Abstract
Aims
Trimethylamine‐N‐oxide (TMAO) is a novel cardiovascular risk marker. We explored the association of commonly used cardiovascular medications with TMAO levels in patients and validated the identified associations in mice.
Methods
Detailed history of drug treatment was recorded in 300 patients with cardiovascular disease without diabetes in an observational, cross‐sectional study. Animal study was performed in CD1 mice.
Results
Median plasma TMAO (interquartile range) level was 2.144 (1.570–3.104) μmol l–1. Among nine cardiovascular drug groups, the use of loop diuretics (0.510 ± 0.296 in users vs. 0.336 ± 0.272 in nonusers, P = 0.008) and mineralocorticoid receptor antagonists (0.482 ± 0.293 in users vs. 0.334 ± 0.272 in nonusers, P = 0.007) was associated with increased log‐TMAO. Acute concomitant administration of furosemide or torasemide with TMAO in mice significantly influenced TMAO pharmacokinetic profile and almost doubled the plasma TMAO area under the curve. Furosemide decreased the TMAO excretion rate by 1.9‐fold during the first 30 min after administration and increased TMAO concentrations in kidney, heart and liver, suggesting the interaction of furosemide and TMAO with efflux transporters. The concentrations of TMAO in blood plasma after the administration of the organic anion transporter inhibitor probenecid were not different from those of the control group, suggesting an effect not mediated by organic anion transporters.
Conclusions
Loop diuretics increased plasma TMAO concentration by decreasing its urinary excretion rate. Loop diuretic use should be considered a potential confounder in TMAO studies.
Keywords: biomarkers, cardiovascular pharmacology, loop diuretics, trimethylamine‐N‐oxide
What is Already Known about this Subject
Trimethylamine‐N‐oxide (TMAO) is a cardiovascular risk marker
Effect of the most cardiovascular drugs on TMAO levels is unknown
What this Study Adds
Loop diuretics increase plasma levels and decrease renal elimination rate of TMAO
Loop diuretics should be taken into account as important confounders in future TMAO studies
The clinical importance of plasma TMAO elevations induced by loop diuretics should be further investigated
Introduction
Trimethylamine‐N‐oxide (TMAO) is a hepatic metabolite of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5521 (TMA) generated by the gut microbiome after the ingestion of dietary precursors http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4780, phosphatidylcholine, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4551 or http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?tab=summary&ligandId=4550 found in products such as red meat and eggs 1. In some products (e.g. fish), however, TMAO is abundant in its preformed state 2. Increased levels of TMAO are shown to have proatherosclerotic effects in animal studies and to correlate with all‐cause mortality as well as major cardiovascular and cerebrovascular events in clinical observational studies 3, 4, 5. In a dose–response meta‐analysis of seven clinical studies, the relative risk for all‐cause mortality increased by 7.6% per 10 μmol l–1 increment of TMAO 6.
Mechanisms explaining the association of TMAO with cardiovascular risk include decreased reverse http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2718 transport and increased inflammation, disturbances in mitochondrial energy metabolism 7, endothelial dysfunction, plaque vulnerability and platelet hyper‐reactivity 3, 8, 9, 10, 11. Although TMAO plasma levels are correlated with several conventional atherosclerotic risk factors such as age, diabetes and impaired renal function, in several studies, TMAO remained an independent risk marker after adjustment for these covariates 4, 5, 12, 13. Adjusted association, however, did not remain independent in other studies, and it is unclear to what extent these associations are subject to unrecognized confounders.
Numerous drugs commonly used for the treatment and prevention of cardiovascular disorders may have off‐target effects on TMAO levels. To the best of our knowledge, meldonium [mildronate; 3‐(2,2,2‐trimethylhydrazinium) propionate dehydrate], which exerts its cardioprotective action through an L‐carnitine‐lowering effect, is currently the only drug known to decrease TMAO plasma levels by increasing the urinary excretion of TMAO as shown in healthy volunteers 14, 15. Effects of other cardiovascular drugs on TMAO levels are unknown. We therefore aimed to analyse the association of commonly used cardiovascular medications with TMAO levels in a cross‐sectional study in patients with cardiovascular diseases and to further investigate the identified associations in experimental animal models.
Methods
Patients
Adult patients were included in an observational, cross‐sectional, single‐centre study at the Latvian Center of Cardiology, Pauls Stradins Clinical University Hospital. The study was approved by the local medical ethics committee and conformed with the principles outlined in the Declaration of Helsinki. All consecutive patients were referred to the hospital by their treating physicians due to suspected or previously established coronary artery disease. The human study was purely observational and the assignment of the medical intervention was not at the discretion of the investigator. All patients signed written informed consent to participate in the study. The following inclusion criteria were employed: (i) age 18–80 years; and (ii) invasive coronary angiography performed within the previous 6 months or planned within the following 1 week. Patients were excluded from the study according to the following criteria: (i) insulin‐dependent diabetes mellitus; (ii) use of oral antidiabetic drug within the prior week; (iii) use of carnitine containing supplements or drinks within the prior week; (iv) acute myocardial infarction or stroke within the prior week; (v) extensive surgery within the prior month (interventional cardiac procedures were not an exclusion criterion); (vi) severe comorbidity associated with significant changes in metabolism (such as active malignancy, hepatic insufficiency or hyperthyroidism) as judged by the study investigator; (vii) extensive use of alcohol (more than seven drinks per day); (viii) anaemia with haemoglobin levels <9 g dl–1; and (ix) consumption of fish, fish products or omega‐3 supplements on the day of blood sampling or on the previous day. Demographic characteristics, medical history, family history, weight, height, waist circumference, smoking status, history of other risk factors and medications used were recorded. Detailed history of drug treatment included name, dose and duration of the all the treatments within the month prior to the study. Patients with unclear history were excluded from respective analysis.
Blood samples were obtained by venepuncture from fasting patients. Plasma was immediately separated in EDTA tubes by centrifugation and stored in a freezer at –80°C until further analysis of TMAO levels by ultra‐high performance liquid chromatography–tandem mass spectrometry (UPLC/MS/MS).
Biochemistry analyses included creatinine, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, total cholesterol, triglycerides, alanine aminotransaminase, bilirubin, creatine kinase and glucose levels. Glomerular filtration rate was calculated according to the 2009 chronic kidney disease (CKD)‐EPI creatinine equation (eGFRcreat) as recommended by the KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease: 141 × min (SCr/k, 1)a × max (SCr/k, 1)–1.209 × 0.993Age [× 1.018 if female] [× 1.159 if black], where SCr is serum creatinine (in mg l–1), k is 0.7 for females and 0.9 for males, a is –0.329 for females and – 0.411 for males, min is the minimum of SCr/k or 1, and max is the maximum of SCr/k or 1 16, 17.
Animals
Seventy‐eight male CD1 mice (age 5–7 weeks; Envigo, the Netherlands) were housed under standard conditions (21–23°C, relative humidity 50% ± 10%, 12 h–12 h light–dark cycle) with unlimited access to food and water. The experimental procedures were approved by the Latvian Animal Protection Ethical Committee, Food and Veterinary Service, Riga, Latvia and were performed in accordance with the guidelines of the European Community (2010/63/EU), local laws and regulations. Performed studies involving animals are reported in accordance with the ARRIVE guidelines 18, 19. Data and statistical analysis were in adherence to British Journal of Pharmacology guidelines 20. Mice were acclimatized to the local conditions for two weeks before the start of treatment. For each experiment, CD1 mice were divided into two or three groups (six mice per group).
Effects of loop diuretics on TMAO excretion
To evaluate the acute effects of loop diuretics, TMAO (dehydrate; Alfa Aesar, Karlsruhe, Germany) was injected intraperitoneally (i.p.) at the dose of 100 and 10 mg kg–1 concomitantly with http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4839 (Sopharma Pharmaceuticals, Sofia, Bulgaria) at the dose of 50 mg kg–1 or http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7312 (A. Menarini Manufacturing Logistics and Services, Florence, Italy) at the dose of 50 mg kg–1, and samples of plasma were collected at baseline (before injection) and after 30, 60, 90, 12 and 240 min of injection. TMAO and saline was i.p. injected into the control group. The inhibitor of organic anion transporters, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4357 (TCI Chemicals, Tokyo, Japan), at a dose of 200 mg kg–1 and furosemide (10 mg kg–1) was i.p. administered with TMAO (10 mg kg–1, i.p.) to clarify the possible mechanism of action of furosemide‐induced effects. Probenecid was dissolved in 8.6% NaHCO3‐containing saline solution and passed through a sterile membrane filter (Filtropur S, 0.2 μm; Sarstedt, Nümbrecht, Germany).
Urine samples were collected after the administration of TMAO (10 mg kg–1, i.p.) or TMAO with furosemide (50 mg kg–1, i.p.) at different time intervals (0–30, 30–90 and 90–120 min) to assess the rate of TMAO excretion in urine. The excretion rate of TMAO in urine was calculated as follows: (concentration of TMAO in urine (μmol ml–1) × urine volume (ml))/time (min). To determine the tissue content of TMAO, the mice were sacrificed by decapitation, and plasma, heart, kidney and liver tissue samples were collected after 60 min of TMAO (10 mg kg–1, i.p.) or TMAO with furosemide (50 mg kg–1, i.p.) administration.
To evaluate the effects of the furosemide and torasemide on the pharmacokinetic profile of TMAO after long‐term treatment, furosemide (20 mg kg–1, s.c.) and torasemide (10 mg kg–1. s.c.) was administered for 21 days. After 7 and 21 days of diuretic administration, plasma samples were collected at baseline (24 h after the last administration of diuretics) and after 30, 60, 90, 12 and 240 min of TMAO (100 or 10 mg kg–1, i.p.) injection. Additionally, furosemide (10 mg kg–1) was administered with drinking water for 21 days to evaluate the effects of the furosemide on TMAO plasma concentrations after long‐term peroral treatment. Plasma samples were collected at baseline and every week for up to 3 weeks.
The collected samples were stored at –20°C prior to analysis. Plasma, urine and tissue samples were deproteinized using acetonitrile/methanol (3/1, v/v) solution, centrifuged and then analysed by UPLC/MS/MS as described previously 21.
Determination of TMAO levels by UPLC/MS/MS analysis
The chromatographic separation was performed using an ACQUITY UPLC system (Waters, USA) on an ACQUITY UPLC BEH HILIC column (2.1 × 50 mm, 1.7 μm, Waters) with a gradient elution from 75 to 55% acetonitrile in 10 mmol l–1 aqueous ammonium acetate (pH 4) at a flow rate of 0.25 ml min–1. The analyte was ionized by electrospray ionization in positive ion mode on a Quattro Micro triple‐quadrupole mass spectrometer (Water). The mass spectrometer was set up as follows: capillary voltage of 0.3 kV; source and desolvation temperatures of 120 and 350°C, respectively; and desolvation gas (nitrogen) flow of 500 l h–1. Cone voltage was 22 V, and collision energy was 14 eV. TMAO analysis was performed in the MRM mode. Precursor to product ion transition was m/z 75.9 → 58.7. Data acquisition and processing were performed using the MassLynx V4.1 and QuanLynx V4.1 software (Waters). The plasma extracts were kept at 10°C in the autosampler. The calibrators were run in triplicate, and the samples in duplicate. Data analysts were blinded to the information about received treatments.
Materials
TMAO dihydrate was obtained from Alfa Aesar. Furosemide was from Sopharma Pharmaceuticals, torasemide was from A. Menarini Manufacturing Logistics and Services. Probenecid was from TCI Chemicals. Acetonitrile, methanol, ammonium acetate and sodium bicarbonate (NaHCO3) were purchased from Sigma–Aldrich (St Louis, MO, USA). Filtropur S (0.2 μm) membrane filters were supplied by Sarstedt.
Data analysis
For human data, TMAO levels were expressed as median and interquartile range (IQR) due to non‐normal distribution. TMAO values were normalized with decimal logarithm (log‐TMAO) to generate a Gaussian‐distributed data set amenable to parametric analysis. Log‐TMAO and other quantitative variables were described as the mean ± standard deviation (SD). Categorical and ordinal variables were expressed as numbers (n) and percentages. Log‐TMAO levels in users and nonusers of a cardiovascular drugs were compared with t test for independent samples for two groups and with one‐way ANOVA for more than two groups. In posthoc analysis, subgroup comparisons were performed with the Tukey's test. A two‐way ANOVA was performed to analyse effect of two categorical independent variables on log‐TMAO. Adjustment for covariates was performed with backward multiple linear regression analysis.
For animal data, TMAO levels were expressed as the mean ± standard error of mean. Statistically significant differences in the mean values were tested by unpaired t test or one‐way ANOVA with Dunnett's multiple comparison post‐test.
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology. 20 All results were considered significant when P < 0.05. The data were analysed using GraphPad Prism 3.0 statistical software (GraphPad Inc., USA) for animal data and IBM SPSS Statistics software (version 22) for human data.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 22, and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 23, 24, 25.
Results
General characteristics of the patient group
Patient characteristics are summarized in Table 1. Three hundred patients were included in the study, and the mean age was 62.8 ± 9.3 years in men and 68.2 ± 8.3 in women. All patients were without history of diabetes. One patient had GFR category G4 of CKD. No patient had GFR category G5 or class 4 chronic heart failure (according to the New York Heart Association Functional Classification). Median (interquartile range) TMAO levels were 2.144 (1.570–3.104) μmol l–1. Mean ± SD logarithmically transformed TMAO (log‐TMAO) was 0.347 ± 0.277. A majority of patients (n = 266, 88.7%) had signs of coronary atherosclerosis or previous stent on angiography. More detailed history of cardiovascular disease is provided in Table 2.
Table 1.
Demographic and clinical characteristics of the patient group (n = 300)
| Characteristic | Value |
|---|---|
| Age (years), mean (SD) | 65.0 (9.3) |
| Male sex, n (%) | 179 (59.7) |
| History of smoking | |
| Current smoker, n (%) | 71 (23.7) |
| Nonsmoker, n (%) | 214 (71.3) |
| Ex‐smoker, n (%) | 15 (5.0) |
| Positive family history of premature cardiovascular disease, n (%) | 52 (17.3) |
| BMI (kg m –2 ), mean (SD) | 29.1 (4.7) |
| TMAO (μmol l –1 ) median (IQR) | 2.144 (1.570–3.104) |
| log‐TMAO, mean (SD) | 0.347 (0.277) |
| eGFR creat (ml min –1 1.73‐m –2 ), mean (SD) | 81.58 (16.48) |
| eGFR creat categories | |
| G1, n (%) | 102 (34.0) |
| G2, n (%) | 160 (53.3) |
| G3a, n (%) | 32 (10.7) |
| G3b, n (%) | 5 (1.7) |
| G4, n (%) | 1 (0.3) |
| G5, n (%) | 0 (0) |
| Cholesterol (mmol l –1 ), mean (SD) | 4.37 (1.19) |
| Triglycerides (mmol l –1 ), median (IQR) | 1.01 (1.00–1.90) |
| HDL‐C (mmol l –1 ), mean (SD) | 1.22 (0.41) |
| LDL‐C (mmol l –1 ), mean (SD) | 2.54 (1.00) |
| ALT (U l –1 ), mean (SD) | 30.19 (20.76) |
| Conjugated bilirubin (U l –1 ) mean (SD) | 4.82 (3.12) |
| Unconjugated bilirubin (U l –1 ), mean (SD) | 7.93 (4.35) |
| CK (U l –1 ), mean (SD) | 131.07 (90.81) |
ALT, alanine aminotransferase; BMI, body mass index; CK, creatine kinase; eGFRcreat, estimated glomerular filtration rate based on 2009 CKD‐EPI creatinine equation; HDL‐C, high‐density lipoprotein cholesterol; IQR, interquartile range; LDL‐C, low‐density lipoprotein cholesterol; SD, standard deviation; TMAO, trimethylamine‐N‐oxide
Table 2.
History of cardiovascular diseases
| Characteristic | Value |
|---|---|
| Hypertension, n (%) | 263 (87.7) |
| Stable angina, n (%) | 283 (94.3) |
| History of MI, n (%) | 75 (25.0) |
| History of unstable angina, n (%) | 9 (3.0) |
| History of PCI, n (%) | 107 (35.7) |
| History of CABG, n (%) | 6 (2.0) |
| History of stroke, n (%) | 19 (6.3) |
| Known carotid atherosclerosis , n (%) | 37 (12.3) |
| PAD, n (%) | 72 (24.0) |
| Atrial fibrillation | 42 (14.0) |
| Chronic heart failure, n (%) a | 164 (54.7) |
| Class I, n (%) | 37 (12.3) |
| Class II, n (%) | 115 (38.3) |
| Class III, n (%) | 9 (3.0) |
According to the New York Heart Association Functional Classification
CABG, coronary artery bypass grafting; MI, myocardial infarction; PAD, peripheral artery disease; PCI, percutaneous coronary intervention
Association of diuretic use with higher TMAO levels in patients
TMAO levels were compared between users and nonusers of the major cardiovascular drug groups (Table 3). Users of diuretics or http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=626 antagonists (MRA) had significantly higher TMAO levels than did nonusers of these drugs (P = 0.005 and P = 0.007, respectively). Loop diuretics, but not thiazides, were associated with high TMAO levels (P = 0.008 and P = 0.216, respectively), and MRA showed additive effect with loop diuretics (P = 0.024 for ANOVA among the four groups, Figure 1A). There was no significant difference of TMAO levels between two thiazides – http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4836 (n = 22) and http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7203 (n = 44; log‐TMAO 0.355 ± 0.245 and 0.399 ± 0.322, respectively, P = 0.575). Among loop diuretic users, most were on torasemide (n = 15), and only four patients were on furosemide, and the difference in TMAO levels was not statistically significant between the two groups (log‐TMAO 0.530 ± 0.313 and 0.434 ± 0.240, respectively, P = 0.576). All MRA users were on http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2875, and none was on http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2876. Other cardiovascular medications such as statins, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1613 (ACE) inhibitors, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2504 type‐2 receptor (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=35) blockers, β‐blockers, dihydropyridines and P2Y12 platelet ADP receptor (http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=328) inhibitors were not significantly associated with TMAO levels in this cohort.
Table 3.
Trimethylamine‐N‐oxide levels in users and nonusers of various cardiovascular drugs
| Drug group (users vs. nonusers) | log‐TMAO (mean ± SD) | P value | |
|---|---|---|---|
| Users | Nonusers | ||
| Diuretics ( n = 91 vs. n = 209) | 0.414 ± 0.299 | 0.318 ± 0.262 | 0.005 |
| Loop diuretics ( n = 19 vs. n = 281) | 0.510 ± 0.296 | 0.336 ± 0.272 | 0.008 |
| Thiazides ( n = 66 vs. n = 234) | 0.385 ± 0.297 | 0.337 ± 0.271 | 0.216 |
| MRA ( n = 28 vs. n = 272) | 0.482 ± 0.293 | 0.334 ± 0.272 | 0.007 |
| Statins ( n = 225 vs. n = 74) | 0.358 ± 0,257 | 0.314 ± 0.332 | 0.237 |
| ACE inhibitors ( n = 184 vs. n = 113) | 0.368 ± 0.277 | 0.318 ± 0.277 | 0.136 |
| ARB ( n = 37 vs. n = 263) | 0.299 ± 0.331 | 0.354 ± 0.269 | 0.259 |
| Beta blockers ( n = 210 vs. n = 88) | 0.353 ± 0.272 | 0.337 ± 0.294 | 0.657 |
| DHP ( n = 105 vs. n = 194) | 0.356 ± 0.326 | 0.343 ± 0.248 | 0.713 |
| Aspirin ( n = 196 vs. n = 103) | 0.350 ± 0.277 | 0.344 ± 0.279 | 0.872 |
| P2Y 12 receptor inhibitor ( n = 101 vs. n = 199) | 0.366 ± 0.293 | 0.338 ± 0.269 | 0.414 |
ACE, angiotensin‐converting enzyme, ARB, angiotensin II type‐2 receptor (AT2 receptor) antagonist, DHP, dihydropyridine, MRA, mineralocorticoid receptor antagonists. Cases with an unclear history of use of particular drug group were excluded from respective analysis. Log‐TMAO levels were compared with t test for independent samples.
Figure 1.
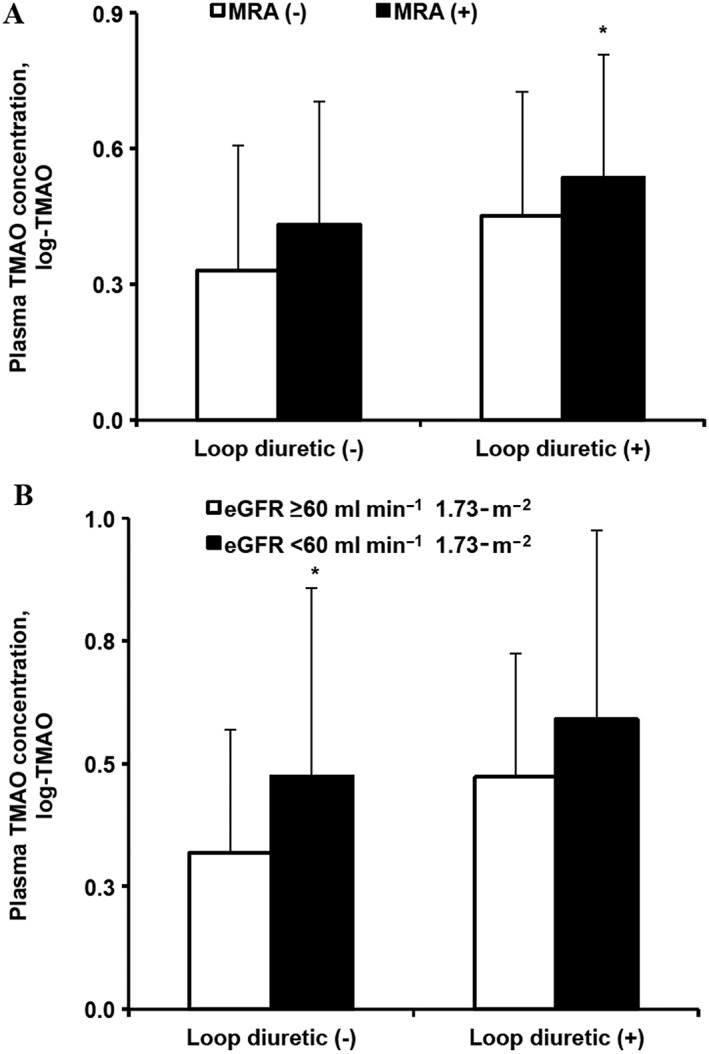
Effect of (A) loop diuretics and mineralocorticoid receptor antagonists (MRAs) and (B) loop diuretics and eGFRcreat < 60 ml min–1 1.73‐m–2 on trimethylamine‐N‐oxide (TMAO) levels in patients. eGFRcreat, estimated glomerular filtration rate based on the 2009 CKD‐EPI creatinine equation. Values are represented as the mean ± standard deviation [number of patients in each subgroup: (A) n = 266, n = 15, n = 6 and n = 13, and (B) n = 249, n = 32, n = 13 and n = 6, respectively]. *P < 0.05 for posthoc subgroup analyses with Tukey's test compared to the reference group (P values: 0.493, 0.716, 0.042 and 0.013, 0.190, 0.073 for (A) and (B), respectively)
The use of meldonium, a drug known to lower TMAO levels, 14 was reported by nine patients, and the majority (n = 8) were using it irregularly. When blood samples were tested for meldonium levels, it was detectable in 43 samples. Neither self‐reported use of meldonium nor the presence of the drug in the blood was significantly associated with log‐TMAO (P = 0.685 and P = 0.678, respectively) in this cohort; therefore, meldonium use was not considered a significant covariate in this study. Moreover, the association of loop diuretics and MRA with lower log‐TMAO values remained significant (P = 0.007 and P = 0.003, respectively) when statistical analysis was confined to meldonium‐naive patients (n = 252).
To adjust for other potential covariates, we performed a backward multiple linear regression analysis with the following independent variables entered in the model: age, sex, eGFRcreat, body mass index, loop diuretic and MRA. In the final model, only the following variables remained significantly correlated with log‐TMAO: eGFRcreat (β = –0.283, P < 0.001) and loop diuretics (β = 0.110, P = 0.049). Users of loop‐diuretics had lower eGFRcreat than nonusers (71.98 ± 16.87 vs. 82.23 ± 16.28, respectively, P = 0.008). Among other studied drug groups, only MRA and beta blockers were associated with lower eGFRcreat: 72.06 ± 17.06 vs. 82.56 ± 16.13 (P = 0.001) and 79.99 ± 16.07 vs. 85.11 ± 17.05 (P = 0.014) for MRA and β‐blockers users and nonusers, respectively. Patients with eGFRcreat < 60 ml min–1 1.73‐m–2 had higher log‐TMAO levels than those with eGFRcreat ≥ 60 ml min–1 1.73‐m–2 (0.493 + 0.362 vs. 0.326 + 0.257, respectively, P < 0.001). A two‐way ANOVA was conducted that examined the effect of loop diuretics and eGFRcreat < 60 ml min–1 1.73‐m–2on log‐TMAO. Both variables were independently associated with higher log‐TMAO (P = 0.028 and P = 0.002 for loop diuretics and eGFRcreat < 60 ml min–1 1.73‐m–2, respectively; P = 0.001 for ANOVA among the four groups, Figure 1B).
Effects of acute administration of loop diuretics on the plasma concentration of TMAO in mice
Studies in mice were performed to further explore the findings from observational data in patients. The concentrations of TMAO in blood plasma after the administration of 10 and 100 mg kg–1 dose in mice are shown in Figures 2A and 3A. The levels of TMAO in blood peaked at 15–30 min after TMAO administration and then decreased to the initial concentrations within 4 h. As shown in Figures 2 and 3, after the acute administration of furosemide, TMAO concentrations in plasma at 30, 60 and 120 min on average were significantly higher by 2‐fold than those in the control group, and the total increase in an area under the curve (AUC) was 1.8‐fold (Figures 2 and 3). Additionally, the acute administration of torasemide significantly increased the TMAO plasma concentrations at 60 and 120 min by 3‐fold and the AUC by 78% (Figures 2A,B).
Figure 2.
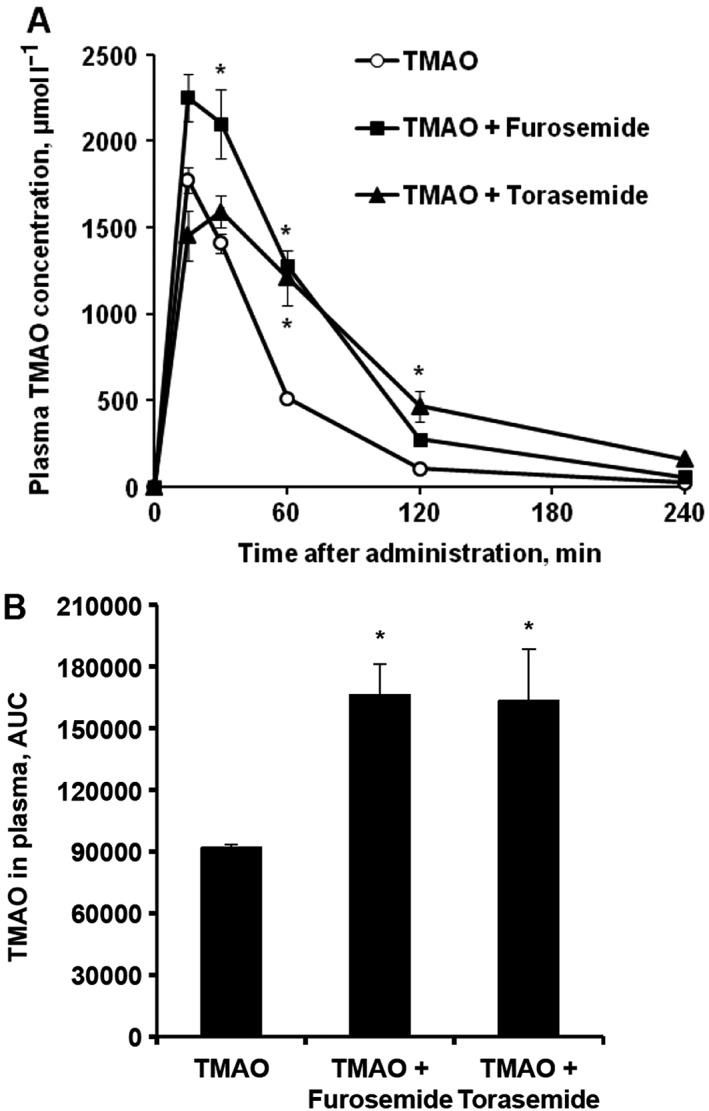
The effects of acute administration of loop diuretics after concomitant trimethylamine‐N‐oxide (TMAO) administration on the plasma concentrations of TMAO in mice. TMAO concentrations were determined in plasma samples up to 4 h after TMAO (100 mg kg–1) administration with furosemide (50 mg kg–1) or torasemide (50 mg kg–1; A) in CD1 mice, and respective areas under the curve (AUCs) of TMAO levels were calculated (B). Values are represented as the mean ± standard error of the mean of 5–6 animals. *Significantly different from the control group (one‐way ANOVA with Dunnett's Multiple Comparison post‐test, P < 0.05)
Figure 3.
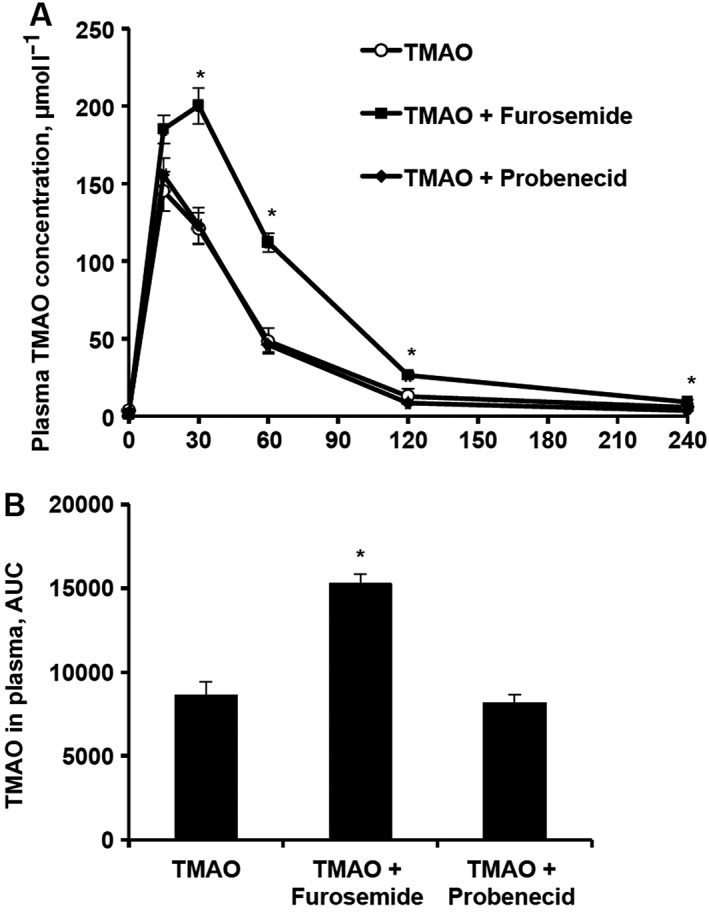
The effects of acute administration of furosemide and probenecid after concomitant trimethylamine‐N‐oxide (TMAO) administration on the plasma concentration of TMAO in mice. TMAO concentrations were determined in plasma samples up to 4 h after TMAO (10 mg kg–1) administration with furosemide (50 mg kg–1) or probenecid (200 mg kg–1; A) in CD1 mice, and respective areas under the curve (AUCs) of TMAO levels were calculated (B). Values are represented as the mean ± standard error of the mean of 5–6 animals. *Significantly different from the control group (one‐way ANOVA with Dunnett's multiple comparison post‐test, P < 0.05)
To clarify whether the increased plasma TMAO concentrations induced by the acute administration of loop diuretics are related to their effects on TMAO transport proteins, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1025 and http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1027, and the influence of the inhibitor of http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=198, probenecid, were examined in parallel to furosemide on the plasma pharmacokinetic profile of TMAO. In contrast to those after furosemide administration, the concentrations of TMAO in blood plasma after probenecid administration were not different from that of the control group (Figure 3).
In another experiment, we measured the TMAO concentration in plasma and TMAO content in kidney, heart and liver tissues after the acute administration of furosemide with TMAO. The concentrations of TMAO in plasma 1 h after the administration of furosemide were increased by 2‐fold (Figure 4A). In comparison, administration of TMAO increased TMAO content in kidney, heart and liver tissues up to 57 ± 5, 10 ± 0.4 and 8 ± 0.5 nmol g–1, respectively. Compared to the control levels, the contents of TMAO in kidney, heart and liver tissues after the acute administration of TMAO were increased by 15‐, 20‐ and 5‐fold, respectively. Furosemide administration induced an even higher increase in TMAO content in kidney, heart and liver tissues by 2.4‐, 1.4‐ and 1.6‐fold, respectively, in comparison to TMAO control administration (Figures 4B,C). This effect might be related to increased plasma concentration and decreased TMAO efflux from cells.
Figure 4.
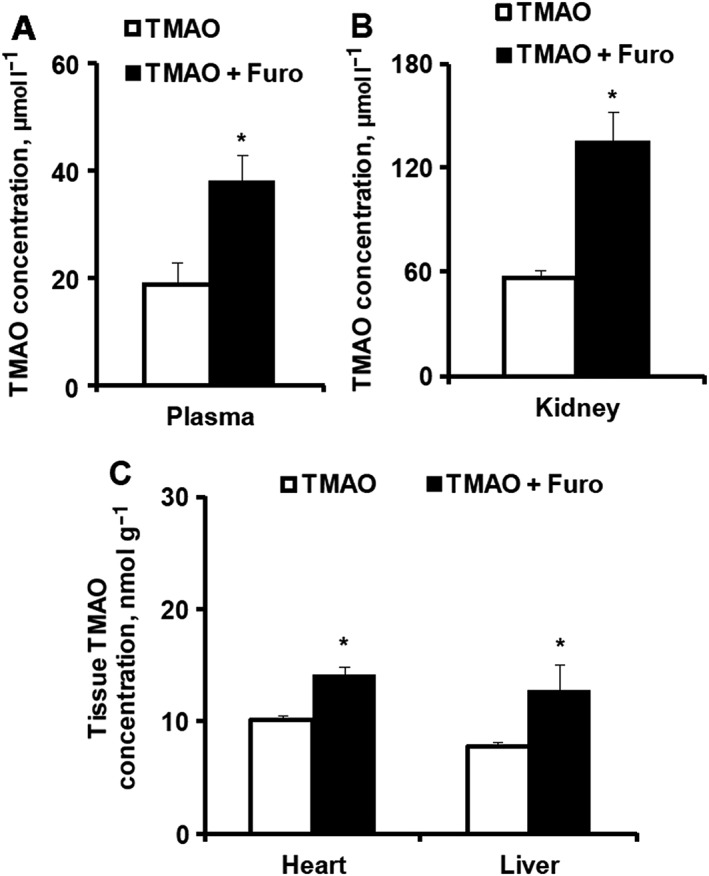
The effects of acute administration of furosemide after concomitant trimethylamine‐N‐oxide (TMAO) administration on the plasma and tissue concentration of TMAO in mice. TMAO concentrations were determined in plasma (A) and in kidney, heart and liver tissues (B) 60 min after TMAO (10 mg kg–1) administration with furosemide (50 mg kg–1) in CD1 mice. Values are represented as the mean ± standard error of the mean of 6 animals. *Significantly different from the control group (Unpaired t test, P < 0.05)
To evaluate whether TMAO levels in blood was increased because of changed urinary excretion rate, we measured TMAO concentrations in urine after its coadministration with furosemide. The average urine concentration of TMAO at baseline was 0.6 μmol ml–1. TMAO administration after 30 min induced a rapid increase in its urine concentration up to 19.4 ± 4.0 μmol ml–1 (Figure 5A). TMAO concentrations in urine were significantly decreased by 8‐ and 4‐fold during the first 30 min and 90–120 min, respectively, after acute furosemide administration relative to those in the TMAO control group (Figure 5A). The acute administration of furosemide during a 2 h period increased urine volume by 4‐fold (on average from 509 ± 109 to 2078 ± 123 mg/120 min), while it significantly decreased the TMAO concentration by up to 8‐fold; thus, the calculated TMAO excretion rate was decreased by 1.9‐fold during the first 30 min after administration (Figure 5B). No significant differences in TMAO urinary excretion rate were observed at later time intervals for 2 h compared to the rate in the control group (Figure 5B).
Figure 5.
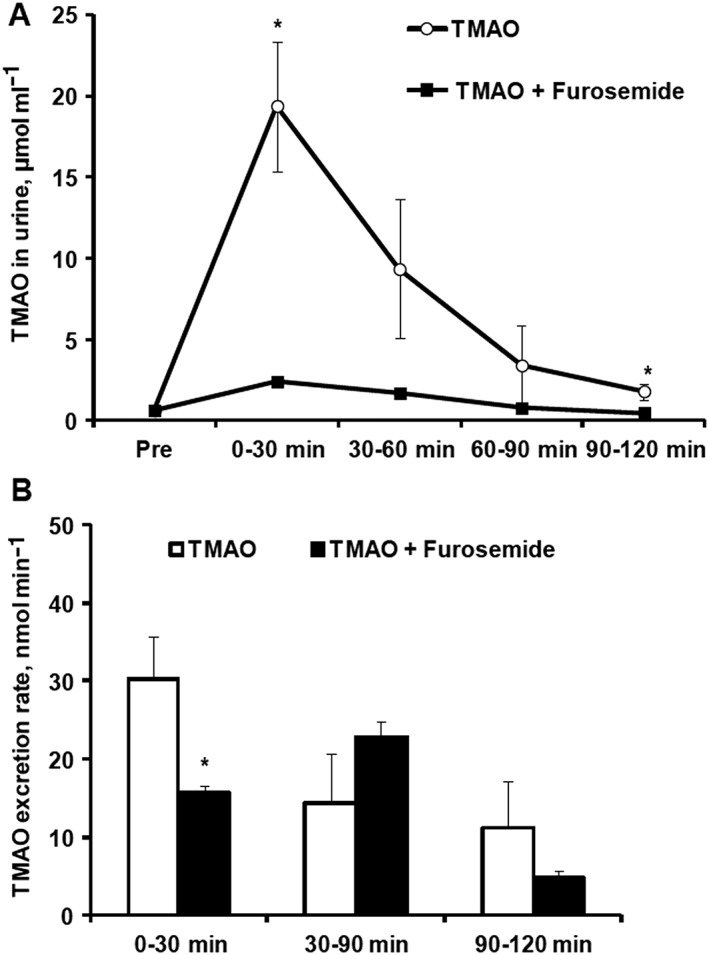
The effects of acute administration of furosemide after concomitant trimethylamine‐N‐oxide (TMAO) administration on the urinary excretion of TMAO in mice. TMAO concentrations were determined in urine (A), and excretion rate of TMAO in urine was calculated (B) at every 30 min interval until 2 h after TMAO (10 mg kg–1) administration with furosemide (50 mg kg–1) in CD1 mice. Values are represented as the mean ± standard error of the mean of 3–6 animals. *Significantly different from the control group (unpaired t test, P < 0.05)
To determine whether the observed functional effects of loop diuretics on TMAO pharmacokinetic profile are due to the long‐term effects of the diuretics on the functional or expressional changes of TMAO transport proteins, we studied TMAO pharmacokinetics on days 7 and 21. To avoid the presence of diuretics, TMAO was administered 24 h after the last administration of furosemide and torasemide. The plasma concentration of TMAO after 21 days of furosemide and torasemide administration was 1 ± 0.1 and 1.2 ± 0.1 μmol l–1, respectively, and it was not significantly different from that in the control group (1 ± 0.1 μmol l–1). The plasma pharmacokinetic profile of TMAO was not different between groups when furosemide and torasemide were administered subcutaneously for 7 (Figure 6A) and 21 days (Figure 6B). Furthermore, peroral administration of furosemide at a dose of 10 mg kg–1 in mice did not significantly increase TMAO concentration in plasma for up to 21 days (the average plasma concentration was 1 ± 0.1 μmol l–1; data not shown).
Figure 6.
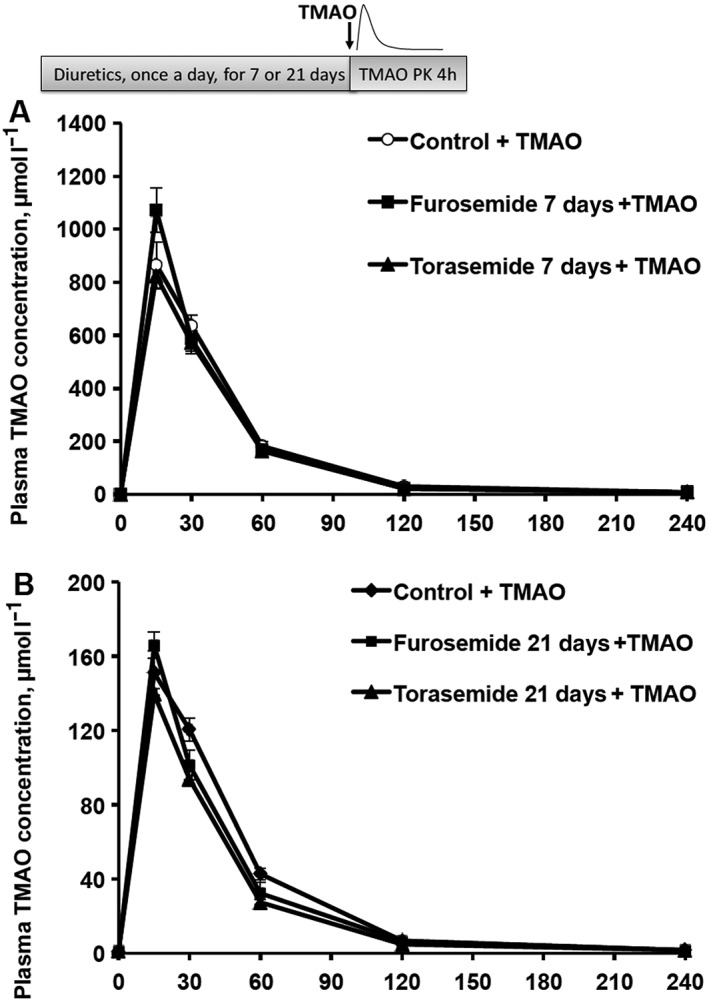
The effects of long‐term administration of loop diuretics after acute trimethylamine‐N‐oxide (TMAO administration on the plasma concentration of TMAO in mice. After 7 (A) and 21 days (B) of furosemide (20 mg kg–1) or torasemide (10 mg kg–1) administration, TMAO concentrations were determined in blood plasma samples up to 4 h after a single dose of TMAO (100 and 10 mg kg–1) administration in CD1 mice. Values are represented as the mean ± standard error of the mean of 6 animals
Discussion
Our findings suggest an association of loop diuretic use with increased TMAO plasma concentrations in patients with cardiovascular diseases. Moreover, we found that the acute concomitant administration of either furosemide or torasemide with TMAO increased plasma TMAO levels in mice by decreasing the elimination rate of TMAO.
In our cross‐sectional observational study, loop diuretics showed strong and independent association with increased TMAO levels in patients with cardiovascular diseases. Only MRAs and none of the other eight commonly used cardiovascular agents were significantly associated with higher TMAO levels, and its effect was additive to loop diuretics. No such effect was seen with other diuretics, namely, hydrochlorothiazide or indapamide. Diuretics are known to have several unfavourable effects on metabolism such as impaired glucose intolerance and dyslipidaemia; and it has been shown that higher TMAO levels in patients with heart failure are associated with diuretic use; however, to the best of our knowledge, this is the first study to demonstrate that loop diuretics increase TMAO plasma levels 26, 27. Clinical implications of this association remain to be determined, but a potential risk of atherogenicity may be implied, although no effect of loop diuretics on atherogenesis has been previously established. In a study by Schartum‐Hansen et al. 28, the use of loop diuretics in patients with suspected coronary artery disease, but without systolic heart failure or renal impairment, was associated with increased risk of all‐cause mortality during a 10‐year follow‐up. Potential unmeasured confounders and other unfavourable effects of loop diuretics such as hypokalaemia, hyponatraemia and hypovolaemia, however, could not be excluded in this observational study and were not explained by atherosclerotic cardiovascular events 28.
Using animal models, we showed that the acute coadministration of TMAO and furosemide or torasemide increased plasma TMAO levels, and this effect was observed as early as within 30 min of administration for furosemide, and it persisted for at least 2 h. Since acute increase in TMAO concentrations was observed after parenteral administration, the effect on TMA production by microbiota in the intestinal compartment was excluded. In addition, chronic peroral administration of furosemide or torasemide for 7 or 21 days did not affect TMAO levels in the absence of drug administration within the prior 24 h, implying that the functional or expressional changes of kidney transport proteins are less likely to play a role. The shift in TMAO plasma pharmacokinetic profile after acute administration suggests a direct interaction of TMAO and loop diuretics on TMAO transporters. Indeed, the acute administration of furosemide decreased the TMAO excretion rate in urine with a fast onset of action. Moreover, furosemide increased TMAO concentrations in renal tissues to a greater extent than in plasma, heart and liver tissues, confirming the involvement of efflux transporters.
Furosemide is known as an inhibitor of OAT1 and OAT3 29, and a recent study reported elevated plasma levels of TMAO in OAT3 knockout mice 30. In our study, we assessed the effect of OAT1/3 inhibitor probenecid, and the lack of its effect on the plasma pharmacokinetic profile of TMAO excluded OATs as a potential mechanism of action. Similarly, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1031, which is common for furosemide and probenecid, is not a transporter for TMAO 31. Recently, multiple transporters involved in TMAO kinetics were elucidated 30, 32, 33. These studies did not confirm OAT‐mediated TMAO transport but showed http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1020 to be involved in the cellular uptake of TMAO. Currently, there is no evidence on the influence of furosemide or torasemide on http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=196#1019 and OCT2 31. In a recent study, it was shown that TMAO is a substrate for multiple efflux transporters http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=768, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=792, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=780, http://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=782, http://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=337) 33, which might also be affected by furosemide. The exact mechanisms by which TMAO levels are increased by loop diuretics remain to be determined, and, although the effect could be multifactorial, our data support the role of renal efflux mechanisms.
The importance of renal function in TMAO metabolism has been demonstrated in several studies. Serum TMAO concentrations are inversely correlated with glomerular filtration rate, are markedly increased in patients with end‐stage renal disease and are substantially decreased after renal transplantation 13, 34. Although eGFRcreat was a strong confounder in the present study, the association of TMAO level with loop diuretic use in patients remained significant after the adjustment for eGFRcreat. Meldonium, a drug previously shown to decrease plasma TMAO concentrations, exhibits its effect via renal mechanisms by enhancing urinary excretion 14. Recently, http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6322 was reported to decrease plasma TMAO levels in Wistar rats, with a trend of increasing urinary TMAO excretion without change in gut microflora 35. In our study, the use of ACE inhibitors was not significantly associated with TMAO levels in humans, but in absolute terms, the TMAO levels tended to be higher in ACE inhibitor users. Such discrepancy could be explained by the following: use of ACE inhibitors other than enalapril in our sample (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6367 or http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6339 among more than 90% of ACE inhibitor users), different effects of the drug class in humans and rats, or the play of chance.
One major advantage of our study was that the same association was seen in human and animal data. The patient selection criteria and preconditions of plasma sampling were specifically determined to evaluate TMAO levels and to minimize several important biases such as consumption of fish, fish products, and carnitine‐ and choline‐containing supplements. Detailed history on cardiovascular drug therapy enabled us to identify specific drug subgroups associated with increased TMAO levels, namely, loop diuretics, but not thiazides.
Some caveats of the study include the retrospective nature of patient data on the use of cardiovascular drugs that may have led to recall and selection biases. Such biases are, however, less likely regarding the effect of loop diuretics, as their effects on TMAO were confirmed in the animal study. Long‐term peroral administration of furosemide in animals did not significantly increase TMAO concentrations, which excluded a potential effect of diuretics on TMA production by microbiome; nevertheless, the acute administration of loop diuretics increased TMAO plasma levels irrespective of their effect on the gut microflora.
Conclusions
Loop diuretics increased plasma TMAO concentrations by decreasing urinary excretion rate, and this effect is not mediated by organic anion transporters. Further clinical studies should evaluate potential TMAO‐mediated detrimental cardiovascular effects of loop diuretics. Loop diuretic use should be considered a potential confounder in TMAO studies.
Competing interests
G.L.: consulting fees/honoraria/speaker bureau: Amgen, Astra‐Zeneca, Bayer, Berlin‐Chemie/Menarini, Boehringer Ingelheim, GlaxoSmithCline, Mylan, Novartis, Novo Nordisk, Pfizer, Sanofi‐Aventis, Servier, Siemens Laboratories; A.E.: grant/research support: Abbott Vascular, Boston Scientific; consulting fees/honoraria/speaker bureau: Abbott Laboratories, Abbott Vascular, Amgen, Astra‐Zeneca, Bayer, Berlin Chemie/Menarini, Biosensors, Biotronik, Boehringer Ingelheim, Boston Scientific, Cordis, GlaxoSmithKline, Merck, Mylan, Olainfarm, Pfizer, Roche, Sandoz, Sanofi, Servier Laboratories, Siemens Laboratories; E.M., M.M., L.B., D.H., H.C., S.G., E.L. and M.D. have no competing interests.
The work was supported by the Ministry of Education and Science of the Republic of Latvia [Latvian National Research Program Biomedicine for Public Health (BIOMEDICINE) 2014–2017].
Contributors
G.L., E.L. and M.D. designed the research study; M.M. and L.B. performed the study in humans; E.M. and H.C. conducted experiments; S.G. and D.H. performed analytical chemistry; G.L. and E.M. performed data analysis; G.L., M.D., E.M., E.L. and A.E. wrote or contributed to the writing of the manuscript.
Latkovskis, G. , Makarova, E. , Mazule, M. , Bondare, L. , Hartmane, D. , Cirule, H. , Grinberga, S. , Erglis, A. , Liepinsh, E. , and Dambrova, M. (2018) Loop diuretics decrease the renal elimination rate and increase the plasma levels of trimethylamine‐N‐oxide. Br J Clin Pharmacol, 84: 2634–2644. 10.1111/bcp.13728.
References
- 1. Zeisel SH, Wishnok JS, Blusztajn JK. Formation of methylamines from ingested choline and lecithin. J Pharmacol Exp Ther 1983; 225: 320–324. [PubMed] [Google Scholar]
- 2. Cho CE, Taesuwan S, Malysheva OV, Bender E, Tulchinsky NF, Yan J, et al Trimethylamine‐N‐oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: a randomized controlled trial. Mol Nutr Food Res 2017; 61 10.1002/mnfr.201600324. [DOI] [PubMed] [Google Scholar]
- 3. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al Intestinal microbiota metabolism of L‐carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013; 19: 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li XS, Obeid S, Klingenberg R, Gencer B, Mach F, Raber L, et al Gut microbiota‐dependent trimethylamine N‐oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J 2017; 38: 814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang Z, Tang WH, Buffa JA, Fu X, Britt EB, Koeth RA, et al Prognostic value of choline and betaine depends on intestinal microbiota‐generated metabolite trimethylamine‐N‐oxide. Eur Heart J 2014; 35: 904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schiattarella GG, Sannino A, Toscano E, Giugliano G, Gargiulo G, Franzone A, et al Gut microbe‐generated metabolite trimethylamine‐N‐oxide as cardiovascular risk biomarker: a systematic review and dose‐response meta‐analysis. Eur Heart J 2017; 38: 2948–2956. [DOI] [PubMed] [Google Scholar]
- 7. Makrecka‐Kuka M, Volska K, Antone U, Vilskersts R, Grinberga S, Bandere D, et al Trimethylamine N‐oxide impairs pyruvate and fatty acid oxidation in cardiac mitochondria. Toxicol Lett 2017; 267: 32–38. [DOI] [PubMed] [Google Scholar]
- 8. Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, et al Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 2016; 165: 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sun X, Jiao X, Ma Y, Liu Y, Zhang L, He Y, et al Trimethylamine N‐oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS‐TXNIP‐NLRP3 inflammasome. Biochem Biophys Res Commun 2016; 481: 63–70. [DOI] [PubMed] [Google Scholar]
- 10. Seldin MM, Meng Y, Qi H, Zhu W, Wang Z, Hazen SL, et al Trimethylamine N‐oxide promotes vascular inflammation through signaling of mitogen‐activated protein kinase and nuclear factor‐kappaB. J Am Heart Assoc 2016; 5: 002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fu Q, Zhao M, Wang D, Hu H, Guo C, Chen W, et al Coronary plaque characterization assessed by optical coherence tomography and plasma trimethylamine‐N‐oxide levels in patients with coronary artery disease. Am J Cardiol 2016; 118: 1311–1315. [DOI] [PubMed] [Google Scholar]
- 12. Dambrova M, Latkovskis G, Kuka J, Strele I, Konrade I, Grinberga S, et al Diabetes is associated with higher trimethylamine N‐oxide plasma levels. Exp Clin Endocrinol Diabetes 2016; 124: 251–256. [DOI] [PubMed] [Google Scholar]
- 13. Stubbs JR, House JA, Ocque AJ, Zhang S, Johnson C, Kimber C, et al Serum trimethylamine‐N‐oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J Am Soc Nephrol 2016; 27: 305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dambrova M, Skapare‐Makarova E, Konrade I, Pugovics O, Grinberga S, Tirzite D, et al Meldonium decreases the diet‐increased plasma levels of trimethylamine N‐oxide, a metabolite associated with atherosclerosis. J Clin Pharmacol 2013; 53: 1095–1098. [DOI] [PubMed] [Google Scholar]
- 15. Dambrova M, Makrecka‐Kuka M, Vilskersts R, Makarova E, Kuka J, Liepinsh E. Pharmacological effects of meldonium: biochemical mechanisms and biomarkers of cardiometabolic activity. Pharmacol Res 2016; 113 (Pt B): 771–780. [DOI] [PubMed] [Google Scholar]
- 16. Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 2013; 158: 825–830. [DOI] [PubMed] [Google Scholar]
- 17. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. J Pharmacol Pharmacother 2010; 1: 94–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol 2010; 160: 1573–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA, et al Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 2015; 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grinberga S, Dambrova M, Latkovskis G, Strele I, Konrade I, Hartmane D, et al Determination of trimethylamine‐N‐oxide in combination with L‐carnitine and gamma‐butyrobetaine in human plasma by UPLC/MS/MS. Biomed Chromatogr 2015; 29: 1670–1674. [DOI] [PubMed] [Google Scholar]
- 22. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al The IUPHAR/BPS guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res 2018; 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E, et al The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 2017; 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD, et al The Concise Guide to PHARMACOLOGY 2017/18: Transporters. Br J Pharmacol 2017; 174: S360–S446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alexander SPH, Cidlowski JA, Kelly E, Marrion NV, Peters JA, Faccenda E, et al The Concise Guide to PHARMACOLOGY 2017/18: Nuclear hormone receptors. Br J Pharmacol 2017; 174: S208–S224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sica DA. Diuretic‐related side effects: development and treatment. J Clin Hypertens (Greenwich) 2004; 6: 532–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tang WH, Wang Z, Fan Y, Levison B, Hazen JE, Donahue LM, et al Prognostic value of elevated levels of intestinal microbe‐generated metabolite trimethylamine‐N‐oxide in patients with heart failure: refining the gut hypothesis. J Am Coll Cardiol 2014; 64: 1908–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schartum‐Hansen H, Loland KH, Svingen GF, Seifert R, Pedersen ER, Nordrehaug JE, et al Use of loop diuretics is associated with increased mortality in patients with suspected coronary artery disease, but without systolic heart failure or renal impairment: an observational study using propensity score matching. PLoS One 2015; 10: e0124611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Konig J, Muller F, Fromm MF. Transporters and drug‐drug interactions: important determinants of drug disposition and effects. Pharmacol Rev 2013; 65: 944–966. [DOI] [PubMed] [Google Scholar]
- 30. Wu W, Bush KT, Nigam SK. Key role for the organic anion transporters, OAT1 and OAT3, in the in vivo handling of uremic toxins and solutes. Sci Rep 2017; 7: 4939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nies AT, Koepsell H, Damme K, Schwab M. Organic cation transporters (OCTs, MATEs), in vitro and in vivo evidence for the importance in drug therapy. Handb Exp Pharmacol 2011; 201: 105–167. [DOI] [PubMed] [Google Scholar]
- 32. Miyake T, Mizuno T, Mochizuki T, Kimura M, Matsuki S, Irie S, et al Involvement of organic cation transporters in the kinetics of trimethylamine N‐oxide. J Pharm Sci 2017; 106: 2542–2550. [DOI] [PubMed] [Google Scholar]
- 33. Teft WA, Morse BL, Leake BF, Wilson A, Mansell SE, Hegele RA, et al Identification and characterization of trimethylamine‐N‐oxide uptake and efflux transporters. Mol Pharm 2017; 14: 310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bain MA, Faull R, Fornasini G, Milne RW, Evans AM. Accumulation of trimethylamine and trimethylamine‐N‐oxide in end‐stage renal disease patients undergoing haemodialysis. Nephrol Dial Transplant 2006; 21: 1300–1304. [DOI] [PubMed] [Google Scholar]
- 35. Konop M, Radkowski M, Grochowska M, Perlejewski K, Samborowska E, Ufnal M. Enalapril decreases rat plasma concentration of TMAO, a gut bacteria‐derived cardiovascular marker. Biomarkers 2018; 380–385. [DOI] [PubMed] [Google Scholar]


