Abstract
Background:
The combination of motor imagery (MI) and afferent input with electrical stimulation (ES) enhances the excitability of the corticospinal tract compared with motor imagery alone or electrical stimulation alone. However, its therapeutic effect is unknown in patients with hemiparetic stroke. We performed a preliminary examination of the therapeutic effects of MI + ES on upper extremity (UE) motor function in patients with chronic stroke.
Methods:
A total of 10 patients with chronic stroke demonstrating severe hemiparesis participated. The imagined task was extension of the affected finger. Peripheral nerve electrical stimulation was applied to the radial nerve at the spiral groove. MI + ES intervention was conducted for 10 days. UE motor function as assessed with the Fugl–Meyer assessment UE motor score (FMA-UE), the amount of the affected UE use in daily life as assessed with a Motor Activity Log (MAL-AOU), and the degree of hypertonia in flexor muscles as assessed with the Modified Ashworth Scale (MAS) were evaluated before and after intervention. To assess the change in spinal neural circuits, reciprocal inhibition between forearm extensor and flexor muscles with the H reflex conditioning-test paradigm at interstimulus intervals (ISIs) of 0, 20, and 100 ms were measured before and after intervention.
Results:
UE motor function, the amount of the affected UE use, and muscle hypertonia in flexor muscles were significantly improved after MI + ES intervention (FMA-UE: p < 0.01, MAL-AOU: p < 0.01, MAS: p = 0.02). Neurophysiologically, the intervention induced restoration of reciprocal inhibition from the forearm extensor to the flexor muscles (ISI at 0 ms: p = 0.03, ISI at 20 ms: p = 0.03, ISI at 100 ms: p = 0.01).
Conclusion:
MI + ES intervention was effective for improving UE motor function in patients with severe paralysis.
Keywords: cerebrovascular disease, hemiparesis, electrical stimulation, motor imagery, rehabilitation
Introduction
Upper motor dysfunction is a common problem in patients with stroke and disrupts activities of daily living and eventually worsens quality of life.1,2 Recently, several rehabilitation approaches have been developed to improve upper extremity (UE) motor function. Previous research has shown that intensive use of the paretic upper limb contributes to improved motor function, even though the motor recovery period has already passed.3–6 However, intensive use of the paretic upper limb is impossible for patients with severe upper limb paralysis, because they cannot voluntarily control the paretic hand. Therefore, other rehabilitative approaches for severely impaired patients are needed. As an alternative approach, motor imagery (MI) can be applied to patients regardless of the degree of motor paralysis. MI is defined as a dynamic state during which the representation of a given motor act is internally rehearsed within working memory without any overt motor output.7 Functional imaging studies have revealed that brain activity during motor execution and MI is largely shared in motor networks, such as the primary motor area, supplementary motor area, and premotor area.8–10 Also, transcranial magnetic stimulation (TMS) studies reported that excitability of the corticospinal tract (CST) is significantly higher during MI in comparison with baseline.11–15 Based on these observations, MI has been applied for rehabilitation of patients with hemiparetic stroke, and the positive therapeutic effects on UE motor function have been reported.16–20 However, the effect size differs among the studies,19 and is lower with regard to motor recovery of the paretic hand.20 To obtain clinically significant improvement, ingenuity to strengthen the therapeutic effect of MI is thought to be necessary.
The combination of MI and afferent input with electrical stimulation (ES) is an approach to enhance the therapeutic effect of MI. The effectiveness of ES for modulation of the excitability of the CST and improvement of dexterity performance of the paretic hand has been reported in patients with mild to moderate paralysis.21,22 Moreover, the additive effect of MI and ES has been reported in healthy adults. Saito and colleagues reported that a combination of MI and peripheral nerve ES enhances the excitability of the CST compared with MI alone or ES alone.23 In addition, Kaneko and colleagues reported that the combination of MI and electrical muscular stimulation reproduces the excitability of the CST at levels similar to voluntary muscle contraction.24 However, its therapeutic effects for motor function in patients with stroke are unknown. Therefore, we performed a preliminary examination of the therapeutic effects of a combination of MI and peripheral nerve ES (MI + ES) on UE motor function in patients with severe paralysis. The aim of this study is to investigate the feasibility and potential of the therapeutic effect for future randomized controlled trials.
Materials and methods
Participants
The participants were 10 patients with hemiparetic stroke who met the following inclusion criteria: (1) a first unilateral stroke as confirmed with brain magnetic resonance imaging or computed tomography; (2) time from stroke onset of more than 150 days; (3) inability to extend the paretic fingers; (4) passive extension range of motion greater than −30 degrees for the metacarpophalangeal joints; (4) no pacemaker or other implanted stimulator. Detailed clinical information of the 10 participants is shown in Table 1. All participants had little or no detectable surface electromyogram activity from the affected extensor digitorum communis (EDC) muscles when they attempted to extend their affected fingers. The study purpose and procedures were explained to the participants, and written informed consent was obtained from each participant. This study was approved by the Institutional Ethics Review Board and was registered at the University Hospital Medical Information Network (UMIN) Clinical Trial Registry (UMIN000023731).
Table 1.
Clinical details of participants.
| Patient | Age (years) | Sex | Stroke type | Stroke location | Paretic side | TFO (years) |
|---|---|---|---|---|---|---|
| A | 51 | Male | CH | Thalamus | Right | 2.2 |
| B | 49 | Male | CI | MCA | Right | 1.6 |
| C | 67 | Male | CI | MCA | Right | 1.4 |
| D | 73 | Male | CH | Thalamus | Right | 7.9 |
| E | 46 | Male | CH | Putamen | Right | 1.0 |
| F | 62 | Female | CI | Corona radiata | Left | 2.7 |
| G | 71 | Male | CI | MCA | Left | 2.8 |
| H | 49 | Female | CH | Frontoparietal lobe | Left | 1.3 |
| I | 62 | Male | CI | Corona radiata | Right | 0.6 |
| J | 68 | Female | CH | Putamen | Right | 0.9 |
CH, cerebral hemorrhage; CI, cerebral infarction; MCA, middle cerebral artery; TFO, time from onset of stroke.
Study design
This feasibility study had a single group, pre- and post-intervention design. The participants trained for 10 days using MI + ES. UE motor function and reciprocal inhibition (RI) were assessed 1 day before and 1 day after the intervention.
Intervention
Each participant was seated in a comfortable chair with his or her affected arm supported and relaxed on the armrest in pronation. The angle of the elbow was kept at 70–90 degrees of flexion. A 14-inch computer monitor was placed about 60 cm in front of the participant. Surface electromyogram was recorded from the affected EDC muscles using Ag-AgCl surface electrodes (9 mm in diameter). The electrodes were applied with center-to-center spacing of 20 mm and were placed parallel to the muscle fibers. The raw signal was amplified and filtered using a band-pass filter of 20 Hz to 3 kHz (Neuropack; Nihon Kohden, Tokyo, Japan).
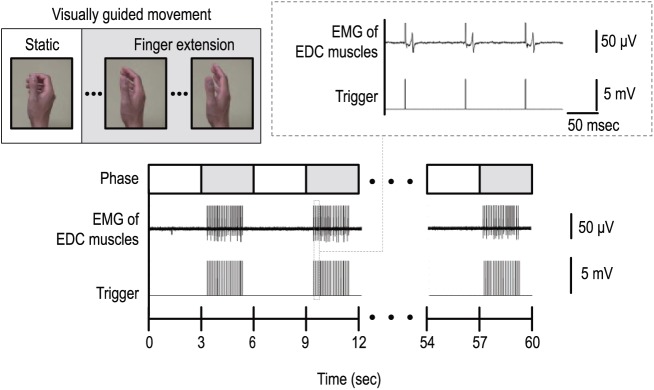
The MI + ES intervention was based on kinesthetic MI visually guided by video animation (Figure 1). Before the first intervention, the participant was instructed to imagine the kinesthetic sensation generated by the actual finger extension. The video animation was composed of a 3-s static phase and a 3-s finger extension phase. The participant was asked to relax during the static phase, and to imagine finger extension during the finger extension phase. This 6-s trial was repeated 10 times and was considered as one session. Moreover, the radial nerve was stimulated at the spiral groove during the finger extension phase. The stimulus timing according to the video animation was controlled by a custom-made computer program (LabVIEW 12.0; National Instruments Corp., Austin, TX, USA). The stimulation was delivered at 10 Hz with a pulse width of 1 ms based on previous reports.23,25,26 For the following four reasons, the stimulus intensity was set at the motor threshold (MT) of the EDC muscles: (1) Previous research has shown that stimulation above the MT is effective to increase excitability of the CST compared with stimulation above the sensory threshold.23 (2) We can confirm that the radial nerve was stimulated by monitoring the compound muscle action potential. (3) Peripheral nerve ES above the MT induces twitching of the wrist extensor muscles and may hinder MI of finger extension. (4) Anatomically, the radial nerve at the spiral groove is located deeper than the nerve at the wrist. Therefore, we need to increase the stimulus intensity more than stimulating the nerve at the wrist, and the participant might feel pain by percutaneous ES above the MT. The MT was defined as the lowest intensity that produced a compound muscle action potential amplitude >50 μV in at least 5 out of 10 stimuli. A total of 15 sessions (150 trials) were performed per day. Additionally, immediately after the MI + ES intervention, conventional occupational therapy targeting the affected UE was administered for 1 h per day. This included gentle stretching exercise, active muscle reeducation exercise, and how to use their paretic hands in actual daily life by a therapist’s manual contact. The training was conducted for 10 days over a 2-week period.
Figure 1.
The experimental setup of the intervention with combination of motor imagery and electrical stimulation (MI + ES).
Schematic diagram of the experimental setup of one session of the MI + ES intervention. Participants were instructed to relax and imagine finger extension movements in accordance with a visual guide. The radial nerve was stimulated at 10 Hz during the finger extension phase. The examiner confirmed that the radial nerve was stimulated by monitoring the compound muscle action potential, and actual muscle contraction of the EDC did not occur.
EDC, extensor digitorum communis; EMG, electromyogram; ES, electrical stimulation; MI, motor imagery.
Assessment
Clinical assessment
Motor function of the affected UE was assessed with the Fugl–Meyer assessment UE motor score (FMA-UE).27 The FMA-UE consists of four categories (A: Shoulder/Elbow/Forearm, B: Wrist, C: Hand/Finger, and D: Coordination) and has a maximum score of 66. Its reliability and validity have been well demonstrated.28 The Motor Activity Log (MAL) is a structured interview used to measure UE disability in activities of daily living. The MAL-14 includes 14 items, scored on an 11-point amount of use (AOU) score (range 0–5) to rate how much the arm is used.29 High construct validity and reliability of the MAL have been demonstrated in patients with chronic stroke.29,30 We calculated the sum of each item as the MAL-AOU. The Modified Ashworth Scale (MAS) grades the degree of hypertonia in individual muscles or muscle groups and is scored with a 6-point rating scale to measure passive muscle resistance.31 The MAS score was summed for the following muscle groups: elbow flexors, wrist flexors, and finger flexors (score 1+ was transformed to 2, and scores 2 and 3 were transformed to 3 and 4). These clinical assessments were performed before and after the intervention and were determined by an independent physiatrist with more than 10 years of experience.
Reciprocal inhibition
RI was assessed to investigate the change in spinal neural circuits using the flexor carpi radialis (FCR) H reflex conditioning test paradigm.32 Percutaneous electrical pulses of 1-ms duration at a frequency of 0.3 Hz were delivered through surface electrodes to the median nerve in the cubital fossa. To determine the stimulus intensity of Hmax, the optimal position for stimulating the median nerve was initially determined by moving the stimulating electrode while checking the waveform. Then, the intensity was gradually increased until an H reflex without an M response was recorded. The response with the largest amplitude was selected as the Hmax. The stimulus intensity was then increased until the Mmax response was obtained. The test FCR H reflex amplitude was maintained at 15–20% of the Mmax for each trial block. Conditioning stimulation to the radial nerve was delivered at the spiral groove. Stimulus intensity was set at a level that was capable of inducing an M response from extensor carpi radialis muscles with peak-to-peak amplitudes of 100 μV. Interstimulus intervals (ISIs) of 0, 20, and 100 ms were selected based on previous reports.33–36 The first phase of RI (ISI at 0 ms) is related to the Ia disynaptic pathway.32 The second phase of RI (ISI at 20 ms) may occur via presynaptic inhibition.37 The inhibitory circuit of the third phase of RI has yet to be clarified. However, the third inhibitory phase may be responsible for descending control of spinal interneurons possibly by the brain stem or cerebral cortex.34,36,38 The size of the conditioned H reflex was expressed as a percentage of the size of the unconditioned H reflex at each interval (e.g. RI 0 ms = conditioned H reflex amplitude of the ISI at 0 ms/test H reflex amplitude).
Statistical analyses
The Wilcoxon signed-rank test was used to compare clinical data (FMA-UE scores, MAL-AOU, MAS) between the pre-intervention and post-intervention periods. The reason of using the nonparametric test is that clinical data are ordinal scales and the normality of the data distribution was not confirmed in some outcomes as a result of the Shapiro–Wilk test. After the normality of the data distribution was confirmed using the Shapiro–Wilk test, the paired Student’s t-test was used to compare RI at each phase between the pre-intervention and post-intervention periods. All reported p-values were two-sided, and p-values < 0.05 were considered significant. Effect sizes for the FMA-UE were calculated using Cohen’s d statistics, with the magnitude of group differences defined as small if d = 0.2, medium if d = 0.5, or large if d = 0.8, considering the clinical significance of the variables.
Results
All the participants completed the intervention without experiencing obvious adverse effects.
Clinical assessments
The changes in clinical assessments are shown in Tables 2 and 3. After MI + ES intervention, the FMA-UE score increased in all participants. The average amount of change was 5.5 points. The MAL-AOU score increased in all participants except one. The average amount of change was 3.4 points. The summed MAS score decreased in six participants. The Wilcoxon signed-rank test showed significant improvement in the FMA-UE score (p < 0.01), the FMA-UE category A score (p < 0.01), the FMA-UE category B score (p = 0.04), the FMA-UE category C score (p = 0.01), and the MAL-AOU (p < 0.01) between the pre-intervention and post-intervention periods. The FMA-UE category D score was not changed in all patients (p = 1.00). The effect size for the FMA-UE was large (d = 0.86). The MAS scores were also reduced significantly (p = 0.02).
Table 2.
Clinical assessments at pre- and post-intervention in 10 patients.
| Patient | FMA-UE |
MAL-AOU |
MAS |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Change | Pre | Post | Change | Pre | Post | Change | |
| A | 28 | 33 | 5 | 5 | 12 | 7 | 5 | 4 | −1 |
| B | 19 | 25 | 6 | 10 | 11 | 1 | 5 | 4 | −1 |
| C | 21 | 31 | 10 | 3 | 8 | 5 | 3 | 0 | −3 |
| D | 15 | 19 | 4 | 0 | 2 | 2 | 4 | 4 | 0 |
| E | 26 | 29 | 3 | 6 | 6 | 0 | 4 | 4 | 0 |
| F | 10 | 14 | 4 | 8 | 20 | 12 | 5 | 3 | −2 |
| G | 23 | 27 | 4 | 8 | 10 | 2 | 3 | 2 | −1 |
| H | 30 | 35 | 5 | 9 | 10 | 1 | 5 | 4 | −1 |
| I | 15 | 21 | 6 | 12 | 13 | 1 | 4 | 4 | 0 |
| J | 13 | 21 | 8 | 3 | 6 | 3 | 2 | 2 | 0 |
| Average | 20.0 | 25.5 | 5.5 | 6.4 | 9.8 | 3.4 | 4.0 | 3.1 | −0.9 |
| SD | 6.4 | 6.4 | 2.0 | 3.5 | 4.6 | 3.5 | 1.0 | 1.3 | 0.9 |
FMA-UE, Fugl–Meyer assessment upper extremity motor score; MAL-AOU, amount of use scores in Motor Activity Log; MAS, Modified Ashworth Scale; SD, standard deviation.
Table 3.
Changes in FMA-UE subscale scores.
| Pre | Post | p-value | |
|---|---|---|---|
| FMA-UE A | 15.7 (5.6) | 18.5 (5.9) | <0.01 |
| B | 0.6 (1.2) | 1.8 (2.0) | 0.04 |
| C | 3.6 (0.7) | 5.1 (0.9) | 0.01 |
| D | 0.1 (0.3) | 0.1 (0.3) | 1.00 |
A, shoulder/elbow/forearm, 36 points; B, wrist, 10 points; C, hand/finger, 14 points; D, coordination, 6 points.
FMA-UE, iFugl–Meyer assessment upper extremity motor score.
Reciprocal inhibition
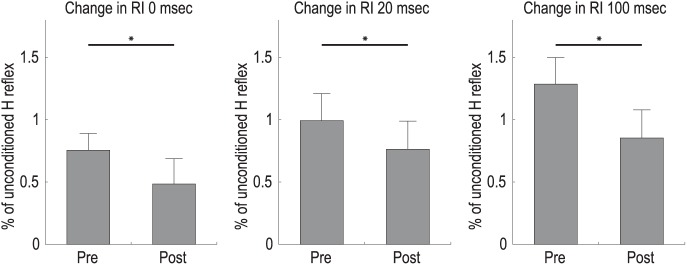
We were able to obtain the H reflex in the affected FCR muscles in seven participants. In the other three patients, the H reflex amplitude was either not observed or too small, so it was impossible to evaluate with an appropriate test stimulus. The paired Student’s t-test showed a significant difference in RI at 0 ms (p = 0.03), at 20 ms (p = 0.03), and at 100 ms (p = 0.01) following the intervention (Figure 2).
Figure 2.
Changes in RI at each phase.
The effects of the intervention with combination of MI and ES on RI at each phase. Error bars indicate the standard error. Asterisks show significant differences between the pre-intervention and post-intervention periods. *p < 0.05.
ES, electrical stimulation; MI, motor imagery; RI, reciprocal inhibition.
Discussion
In this study, we performed a preliminary investigation of the effectiveness of the MI + ES intervention for UE motor function in patients with chronic severe hemiparesis. After the MI + ES intervention, patients showed improved UE motor function, increased use of their paretic UE in activities of daily living, and mitigated spasticity. Furthermore, as a result of evaluation of RI by electrophysiological methods, we found that RI from the forearm extensor to the flexor muscles was increased after the intervention.
All participants showed improved UE motor function after 10 days of MI + ES intervention. Changes in the FMA-UE score were statistically significant and showed a large effect size. The amount of change was beneficial from the perspective of a clinically important difference. Previous research estimated that a clinically important difference in FMA-UE scores was about 5 points in individuals with stable, mild to moderate hemiparesis.39 Six of 10 patients in our study showed an increase in FMA-UE scores of 5 points or more, surpassing the minimal clinically important difference, following intervention for only 10 days. In the results of the subcategories of FMA-UE, Shoulder/Elbow/Forearm, Wrist and Hand/Finger scores were significantly improved between the pre-intervention and post-intervention periods. Especially, FMA-UE category Wrist and Hand/Finger scores reflected the distal UE motor function. We consider that this result demonstrates the effect of MI + ES intervention on distal UE motor function. In addition, the FMA-UE category Shoulder/Elbow/Forearm score was also improved. We speculate that the reasons for improvement were related to the effect of occupational therapy, which consisted of gentle stretching exercise and muscle reeducation exercise and task specific reach-to-grasp training using peg, and increased UE use brought about by the improvement of the Hand/Finger motor function. Indeed, the MAL-AOU scores were significantly increased after the intervention. The improvement in motor function may lead to behavioral changes in activities of daily living.
We believe that the improvement in UE motor function after the intervention with MI + ES was induced by plastic changes at cortical and spinal levels. In healthy adults, MI + ES enhances the excitability of the CST compared with MI alone and ES alone,23 and increases the excitability of the CST at levels similar to motor execution.24 In addition, Bonassi et al. reported that training with MI + ES facilitates motor learning through primary motor cortex plasticity similar to motor execution.40 In this study, plastic changes in movement-related cortical areas may be involved in motor recovery. Furthermore, modulation of the CST excitability facilitates motor learning of a subsequent motor task, based on evidence from neuromodulation with repetitive TMS.41–43 Avenaniti et al. reported that repetitive TMS before UE motor rehabilitation is effective more than repetitive TMS after UE motor rehabilitation for improvement of UE motor function. Thus, MI + ES might facilitate learning of a subsequent UE motor task in occupational therapy by the effect of interventions order.
At the spinal level, we confirmed from RI that plastic changes had occurred after the intervention with MI + ES. It has been reported that RI is modulated by MI alone and ES alone.44–46 MI affects the spinal neural circuits through the descending motor pathway.45–48 Kawakami et al. reported that RI of the antagonist muscle was increased during MI in patients with stroke.45 The effect of ES on RI has also be reported, although the stimulus parameters were different from those used in the present study.44,49 In this study, we consider that RI circuits may be reinforced by repetitive input from the cortex induced by MI, and peripheral nerves induced by ES. As a result, each phase of RI was increased after MI + ES intervention. This change in RI likely contributed to the improvement in UE motor function. RI is an important neural system for selective muscle activity and coordinated movements.50–52 Patients with stroke show reduced or absent RI of the forearm.53–56 Hence, muscle activity shows a synergistic pattern after stroke.57 Additionally, the increment of RI may have contributed to mitigation of spasticity. A previous report showed that RI at 100 ms was correlated with the MAS scores of the wrist and finger flexors.38 Moreover, a change in RI at 100 ms and a change in the MAS scores of the wrist were also correlated after an intervention using integrated volitional control electrical stimulation with a wrist splint.6 We cannot consider the relationship of clinical change and neurophysiological change because of small sample in this study. Further investigation is needed.
Task specific and repetitive training is important for motor recovery after stroke.58,59 However, patients with severe paralysis have difficulties applying these training methods. Therefore, approaches for severe upper limb paralysis are lacking, although several rehabilitation approaches have been developed to improve UE motor function. The advantage of MI + ES interventions is that they can be used in patients with severe stroke who are not capable of moving their affected finger. Kawakami et al. reported that recovery of UE motor function can be enhanced by using a gradual neurorehabilitation approach. If severely paralyzed patients can move their affected fingers even a little, the movement can be amplified by neuromuscular electrical stimulation to further improve the motor function.60 Thus, the MI + ES intervention has the potential to become a new rehabilitation option for severely paralyzed patients.
Study limitations
In this study, we determined the protocol of ES based on previous reports.23,25,26 However, since this is the first study on MI + ES intervention in patients with stroke, it is unknown whether the stimulation protocol was the best for improvements of UE motor function. Further investigation is needed to confirm the appropriate stimulation protocol. In addition, the ability to perform MI and the quality of MI were not evaluated. In future studies, the ability to perform MI should be assessed with a questionnaire (e.g. the Kinesthetic and Visual Imagery Questionnaire). Moreover, we did not utilize a sham treatment group. We cannot draw a conclusion about the effectiveness of the combination of MI and ES, because each intervention has the potential to enhance motor function on its own. Therefore, a randomized controlled study is needed to confirm the effectiveness of the MI + ES intervention. Furthermore, our protocol included not only the MI + ES intervention but also occupational therapy. Therefore, one cannot differentiate the effects of the two interventions. According to the phased approach to the development of clinical rehabilitation evidence, the present study was positioned as a phase I–II clinical trial. This study confirmed that the proposed treatment was clinically feasible from the perspective of both efficacy and safety, and the results ensured that the effects of the treatment are in the desired direction. The results now encourage us to compare its effectiveness with that of existing standard treatments. A phase III clinical trial with a larger sample size is needed for further development of clinical interventions.
Conclusion
As a preliminary result, we have shown that the MI + ES intervention was effective for improving UE motor function and mitigating spasticity in patients with chronic stroke. We showed that one of the reasons for improvement was that RI was increased after MI + ES. Although this study has several limitations and further studies are required, this approach may provide a potential benefit for severely impaired patients who cannot perform intensive motor training accompanied by actual hand movements.
Footnotes
Funding: This research was supported by JSPS KAKENHI Grant Number JP16K19521 from the Ministry of Education, Culture, Sports, Science and Technol-ogy (MEXT), Japan.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Kohei Okuyama, Department of Rehabilitation Medicine, Keio University School of Medicine, Tokyo, Japan.
Miho Ogura, Department of Rehabilitation Medicine, Keio University School of Medicine, Tokyo, Japan.
Michiyuki Kawakami, Department of Rehabilitation Medicine, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan.
Kengo Tsujimoto, Department of Rehabilitation Medicine, Keio University School of Medicine, Tokyo, Japan.
Kohsuke Okada, Department of Rehabilitation Medicine, Keio University School of Medicine, Tokyo, Japan.
Kazuma Miwa, Department of Rehabilitation Medicine, Keio University Hospital, Tokyo, Japan.
Yoko Takahashi, Department of Rehabilitation Medicine, Keio University School of Medicine, Tokyo, Japan.
Kaoru Abe, Department of Rehabilitation Medicine, Keio University Hospital, Tokyo, Japan.
Shigeo Tanabe, Faculty of Rehabilitation, School of Health Sciences, Fujita Health University, Toyoake-shi, Aichi, Japan.
Tomofumi Yamaguchi, Department of Physical Therapy, Yamagata Prefectural University of Health Sciences, Yamagata-shi, Yamagata, Japan.
Meigen Liu, Department of Rehabilitation Medicine, Keio University School of Medicine, Tokyo, Japan.
References
- 1. Hendricks HT, van Limbeek J, Geurts AC, et al. Motor recovery after stroke: a systematic review of the literature. Arch Phys Med Rehabil 2002; 83: 1629–1637. [DOI] [PubMed] [Google Scholar]
- 2. Carod-Artal J, Egido JA, González JL, et al. Quality of life among stroke survivors evaluated 1 year after stroke: experience of a stroke unit. Stroke 2000; 31: 2995–3000. [DOI] [PubMed] [Google Scholar]
- 3. Wolf SL, Lecraw DE, Barton LA, et al. Forced use of hemiplegic upper extremities to reverse the effect of learned nonuse among chronic stroke and head-injured patients. Exp Neurol 1989; 104: 125–132. [DOI] [PubMed] [Google Scholar]
- 4. Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA 2006; 296: 2095–2104. [DOI] [PubMed] [Google Scholar]
- 5. Fujiwara T, Kasashima Y, Honaga K, et al. Motor improvement and corticospinal modulation induced by hybrid assistive neuromuscular dynamic stimulation (HANDS) therapy in patients with chronic stroke. Neurorehabil Neural Repair 2009; 23: 125–132. [DOI] [PubMed] [Google Scholar]
- 6. Fujiwara T, Honaga K, Kawakami M, et al. Modulation of cortical and spinal inhibition with functional recovery of upper extremity motor function among patients with chronic stroke. Restor Neurol Neurosci 2015; 33: 883–894. [DOI] [PubMed] [Google Scholar]
- 7. Decety J, Grèzes J. Neural mechanisms subserving the perception of human actions. Trends Cong Sci 1999; 3: 172–178. [DOI] [PubMed] [Google Scholar]
- 8. Roth M, Decety J, Raybaudi M, et al. Possible involvement of primary motor cortex in mentally simulated movement: a functional magnetic resonance imaging study. Neuroreport 1996; 7: 1280–1284. [DOI] [PubMed] [Google Scholar]
- 9. Ehrsson HH, Geyer S, Naito E. Imagery of voluntary movement of fingers, toes, and tongue activates corresponding body-part-specific motor representations. J Neurophysiol 2003; 90: 3304–3316. [DOI] [PubMed] [Google Scholar]
- 10. Hanakawa T, Dimyan MA, Hallett M. Motor planning, imagery, and execution in the distributed motor network: a time-course study with functional MRI. Cereb Cortex 2008; 18: 2775–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kasai T, Kawai S, Kawanishi M, et al. Evidence for facilitation of motor evoked potentials (MEPs) induced by motor imagery. Brain Res 1997; 744: 147–150. [DOI] [PubMed] [Google Scholar]
- 12. Fadiga L, Buccino G, Craighero L, et al. Corticospinal excitability is specifically modulated by motor imagery: a magnetic stimulation study. Neuropsychologia 1999; 37: 147–158. [DOI] [PubMed] [Google Scholar]
- 13. Hashimoto R, Rothwell JC. Dynamic changes in corticospinal excitability during motor imagery. Exp Brain Res 1999; 125: 75–81. [DOI] [PubMed] [Google Scholar]
- 14. Stinear CM, Byblow WD. Modulation of corticospinal excitability and intracortical inhibition during motor imagery is task-dependent. Exp Brain Res 2004; 157: 351–358. [DOI] [PubMed] [Google Scholar]
- 15. Stinear CM, Byblow WD, Steyvers M, et al. Kinesthetic, but not visual, motor imagery modulates corticomotor excitability. Exp Brain Res 2006; 168: 157–164. [DOI] [PubMed] [Google Scholar]
- 16. Dijkerman HC, Ietswaart M, Johnston M, et al. Does motor imagery training improve hand function in chronic stroke patients? A pilot study. Clin Rehabil 2004; 18: 538–549. [DOI] [PubMed] [Google Scholar]
- 17. Page SJ, Levine P, Leonard AC. Effects of mental practice on affected limb use and function in chronic stroke. Arch Phys Med Rehabil 2005; 86: 399–402 [DOI] [PubMed] [Google Scholar]
- 18. Page SJ, Levine P, Leonard A. Mental practice in chronic stroke: results of a randomized, placebo-controlled trial. Stroke 2007; 38: 1293–1297. [DOI] [PubMed] [Google Scholar]
- 19. Kho AY, Liu KP, Chung RC. Meta-analysis on the effect of mental imagery on motor recovery of the hemiplegic upper extremity function. Aust Occup Ther J 2014; 61: 38–48. [DOI] [PubMed] [Google Scholar]
- 20. Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol 2009; 8: 741–754. [DOI] [PubMed] [Google Scholar]
- 21. Celnik P, Hummel F, Harris-Love M, et al. Somatosensory stimulation enhances the effects of training functional hand tasks in patients with chronic stroke. Arch Phys Med Rehabil 2007; 88: 1369–1376. [DOI] [PubMed] [Google Scholar]
- 22. Liu H, Au-Yeung SSY. Corticomotor excitability effects of peripheral nerve electrical stimulation to the paretic arm in stroke. Am J Phys Med Rehabil 2017; 96: 687–693. [DOI] [PubMed] [Google Scholar]
- 23. Saito K, Yamaguchi T, Yoshida N, et al. Combined effect of motor imagery and peripheral nerve electrical stimulation on the motor cortex. Exp Brain Res 2013; 227: 333–342. [DOI] [PubMed] [Google Scholar]
- 24. Kaneko F, Hayami T, Aoyama T, et al. Motor imagery and electrical stimulation reproduce corticospinal excitability at levels similar to voluntary muscle contraction. J Neuroeng Rehabil 2014; 11: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Collins DF, Burke D, Gandevia SC. Sustained contractions produced by plateau-like behaviour in human motoneurones. J Physiol 2002; 538: 289–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chipchase LS, Schabrun SM, Hodges PW. Peripheral electrical stimulation to induce cortical plasticity: a systematic review of stimulus parameters. Clin Neurophysiol 2011; 122: 456–463. [DOI] [PubMed] [Google Scholar]
- 27. Fugl-Meyer AR, Jääskö L, Leyman I, et al. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med 1975; 7: 13–31. [PubMed] [Google Scholar]
- 28. Platz T, Pinkowski C, van Wijck F, et al. Reliability and validity of arm function assessment with standardized guidelines for the Fugl-Meyer Test, Action Research Arm Test and Box and Block Test: a multicentre study. Clin Rehabil 2005; 19: 404–411. [DOI] [PubMed] [Google Scholar]
- 29. Uswatte G, Taub E, Morris D, et al. Reliability and validity of the upper-extremity Motor Activity Log-14 for measuring real-world arm use. Stroke 2005; 36: 2493–2496. [DOI] [PubMed] [Google Scholar]
- 30. van der Lee JH, Beckerman H, Knol DL, et al. Clinimetric properties of the motor activity log for the assessment of arm use in hemiparetic patients. Stroke 2004; 35: 1410–1414. [DOI] [PubMed] [Google Scholar]
- 31. Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 1987; 67: 206–207. [DOI] [PubMed] [Google Scholar]
- 32. Day BL, Marsden CD, Obeso JA, et al. Reciprocal inhibition between the muscles of the human forearm. J Physiol 1984; 349: 519–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Deuschl G, Seifert C, Heinen F, et al. Reciprocal inhibition of forearm flexor muscles in spasmodic torticollis. J Neurol Sci 1992; 113: 85–90. [DOI] [PubMed] [Google Scholar]
- 34. Huang YZ, Trender-Gerhard I, Edwards MJ, et al. Motor system inhibition in dopa-responsive dystonia and its modulation by treatment. Neurology 2006; 66: 1088–1090. [DOI] [PubMed] [Google Scholar]
- 35. Huang YZ, Rothwell JC, Lu CS, et al. The effect of continuous theta burst stimulation over premotor cortex on circuits in primary motor cortex and spinal cord. Clin Neurophysiol 2009; 120: 796–801. [DOI] [PubMed] [Google Scholar]
- 36. Tsai CH, Chen RS, Lu CS. Reciprocal inhibition in Parkinson’s disease. Acta Neurol Scand 1997; 95: 13–18. [DOI] [PubMed] [Google Scholar]
- 37. Berardelli A, Day BL, Marsden CD, et al. Evidence favouring presynaptic inhibition between antagonist muscle afferents in the human forearm. J Physiol 1987; 391: 71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Okuyama K, Kawakami M, Hiramoto M, et al. Relationship between spasticity and spinal neural circuits in patients with chronic hemiparetic stroke. Exp Brain Res 2018; 236: 207–213. [DOI] [PubMed] [Google Scholar]
- 39. Page SJ, Fulk GD, Boyne P. Clinically important differences for the upper-extremity Fugl-Meyer Scale in people with minimal to moderate impairment due to chronic stroke. Phys Ther 2012; 92: 791–798. [DOI] [PubMed] [Google Scholar]
- 40. Bonassi G, Biggio M, Bisio A, et al. Provision of somatosensory inputs during motor imagery enhances learning-induced plasticity in human motor cortex. Sci Rep 2017; 7: 9300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim YH, Park JW, Ko MH, et al. Facilitative effect of high frequency subthreshold repetitive transcranial magnetic stimulation on complex sequential motor learning in humans. Neurosci Lett 2004; 367: 181–185. [DOI] [PubMed] [Google Scholar]
- 42. Kim YH, You SH, Ko MH, et al. Repetitive transcranial magnetic stimulation-induced corticomotor excitability and associated motor skill acquisition in chronic stroke. Stroke 2006; 37: 1471–1476. [DOI] [PubMed] [Google Scholar]
- 43. Avenanti A, Coccia M, Ladavas E. et al. Low-frequency rTMS promotes use-dependent motor plasticity in chronic stroke: a randomized trial. Neurology 2012; 78: 256–264. [DOI] [PubMed] [Google Scholar]
- 44. Perez MA, Field-Fote EC, Floeter MK. Patterned sensory stimulation induces plasticity in reciprocal ia inhibition in humans. J Neurosci 2003; 23: 2014–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kawakami M, Okuyama K, Takahashi Y, et al. Change in reciprocal inhibition of the forearm with motor imagery among patients with chronic stroke. Neural Plast 2018; 2018: 3946367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Grosprêtre S, Lebon F, Papaxanthis C, et al. New evidence of corticospinal network modulation induced by motor imagery. J Neurophysiol 2016; 115: 1279–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Naseri M, Petramfar P, Ashraf A. Effect of motor imagery on the F-wave parameters in hemiparetic stroke survivors. Ann Rehabil Med 2015; 39: 401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Takemi M, Masakado Y, Liu M, et al. Sensorimotor event-related desynchronization represents the excitability of human spinal motoneurons. Neuroscience 2015; 297: 58–67. [DOI] [PubMed] [Google Scholar]
- 49. Takahashi Y, Fujiwara T, Yamaguchi T, et al. The effects of patterned electrical stimulation combined with voluntary contraction on spinal reciprocal inhibition in healthy individuals. Neuroreport 2017; 28: 434–438. [DOI] [PubMed] [Google Scholar]
- 50. Cavallari P, Fournier E, Katz R, et al. Changes in reciprocal Ia inhibition from wrist extensors to wrist flexors during voluntary movement in man. Exp Brain Res 1984; 56: 574–576. [DOI] [PubMed] [Google Scholar]
- 51. Yang HD, Minn YK, Son IH, et al. Facilitation and reciprocal inhibition by imagining thumb abduction. J Clin Neurosci 2006; 13: 245–248. [DOI] [PubMed] [Google Scholar]
- 52. Yamaguchi T, Fujiwara T, Tsai YA, et al. The effects of anodal transcranial direct current stimulation and patterned electrical stimulation on spinal inhibitory interneurons and motor function in patients with spinal cord injury. Exp Brain Res 2016; 234: 1469–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nakashima K, Rothwell JC, Day BL, et al. Reciprocal inhibition between forearm muscles in patients with writer’s cramp and other occupational cramps, symptomatic hemidystonia and hemiparesis due to stroke. Brain 1989; 112: 681–697. [DOI] [PubMed] [Google Scholar]
- 54. Artieda J, Quesada P, Obeso JA. Reciprocal inhibition between forearm muscles in spastic hemiplegia. Neurology 1991; 41: 286–289. [DOI] [PubMed] [Google Scholar]
- 55. Crone C1, Johnsen LL, Biering-Sørensen F, et al. Appearance of reciprocal facilitation of ankle extensors from ankle flexors in patients with stroke or spinal cord injury. Brain 2003; 126: 495–507. [DOI] [PubMed] [Google Scholar]
- 56. Lamy JC, Wargon I, Mazevet D, et al. Impaired efficacy of spinal presynaptic mechanisms in spastic stroke patients. Brain 2009; 132: 734–748. [DOI] [PubMed] [Google Scholar]
- 57. Dewald JP, Pope PS, Given JD, et al. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain 1995; 118: 495–510. [DOI] [PubMed] [Google Scholar]
- 58. Dobkin BH. Strategies for stroke rehabilitation. Lancet Neurol 2004; 3: 528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kreisel SH, Hennerici MG, Bäzner H. Pathophysiology of stroke rehabilitation: the natural course of clinical recovery, use-dependent plasticity and rehabilitative outcome. Cerebrovasc Dis 2007; 23: 243–255. [DOI] [PubMed] [Google Scholar]
- 60. Kawakami M, Fujiwara T, Ushiba J, et al. A new therapeutic application of brain-machine interface (BMI) training followed by hybrid assistive neuromuscular dynamic stimulation (HANDS) therapy for patients with severe hemiparetic stroke: a proof of concept study. Restor Neurol Neurosci 2016; 34: 789–797. [DOI] [PubMed] [Google Scholar]