Abstract
An infection by Zika virus (ZIKV), a mosquito-borne flavivirus, broke out in South American regions in 2015, and recently showed a tendency of spreading to North America and even worldwide. ZIKV was first detected in 1947 and only 14 human infection cases were reported until 2007. This virus was previously observed to cause only mild flu-like symptoms. However, recent ZIKV infections might be responsible for the increasing cases of neurological disorders such as Guillain-Barré syndrome and congenital defects, including newborn microcephaly. Therefore, researchers have established several animal models to study ZIKV transmission and pathogenesis, and test therapeutic candidates. This review mainly summarizes the reported animal models of ZIKV infection, including mice and non-human primates.
Keywords: Zika virus, Flavivirus, Rodent models, Non-human primate models
Introduction
Zika virus (ZIKV) is a mosquito-borne flavivirus first isolated from a febrile sentinel rhesus macaque in the Zika forest of Uganda in 1947 (Dick et al. 1952). In the same forest, ZIKV was isolated from Aedes africanus mosquitoes in 1948. Although the first case of human infection was documented in 1954 (Macnamara 1954), until the outbreak on Yap Island in 2007 (Duffy et al. 2009), only 14 cases of human infection had been reported. However, in the past 2 years, this virus re-emerged in South American regions, especially in Brazil, and showed a tendency of spreading to North America and even worldwide. ZIKV infection was previously observed to cause only mild symptoms, such as skin rashes, conjunctivitis, muscle and joint pain, or headache, in a part of patients. In contrast, the 2013–2014 ZIKV outbreak was associated with Guillain–Barré syndrome (GBS) in French Polynesia (Bautista and Sethi 2016; Cao-Lormeau et al. 2016). In 2015, ZIKV swept over South and Central America, and infected thousands of people in Brazil and Colombia, where it was responsible for an obvious increase in severe fetal abnormalities, including spontaneous abortion, stillbirth, microcephaly, hydrocephaly, and placental insufficiency (Paploski et al. 2016; Sejvar et al. 2011). Therefore, the World Health Organization (WHO) declared that ZIKV is a global health emergency in February 2016. This sudden challenge urged scientists to establish a series of animal models to explore the transmission and pathogenesis of ZIKV, and to evaluate the vaccines and therapeutics.
Part One: Rodent Models of ZIKV Infection
Immunocompetent Mouse Model
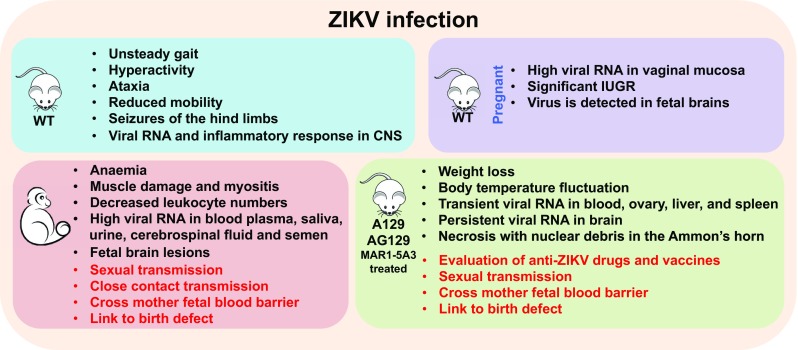
As the most common laboratory animals, mice were first used to establish an animal model of ZIKV infection. Wild-type (WT) C57BL/6 mice (5–6-week-old) exhibited no weight loss, morbidity, or mortality after ZIKV infection. However, 1-day-old C57BL/6 mice subcutaneously inoculated with ZIKV (strain PRVABC59) developed unsteady gait with widening stance, hyperactivity, and ataxia within 2 weeks post-infection. This was followed by reduced mobility, intermittent alternating collapse of the hind limbs, loss of balance, and seizures. Moreover, these mice showed detectable levels of viral RNA in the central nervous system (CNS), especially in the cerebellar white matter and granular layers, as well as in the hippocampal region. However, there is no evidence of viral infection in the spleen or liver, indicating selective infection in the CNS (Manangeeswaran et al. 2016). In the CNS, ZIKV infection profoundly upregulated the genes involved in the recruitment and activation of neutrophils as well as monocytes/macrophages. After virgin female C57BL/6 mice were intravaginally inoculated with ZIKV (strain FSS13025), ZIKV RNA and infectious virus were persistently detectable in the vaginal mucosa (Yockey et al. 2016). In mice, embryonic day 4.5 (E4.5) corresponds to the initiation of embryonic development at the late blastocyst stage, whereas E8.5 corresponds to the late gastrulation and beginning of organogenesis (Ko 2001). ZIKV replicated substantially in the vaginal tract of pregnant C57BL/6 mice that were infected at the two early phases of pregnancy, E4.5 and E8.5. Although ZIKV RNA became undetectable in the placenta at E18.5 (just prior to birth), significant intrauterine growth retardation (IUGR) was observed in fetuses of pregnant mice infected on E4.5, and the presence of ZIKV in the fetal brains was detected by immune-electron microscopy using an antibody specific to flavivirus NS1, suggesting that the sexual transmission of ZIKV probably occurs in mice and leads to more severe effects than those by other transmission routes (Yockey et al. 2016).
Mouse Models with Interferon Pathway Deficiency
An immunocompromised mouse is majorly used to study ZIKV infection (Fig. 1). The innate immune system detects viral infections through the recognition of pathogen-associated molecular patterns (PAMPs) by the host pattern recognition receptors (PRRs) (Medzhitov 2001). Interaction between PRRs and PAMPs turns on signaling cascades, leading to the production of cytokines, including type I interferon (IFN), by the transcription factors NF-κB and interferon regulatory factors (IRFs) (Honda and Taniguchi 2006). Engagement of type I IFNs to their receptor IFNAR1 finally induces a group of IFN-stimulated genes (ISGs) that work in combination to repress viral replication and prime adaptive immune responses to eliminate the virus (Schoggins and Rice 2011). A129 mice (Ifnar1−/−) subcutaneously challenged with ZIKV exhibited weight reduction and abnormal fluctuations in body temperature. ZIKV viral RNA was detected in multiple organs with the highest level in the spleen (Dowall et al. 2016). Additionally, ZIKV persistently infected the testis and epididymis of male mice, resulting in diminished testosterone and inhibin B levels. ZIKV can infect spermatogonia, primary spermatocytes, and Sertoli cells, leading to cell death and destruction of the seminiferous tubules (Govero et al. 2016; Ma et al. 2016). When pregnant Ifnar1−/− mice, which mated with WT mice, were inoculated with ZIKV on E6.5 and E7.5 and sacrificed on E13.5 and E15.5, respectively, most Ifnar1+/− heterozygous fetuses demised and were resorbed on E13.5, leaving only a placental remnant. ZIKV RNA were present in different trophoblast cells in the placenta, including glycogen trophoblasts, spongiotrophoblasts, mononuclear trophoblasts, and syncytiotrophoblasts (Miner et al. 2016). These results are consistent with the cell culture studies that demonstrated that ZIKV could infect human trophoblast cell lines (Bayer et al. 2016). Additionally, ZIKV-infected placentas exhibited severe vascular injury, such as irregularly shaped, reduced fetal capillaries, and destruction of the placental microvasculature (Miner et al. 2016). These results demonstrated that ZIKV could pass the mother fetal blood barrier during pregnancy to cause the potential fetal defects in mice.
Fig. 1.
Animal models of ZIKV infection.
Similar to the A129 mice, MAR1-5A3 (anti-IFNAR1 monoclonal antibody) pre-treated C57BL/6 mice could support ZIKV infection in vivo. ZIKV mRNA was present in multiple organs and caused fatality in these mice (Sheehan et al. 2006). In pregnant mice, MAR1-5A3 treatment did not lead to fetus demise after ZIKV infection, but these fetuses exhibited more significant IUGR (Miner et al. 2016). However, MAR1-5A3-treated mice exhibited no evidence of lesions in the placentas or fetuses, as well as IUGR after DENV serotype 3 (DENV-3) infection (Pinto et al. 2015; Sarathy et al. 2015), suggesting that ZIKV, rather than DENV-3, has a greater tropism for the placental cells. The levels of ZIKV RNA in the fetuses were dependent on the dose of MAR1-5A3 administered in the maternal mice. MAR1-5A3 treatment at a concentration of 1–2 mg led to high viral loads in fetuses (Lazear et al. 2016) and ZIKV RNA persisted in the fetal heads and bodies on E16.5, a crucial period in the early development of the fetus brain (Miner et al. 2016). In another study, MAR1-5A3 treatment resulted in 40% mortality of mice with subcutaneous inoculation of ZIKV, but 100% mortality of mice with intraperitoneal inoculation. Both the exposure routes led to weight loss, high viremia, and severe neuropathological changes in ZIKV-infected mice. More importantly, acute encephalitis and encephalomyelitis were observed in MAR1-5A3 pre-treated mice, suggesting the crucial role of type I IFN in controlling ZIKV pathogenesis (Smith et al. 2017).
AG129 is another immunocompromised mouse model lacking both type I and II IFN receptors, which have been successfully used to imitate infection with flaviviruses such as DENV (Shresta et al. 2004) and yellow fever virus (Thibodeaux et al. 2012). Although both A129 and AG129 mice support ZIKV replication in vivo, the neurological symptoms are much severe in AG129 mice. A129 mice became lethargic and unresponsive to stimuli before death or euthanasia, but AG129 mice were still active and interested in food before death, but remained uncoordinated (Dowall et al. 2016). Therefore, the potential neuroprotective role of IFNγ against ZIKV infection cannot be neglected. In addition, under the same infection conditions, SCID mice (deficient in both T and B lymphocytes) succumbed to infection approximately 26 days later than AG129 did, indicating the vital protective roles of innate immune response against ZIKV infection. Elevated levels of inflammatory cytokines (IFNγ, IL-18, IL-6, and TNF-α) and chemokines (CCL2, CCL5, CCL7, CXCL1, and CXCL10) were detectable in the sera of ZIKV-infected AG129 mice. Among these cytokines, IFNγ and IL-18 levels continuously increased during the course of infection, suggesting their possible roles in evaluation of the disease progression and severity in mouse model. Since ZIKV infection leads to the production of IL-18, it is possible that the inflammasome is activated during the course of infection (Zmurko et al. 2016).
The engagement of PRRs with PAMPs turns on the type I IFN production through the activation of the transcription factors, such as IRF3 and IRF7. Irf3−/− mice exhibited no overt illness during ZIKV infection; however, similar to Ifnar1−/− mice, 4–6-week-old Irf3/Irf5/Irf7 triple knockout (TKO) mice developed neurological disease signs including hindlimb weakness and paralysis. Additionally, the exhibition of clinical signs in TKO mice is much quicker than that in Ifnar1−/− mice, suggesting a possible IFNα/β-independent role of IRFs after ZIKV infection (Miner et al. 2016). Mice deficient in both IRF3 and IRF7 (Irf3−/−Irf7−/−) harbored high titers of ZIKV in the vaginal tract after vaginal infection and continued to persist until 7 days post-infection (dpi). Interestingly, ZIKV RNA level was much higher in the placentas of Irf3−/−Irf7−/− pregnant mice than in those of Ifnar1−/− mice upon infection. Therefore, PRR-signal-induced ISGs are more critical in controlling ZIKV replication in the vagina and placenta, while IFNAR-induced ISGs are more important in blocking systemic spread of the virus from the vagina to the blood stream (Yockey et al. 2016).
Other Rodent Models
Other rodents, such as hamster and guinea pigs, are also used to evaluate ZIKV infection. Subcutaneous ZIKV infection of hamsters caused morbidity and mortality. ZIKV RNA was detectable in multiple organs, including the uterus, placenta, brain, spinal cord, and testicles. Similar to those in ZIKV-infected A129 mice, morphological changes in Sertoli cells and spermatogonia were observed in the testes of male hamsters. Additionally, STAT-2 knockout resulted in a more severe infection in adult and fetal hamsters, suggesting the protective role of STAT-2 against ZIKV infection (Siddharthan et al. 2017). Upon subcutaneous inoculation with ZIKV, guinea pigs demonstrated clinical signs of infection, such as fever, lethargy, hunched back, ruffled fur, and decrease in mobility. ZIKV RNA was also detectable in the whole blood and ZIKV replication was observed in the brain and spleen (Kumar et al. 2017). Intranasal inoculation of ZIKV can also establish the infection in guinea pigs, the viral E antigen is detected in the brain and parotid glands after infection, raising the possibility of close contact transmission of ZIKV in humans (Deng et al. 2017).
Part Two: Non-human Primate Models of ZIKV Infection
Though mouse models are easily accessible, non-human primates (NHPs) are more clinically relevant in ZIKV research. WT mice do not exhibit clinical signs after ZIKV infection; therefore, NHP models could provide more valuable information such as virus pathogenesis, the efficacy and safety of therapeutics, and optimized dose and route for drug administration.
When rhesus macaques were subcutaneously inoculated with ZIKV, five of the six non-pregnant monkeys exhibited mild-to-moderate inappetence as well as mild regenerative anemia. Two of these also developed mild rash around the inoculation site at 1 dpi, which persisted for 4–5 days. In addition, all the six monkeys exhibited elevated serum creatine kinase, which peaked at 5 dpi and was strongly associated with muscle damage and myositis (skeletal, smooth, and cardiac) (Lugo-Roman et al. 2010). Total leukocyte numbers decreased in all the infected monkeys, and then increased almost to pre-infection levels at 10 dpi. Innate and adaptive immunocytes, such as natural killer (NK) cells, CD8+ T cells, and CD4+ T cells, expanded above baseline levels at 6 dpi, followed by the exhibition of high neutralizing antibody (nAb) titers at 14 dpi (Dudley et al. 2016). High ZIKV RNA was detected in the blood plasma, saliva, urine, cerebrospinal fluid, and semen of the cynomolgus macaques after subcutaneous infection, but was only transiently present in the vaginal secretion. Interestingly, viral RNA was cleared from the blood plasma and urine within 10 dpi; however, it was still detectable in the saliva and semen until 4 weeks pi (Osuna et al. 2016). Rechallenge at 10 weeks after the initial ZIKV infection resulted in undetectable viral replication, suggesting the effective immune defense through nAb and ZIKV-specific T-cells (Dudley et al. 2016). In addition, ZIKV infection of pregnant pigtail macaques led to fetal brain lesions, including periventricular white matter gliosis, significant cerebral white matter hypoplasia, axonal and ependymal injury, and asymmetry in the occipital-parietal lobes (Adams Waldorf et al. 2016). High infection rates of adult macaques after intravaginal or intrarectal inoculation with ZIKV have been reported. After intravaginal infection by ZIKV, no clinical signs, such as pyrexia, joint swelling, weight loss, or decreased appetite, were observed in the infected macaques. The body temperature increased, and virus RNA and viremia were detectable in both ZIKV-infected rhesus and cynomolgus macaques. The magnitude and duration of detectable viremia suggested that these infected NHPs could potentially infect mosquito vector species (Haddow et al. 2017). Collectively, these studies demonstrated that ZIKV can pass through the mother fetal blood barrier and damage the fetal brain during pregnancy, and sexual transmission plays potential roles in ZIKV spread in NHPs.
Part Three: Atypical Models of ZIKV Infection
Historically, chick embryos have been extensively used as models in developmental biology, teratology, and virology (Drake et al. 2006; Woodruff and Goodpasture 1931). Embryonated broiler eggs, amniotic infected with ZIKV (strain MEX1-44) on E2.5 or E5, exhibited more than 100-fold increase in viral loads and showed dramatic mortality within 3 dpi. High viral loads were consistently identified within the brain and eyes of chick embryos. Magnetic resonance imaging (MRI) showed a decreased growth of total brain volume (− 18%), telencephalon (− 18%), and brain stem (− 32%). Interestingly, ZIKV-infected chick embryos exhibited enlarged ventricular space (+ 30%), suggesting that ZIKV-infected embryos had less cortical tissue compared to that in sham-infected controls (Goodfellow et al. 2016).
Fission yeast has also been used to analyze the global functions of the ZIKV genome, which encodes 14 proteins or small peptides including capsid protein (C), membrane-anchored capsid (anaC), premembrane protein (prM), membrane protein (M), protein pr (Pr), envelope protein (E), and NS1, NS2A, NS2B, NS3, NS4A, NS4B, 2K, and NS5. Fission yeast strains individually expressing seven ZIKV proteins (anaC, C, prM, M, E, NS2B, and NS4A) exhibited various inhibitory effects on yeast colony formations. Expressions of prM or NS2B almost abolished yeast growth, whereas expressions of anaC, C, M, E, or NS4A attenuated yeast growth. Interestingly, these ZIKV proteins also led to abnormal yeast cell morphology such as cell elongation and hypertrophy. Yeasts expressing NS4A exhibit balloon-like morphology, and NS4A-induced hypertrophy and growth delay are likely mediated by the target of rapamycin (mTOR) cellular stress-response pathway, specifically via Tor1 and Tip41. Expression of prM resulted in cell cycle G1 accumulation, whereas expressions of anaC, M, E, and NS4A caused cell cycle G2/M accumulation, indicating that ZIKV infection leads to cell cycle dysregulation (Li et al. 2017).
Human neural progenitor cells (hNPCs) derived from induced pluripotent stem cells (iPSCs) were utilized for ZIKV infection model in 2D cell culture. ZIKV infection leads to dysregulation of genes associated with cell cycle and apoptotic cell death (Tang et al. 2016). iPSC-derived brain-region-specific organoids have also been used as mini-bioreactors for modeling ZIKV exposure. Among different cell types in the 3D organoid, ZIKV exhibits specific tropism toward NPCs. In addition, the forebrain organoids exhibit many features of microcephaly upon ZIKV exposure, including decreased neuronal layer thickness and overall brain size as well as enlarged lateral ventricles (Qian et al. 2016).
Conclusion
In the past year, ZIKV has attracted attention toward the fields of virology, immunology, and neurology worldwide. Although several animal infection models have been established, the molecular mechanisms underlying ZIKV immune evasion and pathogenesis are still largely unknown. Most of these animal model studies focus only on the changes in phenotype after ZIKV infection, without including another closely related virus, such as DENV or West Nile virus, as a control. The key question, why ZIKV causes newborn microcephaly, but other close flavivirus family members do not, remains unanswered. The unique molecular mechanisms of ZIKV pathogenesis still need to be further explored by molecular and biochemistry approaches. Additionally, these animal models will not only play a key role in understanding the pathological mechanism of ZIKV infection, but also be useful for evaluation of therapies and candidate vaccines.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31770176), the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher learning, and the Shanghai Rising-Star Program (17QA1403200) for QL.
Conflict of interest
The authors declare that they have no conflict of interest.
Animal and Human Rights Statement
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- Adams Waldorf KM, Stencel-Baerenwald JE, Kapur RP, Studholme C, Boldenow E, Vornhagen J, Baldessari A, Dighe MK, Thiel J, Merillat S, Armistead B. Fetal brain lesions after subcutaneous inoculation of Zika virus in a pregnant nonhuman primate. Nat Med. 2016;22:1256–1259. doi: 10.1038/nm.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista LE, Sethi AK. Association between Guillain–Barré syndrome and Zika virus infection. Lancet. 2016;387:2599–2600. doi: 10.1016/S0140-6736(16)30844-3. [DOI] [PubMed] [Google Scholar]
- Bayer A, Lennemann NJ, Ouyang Y, Bramley JC, Morosky S, Marques ET, Jr, Cherry S, Sadovsky Y, Coyne CB. Type III interferons produced by human placental trophoblasts confer protection against Zika virus infection. Cell Host Microbe. 2016;19:705–712. doi: 10.1016/j.chom.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao-Lormeau VM, Blake A, Mons S, Lastère S, Roche C, Vanhomwegen J, Dub T, Baudouin L, Teissier A, Larre P, Vial AL. Guillain–Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: a case–control study. Lancet. 2016;387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng YQ, Zhang NN, Li XF, Wang YQ, Tian M, Qiu YF, Fan JW, Hao JN, Huang XY, Dong HL, Fan H, Wang YG, Zhang FC, Tong YG, Xu Z, Qin CF. Intranasal infection and contact transmission of Zika virus in guinea pigs. Nat Commun. 2017;8:1648. doi: 10.1038/s41467-017-01923-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- Dowall SD, Graham VA, Rayner E, Atkinson B, Hall G, Watson RJ, Bosworth A, Bonney LC, Kitchen S, Hewson R. A susceptible mouse model for Zika virus infection. PLoS Negl Trop Dis. 2016;10:e0004658. doi: 10.1371/journal.pntd.0004658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake VJ, Koprowski SL, Lough JW, Smith SM. Gastrulating chick embryo as a model for evaluating teratogenicity: a comparison of three approaches. Birth Defects Res A Clin Mol Teratol. 2006;76:66–71. doi: 10.1002/bdra.20202. [DOI] [PubMed] [Google Scholar]
- Dudley DM, Aliota MT, Mohr EL, Weiler AM, Lehrer-Brey G, Weisgrau KL, Mohns MS, Breitbach ME, Rasheed MN, Newman CM, Gellerup DD. A rhesus macaque model of Asian-lineage Zika virus infection. Nat Commun. 2016;7:12204. doi: 10.1038/ncomms12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, Pretrick M, Marfel M, Holzbauer S, Dubray C, Guillaumot L. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- Goodfellow FT, Tesla B, Simchick G, Zhao Q, Hodge T, Brindley MA, Stice SL. Zika virus induced mortality and microcephaly in chicken embryos. Stem Cells Dev. 2016;25:1691–1697. doi: 10.1089/scd.2016.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govero J, Esakky P, Scheaffer SM, Fernandez E, Drury A, Platt DJ, Gorman MJ, Richner JM, Caine EA, Salazar V, Moley KH. Zika virus infection damages the testes in mice. Nature. 2016;540:438–442. doi: 10.1038/nature20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddow AD, Nalca A, Rossi FD, Miller LJ, Wiley MR, Perez-Sautu U, Washington SC, Norris SL, Wollen-Roberts SE, Shamblin JD, Kimmel AE. High infection rates for adult macaques after intravaginal or intrarectal inoculation with Zika virus. Emerg Infect Dis. 2017;23:1274–1281. doi: 10.3201/eid2308.170036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6:644–658. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- Ko MS. Embryogenomics: developmental biology meets genomics. Trends Biotechnol. 2001;19:511–518. doi: 10.1016/S0167-7799(01)01806-6. [DOI] [PubMed] [Google Scholar]
- Kumar M, Krause KK, Azouz F, Nakano E, Nerurkar VR. A guinea pig model of Zika virus infection. Virol J. 2017;14:75. doi: 10.1186/s12985-017-0750-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, Diamond MS. A mouse model of Zika virus pathogenesis. Cell Host Microbe. 2016;19:720–730. doi: 10.1016/j.chom.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Poulsen M, Fenyvuesvolgyi C, Yashiroda Y, Yoshida M, Simard JM, Gallo RC, Zhao RY. Characterization of cytopathic factors through genome-wide analysis of the Zika viral proteins in fission yeast. Proc Natl Acad Sci USA. 2017;114:E376–E385. doi: 10.1073/pnas.1619735114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo-Roman LA, Rico PJ, Sturdivant R, Burks R, Settle TL. Effects of serial anesthesia using ketamine or ketamine/medetomidine on hematology and serum biochemistry values in rhesus macaques (Macaca mulatta) J Med Primatol. 2010;39:41–49. doi: 10.1111/j.1600-0684.2009.00394.x. [DOI] [PubMed] [Google Scholar]
- Ma W, Li S, Ma S, Jia L, Zhang F, Zhang Y, Zhang J, Wong G, Zhang S, Lu X, Liu M. Zika virus causes testis damage and leads to male infertility in mice. Cell. 2016;167(1511–1524):e10. doi: 10.1016/j.cell.2016.11.016. [DOI] [PubMed] [Google Scholar]
- Macnamara FN. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg. 1954;48:139–145. doi: 10.1016/0035-9203(54)90006-1. [DOI] [PubMed] [Google Scholar]
- Manangeeswaran M, Ireland DD, Verthelyi D. Zika (PRVABC59) infection is associated with T cell infiltration and neurodegeneration in CNS of immunocompetent neonatal C57Bl/6 mice. PLoS Pathog. 2016;12:e1006004. doi: 10.1371/journal.ppat.1006004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- Miner JJ, Cao B, Govero J, Smith AM, Fernandez E, Cabrera OH, Garber C, Noll M, Klein RS, Noguchi KK, Mysorekar IU. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell. 2016;165:1081–1091. doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osuna CE, Lim SY, Deleage C, Griffin BD, Stein D, Schroeder LT, Omange R, Best K, Luo M, Hraber PT, Andersen-Elyard H. Zika viral dynamics and shedding in rhesus and cynomolgus macaques. Nat Med. 2016;22:1448–1455. doi: 10.1038/nm.4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paploski IA, Prates AP, Cardoso CW, Kikuti M, Silva MM, Waller LA, Reis MG, Kitron U, Ribeiro GS. time lags between exanthematous illness attributed to Zika virus, Guillain–Barré Syndrome, and microcephaly, Salvador, Brazil. Emerg Infect Dis. 2016;22:1438–1444. doi: 10.3201/eid2208.160496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto AK, Brien JD, Lam CY, Johnson S, Chiang C, Hiscott J, Sarathy VV, Barrett AD, Shresta S, Diamond MS. Defining new therapeutics using a more immunocompetent mouse model of antibody-enhanced dengue virus infection. MBio. 2015;6:e01316–1315. doi: 10.1128/mBio.01316-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, Yoon KJ. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell. 2016;165:1238–1254. doi: 10.1016/j.cell.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarathy VV, White M, Li L, Gorder SR, Pyles RB, Campbell GA, Milligan GN, Bourne N, Barrett AD. A lethal murine infection model for dengue virus 3 in AG129 mice deficient in type I and II interferon receptors leads to systemic disease. J Virol. 2015;89:1254–1266. doi: 10.1128/JVI.01320-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoggins JW, Rice CM. Interferon-stimulated genes and their antiviral effector functions. Curr Opin Virol. 2011;1:519–525. doi: 10.1016/j.coviro.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sejvar JJ, Baughman AL, Wise M, Morgan OW. Population incidence of Guillain–Barré syndrome: a systematic review and meta-analysis. Neuroepidemiology. 2011;36:123–133. doi: 10.1159/000324710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan KC, Lai KS, Dunn GP, Bruce AT, Diamond MS, Heutel JD, Dungo-Arthur C, Carrero JA, White JM, Hertzog PJ, Schreiber RD. Blocking monoclonal antibodies specific for mouse IFN-alpha/beta receptor subunit 1 (IFNAR-1) from mice immunized by in vivo hydrodynamic transfection. J Interferon Cytokine Res. 2006;26:804–819. doi: 10.1089/jir.2006.26.804. [DOI] [PubMed] [Google Scholar]
- Shresta S, Kyle JL, Snider HM, Basavapatna M, Beatty PR, Harris E. Interferon-dependent immunity is essential for resistance to primary dengue virus infection in mice, whereas T- and B-cell-dependent immunity are less critical. J Virol. 2004;78:2701–2710. doi: 10.1128/JVI.78.6.2701-2710.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddharthan V, Van Wettere AJ, Li R, Miao J, Wang Z, Morrey JD, Julander JG. Zika virus infection of adult and fetal STAT2 knock-out hamsters. Virology. 2017;507:89–95. doi: 10.1016/j.virol.2017.04.013. [DOI] [PubMed] [Google Scholar]
- Smith DR, Hollidge B, Daye S, Zeng X, Blancett C, Kuszpit K, Bocan T, Koehler JW, Coyne S, Minogue T, Kenny T. Neuropathogenesis of Zika virus in a highly susceptible immunocompetent mouse model after antibody blockade of type I interferon. PLoS Negl Trop Dis. 2017;11:e0005296. doi: 10.1371/journal.pntd.0005296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, Yao B, Shin J, Zhang F, Lee EM, Christian KM. Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell. 2016;18:587–590. doi: 10.1016/j.stem.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeaux BA, Garbino NC, Liss NM, Piper J, Blair CD, Roehrig JT. A small animal peripheral challenge model of yellow fever using interferon-receptor deficient mice and the 17D-204 vaccine strain. Vaccine. 2012;30:3180–3187. doi: 10.1016/j.vaccine.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff AM, Goodpasture EW. The susceptibility of the chorio-allantoic membrane of chick embryos to infection with the Fowl-Pox virus. Am J Pathol. 1931;7(209):222.5. [PMC free article] [PubMed] [Google Scholar]
- Yockey LJ, Varela L, Rakib T, Khoury-Hanold W, Fink SL, Stutz B, Szigeti-Buck K, Van den Pol A, Lindenbach BD, Horvath TL, Iwasaki A. Vaginal exposure to Zika virus during pregnancy leads to fetal brain infection. Cell. 2016;166(1247):1256.e4. doi: 10.1016/j.cell.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmurko J, Marques RE, Schols D, Verbeken E, Kaptein SJ, Neyts J. The viral polymerase inhibitor 7-deaza-2′-C-methyladenosine is a potent inhibitor of in vitro Zika virus replication and delays disease progression in a robust mouse infection model. PLoS Negl Trop Dis. 2016;10:e0004695. doi: 10.1371/journal.pntd.0004695. [DOI] [PMC free article] [PubMed] [Google Scholar]


