Dear Editor,
Natural killer (NK) cells are lymphocytes that play important roles in the host defense against hepatitis C virus (HCV) infection. Killer cell immunoglobulin-like receptors (KIRs) are a group of regulatory molecules expressed on NK cells and a subset of T cells (Parham 2005). Ligands for KIRs are human leukocyte antigen (HLA) class I molecules, and HLA-C1 is a ligand for the inhibitory receptors KIR2DL2, KIR2DL3 and the activating receptor KIR2DS2 (Robinson et al. 2003; Du et al. 2007). In 2002, the National Institutes of Health Consensus Development Conference concluded that a combination therapy of pegylated alpha interferon (PEG-IFN) with ribavirin (RBV) manages HCV infections effectively (Gebo and Bartlett 2002). Before the direct-acting antiviral agent treatment was approved, PEG-IFN and RBV were the main antiviral treatments for chronic HCV in China (Chinese Society of Hepatology et al. 2015). Patients that receive the same standard combination therapies are classified as non-responders (NR) and sustained virological responders (SVR) according to their responses to the treatment by detecting HCV RNA 24 weeks after treatment (Asselah 2012). The NR do not mount a sufficient anti-HCV response, which is defined by a consistent positive viral load during treatment, at its end, or at 24-weeks post-treatment. The SVR are defined by consistent undetectable HCV RNA levels in serum at 24-weeks post-treatment (Fried et al. 2002).
To investigate the association of the KIR frequencies with HCV infection and therapy responses in Chinese Han population, we recruited 333 patients infected with HCV-1b and 320 healthy individuals in Hubei Province between October 2010 and August 2012. In 333 HCV patients, 98 treated by PEG-IFN and RBV were successfully tracked and divided into NR and SVR groups, which were detected with Real-Time Quantitative PCR Detection System and ELISA (Kehua Bio-Engineering Co, Shanghai, China). All subjects were negative from other disorders, such as infection with hepatitis B virus, hepatitis D virus, and HIV, which were detected by quantitative PCR and ELISA, as well as diabetes, malignant tumor, or any autoimmune diseases.
We extracted the genomic DNA from the blood of donors with a SE Blood DNA kit (Omega Bio-Tek Inc, Norcross, GA, USA), and the KIR alleles were determined by the sequence-specific PCR primers (Bunce et al. 1995; Hsu et al. 2002). The results were analyzed with a Chi square test based on P values and odds ratios (OR). The gene frequencies of KIR2DL2 and KIR2DS3 were significantly higher in HCV patients (Supplementary Table S1, Table 1) (P < 0.01), and only the frequency of KIR2DL2 was significantly higher in the SVR group (Supplementary Table S2). The gene frequency of full-length KIR2DS4 was significantly lower in HCV patients and the SVR group. HLA-C1, as a ligand, is necessary for the function of KIR2DL2 (Bashirova et al. 2006; Du et al. 2007).
Table 1.
Clinical characteristics and occurrence of KIR2DL2 and HLA-C1 in HCV and healthy patients.
| Characteristic | HCV patients (n = 333) | Healthy controls (n = 320) | P valuea | Odds ratioa (95% CI) |
|---|---|---|---|---|
| Sex (M:F) | 1.41:1 | 1.22:1 | ||
| Mean age (span; median) | (24–55); 40 | (27–55); 41 | ||
| Mean ALT ± SD (IU/L) | 101.11 ± 28.22 | 25.2 ± 12.11 | ||
| HCV RNA (copies/mL) | (5.13 ± 1.56) × 105 | – | ||
| Anti-HCV (detected by ELISA) | + | – | ||
| HLA-C1 | 307 (92.19%) | 289 (90.31%) | 0.40 | 1.26 (0.73–2.18) |
| KIR2DL2 | 186 (55.86%) | 94 (29.38%) | < 0.01 | 3.04 (2.20–4.20) |
| KIR2DL2+/HLA-C1− | 6 (1.80%) | 10 (3.12%) | 0.27 | 0.57 (0.20–1.58) |
| KIR2DL2+/HLA-C1+ | 180 (54.05%) | 84 (26.25%) | < 0.01 | 3.31 (2.38–4.60) |
aP value of Z-test for pooled odds ratio.
Therefore, we analyzed the association among KIR2DL2, its ligand HLA-C1, HCV-1b infection, and treatment response. As shown in Table 1, although the frequency of HLA-C1 was not different compared with healthy controls, both KIR2DL2 and HLA-C1 positive (KIR2DL2+/C1+ pairs) showed a risk association with HCV-1b infection. As shown in Table 2, in contrast to the SVR group, the gene frequency of KIR2DL2 was significantly higher in the NR group, whereas the frequency of HLA-C1 was not different. The joint analysis revealed that the frequency of KIR2DL2+/C1+ pairs in the NR group was significantly higher than the SVR group (P < 0.05), and the KIR2DL2+/C1− pairs showed no difference. This suggests that the patients carrying KIR2DL2/C1 genes have a high risk of being infected by HCV-1b and they are not beneficial for HCV treatment. But 186 of the 333 HCV patients had the KIR2DL2 gene, of which 180 had HLA-C1 group genes and 6 did not. Ninety-four of the 320 healthy control participants had the KIR2DL2 gene, of which 84 had the HLA-C1 gene and 10 did not. There was no statistical difference in the P values and OR values of KIR2DL2+/C1− between HCV patients and the healthy controls.
Table 2.
Clinical characteristics and occurrence of KIR2DL2 and HLA-C1 in NR and SVR HCV patients.
| Characteristic | NR patients (n = 36) | SVR patients (n = 62)a | P valueb | Odds ratiob (95% CI) |
|---|---|---|---|---|
| Sex (M:F) | 1.25:1 | 1.69:1 | ||
| Mean age (span; median) | (28–55); 41 | (27–51); 41 | ||
| Mean ALT ± SD (IU/L) | 72.5 ± 17.6 | 45.2 ± 11.3 | ||
| HCV RNA (copies/mL) | (4.76 ± 1.34) × 104 | – | ||
| Anti-HCV (detected by ELISA) | + | – | ||
| HLA-C1 | 34 (94.44%) | 57 (91.94%) | 0.64 | 1.49 (0.27–8.11) |
| KIR2DL2 | 26 (72.22%) | 32 (51.61%) | < 0.05 | 2.44 (1.01–5.90) |
| KIR2DL2+/HLA-C1− | 2 (5.56%) | 4 (6.45%) | 0.86 | 0.83 (0.15–4.90) |
| KIR2DL2+/HLA-C1+ | 24 (66.67%) | 28 (45.16%) | < 0.05 | 2.43 (1.03–5.71) |
aThere is a KIR2DL2-/C1- in SVR patients.
bP value of Z-test for pooled odds ratio.
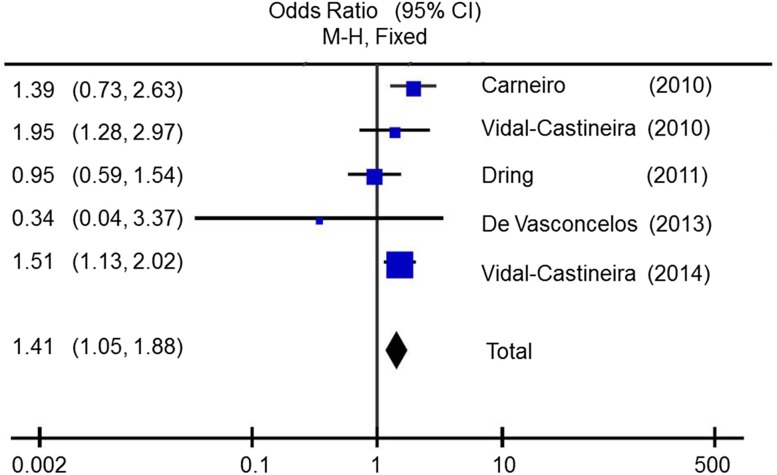
We further performed meta-analysis to assess the association between KIR2DL2 and the response to therapy. Twelve studies were identified through databases searching (e.g., PubMed, Science Direct) for all case-control studies evaluating KIRs and HCV-1b treatment in humans (up to April 2017). Studies were further selected if they fulfilled the following criteria: (1) have a NR-SVR design, used the same therapy strategy, reported the KIR2DL2 genotype frequencies, and confirmed that the recruited HCV patients had no other disease; (2) supplied sufficient information to calculate the OR in a peer-reviewed journal. Finally, five articles covering populations in three countries (Brazil, Spain, Ireland) were eligible for meta-analysis (Carneiro et al. 2010; Vidal-Castineira et al. 2010; Dring et al. 2011; de Vasconcelos et al. 2013; Vidal-Castineira et al. 2014). A significant association of the KIR2DL2 gene with the response to therapy was detected under the fixed effects model, including 934 NRs and 732 SVRs (shown in Supplementary Table S3 and Fig. 1). The aggregated OR was 1.41 (P < 0.01, 95% CI = 1.05–1.88), and the heterogeneity was moderate (P = 0.16, I2 = 39%). The publication bias of the literature was estimated by funnel plots (shown in Supplementary Figure S1). The shapes of funnel plots appeared symmetrical for NR versus SVR in the studies. Together with our data, KIR2DL2 is not beneficial for HCV therapy in multiple populations. However, none of the above papers discussed the relationship between the KIR2DL2/C1 pair and HCV patients.
Fig. 1.
Forest plot of the meta-analysis for KIR2DL2 in NR and SVR HCV patients under the fixed effects model.
This study displayed a significantly higher frequency of the KIR2DL2 gene among patients infected with HCV-1b compared with healthy controls in a Chinese Han population. As previously mentioned, the frequency of the KIR2DL2 gene in this Chinese Han population was 10%–30% and is present in 40%–60% of Caucasians (Single et al. 2007). In this study, the KIR2DL2 gene frequency in Chinese Han HCV patients (55.86%) is similar to that in Caucasian patients, which also implies that KIR2DL2 is a risk factor for HCV-1b infection in the Chinese Han population.
KIR2DL2 is an inhibitory receptor on the surface of NK cells, which can transmit inhibitory signals to the cell, but all these require the involvement of HLA-C1. In the early stages of virus infection and development, the elimination of viruses in the body also depends on innate immunity, including NK cells. The killing ability of NK cells carrying KIR2DL2 may be impaired, which has also been verified in studies on KIR2DL2 and HIV (Zwolinska et al. 2016). In this study, one possibility is that the inhibition of virus infection mediated by KIR2DL2 can overcome the activation of NK cells by IFN-alpha and decrease the antiviral ability of NK cells.
In conclusion, KIR2DL2/C1 is a risk factor of HCV-1b infection and is associated with nonresponse to PEG-IFN and RBV combination therapy in Chinese Han patients. These findings might contribute to our understanding of the pathogenic mechanisms of HCV infection and to the development of more efficient therapeutic strategies for HCV that consider host genetic factors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all subjects who donated samples for this study. This work was supported by Grants from the National Major Science and Technology Project for Infectious Diseases of China (2012ZX10004503), and Major State Basic Research Development Program of China (973 Program; No. 2013CB530505).
Conflict of interest
The authors declare that they have no conflicts of interest.
Animal and Human Rights Statement
This study conformed to the 1975 Declaration of Helsinki guidelines and permission was obtained from the Ethics Committee of Tongji Medical College, Huazhong University of Science and Technology. Prior to the study, informed consent was obtained from each individual.
References
- Asselah T. A revolution in hcv treatment with direct-acting antivirals: from non-response to eradication. J Hepatol. 2012;57:455–457. doi: 10.1016/j.jhep.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Bashirova AA, Martin MP, McVicar DW, Carrington M. The killer immunoglobulin-like receptor gene cluster: tuning the genome for defense. Annu Rev Genomics Hum Genet. 2006;7:277–300. doi: 10.1146/annurev.genom.7.080505.115726. [DOI] [PubMed] [Google Scholar]
- Bunce M, O’Neill CM, Barnardo MC, Krausa P, Browning MJ, Morris PJ, Welsh KI. Phototyping: comprehensive DNA typing for HLA-A, B, C, DRB1, DRB3, DRB4, DRB5 & DQB1 by PCR with 144 primer mixes utilizing sequence-specific primers (PCR-SSP) Tissue Antigens. 1995;46:355–367. doi: 10.1111/j.1399-0039.1995.tb03127.x. [DOI] [PubMed] [Google Scholar]
- Carneiro VL, Lemaire DC, Bendicho MT, Souza SL, Cavalcante LN, Angelo AL, Freire SM, Mendes CM, Santana N, Lyra LG, Lyra AC. Natural killer cell receptor and HLA-C gene polymorphisms among patients with hepatitis C: a comparison between sustained virological responders and non-responders. Liver Int. 2010;30:567–573. doi: 10.1111/j.1478-3231.2010.02212.x. [DOI] [PubMed] [Google Scholar]
- Chinese Society of Hepatology CMA. Wei L, Chinese Society of Infectious Diseases CMA. Hou JL. The guideline of prevention and treatment for hepatitis C: a 2015 update. Zhonghua Gan Zang Bing Za Zhi. 2015;23:906–923. doi: 10.3760/cma.j.issn.1007-3418.2015.12.003. [DOI] [PubMed] [Google Scholar]
- de Vasconcelos JM, de Jesus Maues Pereira Moia L, Amaral IS, Miranda EC, Cicalisetakeshita LY, de Oliveira LF, de Araujo Melo Mendes L, Sastre D, Tamegao-Lopes BP, de Aquino Pedroza LS, Batista Dos Santos SE, Soares MC, de Araujo MT, Bandeira CL, de Sousa da Silva AM, de Medeiros ZL, Sena L, Demachki S, Dos Santos EJ. Association of killer cell immunoglobulin-like receptor polymorphisms with chronic hepatitis c and responses to therapy in Brazil. Genet Mol Biol. 2013;36:22–27. doi: 10.1590/S1415-47572013000100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dring MM, Morrison MH, McSharry BP, Guinan KJ, Hagan R, Irish HCVRC, O’Farrelly C, Gardiner CM. Innate immune genes synergize to predict increased risk of chronic disease in hepatitis C virus infection. Proc Natl Acad Sci U S A. 2011;108:5736–5741. doi: 10.1073/pnas.1016358108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z, Gjertson DW, Reed EF, Rajalingam R. Receptor-ligand analyses define minimal killer cell ig-like receptor (KIR) in humans. Immunogenetics. 2007;59:1–15. doi: 10.1007/s00251-006-0168-4. [DOI] [PubMed] [Google Scholar]
- Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr, Haussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- Gebo KA, Bartlett JG. Management of hepatitis C: a review of the NIH consensus development conference. Hopkins HIV Rep. 2002;14:1–4. [PubMed] [Google Scholar]
- Hsu KC, Liu XR, Selvakumar A, Mickelson E, O’Reilly RJ, Dupont B. Killer ig-like receptor haplotype analysis by gene content: evidence for genomic diversity with a minimum of six basic framework haplotypes, each with multiple subsets. J Immunol. 2002;169:5118–5129. doi: 10.4049/jimmunol.169.9.5118. [DOI] [PubMed] [Google Scholar]
- Parham P. MHC class I molecules and KIRs in human history, health and survival. Nat Rev Immunol. 2005;5:201–214. doi: 10.1038/nri1570. [DOI] [PubMed] [Google Scholar]
- Robinson J, Waller MJ, Parham P, de Groot N, Bontrop R, Kennedy LJ, Stoehr P, Marsh SG. IMGT/HLA and IMGT/MHC: sequence databases for the study of the major histocompatibility complex. Nucleic Acids Res. 2003;31:311–314. doi: 10.1093/nar/gkg070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Single RM, Martin MP, Gao X, Meyer D, Yeager M, Kidd JR, Kidd KK, Carrington M. Global diversity and evidence for coevolution of KIR and HLA. Nat Genet. 2007;39:1114–1119. doi: 10.1038/ng2077. [DOI] [PubMed] [Google Scholar]
- Vidal-Castineira JR, Lopez-Vazquez A, Diaz-Pena R, Alonso-Arias R, Martinez-Borra J, Perez R, Fernandez-Suarez J, Melon S, Prieto J, Rodrigo L, Lopez-Larrea C. Effect of killer immunoglobulin-like receptors in the response to combined treatment in patients with chronic hepatitis C virus infection. J Virol. 2010;84:475–481. doi: 10.1128/JVI.01285-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Castineira JR, Lopez-Vazquez A, Martinez-Borra J, Martinez-Camblor P, Prieto J, Lopez-Rodriguez R, Sanz-Cameno P, de la Vega J, Rodrigo L, Perez-Lopez R, Perez-Alvarez R, Lopez-Larrea C. Diversity of killer cell immunoglobulin-like receptor (KIR) genotypes and KIR2DL2/3 variants in HCV treatment outcome. PLoS ONE. 2014;9:e99426. doi: 10.1371/journal.pone.0099426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwolinska K, Blachowicz O, Tomczyk T, Knysz B, Gasiorowski J, Zalewska M, Orzechowska BU, Sochocka M, Piasecki E. The effects of killer cell immunoglobulin-like receptor (KIR) genes on susceptibility to HIV-1 infection in the polish population. Immunogenetics. 2016;68:327–337. doi: 10.1007/s00251-016-0906-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.