one indication, the drug class was included only once (anti‐histamine anti‐emetics and H1 receptor antagonists were separate in the old list and combined in the new list). Vaccines and antisera were excluded because, although they fall within the definition of medicinal products, we judged that they were educationally distinct. Electrolytes were split and analysed as their constituents (e.g. oral potassium, oral magnesium, intravenous electrolytes), which didn't individually make the list based on prescribing frequency.
Table 5.
Changes in core drug list between 2006–9 and 2015
| Drugs dropping out of the core list | New entrants to the list |
|---|---|
| Anti‐emetics, phenothiazinesa | Acetylcholinesterase inhibitors |
| Compound (beta‐2 agonist corticosteroid) inhalersb | Antiproliferative immunosuppressants |
| Dipyridamolea | Antivirals |
| Electrolytes, e.g. potassium, magnesiumb | Sex hormone antagonists for breast cancer |
| Laxatives, bulk forminga | Chloramphenicol |
| Nicorandilc | Dipeptidyl peptidase‐4 inhibitors |
| Opioids, compound preparationsb | Lamotrigine |
| Phenytoina | Leukotriene receptor antagonists |
| Potassium sparing diuretics with other diuretics (e.g. co‐amilofruse)a | Levetiracetam |
| Thiazolidinedionesa | Direct oral anticoagulants |
| Vaccines and antiserab | Serotonin (5HT1)‐receptor agonists |
| Anti‐histamine anti‐emetics combined with H1 receptor antagonists in the new listb | Mucolytics |
Drugs dropping out of the list due to reduction in relative prescribing or dispensing frequency
Drugs dropping out of the list due to changes in qualification rules
Drug with more specialist use making way for drugs for more generalist use
Other drugs dropping out of the core list did so due to a fall in prescribing frequency relative to new entrants. These were anti‐emetics, phenothiazines; dipyridamole; diuretics, potassium‐sparing diuretics with other diuretics; laxatives, bulk forming, phenytoin and thiazolidinediones. Nicorandil was borderline for inclusion on the basis of prescribing frequency, but was excluded from the final list to make room for emergency medicines as it was judged more specialist than generalist compared to other borderline drugs.
All new entrants to the list qualified through an increase in relative prescribing frequency. For some drugs, this represents a genuine increase in use, e.g. direct oral anticoagulants, DPP‐4 inhibitors, levetiracetam. For others, drug use may have remained constant but increased relative to some of those leaving the list (e.g. thiazolidinediones, phenytoin) where use has decreased.
Comparison of core drugs list to the World Health Organization list of essential medicines
The World Health Organization (WHO) compiles and updates a core list of minimum medicines required for a basic healthcare system and a complementary list of essential medicines for priority diseases where some specialist facilities, care or training are needed for their use 12. Together these lists contain around 438 individual drugs. To determine the applicability of the core drug list to trainee prescribers working in healthcare systems outside England, we compared our list to the WHO list of essential medicines 12. Seventy‐eight per cent of our core drugs were on the WHO essential list and 4% were on the complementary list. Drugs not on the WHO list or on the complementary list only are shown in Table 6.
Table 6.
Drugs in the English top 100 starter formulary that are not in the World Health Organization's essential medicines list
| 1 | Alpha‐adrenoceptor blocking drugs |
| 2 | Gabapentin and pregabalin |
| 3 | Emollients |
| 4 | Alginates and antacids |
| 5 | Anti‐depressant drugs, other (venlafaxine, mirtazapine) |
| 6 | Z drugs |
| 7 | Ocular lubricants (artificial tears) |
| 8 | Anti‐muscarinics, genitourinary uses |
| 9 | Dipeptidyl peptidase‐4 inhibitors |
| 10 | Direct oral anticoagulants |
| 11 | Mucolytics, e.g. carbocisteine |
| 12 | Levetiracetam |
| 13 | 5α‐reductase inhibitors |
| 14 | Phosphodiesterase (type 5) inhibitors |
| 15 | Acetylcholinesterase inhibitors |
| 16 | Serotonin (5HT1)‐receptor agonists |
| 17 | Leukotriene receptor antagonists |
| 18 | Adenosine |
| On the complementary list | |
| 19 | Anti‐proliferative immunosuppressants, e.g. azathioprine |
| 20 | Amiodarone |
| 21 | Drugs for breast cancer, e.g. tamoxifen |
| 22 | Fibrinolytics, e.g. streptokinase |
Discussion
We have identified the drug groups most commonly prescribed in England in primary and secondary care settings in 2015. We have used this analysis to develop a ‘top 100 drugs’ list to provide a starting point for trainee prescribers being introduced to pharmacology for the first time. This new list updates our previous analysis of 2006–9 prescribing data 6. Reassuringly, only 12% of drugs in the list have changed, indicating that learning based on this resource could have long‐term relevance for prescribing in practice.
Some of the changes in the updated list reflect changes in qualification rules, such as removal of separate entries for compound preparations and drug groups used in more than one therapeutic area. Other changes, however, are likely to reflect genuine changes in prescribing guidelines and practice. For example, in 2010 the European Committee on Medicinal Products for Human Use recommended suspension of the marketing authorization of rosiglitazone, a thiazolidinedione, due to emerging evidence of cardiovascular risk 13. Another thiazolidinedione, troglitazone, had previously been withdrawn from the British market in 1997 due to hepatotoxicity 14. Although pioglitazone remains available for prescription and is still included in English guidelines produced by the National Institute for Health and Care Excellence (NICE) for the management of type 2 diabetes 15, concerns about the safety of this drug class and adoption of alternatives, including the dipeptidyl peptidase‐4 inhibitors (entering the list in 2015), likely account for the fall in thiazolidinedione prescribing. Another example is change in antiepileptic drug prescribing. Phenytoin, which was included in the 2006–9 list, was put on a ‘potential signals of serious risks’ list by the US Food and Drug Administration (FDA) in 2008 and is no longer recommended as either first line or adjunctive therapy for the prevention of any seizure type by NICE 16. Carbamazepine and sodium valproate (in both old and new lists), as well as lamotrigine and levetiracetam (entering the list in 2015), are preferred. Phenytoin remains on the WHO List of essential medicines 12 and is still listed in NICE guidelines as adjunctive treatment to benzodiazepines for status epilepticus. There is therefore a case to include it in the top 100 list as an emergency drug. As trials seek to replace its use even for status epilepticus with safer alternatives 17, we have made the judgement to leave it out of our list. Other educators and learners may wish to include it in theirs. Other changes in the list may be due to increasing disease prevalence or diagnosis. For example, increasing rates of diagnosis of dementia and prescription of anti‐dementia drugs 18 could be responsible for the entry of acetylcholinesterase inhibitors to the list. Differences in data collection between the two analyses may also have had an effect. In 2006–9, secondary care prescribing data was collected by hand and so only included approximately 7500 prescriptions, whereas in 2015 use of electronic prescribing data allowed inclusion of nearly 1.8 million secondary care prescriptions.
Our list was developed using prescribing and dispensing data from England. To determine its relevance to an international audience, we reviewed it against the WHO essential and complementary medicines lists 12. Over three quarters of drugs on our list are considered essential for a basic healthcare system and are therefore likely to be used worldwide. We considered the WHO list in its entirety (438 drugs) to be overwhelming for a beginner prescriber and feel that our core list has an important place in helping novice prescribers to direct most of their initial attention to the most commonly prescribed drugs.
A list of drugs to learn about perhaps seems an old fashioned concept in an era where healthcare education seeks to be patient‐centred, integrated and problem‐based, and curricula are moving to define and assess higher level competencies. Learning to prescribe is a complex process, well suited to a spiral curriculum where learners acquire understanding of the principles of clinical pharmacology, knowledge of drugs and therapeutics, and skills in prescribing in parallel, through multiple ‘visits’ to the topic of increasing complexity 19. A core drug list gives trainee prescribers a tool to focus their acquisition of knowledge around drugs that they will use in early clinical practice. It allows them to build their learning from knowledge of the pharmacology of individual drugs, through understanding how these drugs are used in the management of common diseases to prescribing them in simulated, then real, clinical scenarios. The principles and skills developed can then be applied to unfamiliar drugs encountered in practice. A core drug list can also help educators to design useful learning resources 7 and assessments that are relevant to practice. For example, learners could be assessed not only on their knowledge of drugs on the core list, but also on their skills in information gathering to support safe prescribing of an unfamiliar drug.
Limitations
Our analysis has several limitations. The primary care data reflects English prescribing practice only, although we consider that it should be broadly representative of UK practice. With an appropriate overlay of local clinical–educational judgement, it may have broader generalizability. Our finding that over three quarters of drugs on the core list were also on the WHO essential medicines list supports this. Secondary care data was obtained from a single hospital, and may therefore be affected by local prescribing patterns, population characteristics, and specialist services. However, it is reassuring that the large majority of items on the list were prescribed frequently in both primary and secondary care, suggesting that most do not reflect specialist or centre‐specific practice. Moreover, we applied clinical–educational judgement to exclude drugs considered to be mainly for specialist use and beyond the scope of a new prescriber.
The method of analysis and definition of drug groupings also had potential to influence the results. The complex process of screening BNF sub‐paragraphs, classes and individual drugs requires some subjective judgement. However, this was informed by considerable experience of both clinical practice and prescriber training, aiming to produce educationally useful, clinically relevant groups. These are fully described so that educators using the list may also apply their own judgement.
Conclusion
Personal formularies are valuable tools to improve prescribing skills, but can be difficult to develop without help for the trainee prescriber. We have produced a core drug list of the most commonly prescribed drug groups in England to assist in this process. We consider that it should be generalizable to UK practice and – if supported by appropriate clinical–educational judgement – more widely. Updating this formulary has resulted in 12 changes from 2006–9, keeping the list up to date with contemporary prescribing practice. This core drug list is not intended to restrict the scope of teaching or to stifle students' inquisitiveness. Rather, it should be considered as a ‘starter formulary’ to help novice prescribers to direct most of their early attention to the most commonly prescribed drugs.
Competing Interests
E.H.B. described the top 100 most commonly prescribed drugs in 2006–9 in the British Journal of Clinical Pharmacology 6. Subsequently, A.W.H., D.O.L., D.R.B. and E.H.B. published a textbook with Elsevier entitled The Top 100 Drugs: Clinical Pharmacology and Practical Prescribing 7. This was based on the 2006–9 top 100 drugs list and these authors were paid royalties by the publisher. The same authors have already produced a second edition of this book, based on the updated 2015 analysis reported in this paper. The second edition of The Top 100 Drugs will be published in 2018 and these same authors will receive further royalties for this work. S.A., S.P. and J.J.C. have no competing interests to declare.
The analysis presented in this paper used the ‘NHS Business Services Authority Prescription cost analysis data 2015, NHSBSA Copyright 2018’. This information is licensed under the terms of the Open Government Licence.
Audi, S. , Burrage, D. R. , Lonsdale, D. O. , Pontefract, S. , Coleman, J. J. , Hitchings, A. W. , and Baker, E. H. (2018) The ‘top 100’ drugs and classes in England: an updated ‘starter formulary’ for trainee prescribers. Br J Clin Pharmacol, 84: 2562–2571. 10.1111/bcp.13709.
References
- 1. General Medical Council . Outcomes for graduates [online], 2015. Available at https://www.gmc-uk.org/education/undergraduate/undergrad_outcomes.asp (last accessed 23 February 2018).
- 2. MSC Assessment and British Pharmacological Society . Prescribing Safety Assessment [online], 2016. Available at https://prescribingsafetyassessment.ac.uk/ (last accessed 23 February 2018).
- 3. British Medical Association . The prescribing safety assessment [online], 2017. Available at https://www.bma.org.uk/advice/career/applying-for-training/prescribing-safety-assessment (last accessed 23 February 2018).
- 4. World Health Organization Guide to good prescribing [online]. Available at http://whqlibdoc.who.int/hq/1994/WHO_DAP_94.11.pdf (last accessed 23 February 2018).
- 5. De Vries TP, Daniels JM, Mulder CW, Groot OA, Wewerinke L, Barnes KI, et al Should medical students learn to develop a personal formulary? An international, multicentre, randomised controlled study. Eur J Clin Pharmacol 2008; 64: 641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baker E, Roberts AP, Wilde K, Walton H, Suri S, Rull G, et al Development of a core drug list towards improving prescribing education and reducing errors in the UK. Br J Clin Pharmacol 2011; 71: 190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hitchings AW, Lonsdale DO, Burrage DR, Baker EH. The Top 100 Drugs: Clinical Pharmacology and Practical Prescribing, 1st edn. Edinburgh, Scotland: Churchill Livingston, 2014. [Google Scholar]
- 8. NHS Digital . Prescription Cost Analysis – England, 2015 [online], 2016. Available at https://digital.nhs.uk/catalogue/PUB20200 (last accessed 7 February 2017).
- 9. British National Formulary . British National Formulary [online]. Available at https://bnf.nice.org.uk/ (last accessed 23 February 2018).
- 10. Guidelines for the Management of Common Medical Emergencies and for the Use of Antimicrobial Drugs. St George's University Hospitals NHS Foundation trust [online], 2017. Available at http://www.greybook.sgul.ac.uk/ (last accessed 23 February 2018).
- 11. Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use [online]. Available at http://ec.europa.eu/health/sites/health/files/files/eudralex/vol-1/dir_2001_83_consol_2012/dir_2001_83_cons_2012_en.pdf (last accessed 23 February 2018).
- 12. World Health Organization . Model lists of essential medicines, 20th edition, updated March 2017 [online]. Available at http://www.who.int/medicines/publications/essentialmedicines/en/ (last accessed 27 July 2018).
- 13. Medicines and Healthcare products Regulatory Agency . Rosiglitazone: recommended withdrawal from clinical use. Drug Saf Update 2010; 4: S1. [Google Scholar]
- 14. Lebovitz HE. Differentiating members of the thiazolidinedione class: a focus on safety. Diabetes Metab Res Rev 2002; 18 (Suppl. 2): S23–S29. [DOI] [PubMed] [Google Scholar]
- 15. National Institute for Health and Care Excellence . Type 2 diabetes in adults: management. May 2017 [online]. Available at https://www.nice.org.uk/guidance/ng28 (last accessed 27 July 2018).
- 16. National Institute for Health and Care Excellence . Epilepsies: diagnosis and management. April 2018. Available at https://www.nice.org.uk/guidance/cg137/chapter/Appendix-E-Pharmacological-treatment (last accessed 27 July 2018).
- 17. Bleck T, Cock H, Chamberlain J, Cloyd J, Connor J, Elm J, et al The established status epilepticus trial 2013. Epilepsia 2013; 54 (Suppl. 6): 89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mukadam N, Livingston G, Rantell K, Rickman S. Diagnostic rates and treatment of dementia before and after launch of a national dementia policy: an observational study using English national databases. BMJ Open 2014; 4: e004119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baker E, Lonsdale D, Burrage D, Hitchings A. Design of a spiral curriculum to develop prescribing skills in undergraduate medical students. Proc Br Pharmacol Soc 2016; 16: abst095p. [Google Scholar]


