Abstract
There has been a resurgence in interest and use of the cannabis plant for medical purposes. However, an in‐depth understanding of plant contaminants and toxin effects on stability of plant compounds and human bioavailability is needed. This systematic review aims to assess current understanding of the contaminants of cannabis and their effect on human health, leading to the identification of knowledge gaps for future investigation. A systematic search of seven indexed biological and biomedical databases and the Cochrane library was undertaken from inception up to December 2017. A qualitative synthesis of filtered results was undertaken after independent assessment for eligibility by two reviewers. The common cannabis contaminants include microbes, heavy metals and pesticides. Their direct human toxicity is poorly quantified but include infection, carcinogenicity, reproductive and developmental impacts. Cannabis dosing formulations and administration routes affect the transformation and bioavailability of contaminants. There may be important pharmacokinetic interactions between the alkaloid active ingredients of cannabis (i.e. phytocannabinoids) and contaminants but these are not yet identified nor quantified. There is significant paucity in the literature describing the prevalence and human impact of cannabis contaminants. Advances in the availability of cannabis globally warrant further research in this area, particularly when being used for patients.
Keywords: addiction medicine, cannabinoids, molecular biology, pharmacokinetics
Introduction
Cannabis, also known as marijuana, is defined by the United Nations Single Convention on Narcotic Drugs 1961 as ‘the flowering of fruiting tops of the Cannabis plant (of the genus Cannabis)’ 1. The therapeutic application of cannabis and its constituent phytocannabinoids, particularly http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2424, continues to garner significant clinical and public attention. Yet, medicinal cannabis remains a relatively new clinical pharmacology frontier, and our understanding of its human toxicity profile is incomplete. The pharmaceutical approach to the development of therapeutic cannabis requires that the full gamut of toxicity resulting from contaminants be quantified and preparations standardized to minimize adverse events 2. The non‐medical community generally considers unadulterated cannabis a relatively safe drug with a tolerable adverse effects profile 3. There are, however, significant uncertainties surrounding the prevalence and effects of toxic abiotic and biotic contaminants.
This review aims to aggregate and critically appraise the literature pertaining to: the sources of cannabis contaminants and their distribution, their human toxicity, the effect of different routes of administration on contaminant bioavailability and the potential interactions with the pharmacokinetic (PK) and pharmacodynamic (PD) profile. Sitting at the nexus between pharmacology and toxicology, it investigates the contaminants of cannabis and considers how they might affect the patient. This knowledge has important implications for policy makers, regulators, clinicians and, most importantly, patients.
Methodology
Search strategy
We intended to undertake a systematic review of the contaminants of cannabis and their effect on the human, with a meta‐analysis if appropriate. The thematic survey of the selected literature returned insufficient results to permit a quantitative analysis of grouped outcome measures, in part due to the large spread of subtopics and contaminants identified. A qualitative systematic review, in the form of a narrative synthesis of the literature was thus undertaken across the seven indexed databases relating to medicinal and biological sciences via the Ovid Platform. These included: Allied and Complementary Medicine, Biological Abstract, BIOSIS Previews, Embase, International Pharmaceutical Abstracts, Medline, and Ovid Medline. Further, a search of the Cochrane Library did not isolate any pertinent systematic reviews or meta‐analyses. Additional English language and human focused clinical studies relevant to the review research question were reviewed and included if identified during the review of the selected manuscripts.
The Australian Therapeutic Goods Order 93 (TGO 93), published by the Australian Therapeutic Goods Administration under subsection 10(4) of the Therapeutic Goods Act 1989, governs the quality standards of medicinal cannabis administered in Australia. The cannabis plant material used to manufacture the medicinal product must meet the requirements of Schedule 1. This Schedule, founded on the European Pharmacopoeia general monograph Herbal Drugs (1433) and Herbal Drug Preparations (1434), identifies six environmental contaminants that need to be specifically assayed in medicinal cannabis preparations 4. These include: aflatoxins, foreign matter, heavy metals (arsenic, cadmium, lead, and mercury), ochratoxin A, pesticides and total ash. Similar production directives and contaminant analysis guidelines are found in the Dutch Cannabis Analytical Monograph 5 and American Herbal Pharmacopeia 6.
The search keyword terms were guided by the provisions of the TGO 93 4 and included: Cannabis.mp. or marijuana.mp. crosslinked with specified contaminants (aflatoxins.mp., foreign matter.mp., heavy metals.mp., arsenic.mp., cadmium.mp., lead.mp., mercury.mp., ochratoxin.mp., pesticides.mp., and total ash.mp.). The cumulative results were then crosslinked with routes of administration (inhalation.mp., ingestion.mp., oral intake.mp., vaporisation.mp., vaporization.mp., injection.mp., absorption.mp., smoking.mp., dabbing.mp., eating.mp., dosing formulation.mp., and administration route.mp.) or pharmacokinetics.mp. Please refer to the Supporting Information Appendix S1 for the search strategy. A title screening and subsequent abstract screening were undertaken by two reviewers independently. Inclusion criteria included: cannabis (sativa) described as the pharmaceutical or drug of interest and premise or outcome of interest concerned with one of (contaminants of cannabis, differing routes of administration of cannabis, issues regarding the legalization process of medicinal cannabis or the Pharmacokinetic or Pharmacodynamic profile of cannabis). Exclusion criteria included: substance of interest other than cannabis, outcome of interest; non‐contaminant mediated adverse effects of cannabis or therapeutic effects of cannabis on specific population groups and non‐English publications. Any disagreements were discussed and resolved based on their relevance to the research question. External to the aforementioned databases, 15 further references were sourced from citation referencing of eligible studies. The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) diagram (Figure 1 ) provides a numerical breakdown of the search strategy.
Figure 1.
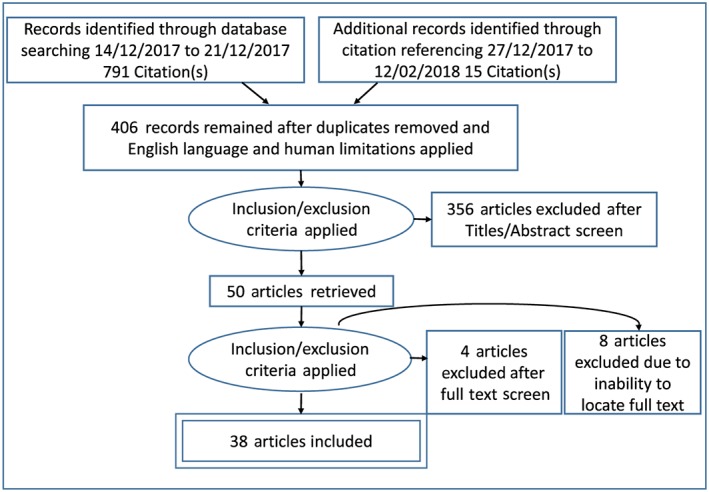
PRISMA diagram of the literature search for information regarding contaminants of cannabis
Results
Sources of contaminants and their distribution
From the review of the literature the most commonly reported contaminants of cannabis preparations were microbes, heavy metals and pesticides. Similar types of contaminants have been reported in complementary medicines including herbal, Ayurvedic and Chinese traditional medicines 7.
Microbial contamination
The Cannabis sativa plant provides host to a variety of organisms and its complex microbiome continues to be deciphered 8. Grey and academic literature highlight the presence of pathogenic microbial contaminants, particularly bacteria and fungi (mould), within cannabis preparations. Most of the microbial contamination occurs during the improper preparation and storage of cannabis products. For example, harvesting whilst wet, drying and storage under wet, humid conditions can lead to fungal infections such as powdery mildew and botrytis, and budworm or mite infestations. Historically, there have been reports of bacterial contamination with Salmonella 9 and Enterobacter, Streptococcus and Klebsiella 10. There are various case reports of fungal spore contaminants, including mycotoxin‐producing strains of Aspergillus 11, 12, 13. Much of the information regarding microbial contamination emanates from isolated and uncontrolled case reports and series, as well as growers' personal communication. In the absence of quantification and effects on health, as the medical cannabis industry moves forward, thresholds for clean medicine, standards and procedures, such as TGO 93, need to be developed and standardized internationally.
McKernan et al. recently reported their analysis of microbial toxins in dispensary sourced cannabis samples (n = 17) from Amsterdam and Massachusetts 8. In this observational study, a comparison of fungal populations isolated from cannabis on commercial culture‐based assays and a variety of metagenomic techniques, including the use of DNA sampling to achieve a culture independent analysis for microorganisms, were described. Evidence of multiple strains of fungi was found in six samples, including several toxigenic species of Aspergillus and Penicillium as well as Cryptococcus liquefaciens 8. These results support the findings of clinical case reports of fungal contaminants 11, 12, 13. Although the study did not clarify whether the contaminants were present at a concentration that could cause harm to a patient, vaporized fungal spores at even very low concentrations are likely to cause fungal pneumonia 14, 15. Importantly, the commercial culture‐based analyses were unable to identify many of the toxigenic species that metagenomic techniques elicited. The evident superiority of the metagenomics approach for fungal species identification, indicates that it should be adopted routinely to comprehensively test medicinal cannabis preparations. No breakdown addressing region‐specific microbial infestation loads was included in the study and the results should be extrapolated with care for crops of geographically distant provenance.
Aflatoxins, carcinogenic mycotoxins, are produced as a metabolite by certain species of Aspergillus and have been detected in cannabis preparations and smoke 16. In a case control study, the comparison of two species of Aspergillus, flavus and parasiticus, cultured either with an American cannabis or a natural flora substrate, demonstrated that growth on the cannabis substrate produced aflatoxins B1 and G1 17. Hence it was concluded that cannabis, when hosting certain Aspergilli species, may produce aflatoxins. Although the experimental design and lack of methodological clarity significantly impacted on the study's validity, the finding of aflatoxins associated with cannabis is important, especially as it is suggested that they may survive pasteurization and smoking processes 16. Given the recent isolation of Aspergillus species from cannabis 8, contemporary investigation of aflatoxins is warranted.
Heavy metals
There are three pathways through which cannabis may be contaminated with heavy metal substances. Firstly, cannabis is able to remove heavy metals from substrate soils and deposit these in its tissues, by virtue of its bioaccumulative capacity 18. Secondly, cross‐contamination may occur during processing (e.g., during drying). Thirdly, post‐processing adulteration may occur, whereby metals may be added to the preparation to increase weight and thereby appreciate its street value 19.
Fertilizer uptake from soil is an important source of heavy metal contamination in cannabis. For example, phosphate fertilizers rich in cadmium are readily taken up by most plants including cannabis 20. Although there have not been any major reports on heavy metal uptake by medicinal cannabis, there have been a number of studies on the uptake of heavy metals by industrial hemp (Cannabis sativa L.). This indicates that the cannabis plant is very efficient in the uptake of heavy metals from contaminated sites and can be considered as a potential candidate for phytoremediation of contaminated soils 21, 22.
There is a paucity of serial and systematic analysis of heavy metal contaminants of cannabis preparations. There are case reports of possible arsenic contamination related to the disease cannabis arteritis, a form of thrombitis obliterans 23, 24. Another case series reported on 95 cases of lead poisoning due to lead adulteration of illegal preparations to increase the weight and thus market value of cannabis 19. These uncontrolled case reports and series form the bulk of the academic literature pertaining to this topic. Future investigations into the prevalence and concentration of heavy metals, particularly arsenic, in cannabis preparations is warranted.
Pesticides
Pesticide use for the cultivation of cannabis crops is well established 2, 16, 25. Human consumption of pesticides may confer substantial sequelae, including malignancy, developmental issues, reproductive, neurological and endocrine disorders. Russo recently reported on the significant prevalence of pesticide contamination in Washington State, where laboratory analysis revealed that 84.6% (n = 26 samples) of legalized cannabis products contained significant quantities of pesticides including insecticides, fungicides, miticides and herbicides. These comprised a wide array of different substances and encompassed proven carcinogens (Carbaryl, Diuron, Ethoprophos, Permethrin, and Propargite), endocrine disruptors, as well as a variety of developmental, reproductive and neurological toxins 2. These findings corroborate an earlier analysis by the Los Angeles City Attorney's office, who found excessive quantities of the pesticide Bifenthrin in medicinal cannabis samples 26. The prevalence, constituents and concentration of pesticides above maximum residue levels in cannabis preparations remains incompletely described in the scientific literature.
Human toxicity of cannabis contaminants
Many deleterious effects of cannabis consumption have been noted and posited, though not always consistently substantiated. The potential human toxicity profile encompasses acute morbidity; acute myocardial infarction, cerebellar infarction, infections, or psychomotor changes, as well as longer‐term morbidity; pulmonary disease, immune dysfunction, testicular cancer, reproductive issues, teratogenicity, and psychiatric disease 27, 28, 29. Plausibly, some of these may aetiologically derive from contaminants. There are difficulties in establishing a direct causal relationship between cannabis and sequelae, as users commonly smoke tobacco and consume other drugs concurrently. It is further complicated by the significant time‐lag between consumption and diagnoses. This is compounded by the lack of adequately powered and well‐designed epidemiological or experimental studies assessing the human effect of contaminants 30. A broad analysis of the entire human toxicity of cannabis is beyond the scope of this review and it will focus on those attributable to identified contaminants.
Infection
The pathogenic microbial species isolated from cannabis preparations can result in infections and present a potential outbreak risk. Taylor et al. retrospectively reported on an outbreak of Salmonella enteritis which was traced back to cannabis harbouring Salmonella Muenchen (antigenic formula 6,8:d:1,2) 9. More hazardous are fungal spores that can directly cause infection or may produce mycotoxin secondary metabolites. There have been several case reports of opportunistic infection with fungi, commonly Aspergilli, in immunocompromised patients linked to findings of cannabis contaminated by fungal spores 11, 12, 13.
Tashkin et al. highlighted the importance of eradicating bacterial and fungal species from medicinal preparations of cannabis due to the potential immunocompromised state of patients receiving prescribed medicinal cannabis 31. This may be achieved at multiple points in the production process; by ensuring clean growing media, handling and storing preparations hygienically, and gamma‐irradiation of the final product 2, 16. Gamma‐irradiation has been tested successfully as a standard technique for sterilization of medicinal cannabis preparations 32.
Carcinogenicity
The carcinogenic load of non‐medicinal cannabis, particularly when consumed via smoking, is significant 27, 33. Identified carcinogens include vinyl chloride, nitrosamines, reactive oxygen species and polycyclic aromatic hydrocarbons, notably benzo[a]pyrene and benzathracene 27, 30, as well as arsenic and aflatoxins. Many of these are pesticides with the lattermost arising from species of Aspergilli. Surprisingly, given the carcinogenic load of cannabis smoke and the effectiveness of their delivery via smoking, the link between cancer of the respiratory tract and cannabis smoking is equivocal 30. Cannabis use has also been implicated as a possible aetiological agent for the development of testicular cancer 29. The International Agency for Research on Cancer classifies aflatoxins and arsenic, both of which have been associated with cannabis, as Group 1 substances (carcinogenic to humans) due to their causal association with hepatocellular carcinoma 34 and bladder, kidney, skin, liver, and prostate 35 cancers, respectively.
Pertinent to the potential carcinogenicity of cannabis is uncertainty surrounding the presence and concentration of pesticide residues. Food Standards Australia and New Zealand sets maximum residue limits (MRL) of pesticides and other chemicals for food consumed in Australia, as do similar authorities globally. Medicinal cannabis was only made federally legal in Australia in 2016 36 and, accordingly, prior to this no pesticides were declared for use on cannabis crops. A similar legislative abyss existed in the United States 16, 25. This created a three‐fold problem. Firstly, regulators had not specified pesticides deemed safe, resulting in a wide array of potentially noxious substances being utilized, and secondly, no regulator routinely monitored the MRL of cannabis products. Finally, laboratories may not have utilized assay panels with the breadth required to isolate all potential pesticide contaminants. All of these factors may contribute to the lack of reliable data on the concentration and toxicity of pesticides used on cannabis crops. This highlights the need for uniform and robust methods to establish whether pesticides used on cannabis crops exceed equivalent MRLs and whether carcinogenic substances are employed.
Reproductive issues and development
Endocrine disruptors refer to a group of bioactive substances that interfere with the function of the endocrine system via either interruption of signalling or alteration of hormone synthesis and secretion 37. Various pesticides, particularly organophosphate insecticides 37, and most heavy metals 38 are recognized endocrine disruptors. These have the ability to interfere with normal fertility function and developmental processes. Additionally, mercury and lead, when consumed in sufficient quantities, also affect development. The presence of these contaminants in cannabis preparations consumed by individuals within their fertile window or children would be of particular concern.
Toxicity thresholds of contaminants
The body of currently available evidence insufficiently investigates and quantifies the levels of contaminants found in cannabis preparations or cannabis users. This would be a valuable line of inquiry and a comparison of calculated weekly intakes with safe limits stipulated by the World Health Organization and domestic authorities would help inform dose limits. Importantly, the patient population who seek the prescription of medicinal cannabis may have a decreased physiological tolerance to deal with contaminants and their toxicity 20. Given the multiple impingements on the immune system of prospective patients, the potential toxicity of cannabis contaminants warrants a more thorough investigation.
Minimizing potential deleterious effects
Use of purified single cannabinoid extracts might hold the potential to circumvent some of the human toxicity associated with consumption of whole cannabis extracts. This is the subject of polemic debate, as many believe that the consumption of holistic extracts to be therapeutically superior to cannabinoid isolates, stemming from pre‐clinical studies which posited superior symptomatic outcomes with the consumption of whole cannabis extracts as compared to purified cannabinoids (primarily THC). This synergistic phenomenon has been termed the ‘entourage effect’ 39, 40, 41. Our current search did not identify any comparative analyses quantifying levels of contaminants between whole and purified cannabis preparations. Given that there are many different medicinal cannabis formulations containing different amounts and types of both active pharmaceutical ingredients and potential contaminants, future research should seek to provide evidence of whether there are optimal blends of cannabis components.
Interestingly, there is evidence to suggest that the preparation and extraction processes may impact the level of contaminants in the final product. For example, it has been found that boiling medicinal plants in water extracted greater amounts of heavy metals than immersing in hot water, thereby reducing heavy metal contamination of the end product 42. Information on which types of formulations are more prone to containing contaminants, and methods of preparation that minimize the potential of contaminants, should also be obtained. Further, when considering the writing and implementation of policies surrounding the safe production of medicinal cannabis, the relative contaminant concentrations between different formulations is important. This is because, as suggested above, the level of contaminants varies along the production process and clear directives need to establish at what stage contaminant sampling should be completed to ensure a safe product is delivered to consumers.
Routes of administration
Cannabis is commonly consumed via inhalation or ingestion and less frequently through ophthalmic, rectal, sublingual and dermal preparations 43, 44. Different routes of administration and dosing formulations give rise to a varied bioavailability of constituent compounds. Specifically, it is well established that cannabinoids are absorbed differently and have variable effects, dependent upon whether cannabis is inhaled or ingested 44, 45. It logically follows that the bioavailability of contaminants may be contingent on the administration route with the pyrolytic effect of heating contaminants an important issue. Additionally, there are questions surrounding the altered pharmacokinetics of cannabis and cannabinoids in the presence of contaminants. The literature has not systematically addressed the effects of administration routes on the bioavailability and pharmacokinetic properties of contaminants at this point.
Inhalation
Inhalation techniques of cannabis (smoking, vaporizing and dabbing) deliver cannabinoids efficiently to the respiratory capillary membrane and peak THC plasma concentration is rapidly achieved in between 3 and 10 minutes 3. These preparations avoid first pass metabolism resulting in a high but variable systematic THC bioavailability of 10–35% 44. This also means that contaminants are subject to little metabolism or degradation before they reach the systemic circulation. Heating of preparations also alters the activity and potency of cannabinoids and contaminants. Given the wide variety of contaminants it can be expected that their behaviour and potency will be affected differently.
Smoking
Smoking is the most common recreational consumption technique and involves the combustion of cannabis and other compounds. The application of sufficient heat to cannabis causes decarboxylation of delta‐9‐tetrahydrocannabinoic acid (THCA) to its active form THC 46 but, concomitantly, the process of pyrolysis transforms some contaminants into more toxic forms 25. Heavy metals such as cadmium and arsenic and some pesticides are highly volatile and become carcinogenic under pyrolytic conditions 47. Direct studies of pyrolytic products in cannabis smoke are lacking.
Sullivan et al. performed an in vitro experimental study to quantify the concentration of pesticide residues from contaminated cannabis liberated by smoking 25. Pesticide‐spiked cannabis samples (bifenthrin, diazinon, paclobutazol and permethrin) were mechanically smoked via three separate devices (glass pipe, water pipe, and water pipe with filter) with pesticide residues of the smoke stream measured by gas chromatography. Comparatively, the water pipe with filter had the lowest detected residue concentrations (<11% recovery for all four pesticides) whereas the glass pipe exhibited the highest (>60% recovery for all four pesticides). This was a methodologically rigorous study and the generalization of its results suggest that smoking of pesticide contaminated cannabis, particularly without a filter, efficiently transfers these contaminants to the end user.
Vaporization
Recently, the use of cannabis vaporization apparatuses 48 has increased in conjunction with the expansion of the ‘heat not burn’ products in the tobacco market. This is established on the popular perception that products consumed in this way expose the individual to a lower level of contaminant particulate matter, particularly carcinogens 49. To date, there is no robust evidence that support these anecdotal statements, as the requisite randomized controlled trials or epidemiological studies are lacking.
Dabbing
Dabbing is another inhalation technique that is gaining popularity in medical and recreational cannabis users 50. It refers to the use of vaporization of concentrated butane hash oil, containing high THC concentrations, with a blowtorch. Loflin et al. found in their preliminary qualitative study of cannabis dab users (n = 357) that there is a perception amongst the cannabis using community that dabbing is considered significantly more dangerous than consumption of flower extracts 50. This was most probably due to anticipated withdrawal and tolerance differences rather than the transmission of contaminants. There exist no quantitative data to validate this perception or to ascertain the contaminants of butane hash oil.
The relative safety of the inhalation techniques depends partially on the burn temperatures of any present contaminants as well as the use of a particulate matter filter. Ultimately, all inhalation preparations avoid first‐pass metabolism which may have important implications for detoxification of any constituent contaminants. Importantly, while heat application can volatilize some contaminants, it may also aid in the decontamination of microorganisms from cannabis preparations. Further primary research is required to characterize the behaviour of contaminants when inhaled in order to inform patients about the relative hazards and risk reduction of the different inhalation techniques.
Oral intake
Preparations taken orally (capsules, oral‐mucosal sprays and imbued food/liquid consumables) have a much lower peak THC plasma concentration compared to inhalation 51 and a longer lag to peak concentration (t max) of between 60 and 120 minutes 44. They are subject to both gastric hydrochloric acid degradation and extensive first pass metabolism 52 resulting in a much lower systematic bioavailability of THC compared with inhaled preparations 44. A large number of studies have examined the gastrointestinal bioavailability of oral intake heavy metals through food, drinking water and complementary medicine. These have concluded, broadly, that heavy metals cross from the gastrointestinal lumen via both paracellular and transcellular routes into the circulation where they may accumulate and affect the human organism 53, 54. There is, however, very little information in the scientific literature pertaining to the concentration and behaviour of contaminants in oral preparations of cannabis.
Pharmacologic effects of contaminants
A myriad of phytocannabinoids that interact with the human http://www.guidetopharmacology.org/GRAC/FamilyIntroductionForward?familyId=13 have been isolated in cannabis. Of these, the profile of THC is the most comprehensively documented and a brief outline of its properties is provided for contextual sake. THC is lipophilic and absorbs efficiently across body membranes. The majority is transported bound to plasma proteins 51, with a small portion by the red blood cells. It distributes rapidly to vascular organs and is accumulated and stored in adipose tissue. Importantly, it is able to cross the placenta and is conveyed in breast milk. THC is metabolized predominantly by the phase 1 oxidative metabolism in the liver through the cytochrome P450 system (CYP), via the isoenzymes CYP2C9, CYP2C19 and CYP3A4 44, 51, of which it is an inducer. The resultant metabolites 11‐hydroxy‐Δ9‐tetrahydrocannabinol and 11‐nor‐9‐carboxy‐Δ9‐tetrahydrocannabinol are then conjugated before being predominantly excreted in faeces, with approximately one third excreted in the urine 52.
Cytochrome P450 and contaminants
The CYP isoenzymes may interact with contaminants and affect the metabolism of THC and other cannabinoids. Lead has been shown in animal studies to inhibit the activity of CYP 55, 56, although the evidence of a similar effect in humans is equivocal 57, 58. Some pesticides are metabolized through the CYP system and alter the expression of isoenzymes 59. Rose et al. performed an in vitro experimental analysis of pesticides (chlorpyrifos, carbaryl and permethrin) that were added to a substrate of cytosolic and microsomal pooled human liver fractions. They found that the CYP system was responsible for the detoxification of chlorpyrifos, carbaryl and an isomer of permethrin. It was also found that permethrin and particularly chlorpyrifos induced multiple CYP isoenzymes 59. This suggests there may be an interaction between the metabolic processes of pesticides and cannabinoids which warrants further investigation.
Clinical effects of contaminants
In addition to the various host factors that affect the PK and PD profile, contaminants, as bioactive substances, may affect the absorption, distribution, metabolism and excretion of phytocannabinoids and thus potentially alter their clinical effect. The interactions between phytocannabinoid and contaminants are likely to modulate the bioavailability of both the cannabinoid and contaminants. No formal research investigating this was isolated from the literature. However, McPartland et al. reported a small case series (n = 5) depicting the PD effects of cholinergic adulteration of cannabis preparations 60. Their findings suggest that the addition of cholinergic compounds (nicotinic agonists, muscarinic antagonists and anti‐acetylcholinesterase substances) is associated with an enhancement of the cannabimimetic effects of THC. This represents an uncontrolled, retrospective case series that is insufficiently powered and causation cannot be determined. It casts no light on the PK and PD effects of the aforementioned contaminants; rather, it serves to highlight that co‐administered substances can interact with THC and other cannabinoids in a way that modulates both their PK and clinical effect. Further investigation into the pharmacological effects of contaminants is required.
Discussion
The re‐emergence of cannabis for medicinal use represents an unusual situation where, instead of investigating single drug compounds isolated or synthesized in a controlled laboratory setting, we must now consider the potential for a variety of contaminants to be present in products as a result of the botanical source of the starting material. The lack of a comprehensive evidence base suggests that the study of cannabis contaminants is an emerging field of clinical pharmacology. It is likely that contaminants may not only affect human health directly, they also have the potential to modulate bioavailability and other pharmacological parameters, dependent upon the method of preparation of the product and route of administration. In Australia, the TGO93 standard is regulating the production of medicinal cannabis; however, recreational use outside of this (‘black market’) is not covered and tested in this way.
Overall completeness, applicability and quality of evidence
Understandably, a legacy of illegality may have historically stymied rigorous scientific investigation in this arena; however, high‐quality, pesticide‐, microbe‐ and heavy metal‐free products are essential for patient care. This review confirms there are significant knowledge gaps regarding the sources and effects of contaminants, which manifest as a lack of safety and human pharmacokinetic and pharmacoydynamic data. Given the potential for harmful effects of these contaminants, this aspect of cannabis products will need to be addressed as therapeutic indications expand and medicinal cannabis markets mature, particularly in jurisdictions outside Australia where cannabis is not treated as a ‘therapeutic good’ but rather as a herbal supplement or nutraceutical.
To date, for medical use, too little quantitative data has been published to address all pharmaceutical and clinical health issues relating to cannabis contaminants. Further, as the legalization of medicinal cannabis spreads, public acceptance of its recreational use can be expected to increase. Indeed, it is already the most popular recreational drug in Australia, with an estimated 6.6 million or 35% of the population surveyed as having used cannabis at some point during their life 61. Beyond the delivery of a safe and uncontaminated product to patients, the understanding of the prevalence and effects of cannabis contaminants is a broader public health matter.
This review identifies that there is a dearth of well‐designed scientific studies investigating the contaminants of cannabis. The lack of cross‐sectional studies hampers accurate quantification and identification of contaminants. Causality between contaminants and human toxicity is speculative due to the absence of sufficiently powerful longitudinal studies or pharmacologic analyses. Finally, the health effects of different delivery routes on contaminant bioavailability pharmacokinetic and pharmacodynamic effects are poorly understood owing to the lack of primary research in this area. Therefore, we propose the following areas that require further investigation:
Quantification of heavy metals, pesticide residues and microbes (particularly aflatoxin‐producing Aspergillus species) in dispensary grade cannabis preparations.
Comparative contaminant profiles of purified single cannabinoid extracts with herbal cannabis extracts and powdered cannabis plants.
Volatility and pyrolysis of pesticides and heavy metals between different inhalation methods of cannabis.
Effect of oral intake on contaminant bioavailability and metabolism.
Cannabinoid‐metal interactions in relation to bioavailability of both the cannabinoid and metals.
Interaction of contaminants with hepatic CYP isoenzymes and metabolism of THC.
Potential biases in the review process
This review aggregated and interpreted all available academic literature. Unpublished data was not sought from individual pharmaceutical companies. A key assumption inherent in this approach is that all pertinent information has been published and available through the databases identified in the methodology section. The authors cannot be sure that unpublished data pertinent to this review does not exist. Further, the contaminants articulated in the search of the literature were based on monographs. It is possible that there are additional contaminants that consequently fall outside the premise of this search. Due to the paucity of data, it aggregates studies interrogating street‐grade and dispensary‐grade cannabis products. These products have different growing and processing procedures and conceivably could be considered as different products.
The narrative review form of a systematic review is a relatively novel and incompletely developed methodology. When compared to the more developed quantitative systematic review, it introduces greater scope for interpreter bias. Further, there are a number of uncertainties raised in this review. They predominantly arise from the emergent nature of this field of pharmacology and the previous illegal paradigm of cannabis. Ultimately, this review identifies that significant further research in this field needs to be undertaken to ameliorate the uncertainties regarding the prevalence and effects of medicinal cannabis contaminants.
Conclusion and future directions
Cannabis has significant therapeutic potential for many distressing symptoms and diseases. This qualitative narrative synthesis aggregates and interprets the available clinical evidence of cannabis contaminants and their potential human effects. Its central narrative is that the current academic literature does not deliver a clear understanding of the sources, distribution and pharmacologic effects of cannabis contaminants. Further research is required to bridge the remaining knowledge gaps in these areas.
Many countries are looking towards increasing the size and scope of their medicinal cannabis operations with the aim of consistently producing high‐quality, pure cannabis products for clinical applications. Until recently, most stakeholders have participated in small‐scale production processes focused on domestic clinical supply. This is forecast to change significantly over the coming years, with various countries expanding towards export trade in medicinal cannabis. International consensus is needed to establish quality control and product standardization. The development of the requisite analytical standards for quality testing of global medicinal cannabis preparations requires a more comprehensive evidence base of the contaminants of cannabis.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 62 and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 63.
Competing Interests
There are no competing interests to declare.
The authors N.S.B., P.G., C.P.L.G. and J.H.M. are investigators on the Australian Centre for Cannabinoid Clinical and Research Excellence (ACRE), funded by the Federal Government through the National Health and Medical Research Council Centres of Research Excellence Scheme (2017–2022). ACRE also receives funding from the NSW Ministry of Health as part of the NSW Clinical Cannabis Medicines Program.
Supporting information
Appendix S1 Search strategy
Dryburgh, L. M. , Bolan, N. S. , Grof, C. P. L. , Galettis, P. , Schneider, J. , Lucas, C. J. , and Martin, J. H. (2018) Cannabis contaminants: sources, distribution, human toxicity and pharmacologic effects. Br J Clin Pharmacol, 84: 2468–2476. 10.1111/bcp.13695.
References
- 1. United Nations . Single Convention on Narcotic Drugs, 1961. New York: United Nations, 1961. [Google Scholar]
- 2. Russo EB. Current therapeutic cannabis controversies and clinical trial design issues. Front Pharmacol 2016; 7 (309): 10.3389/fphar.2016.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grotenhermen F. Clinical pharmacodynamics of cannabinoids. J Cannabis Ther 2004; 4: 29–78. [Google Scholar]
- 4. Therapeutic Goods Order Number 93 – Standard for Medicinal Cannabis. Therapeutic Goods Act 1989, 2017.
- 5. Office of Medicinal Cannabis . Analytical Monograph Cannabis Flos. Den Haag. The Netherlands: Office of Medicinal Cannabis, 2014. [Google Scholar]
- 6. American Herbal Pharmacopeia . Cannabis Inflorescence: Cannabis spp.; Standards of Identity, Analysis and Quality Control. Scotts Valley, CA: American Herbal Pharmacopeia, 2013. [Google Scholar]
- 7. Bolan S, Kunhikrishnan A, Seshadri B, Choppala G, Naidu R, Bolan NS, et al Sources, distribution, bioavailability, toxicity, and risk assessment of heavy metal(loid)s in complementary medicines. Environ Int 2017; 108: 103–118. [DOI] [PubMed] [Google Scholar]
- 8. McKernan K, Spangler J, Zhang L, Tadigotla V, Helbert Y, Foss T, et al Cannabis microbiome sequencing reveals several mycotoxic fungi native to dispensary grade Cannabis flowers. F1000Res 2015; 4: 1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taylor D, Wachsmuth K, Shangkuan Y, Schmidt E, Barrett T, Schrader J, et al Salmonellosis associated with marijuana. N Engl J Med 1982; 306: 1249–1253. [DOI] [PubMed] [Google Scholar]
- 10. Ungerleider JT, Andrysiak T, Tashkin DP, Gale RP. Contamination of marijuana cigarettes with pathogenic bacteria – possible source of infection in cancer patients. Cancer Treat Rep 1962; 66: 589. [PubMed] [Google Scholar]
- 11. Llamas R, Hart DR, Schneider NS. Allergic bronchopulmonary aspergillosis associated with smoking moldy marihuana. Chest 1978; 73: 871–872. [DOI] [PubMed] [Google Scholar]
- 12. Hamadeh R, Ardehali A, Locksley RM, York MK. Fatal aspergillosis associated with smoking contaminated marijuana, in a marrow transplant recipient. Chest 1988; 94: 432–433. [DOI] [PubMed] [Google Scholar]
- 13. Remington TL, Fuller J, Chiu I. Chronic necrotizing pulmonary aspergillosis in a patient with diabetes and marijuana use. CMAJ 2015; 187: 1305–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thompson GR, Tuscano JM, Dennis M, Singapuri A, Libertini S, Gaudino R, et al A microbiome assessment of medical marijuana. Clin Microbiol Infect 2017; 23: 269–270. [DOI] [PubMed] [Google Scholar]
- 15. Gargani Y, Bishop P, Denning D. Too many mouldy joints – marijuana and chronic pulmonary aspergilliosis. Mediterr J Hematol Infect Dis 2011; 3: e2011005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stone D. Cannabis, pesticides and conflicting laws: the dilemma for legalized states and implications for public health. Regul Toxicol Pharmacol 2014; 69: 284–288. [DOI] [PubMed] [Google Scholar]
- 17. Llewellyn GC, O'Rear CE. Examination of fungal growth and aflatoxin production on marihuana. Mycopathologia 1977; 62: 109–112. [DOI] [PubMed] [Google Scholar]
- 18. McPartland JM, McKernan KJ. Contaminants of concern in cannabis: microbes, heavy metals and pesticides In: Cannabis sativa L – Botany and Biotechnology, eds Chandra S, Lata H, Elsohly M. Cham, Switzerland: Springer, 2017. [Google Scholar]
- 19. Busse FP, Fiedler GM, Leichtle A, Hentschel H, Stumvoll M. Lead poisoning due to adulterated marijuana in Leipzig. Dtsch Arztebl Int 2008; 105: 757–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bolan N, Makino T, Kunhikrishnan A. Cadmium contamination and its risk management in rice ecosystems. Adv Agron 2013; 119: 183–273. [Google Scholar]
- 21. Linger P, Müssig J, Fischer H, Kobert J. Industrial hemp (Cannabis sativa L.) growing on heavy metal contaminated soil: fibre quality and phytoremediation potential. Ind Crops Prod 2002; 16: 33–42. [Google Scholar]
- 22. Girdhar M, Sharma N, Rehman H, Kumar A, Mohan A. Comparative assessment for hyperaccumulatory and phytoremediation capability of three wild weeds. 3 Biotech 2014; 4: 579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Combemale P, Consort T, Denis‐Thelis L, Estival J‐L, Dupin M, Kanitakis J. Cannabis arteritis. Br J Dermatol 2005; 152: 166–169. [DOI] [PubMed] [Google Scholar]
- 24. Noel B. Regarding ‘Cannabis arteritis revisited – ten new case reports’. Angiology 2001; 52: 505–506. [DOI] [PubMed] [Google Scholar]
- 25. Sullivan N, Elzinga S, Raber JC. Determination of pesticide residues in cannabis smoke. J Toxicol 2013; 2013 (378168): 10.1155/2013/378168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Skeet N. City Attorney explains medical marijuana issue on NBC. Los Angeles City Attorney website, 10 October 2009 [online]. Available at http://lacityorgatty.blogspot.com/2009/10/city‐attorney‐explains‐medical.html (last accessed 8 January 2018).
- 27. McGuinness TM. Update on marijuana. J Psychosoc Nurs Ment Health Serv 2009; 47: 19–22. [DOI] [PubMed] [Google Scholar]
- 28. Tashkin DP. Smoked marijuana as a cause of lung injury. Monaldi Arch Chest Dis Pulmonary Series 2005; 63: 93–100. [DOI] [PubMed] [Google Scholar]
- 29. Smith ND. Environmental exposures and genitourinary malignancies. Urol Oncol Semin Original Invest 2012; 30: 196–198. [DOI] [PubMed] [Google Scholar]
- 30. Tashkin DP. Effects of marijuana smoking on the lung. Ann Am Thorac Soc 2013; 10: 239–247. [DOI] [PubMed] [Google Scholar]
- 31. Tashkin DP, Baldwin GC, Sarafian T, Dubinett S, Roth MD. Respiratory and immunologic consequences of marijuana smoking. J Clin Pharmacol 2002; 42(S1): 71S–81S. [DOI] [PubMed] [Google Scholar]
- 32. Hazekamp A. Evaluating the effects of gamma‐irradiation for decontamination of medicinal cannabis. Front Pharmacol 2016; 7: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moir D, Rickert W, Levasseur G, Larose Y, Maertens R, White P, et al A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chem Res Toxicol 2008; 21: 494–502. [DOI] [PubMed] [Google Scholar]
- 34. International Agency for Research on Cancer . Aflatoxins. IARC monographs on the evaluation of carcinogenic risks to humans [online]. 2018. Available at https://monographs.iarc.fr/ENG/Monographs/vol100F/mono100F-23.pdf (last accessed 4 January 2018).
- 35. International Agency for Research on Cancer . Arsenic and arsenic compounds. IARC monographs on the evaluation of carcinogenic risks to humans [online]. 2012. Available at http://monographs.iarc.fr/ENG/Monographs/vol100C/ (last accessed 5 January 2018).
- 36. Narcotic Drugs Amendment Act 2016 (Cth), Stat . C2016A00012 (2016).
- 37. Stoker TE, Kavlock RJ. Pesticides as endocrine‐disrupting chemicals In: Hayes' Handbook of Pesticide Toxicology, 3rd edn, ed Krieger R. Amsterdam: Elsevier, 2010. [Google Scholar]
- 38. Dyer CA. Heavy metals and endocrine disrupting chemicals In: Endocrine‐disrupting Chemicals: From Basic Research to Clinical Practice, ed Gore A. Totowa, NJ: Humana Press, 2007. [Google Scholar]
- 39. Ben‐Shabata S, Fridea E, Sheskina T, Tamirib T, Rheec MH, Vogelc Z, et al An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2‐arachidonoyl‐glycerol cannabinoid activity. Eur J Pharmacol 1998; 353: 23–31. [DOI] [PubMed] [Google Scholar]
- 40. Mechoulam R, Ben‐Shabat S. From gan‐zi‐gun‐nu to anandamide and 2‐arachidonoylglycerol: the ongoing story of cannabis. Nat Prod Rep 1999; 16: 131–143. [DOI] [PubMed] [Google Scholar]
- 41. Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid‐terpenoid entourage effects. Br J Pharmacol 2011; 163: 1344–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Abou‐Arab AAK, Abou Donia MA. Heavy metals in Egyptian spices and medicinal plants and the effect of processing on their levels. J Agric Food Chem 2000; 48: 2300–2304. [DOI] [PubMed] [Google Scholar]
- 43. Madras K. Update of Cannabis and its Medical Use. Geneva, Switzerland: World Health Organisation Expert Committee on Drug Dependence, 2015. [Google Scholar]
- 44. Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet 2003; 42: 327–360. [DOI] [PubMed] [Google Scholar]
- 45. Lucas C, Galettis P, Song S, Solowij N, Reuter S, Schneider J, et al Cannabinoid disposition after human intraperitoneal use: an insight into intraperitoneal pharmacokinetic properties in metastatic cancer. Clin Ther 2018; 10.1016/j.clinthera.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 46. Pizzorno J. What should we tell our patients about marijuana (Cannabis indica and Cannabis sativa)? Dermatol Int 2016; 15: 8–12. [PMC free article] [PubMed] [Google Scholar]
- 47. Lorenz W, Bahadir M, Korte F. Thermolysis of pesticide residues during tobacco smoking. Chemosphere 1987; 16: 521–522. [Google Scholar]
- 48. Gartner CE. Mull it over: cannabis vaporizers and harm reduction. Addiction 2015; 110: 1709–1710. [DOI] [PubMed] [Google Scholar]
- 49. Budney AJ, Sargent JD, Lee DC. Vaping cannabis (marijuana): parallel concerns to e‐cigs? Addiction 2015; 110: 1699–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Loflin M, Earleywine M. A new method of cannabis ingestion: the dangers of dabs? Addict Behav 39: 1430–1433. [DOI] [PubMed] [Google Scholar]
- 51. Dinis‐Oliveira RJ. Metabolomics of Δ9‐tetrahydrocannabinol: implications in toxicity. Drug Metab Rev 2016; 48: 80–87. [DOI] [PubMed] [Google Scholar]
- 52. Elchier M, Spinedi L, Uinfer Grauwiler S, Bodmer M, Surber C, Luedi M, et al Heat exposure of Cannabis sativa extracts affects the pharmacokinetic and metabollc profile in healthy male subjects. Planta Med 2012; 78: 686–691. [DOI] [PubMed] [Google Scholar]
- 53. Buettner C, Mukamal KJ, Gardiner P, Davis RB, Phillips RS, Mittleman MA. Herbal supplement use and blood lead levels of United States adults. J Gen Intern Med 2009; 24: 1175–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Grøn C, Andersen L. Human Bioaccessibility of Heavy Metals and PAH from Soil. Hørsholm, Denmark: Danish Environmental Protection Agency, 2003. [Google Scholar]
- 55. Goldberg A, Meredith PA, Miller S, Moore MR, Thompson GG. Hepatic drug metabolism and haem biosynthesis in lead poisoned rats. Br J Pharmacol 1978; 65: 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Degawa M, Aria H, Kubota M, Hashimoto Y. Ionic lead, a unique metal ion as an inhibitor for cytochrome P4501A2 (CYP1A2) expression in the rat liver. Biochem Biophys Res Commun 1994; 200: 1086–1092. [DOI] [PubMed] [Google Scholar]
- 57. Alvares AP, Fischbein A, Sassa S, Anderson KE, Kappas A. Lead intoxication: effects on cytochrome P‐450 mediated hepatic oxidases. Clin Pharmacol Ther 1976; 19: 183–190. [DOI] [PubMed] [Google Scholar]
- 58. Lowry JA, Pearce RE, Gaedigk A, Venneman M, Talib N, Shaw P, et al Lead and its effects on cytochromes P450. J Toxicol 2006; 5: 1–4. [Google Scholar]
- 59. Rose RL, Tang J, Choi J, Cao Y, Usmani A, Cherrington N, et al Pesticide metabolism in humans, including polymorphisms. Scand J Work Environ Health 2005; 31: 156–163. [PubMed] [Google Scholar]
- 60. McPartland JM, Blanchon DJ, Musty RE. Cannabimimetic effects modulated by cholinergic compounds. Addict Biol 2008; 13: 411–415. [DOI] [PubMed] [Google Scholar]
- 61. Australian Institute of Health and Welfare . Australia's Health 2016 – Illicit Drug Use. Canberra: Australian Institute of Health and Welfare, 2016. [Google Scholar]
- 62. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res 2018; 46 (D1): D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA, et al The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 2017; 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Search strategy


