Abstract
A novel PRRSV strain was isolated in China that was genetically similar to the NADC30 strain which is reported to have spread throughout China. The objective of the present study was to evaluate the cross-protective efficacy of the live vaccine TJM-F92 in young pigs against challenge with a NADC30-like strain, HN201605. Twenty-five PRRSV- and antibody-free pigs were randomly divided into the following five groups: Vac/ChA, Unvac/ChA, Vac/ChB, Unvac/ChB and the mock. The pigs in groups Vac/ChA and Vac/ChB were inoculated intramuscularly with 1 mL TJM-F92 (105.0 TCID50/mL). At 28 days post vaccination (0 days post challenge), groups Vac/ChA and Unvac/ChA were inoculated intranasally with 104.5 TCID50/mL PRRSV strain TJ F3 (2 mL/pig), while groups Vac/ChB and Unvac/ChB were inoculated, using the same route, with the same dose of the NADC30-like strain HN201605 F3. Protective effects of the PRRSV strain were observed in all pigs in the Vac/ChA and Vac/ChB groups. Neither high fever nor signs of clinical disease were observed through the experiment in these groups, whereas pigs in Unvac/ChA group exhibited serious clinical symptoms, pathological lesions, and weight loss. In Unvac/ChB group, pigs developed milder clinical symptoms, which demonstrated that the NADC30-like strain HN201605 had moderate pathogenicity. The results suggest that the MLV vaccine strain TJM-F92 is an effective and safe vaccine candidate for use in China.
Electronic supplementary material
The online version of this article (10.1007/s12250-018-0027-0) contains supplementary material, which is available to authorized users.
Keywords: Porcine reproductive and respiratory syndrome virus (PRRSV), NADC30-like, Vaccine, Cross protection
Introduction
Porcine reproductive and respiratory syndrome virus (PRRSV) is a member of the family Arteriviridae, order Nidovirales, and causes both reproductive failure in sows and respiratory dyspnea in piglets. This virus is responsible for tremendous financial losses worldwide (Liu et al. 2016). The viral genome consists of at least 10 open reading frames (ORFs), with a length of 14.9–15.5 kb (Huang et al. 2015). The virus is classified into two major genotypes, genotype 1 (European) and genotype 2 (North American), based on genetic and antigenic analyses. There is a 36.6% nucleotide sequence difference between the prototypes and considerable genetic variation in each genotype (up to 30% for Type 1 and 21% for Type 2 PRRSV) (Allende et al. 1999). In 2006, a new lineage of genotype 2 PRRSV was reported, which is described as being a highly pathogenic PRRS (HP-PRRS) and has a unique 30-amino-acid deletion in its nsp2 coding region. This strain has spread to most provinces of China and neighboring countries (Tian et al. 2007). Clinical symptoms include high-grade fever, anorexia, and listlessness, which contribute to the development of high morbidity and mortality in the HP-PRRSV-infected pigs. HP-PRRS has caused significant financial losses to the swine industry in Asia.
Vaccination is a fundamental and important strategy to control and prevent PRRS. A modified live PRRSV vaccine has been confirmed to be an efficient measure against PRRS in nursery and finishing pigs (Allende et al. 1999). The commercial modified live PRRSV vaccine (strain TJM-F92, LAN Er Dai™, PRRS MLV, Sinovet Co., Ltd, China) has a spontaneous deletion of 120 amino acids (360 nucleotides) within the nsp2 gene (Leng et al. 2012a). Studies have indicated that the vaccine can provide protection against homologous challenge with their respective wild-type isolates. The reduction of clinical signs such as lung lesions, morbidity, viremia, and weight loss, improvement in overall health and production under field conditions have also been reported with the use of vaccines from North America and local virus derived MLV vaccines (Tian et al. 2009; Wei et al. 2013; Renukaradhya et al. 2015). As all PRRSV-MLV vaccine strains replicate in the host, every vaccine strain has the potential for reversion to virulence. The standard for evaluating the safety profile of PRRSV-MLV vaccines has been challenged by some vaccine regulation agencies, and includes increased back-passage studies and high-dose safety testing in the most susceptible populations (young pigs and pregnant sows at 90 days gestation). However, inoculation of PRRSV antigen–antibody free pigs intramuscularly with TJM-F92 (105.0 TCID50/mL) and challenged at 28, 60, 120, and 180 days post immunization elicited a high rate of protection, and the absence of fever and clinical disease was also noted (Leng et al. 2012a). Although the PRRSV-MLV vaccine can induce a protective immune response, further studies are required to identify whether this vaccine provides broad cross-protection against all isolates.
In 2008, the NADC30 strain was first isolated in the United States and was determined to be more virulent than other isolates (MN184, SDSU73, and NADC31), leading to the development of viremia, dyspnea, mild lethargy, and moderate proliferative interstitial pneumonia in infected pigs (Brockmeier et al. 2012). Since 2013, a novel PRRSV strain has been reported with three discontinuous deletions (a total of 131 amino acids) in the nsp2 gene that has been shown to be genetically similar to the NADC30 strain but which underwent genetic exchange with the classic HP-PRRSV strains in China. A widespread outbreak of this strain with a high morbidity rate of 100% and a mortality rate of 76.6% (230/300) was reported in Jilin province, China (Zhao et al. 2015). This highlights the problem of whether the commercial HP-PRRSV live vaccine can provide cross-protection against the NADC30-like strains. The purpose of this study was to evaluate the efficacy of the TJM-F92 vaccine against the NADC30-like strain and also to analyze the pathogenicity of the HP-PRRSV and NADC30-like strains.
Materials and Methods
Cells and Viral Strains
MARC-145 cells were maintained in modified Eagle’s medium (MEM) supplemented with 10% fetal bovine serum (FBS), and viral infection and titration were performed in MEM supplemented with 2% FBS as described previously (Li et al. 2013). The HP-PRRSV strain TJ was isolated from the serum of an infected pig in Tianjin province and sequenced in 2012 (GenBank accession no. EU860248) (Leng et al. 2012b). Strain HN201605 was isolated and identified from a field case of PRRSV in the Henan province of China in 2016 and harvested after passage three (F3) from MARC-145 cells. This strain was used as the challenge virus in this study. A HP-PRRSV MLV vaccine TJM-F92 (Lan Er Dai) was manufactured by Sinovet (Beijing) Biotechnology Co. Ltd.
Experimental Animals
A total of 25 pigs, 4 weeks of age, were purchased from a pig farm. All animals were confirmed to be free of PRRSV, porcine circovirus type 2 (PCV2), classical swine fever virus, porcine parvovirus, pseudorabies virus, swine influenza virus, and Mycoplasma hyopneumoniae infections. All animals were housed in separate units in a biosafety level 2 (BSL2) facility, and prior to challenge, all animals were transferred to isolation units at the Sinovet (Jiangsu) Biotechnology Co. Ltd.
Vaccination and Challenge
Twenty-five PRRSV antigen–antibody free pigs were randomly divided into the following five groups (n = 5 per group): Vac/ChA, Vaccinated + Challenged TJ strain; Unvac/ChA, Challenged TJ strain; Vac/ChB, Vaccinated + Challenged HN 201605 strain; Unvac/ChB, Challenged HN 201605 strain; and the Mock. The pigs in Vac/ChA and Vac/ChB group were inoculated intramuscularly with 1 mL TJM (105.0 TCID50/mL) according to the manufacturer’s recommendations. Twenty-eight days post vaccination (dpv), groups Vac/ChA and Unvac/ChA were inoculated intranasally with 104.5 TCID50/mL PRRSV strain TJ F3 (2 mL/pig), while groups Vac/ChB and Unvac/ChB were inoculated with the same route and dose of the NADC30-like strain HN201605 F3. Finally, the Mock group was challenged with the same dose of PBS. At 21 days post challenge (dpc), all surviving pigs were humanely euthanized.
Clinical Assessment
Pigs were observed every day for clinical signs including depression, cough, excessive lacrimation, and rectal temperature, and they were scored daily for clinical respiratory disease severity using scores ranging from 0 (normal) to 6 (severe dyspnea and abdominal breathing) (Halbur et al. 1995a). Rectal temperatures were recorded daily at the same time by the same personnel, and the clinical threshold for fever associated with HP-PRRSV and NADC30-like strains were set at 41 and 40 °C, respectively, as previously described (Li et al. 2016a, b). Pigs were weighed on 0, 7, 14, and 21 dpv and 0, 7, 14, and 21 dpc.
Serological Testing and Assessment of the Virus Neutralizing Titer
Blood samples were collected on 0, 7, 14, 21, and 28 dpv and 2, 4, 6, 8, 10, 12, 14, 16, 18, and 21 dpc. Serum samples were isolated from clotted blood and preserved at − 80 °C. Serum samples were tested for PRRSV antibody using the Herdcheck Porcine Reproductive and Respiratory Syndrome X3 Antibody Test (IDEXX Laboratories, Westbrook, ME). The samples were considered positive for PRRSV antibody if the sample-to-positive (S/P) ratio was equal to or greater than 0.4. Virus neutralizing titer in the serum was determined by a virus serum neutralizing test. Serum samples were heat-inactivated at 56 °C for 30 min and serially diluted with equal volumes of the PRRSV strains: HN201605 and TJ, respectively. After incubation, the mixtures were transferred to Marc-145 monolayers in 96-well plates and incubated for an additional 72 h at 37 °C in an incubator containing 5% CO2. Cells were examined for cytopathic effects (CPE) with end-point titers as described previously (Calzada-Nova et al. 2012; Galliher-Beckley et al. 2015).
Viremia Detection by RT-PCR
Blood samples were collected at 0, 2, 4, 6, 8, 10, 12, 14, 16, 18, and 21 dpc. Blood samples were analyzed by PCR using primers for the nsp2 gene (forward primer 5′-CACCCTTCCYGAAAGAGTRA-3′; reverse primer 5′-CCTCATATTCMGTCTTGAGGA H-3′, designed according to PRRSV NADC30, GenBank No: JN654459, and TJ strain, GenBank No: EU860248. A 1425-bp target gene can be amplified from the HP-PRSSV strain compared with the length of 1122-bp from the NADC30-like strain. Total viral RNA was extracted from 300 µL samples with TRIzol (Invitrogen, USA). PCR was performed in a 50-µL volume containing 25 µL 2 × Reaction Mix (Invitrogen, USA), 6 µL Template RNA, 1 µL 10 µmol/L of each primer, 2 µL SuperScript® III/Platinum® Taq Mix, and 15 µL autoclaved distilled water. Thermal-cycling conditions were 55 °C for 30 min, 94 °C for 2 min; followed by 40 cycles of 94 °C for 15 s, 55 °C for 30 s, 68 °C for 90 s; and a final extension step at 68 °C for 5 min. PCR products were visualized on a 1.2% agarose gel (Zhou et al. 2017).
Macroscopic and Microscopic Analysis
The lungs and lymph nodes were resected from all the pigs. Samples were fixed in 10% buffered formalin and were macroscopically evaluated as previously described (Halbur et al. 1995b). The selected samples were processed for hematoxylin and eosin (H&E) staining as per the previously described protocol (Halbur et al. 1996). Pathological lesions were assessed by the veterinary college of Inner Mongolia Agricultural University. The mouse monoclonal antibody specific PRRSV nucleocapsid (N) protein was used as detecting antibody and immunohistochemistry staining (IHC) was performed according to the published protocol (Zhang et al. 2015). The results were assessed by the China Institute of Veterinary Drug Control.
Statistical Analysis
All data were presented as mean ± SEM. The difference between the Vac/Ch and Unvac/Ch groups were analyzed by the t test by using GraphPad Prism software (version 6.01, SanDiego, CA). Differences were considered statistically significant when P < 0.05.
Results
Clinical Assessment of Pigs Post Challenge
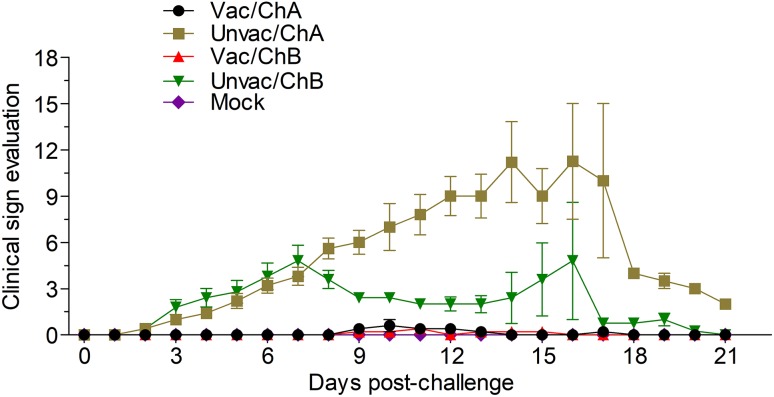
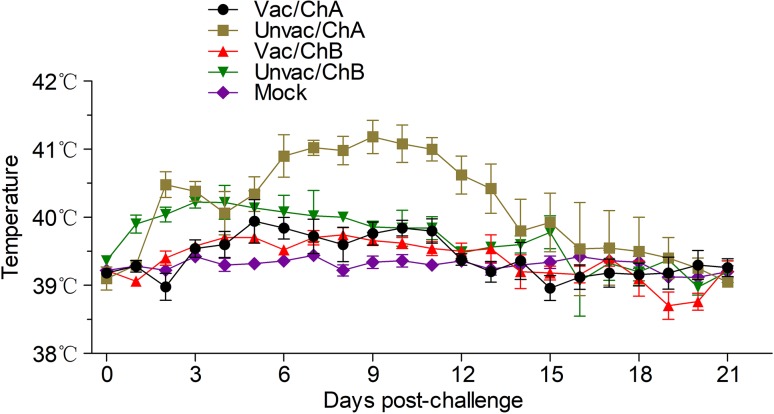
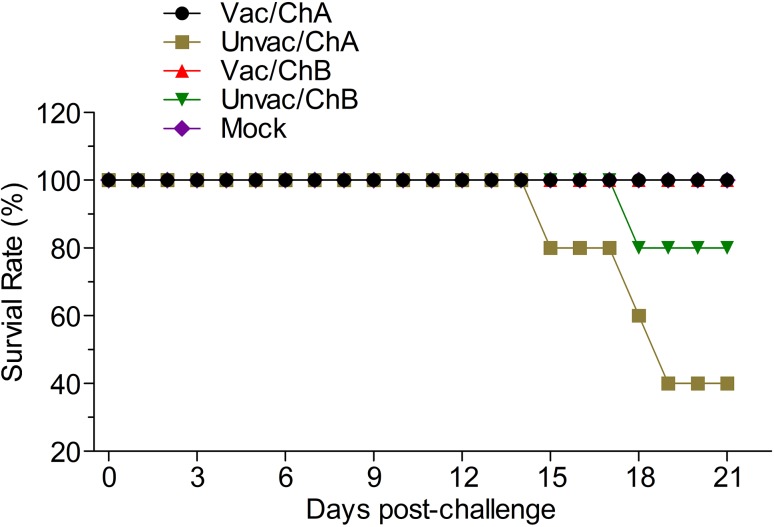
Pigs in groups Vac/ChA, Vac/ChB, and Mock were clinically normal from the beginning of the study (0 dpv) to the end of challenge (21 dpc); this included the respiratory scores and rectal temperatures. However, the pigs from the unvaccinated groups, Unvac/ChA and Unvac/ChB, showed clear clinical symptoms. In the TJ strain challenge group, the vaccinated pigs in group Vac/ChA remained normal throughout the study, as evaluated by the clinical symptom scores (Fig. 1) and rectal temperatures (Fig. 2). However, in group Unvac/ChA (unvaccinated pigs), all pigs developed typical clinical signs of HP-PRRSV, such as severe depression and anorexia, lameness and shivering, dyspnea, skin cyanosis, and death (Li et al. 2016b). All pigs in group Unvac/ChA displayed persistently high fever (≥ 41 °C) for 6 days from 6 dpc. The mean rectal temperature and clinical score were significantly higher in group 2 than in group Vac/ChA (P < 0.05). At 15, 18, and 19 dpc, three of five unvaccinated pigs that had been challenged in group Unvac/ChA died of acute respiratory disease (mortality rate of 60%) (Fig. 3). Unlike in pigs in the Unvac/ChB group, no clinical symptoms were observed in group Vac/ChB. In group Unvac/ChB, four of five infected pigs presented with persistent high temperature (≥ 40 °C) beginning 3 dpc that lasted for 7 days. However, one pig experienced persistent high fever (≥ 40 °C) between 11 and 15 dpc, and then died 18 dpc. Clinical differences between groups Vac/ChB and Unvac/ChB were reflected in the rectal temperatures and clinical scores (Lager et al. 2014).
Fig. 1.
Clinical scores in vaccinated or unvaccinated pigs. Pigs were observed every day for clinical signs including depression, cough, excessive lacrimation, and rectal temperature, and they were scored daily for clinical respiratory disease severity using scores ranging from 0 (normal) to 6 (severe dyspnea and abdominal breathing). Vac/ChA: Vaccinated + Challenged TJ strain; Unvac/ChA: Challenged TJ strain; Vac/ChB: Vaccinated + Challenged HN 201605 strain; Unvac/ChB: Challenged HN 201605 strain; Mock: challenged with the same dose of PBS.
Fig. 2.
Daily monitoring of rectal temperature of all the pigs post challenge. The clinical threshold for “fever” in the NADC30-like strain and HP-PRRSV strain was set at 40 and 41 °C, respectively. Vac/ChA: Vaccinated + Challenged TJ strain; Unvac/ChA: Challenged TJ strain; Vac/ChB: Vaccinated + Challenged HN 201605 strain; Unvac/ChB: Challenged HN 201605 strain; Mock: challenged with the same dose of PBS.
Fig. 3.
The survival rate for each challenged group. At 15, 18, and 19 dpc, three of the five unvaccinated pigs in group Unvac/ChA that had been challenged died of acute respiratory disease (mortality rate of 60%) and one pig died at 18 dpc in group Unvac/ChB. Vac/ChA: Vaccinated + Challenged TJ strain; Unvac/ChA: Challenged TJ strain; Vac/ChB: Vaccinated + Challenged HN 201605 strain; Unvac/ChB: Challenged HN 201605 strain; Mock: challenged with the same dose of PBS.
There was no noticeable difference in clinical symptoms between Vac/ChA and Vac/ChB animals. In contrast, clear differences in clinical symptoms were observed between the Unvac/ChA and Unvac/ChB animals. The pigs in the Unvac/ChB group presented with mild clinical symptoms as evidenced by the mean rectal temperature, clinical score, and mortality rate. The results were consistent with those of a previous study (Sun et al. 2016). The pigs in the Mock group maintained normal temperatures without respiratory symptoms throughout the experiment. No bacterial pathogens were isolated at necropsy.
Antibody Responses in Pigs
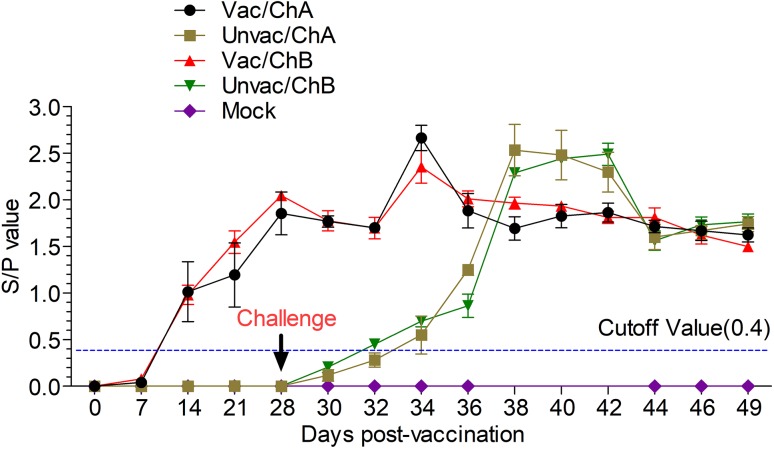
All pigs in all five groups were seronegative for PRRSV-specific antibody at 0 dpv. Pigs in the Vac/ChA and Vac/ChB groups developed antibodies that were detected using ELISA from 14 dpv onwards; in the Unvac/ChA and Unvac/ChB groups, these were detected from 6 dpc onwards (Fig. 4) The S/P ratio reached the highest level at 6 dpc, following which it gradually decreased in the vaccinated groups from the highest level at 10 dpc in the Unvac/ChA group and 14 dpc in the Unvac/ChB group. No anti-PRRSV antibodies were detected in mock group throughout the experiment. The results of virus neutralization assays showed that NAs were not detected in any groups from 0 to 21 dpc (data not shown).
Fig. 4.
Commercial ELISA kits were used to test the PRRSV-specific antibodies. S/P value = sample OD650 divided by negative control OD650. S/P ≥ 0.4, positive; S/P < 0.4, negative. Vac/ChA; Vaccinated +Challenged TJ strain; Unvac/ChA: Challenged TJ strain; Vac/ChB: Vaccinated +Challenged HN 201605 strain; Unvac/ChB: Challenged HN 201605 strain; Mock: challenged with the same dose of PBS.
Viremia in Challenged Pigs
No virus was detected in the Vac/ChA and Vac/ChB groups (Table 1). However, the groups that were challenged (but received no vaccine) did shed virus. Three pigs in the Unvac/ChA groups shed virus for 12, 14, and 16 days, respectively from 2 dpc onwards, as against the other two pigs, which that stopped shedding virus after 10 dpc. In the Unvac/ChB group, four pigs shed virus for 4–6 days, and one pig shed virus from 8 to 16 dpc, at which point it died. Furthermore, the virus shedding rate per day in the Unvac/ChA group was higher than that in the Unvac/ChB group.
Table 1.
Viremia from post-challenge pigs.
| Groups | Days post challenge (Number of viremia/Animals with detected) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 | 21 | |
| Vaccinated + Challenged TJ strain | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| Challenged TJ strain | 0/5 | 4/5 | 5/5 | 5/5 | 5/5 | 5/5 | 3/5 | 2/4 | 1/3 | 0/2 | 0/2 |
| Vaccinated + Challenged HN201605 strain | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| Challenged HN201605 strain | 0/5 | 3/5 | 4/5 | 4/5 | 3/5 | 2/5 | 1/5 | 1/5 | 1/5 | 0/4 | 0/4 |
| Mock group | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
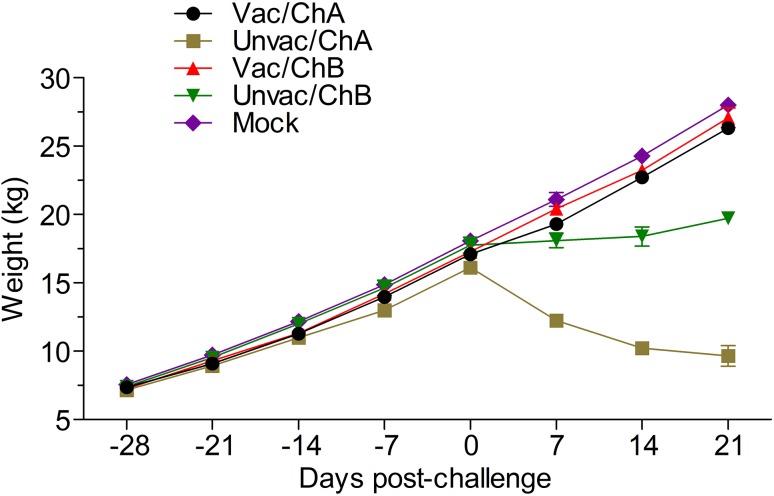
Body Weight Gain in Pigs
After all four groups were challenged with the PRRSV, pigs in the vaccinated groups continued to gain body weight until euthanized. There was no significant difference in body weight between the vaccinated-challenged groups and the mock group. However, all the pigs in the unvaccinated-challenged groups experienced weight loss to a greater extent than did pigs in the vaccinated and mock groups. The pigs in group Unvac/ChA lost significantly more body weight than those in group Unvac/ChB (Fig. 5).
Fig. 5.
Body weight gain of pigs in all the groups. All the pigs in the unvaccinated-challenged groups experienced weight loss compared with those in the vaccinated and mock groups. Vac/ChA: Vaccinated + Challenged TJ strain; Unvac/ChA: Challenged TJ strain; Vac/ChB: Vaccinated + Challenged HN 201605 strain; Unvac/ChB: Challenged HN 201605 strain; Mock: challenged with the same dose of PBS.
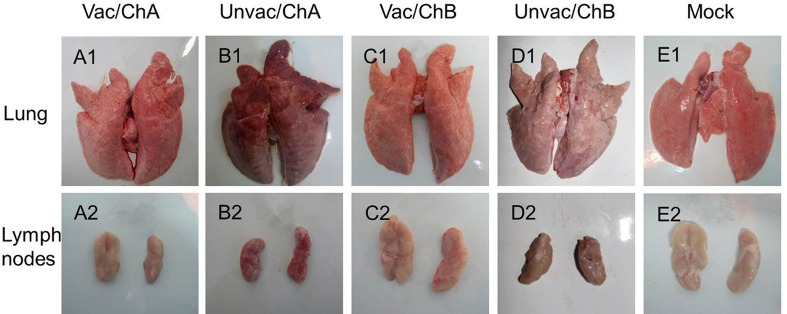
Pathological Examination
All surviving pigs were euthanized at 21 dpc and subjected to necropsy. In the unvaccinated-challenged group, Unvac/ChA pigs exhibited severe gross lesions with consolidation, hemorrhage, firmer and heavier parenchyma in the lung tissues with hemorrhage, and swelling of the lymph nodes. This differed from observations of lighter parenchyma of the lung and fewer pathological changes in the Unvac/ChB pigs (Fig. 6). However, there were no visible gross pathological changes in the vaccinated-challenged groups, nor were there any lesions in the tissues of the mock group.
Fig. 6.
Macroscopic examination of the lungs (A1–E1) and lymph nodes (A2–E2). A1 and A2 (Vac/ChA group), C1 and C2 (Vac/ChB group), and E1 and E2 (mock group) indicate the normal lungs and lymph nodes. B1 indicates extensive pneumorrhagia and lung consolidation. B2: Extensive hemorrhage in the lymph nodes. D1 indicates mild lung consolidation. D2 indicates slight hemorrhage in the lymph nodes. Vac/ChA: Vaccinated + Challenged TJ strain; Unvac/ChA: Challenged TJ strain; Vac/ChB: Vaccinated + Challenged HN 201605 strain; Unvac/ChB: Challenged HN 201605 strain; Mock: challenged with the same dose of PBS.
Microscopic pulmonary lesions were typical of those associated with PRRSV infection. Therefore, pathological evaluation that includes detection of the PRRSV antigen within these lesions is also critical for determining the efficacy of PRRSV vaccines (Opriessnig et al. 2007). Consistent with the macroscopic findings, the histological examination results also revealed that the Unvac/ChB pigs exhibited mild interstitial pneumonia with symptoms of epithelial proliferation and fewer fibroblasts. Furthermore, the lymph nodes of these pigs exhibited less proliferation of cortical lymphocytes. However, severe interstitial pneumonia and acute hemorrhage were observed in the Unvac/ChA pigs (Fig. 7). IHC staining results revealed different intensities of positive staining in the lung tissues of the Unvac/ChA and Unvac/ChB pigs. However, no positive staining was detected in the mock group (Fig. 7).
Fig. 7.
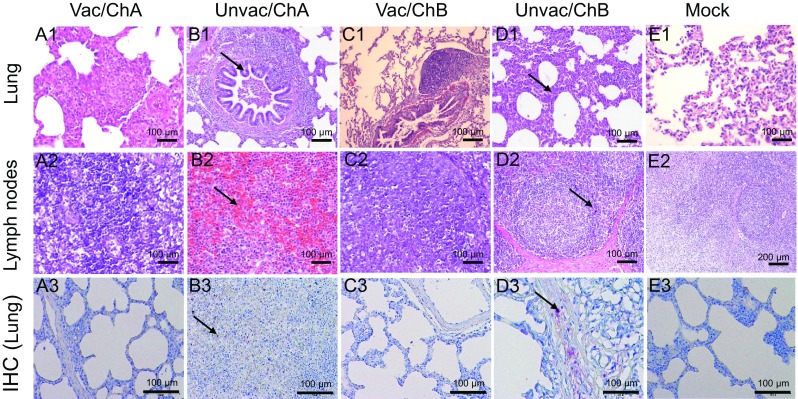
Histopathological examination of the lungs (A1–E1) and lymph nodes (A2–E2) and IHC examination of the lungs (A3–E3). A1–A3, C1–C3, and E1–E3 indicate the normal lungs and lymph nodes. B1 indicates serious bronchopneumonia. B2 indicates serious hemorrhage in the lymph nodes and lymphopenia. Positive staining was detected in B3. D1 indicates milder interstitial pneumonia and lymphoreticulosis, D2 indicates slight lymphoproliferation and milder subcortical hyperplasia. Positive staining was detected in D3. No positive staining was detected in E3. Vac/ChA: Vaccinated + Challenged TJ strain; Unvac/ChA: Challenged TJ strain; Vac/ChB: Vaccinated + Challenged HN 201605 strain; Unvac/ChB: Challenged HN 201605 strain; Mock: challenged with the same dose of PBS.
Discussion
An outbreak of the highly pathogenic PRRSV caused significant economic losses in China in 2006 (Zhao et al. 2015). This virus causes severe morbidity and mortality in pigs of all ages. Several field isolates of PRRSV have been identified with different genetic backgrounds and exhibiting 131 discontinuous amino acid deletions in the genome. Clinical symptoms associated with low mortality are consistent with infection with a NADC30 strain that was isolated in the United States in 2008 (Brockmeier et al. 2012; Zhou et al. 2015). Therefore, these new PRRSV isolates were termed as NADC30-like PRRSV in China.
A comparison of the deduced amino acids of the nsp2 proteins of NADC30-like isolates with the classical PRRSV strain (VR2332, CH-1a) showed amino acid deletions at positions 322–432 (111 aa), 481 (1 aa), and 504–522 (19 aa) in the nsp2 of HN201605, HENAN-XINX, and HNjz15, respectively (Supplementary Figure S1). An amino acid homology analysis indicated that the HN201605 isolate shared 83.2%–92.4%, 76.4%–77.1%, and 76.3%–77% (data not shown) homology to NADC30-like, HP-PRRSV, and classical PRRSV isolates, respectively. As reported previously, NADC30-like strains showed moderate pathogenicity in experimental studies, and its different isolates shared more than 80% homology for the nsp2 gene (Li et al. 2016a). In order to evaluate whether the commercial vaccines in the market could provide protection against the NADC30-like PRRSV infection, an attenuated Chinese highly pathogenic PRRSV vaccine strain TJM-F92 made in China was used. The deletion of 120 amino acids from positions 628 to 747 in the nsp2 region was specific to the TJM-F92 strain compared with other commercial strains (Leng et al. 2012a). The nsp2 is the most variable gene in the PRRSV genome and can endure a number of mutations, insertions, and deletions. Faaberg found that pigs that received the recombinant virus with rΔ727–813 nsp2 deletion mutants showed significant reduction in lymph node enlargement (Faaberg et al. 2010). A recombinant virus with 131 aa (628–759) deletions of the nsp2 gene was found to be less virulent in pigs (Kim et al. 2009). These results show that disruption of amino acids in the nsp2 domain had a lethal effect on viral replication.
In this study, the pigs in the Unvac/ChA group exhibited severe clinical symptoms including persistently high fever (≥ 41 °C), cyanopathy of the ears, red coloration of the body, conjunctivitis, dyspnea, and severe diffuse pulmonary lesions that were consolidated, consistent with those reported in a previous study (Zhou et al. 2008). Three pigs died at 15, 17, and 18 dpc, respectively. The pigs in the Unvac/ChB group developed fever (body temperature ≥ 40 °C) at 3 dpc, which lasted for 7 days. Cough, anorexia, shivering, and respiratory distress were observed before the pigs recovered to normal conditions. The pigs in the Vac/ChA, Vac/ChB, and mock groups showed normal conditions throughout the study. These clinical and pathological results revealed that compared to the TJ strain (HP-PRRSV strain), the NADC30-like HN201605 strain had less pathogenicity. Pigs immunized with the TJM-F92 vaccine were effectively protected against the challenge of HP-PRRSV and NADC30-like strains. The antibody response detected by ELISA showed that the S/P ratio was above 0.4 in the vaccinated groups from 14 dpv to 21 dpc. However, although all vaccinated–challenged pigs were protected, not all the neutralizing antibodies have yet been detected. This may be because PRRSV viremia often resolved before neutralizing antibodies were detected in both infected and vaccinated pigs (Nelson et al. 1994; Mengeling et al. 2003; Mateu and Diaz 2008). This finding is identical to the result of a study that showed that neutralizing antibodies were detected only at 91 dpv but still protected against PRRSV challenge (Park et al. 2014).
As a direct and objective indicator, gain in body weight is considered to be the most important parameter of vaccine efficacy, and vaccination was effective in reducing financial losses due to the PRRSV infection (Mengeling et al. 2003). In this study, all the vaccinated–challenged pigs were at least 5 kg heavier than the pigs of the challenged-only groups. The body weight of pigs in the Unvac/ChA group clearly decreased after challenge. Compared with the vaccinated group, pigs of the Unvac/ChB lost more weight and grew more slowly. No viral shedding was detected in the vaccinated groups compared with a long period of viral shedding in the unvaccinated groups. These results suggest that TJM-F92 vaccine can protect against PRRSV infection.
Galliher-Beckley found that the pigs immunized with modified HP-PRRS live vaccine were protected from challenge with the North American PRRSV NADC strain (Galliher-Beckley et al. 2015). Nearly 97% of lesions were reduced in the pigs vaccinated with Ingelvac PRRSV (VR2332) after challenge with the NADC8, NADC9, or NVSL strains compared with the challenge control, (Roof et al. 2003). Furthermore, gross lung pathology reduced. Experiments in piglets showed that the commercial PRRSV modified live vaccines TJM-F92 and R98 provided partly protective efficacy against FJ1402 challenge (Zhang et al. 2016). The different protection rates of TJM-F92 might be due to the different challenge strains, but further studies are necessary to confirm this. Some studies have shown that the current vaccine provided limited protection against NADC30-like infection (Bai et al. 2016). The failure of vaccination could be due to co-infection with other swine viruses or improper immunization schedules.
In the United States, the total losses in the swine industry approached $664 million annually due to PRRSV infection (Holtkamp et al. 2013). An effective PRRSV vaccine has been estimated to increase the market weight of pigs by 1.26 kg/pig (increasing the revenue by $3.25/pig) and decrease mortality by 17% (a saving of $45/pig). Thus, the economic benefit with introduction of the new modified live PRRSV vaccine is considered to be $48.25/pig (Park et al. 2014). The TJM-F92 strain has a unique, continuous 360 nucleotide (120 aa) deletion in the nsp2 gene and provides protection against PRRSV infection (Leng et al. 2012b). This could be used as a safe and effective PRRSV candidate vaccine, which protects pigs against the NADC30-like strain. However, we should not solely rely on the PRRSV vaccine. Modified HP-PRRSV live vaccine is not recommended for use in PRRSV-free hog farms.
This study showed that compared to the HP-PRRSV strain, the NADC30-like strain HN201605 had lower pathogenicity. We also demonstrated that the MLV vaccine strain TJM-F92 provided cross protection against PRRSV NADC30-like strain HN201605 infection, suggesting that it is an effective and safe vaccine candidate in China. In order to better evaluate this vaccine, further studies are needed to test its efficiency against other PRRSV NADC30-like isolates.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Alignment of amino acid sequences of nonstructural protein of HN201605 strain with isolates. CHsx1401 (KP861625), HENAN-XINX (KF611905.1), HENXX-9 (KY290748.1), HNjz15 (KT945017.1), JL580 (KR706343.1), NADC30 (JN654459.1), HUN4 (EF635006.1), JXA1 (EF112445.1), TJ (E U860248.1), VR2332 (EF536003.1), CH-1a (AY03 2626.1). En dashes indicate deleted residues. Positions of deleted amino acids were determined on the basis of the genome of PRRSV strains, CH-1a and VR2332. (PDF 75 kb)
Acknowledgements
The authors thank all the technicians who were involved in animal care and sample collection. The research results belonged to the Sinovet (Beijing) Biotechnology Co. Ltd.
Author Contributions
HWZ, MQX, WW, DCJ, LC, BW, XW, YW, and NS performed the study, HWZ wrote the manuscript, and JXH and CXT participated in the collection of samples. HW and SCZ participated in revision of the manuscript. HW is the leader of the project. All authors read and approved the final manuscript.
Conflict of interest
The authors declare that they have no competing interests.
Animal and Human Rights Statement
All animal experimental procedures have been reviewed and approved by the Animal Care and Use Committee of Sinovet (Jiangsu) Biotechnology Co. Ltd. (approval ID: SYXK(Su) 2013-0032). All pigs were supplied with water, food, and healthcare throughout the study period.
References
- Allende R, Lewis T, Lu Z, Rock D, Kutish G, Ali A, Doster A, Osorio F. North American and European porcine reproductive and respiratory syndrome viruses differ in non-structural protein coding regions. J Gen Virol. 1999;80:307–315. doi: 10.1099/0022-1317-80-2-307. [DOI] [PubMed] [Google Scholar]
- Bai X, Wang Y, Xu X, Sun Z, Xiao Y, Ji G, Li Y, Tan F, Li X, Tian K. Commercial vaccines provide limited protection to NADC30-like PRRSV infection. Vaccine. 2016;34:5540–5545. doi: 10.1016/j.vaccine.2016.09.048. [DOI] [PubMed] [Google Scholar]
- Brockmeier SL, Loving CL, Vorwald AC, Kehrli ME, Baker RB, Nicholson TL, Lager KM, Miller LC, Faaberg KS. Genomic sequence and virulence comparison of four Type 2 porcine reproductive and respiratory syndrome virus strains. Virus Res. 2012;169:212–221. doi: 10.1016/j.virusres.2012.07.030. [DOI] [PubMed] [Google Scholar]
- Calzada-Nova G, Husmann RJ, Schnitzlein WM, Zuckermann FA. Effect of the host cell line on the vaccine efficacy of an attenuated porcine reproductive and respiratory syndrome virus. Vet Immunol Immunopathol. 2012;148:116–125. doi: 10.1016/j.vetimm.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Faaberg KS, Kehrli ME, Lager KM, Guo B, Han J. In vivo growth of porcine reproductive and respiratory syndrome virus engineered nsp2 deletion mutants. Virus Res. 2010;154:77–85. doi: 10.1016/j.virusres.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliher-Beckley A, Li X, Bates JT, Madera R, Waters A, Nietfeld J, Henningson J, He D, Feng W, Chen R. Pigs immunized with Chinese highly pathogenic PRRS virus modified live vaccine are protected from challenge with North American PRRSV strain NADC-20. Vaccine. 2015;33:3518–3525. doi: 10.1016/j.vaccine.2015.05.058. [DOI] [PubMed] [Google Scholar]
- Halbur P, Miller L, Paul P, Meng X-J, Huffman E, Andrews J. Immunohistochemical identification of porcine reproductive and respiratory syndrome virus (PRRSV) antigen in the heart and lymphoid system of 3-week-old colostrum-deprived pigs. Vet Pathol. 1995;32:200–204. doi: 10.1177/030098589503200218. [DOI] [PubMed] [Google Scholar]
- Halbur P, Paul P, Frey M, Landgraf J, Eernisse K, Meng X-J, Lum M, Andrews J, Rathje J. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Pathol. 1995;32:648–660. doi: 10.1177/030098589503200606. [DOI] [PubMed] [Google Scholar]
- Halbur PG, Paul PS, Meng XJ, Lum MA, Andrews JJ, Rathje JA. Comparative pathogenicity of nine US porcine reproductive and respiratory syndrome virus (PRRSV) isolates in a 5-week-old cesarean-derived, colostrum-deprived pig model. J Vet Diagn Invest. 1996;8:11–20. doi: 10.1177/104063879600800103. [DOI] [PubMed] [Google Scholar]
- Holtkamp DJ, Kliebenstein JB, Neumann EJ, Zimmerman JJ, Rotto HF, Yoder TK, Wang C, Yeske PE, Mowrer CL, Haley CA. Assessment of the economic impact of porcine reproductive and respiratory syndrome virus on United States pork producers. J Swine Health Prod. 2013;21:72–84. [Google Scholar]
- Huang C, Zhang Q, W-h Feng. Regulation and evasion of antiviral immune responses by porcine reproductive and respiratory syndrome virus. Virus Res. 2015;202:101–111. doi: 10.1016/j.virusres.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Kaiser TJ, Horlen K, Keith ML, Taylor LP, Jolie R, Calvert JG, Rowland RR. Insertion and deletion in a non-essential region of the nonstructural protein 2 (nsp2) of porcine reproductive and respiratory syndrome (PRRS) virus: effects on virulence and immunogenicity. Virus Genes. 2009;38:118–128. doi: 10.1007/s11262-008-0303-4. [DOI] [PubMed] [Google Scholar]
- Lager KM, Schlink SN, Brockmeier SL, Miller LC, Henningson JN, Kappes MA, Kehrli ME, Loving CL, Guo B, Swenson SL. Efficacy of Type 2 PRRSV vaccine against Chinese and Vietnamese HP-PRRSV challenge in pigs. Vaccine. 2014;32:6457–6462. doi: 10.1016/j.vaccine.2014.09.046. [DOI] [PubMed] [Google Scholar]
- Leng X, Li Z, Xia M, He Y, Wu H. Evaluation of the efficacy of an attenuated live vaccine against highly pathogenic porcine reproductive and respiratory syndrome virus in young pigs. Clin Vaccine Immunol. 2012;19:1199–1206. doi: 10.1128/CVI.05646-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng X, Li Z, Xia M, Li X, Wang F, Wang W, Zhang X, Wu H. Mutations in the genome of the highly pathogenic porcine reproductive and respiratory syndrome virus potentially related to attenuation. Vet Microbiol. 2012;157:50–60. doi: 10.1016/j.vetmic.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Li X, Galliher-Beckley A, Huang H, Sun X, Shi J. Peptide nanofiber hydrogel adjuvanted live virus vaccine enhances cross-protective immunity to porcine reproductive and respiratory syndrome virus. Vaccine. 2013;31:4508–4515. doi: 10.1016/j.vaccine.2013.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Zhuang J, Wang J, Han L, Sun Z, Xiao Y, Ji G, Li Y, Tan F, Li X. Outbreak Investigation of NADC30-like PRRSV in South-East China. Transbound Emerg Dis. 2016;63:474–479. doi: 10.1111/tbed.12530. [DOI] [PubMed] [Google Scholar]
- Li Z, He Y, Xu X, Leng X, Li S, Wen Y, Wang F, Xia M, Cheng S, Wu H. Pathological and immunological characteristics of piglets infected experimentally with a HP-PRRSV TJ strain. BMC Vet Res. 2016;12:230. doi: 10.1186/s12917-016-0854-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Ning Y, Xu B, Gong W, Zhang D. Analysis of genetic variation of porcine reproductive and respiratory syndrome virus (PRRSV) isolates in Central China. J Vet Med Sci. 2016;78:641–648. doi: 10.1292/jvms.15-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateu E, Diaz I. The challenge of PRRS immunology. Vet J. 2008;177:345–351. doi: 10.1016/j.tvjl.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengeling WL, Lager KM, Vorwald AC, Clouser DF. Comparative safety and efficacy of attenuated single-strain and multi-strain vaccines for porcine reproductive and respiratory syndrome. Vet Microbiol. 2003;93:25–38. doi: 10.1016/S0378-1135(02)00426-1. [DOI] [PubMed] [Google Scholar]
- Nelson EA, Christopher-Hennings J, Benfield DA. Serum immune responses to the proteins of porcine reproductive and respiratory syndrome (PRRS) virus. J Vet Diagn Invest. 1994;6:410–415. doi: 10.1177/104063879400600402. [DOI] [PubMed] [Google Scholar]
- Opriessnig T, Baker RB, Halbur PG. Use of an experimental model to test the efficacy of planned exposure to live porcine reproductive and respiratory syndrome virus. Clin Vaccine Immunol. 2007;14:1572–1577. doi: 10.1128/CVI.00332-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C, Seo HW, Kang I, Jeong J, Choi K, Chae C. A new modified live porcine reproductive and respiratory syndrome vaccine improves growth performance in pigs under field conditions. Clin Vaccine Immunol. 2014;21:1350–1356. doi: 10.1128/CVI.00377-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renukaradhya GJ, Meng X-J, Calvert JG, Roof M, Lager KM. Live porcine reproductive and respiratory syndrome virus vaccines: current status and future direction. Vaccine. 2015;33:4069–4080. doi: 10.1016/j.vaccine.2015.06.092. [DOI] [PubMed] [Google Scholar]
- Roof M, Vaughn E, Burkhart K, Faaberg K (2003) Efficacy of modified live virus porcine reproductive and respiratory virus vaccines against heterologous respiratory challenge. In: Proceedings—4th international symposium on emerging and re-emerging pig diseases, Rome, pp 117–118
- Sun Z, Wang J, Bai X, Ji G, Yan H, Li Y, Wang Y, Tan F, Xiao Y, Li X. Pathogenicity comparison between highly pathogenic and NADC30-like porcine reproductive and respiratory syndrome virus. Arch Virol. 2016;161:2257–2261. doi: 10.1007/s00705-016-2883-y. [DOI] [PubMed] [Google Scholar]
- Tian K, Yu X, Zhao T, Feng Y, Cao Z, Wang C, Hu Y, Chen X, Hu D, Tian X. Emergence of fatal PRRSV variants: unparalleled outbreaks of atypical PRRS in China and molecular dissection of the unique hallmark. PLoS ONE. 2007;2:e526. doi: 10.1371/journal.pone.0000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian ZJ, An TQ, Zhou YJ, Peng JM, Hu SP, Wei TC, Jiang YF, Xiao Y, Tong GZ. An attenuated live vaccine based on highly pathogenic porcine reproductive and respiratory syndrome virus (HP-PRRSV) protects piglets against HP-PRRS. Vet Microbiol. 2009;138:34–40. doi: 10.1016/j.vetmic.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Wei Z, Zhang J, Zhuang J, Sun Z, Gao F, Yuan S. Immunization of pigs with a type 2 modified live PRRSV vaccine prevents the development of a deadly long lasting hyperpyrexia in a challenge study with highly pathogenic PRRSV JX143. Vaccine. 2013;31:2062–2066. doi: 10.1016/j.vaccine.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Zhang C, Guo L, Jia X, Wang T, Wang J, Sun Z, Wang L, Li X, Tan F, Tian K. Construction of a triple gene-deleted Chinese Pseudorabies virus variant and its efficacy study as a vaccine candidate on suckling piglets. Vaccine. 2015;33:2432–2437. doi: 10.1016/j.vaccine.2015.03.094. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Jiang P, Song Z, Lv L, Li L, Bai J. Pathogenicity and antigenicity of a novel NADC30-like strain of porcine reproductive and respiratory syndrome virus emerged in China. Vet Microbiol. 2016;197:93–101. doi: 10.1016/j.vetmic.2016.11.010. [DOI] [PubMed] [Google Scholar]
- Zhao K, Ye C, Chang XB, Jiang CG, Wang SJ, Cai XH, Tong GZ, Tian ZJ, Shi M, An TQ. Importation and recombination are responsible for the latest emergence of highly pathogenic porcine reproductive and respiratory syndrome virus in China. J Virol. 2015;89:10712–10716. doi: 10.1128/JVI.01446-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou YJ, Hao XF, Tian ZJ, Tong GZ, Yoo D, An TQ, Zhou T, Li GX, Qiu HJ, Wei TC. Highly virulent porcine reproductive and respiratory syndrome virus emerged in China. Transbound Emerg Dis. 2008;55:152–164. doi: 10.1111/j.1865-1682.2008.01020.x. [DOI] [PubMed] [Google Scholar]
- Zhou L, Wang Z, Ding Y, Ge X, Guo X, Yang H. NADC30-like strain of porcine reproductive and respiratory syndrome virus, China. Emerg Infect Dis. 2015;21:2256. doi: 10.3201/eid2112.150360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Li S, Wang X, Zou M, Gao S. Bartha-k61 vaccine protects growing pigs against challenge with an emerging variant pseudorabies virus. Vaccine. 2017;35:1161–1166. doi: 10.1016/j.vaccine.2017.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Alignment of amino acid sequences of nonstructural protein of HN201605 strain with isolates. CHsx1401 (KP861625), HENAN-XINX (KF611905.1), HENXX-9 (KY290748.1), HNjz15 (KT945017.1), JL580 (KR706343.1), NADC30 (JN654459.1), HUN4 (EF635006.1), JXA1 (EF112445.1), TJ (E U860248.1), VR2332 (EF536003.1), CH-1a (AY03 2626.1). En dashes indicate deleted residues. Positions of deleted amino acids were determined on the basis of the genome of PRRSV strains, CH-1a and VR2332. (PDF 75 kb)