Abstract
Importance
Orbital floor fracture is common among patients suffering from facial trauma. Open reduction and reconstruction of the orbital floor with Medpor is the treatment of choice in our centre to correct diplopia and enophthalmos.
Objective
Application of locally available 3D printing service in perioperative planning of orbital floor reconstruction with porous polyethylene.
Design
We present two patients who suffered from orbital floor fracture complicated by diplopia. Open reduction and orbital floor reconstruction with Medpor was performed with the guidance of a 3D printed customized model of the orbital floor defect.
Participants
Both patients were admitted through the Emergency Department to surgical ward after facial trauma. CT scan of the face showed orbital floor fracture with entrapment of inferior rectus muscle. Clinically patients also suffered from diplopia on extreme gaze.
Results
With the aid of 3D printed model, it shortened operative time and duration of anaesthesia. Defect-specific Medpor could be trimmed and molded easily from the model and thus reduced fatigue of the material. Furthermore, the model was helpful in patient education and explanation of the surgical procedure.
Conclusions and relevance
Application of 3D printing in medical specialties is rapidly developing in the past few years. In orbital floor fracture reconstruction, 3D printed model provides a customized solution, decreases operative time and duration of anaesthesia.
Keywords: Three-dimensional printing, Rapid prototyping, Orbital floor reconstruction, Blowout fracture
Introduction
Three-dimensional printing (3DP) technology, also termed rapid prototyping, has been widely applied in a variety of medical specialties especially in craniofacial, plastic and reconstructive and orthopaedic surgery. The number of publications focusing on medical applications of 3DP has increased exponentially over the past few years [1]. Compared to the technology in the 1980s when 3DP was first developed, nowadays it is considerably cheaper, easily accessible and less time-consuming. A great variety of printing materials allows for mechanical properties and appearance accustomed to specific applications. More importantly, accessibility of robust 3D modelling software and powerful computer processors enables 3D models to be created at ease with typical desktop workstations.
Orbital floor involvement is common in orbital blowout fracture (around 50%) [2,3]. Diplopia and enophthalmos are the indication for surgical reconstruction of floor defect. Autologous calvarial bone grafts, porous polyethylene, and polydioxanone are most widely used for orbital floor reconstructions [4]. In our centre, porous polyethylene sheets (MEDPOR®, Stryker, MI, USA) is the treatment of choice for orbital floor reconstructions via a transconjunctival approach after reduction of orbital contents. In this technique, it is imperative that the implant is precisely shaped to securely seal off the orbital floor defect.
3DP provides customized patient-specific solutions to surgical challenges. In this article, we describe two patients with orbital floor fracture managed by porous polyethylene reconstruction precisely shaped with the aid of 3DP bone models.
Patients and methods
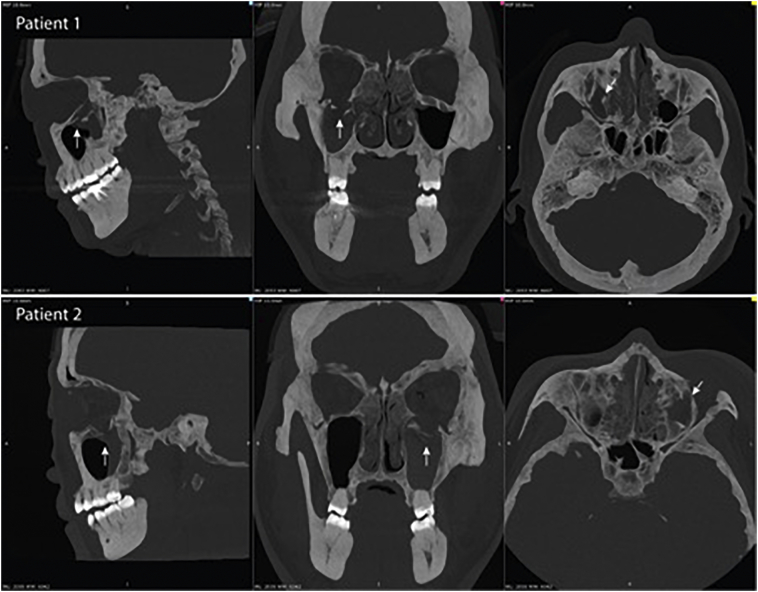
Two patients suffering acute blunt facial trauma were included. The first patient was a 37 years old male who received a direct blow to the face by a glass bottle during an assault. He presented with diplopia and restricted extraocular muscle movements. Computer tomography (CT) scan revealed right orbital floor fracture with herniation of inferior rectus muscle (Fig. 1-Patient 1). The second patient was a 50 years old female presented with diplopia on upward gaze after falling on her face at level ground. CT scan showed isolated left orbital floor fracture with muscle herniation (Fig. 1-Patient 2). Both patients have consent to publishing their results in this article.
Fig. 1.
CT scan with 10 mm maximum intensity projection (MIP) views in three planes showing (Patient 1) right sided fractured orbital floor in first patient and (Patient 2) left sided fracture in second patient. Both having caudal herniation of orbital contents.
Digital imaging and communications in medicine (DICOM) data of the CT scans were obtained in 0.5 mm layer thickness and region of interest (ROI) resolution of 512 × 512 corresponding to a voxel size of 0.331 × 0.331 × 0.5 mm. The skull bone was segmented from DICOM volumetric data using 3DSlicer software [5] (Version 4.62, http://www.slicer.org) by Hounsfield unit thresholding. A 3D skull model was created in standard triangle language (STL) format (Fig. 3-A) and refined with 3D modelling software Meshmixer (Version 3.2, Autodesk, CA, USA) by following techniques previously described for other medical applications [6]. The relevant area including the orbital floor defect and the contralateral intact orbital floor was cropped from the skull (Figs. 2-A, 3-B). Afterwards, mesh reduction (decimation), smoothing and defect filling techniques were applied to the 3D models to reduce its triangular complexity (Figs. 2-B, 3-C). These mesh optimization steps were aimed at reducing the computational burden and time required for printing.
Fig. 3.
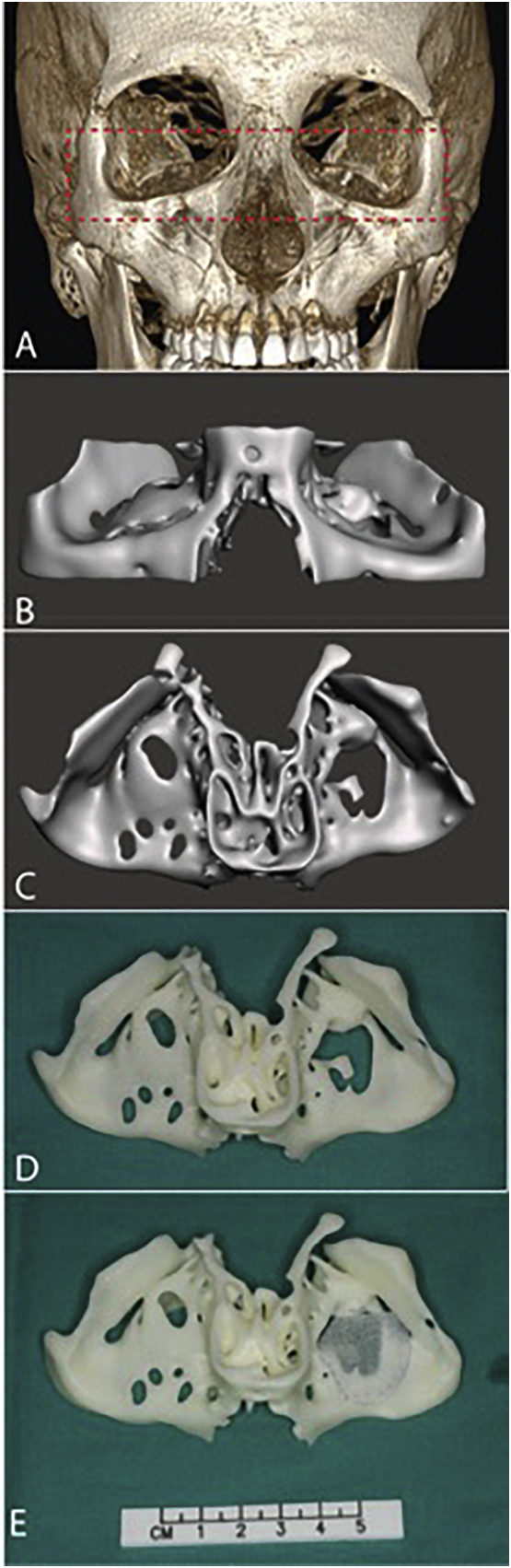
(A) 3D skull model of the second patient created from CT data showing the crop margin. (B) Frontal and (C) cranial views of the optimized and cropped STL 3D model with right orbital defect, note that perforations on the right orbital floor are artefacts and not true defects. (D) The 3DP bone model template with (E) Medpor trimmed and fit to cover the defect.
Fig. 2.
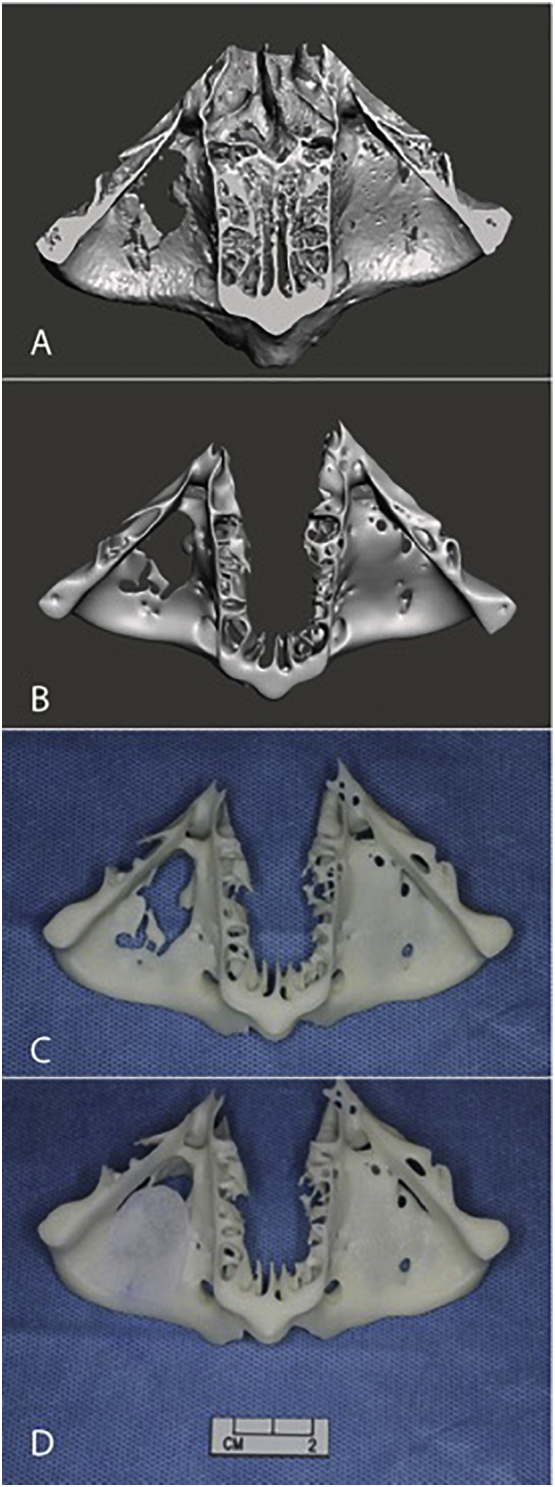
(A) A cropped digital 3D bone model of the first patient with right orbital floor fracture before optimization viewed from above. (B) STL model after optimization by smoothing, triangular decimation and removal of intranasal structures. (C) The 3DP bone model with (E) Medpor trimmed and templated to complete cover the defect.
3DP models were produced with medically designated (ISO 10993 and USP Class VI biocompatibility certified) acrylonitrile butadiene styrene (ABS-M30i, Stratasys, MI, USA) using an industrial grade fused deposition modelling (FDM) printer (Fortus 450mc, Stratasys, MI, USA). The model was created at a layer thickness of 0.178 mm and sterilized by low temperature hydrogen peroxide gas plasma (ASP Sterrad NX, Cilag GMBH, Schaffhausen, Switzerland) following published recommendations [7] before operation (Figs. 2-C, 3-D).
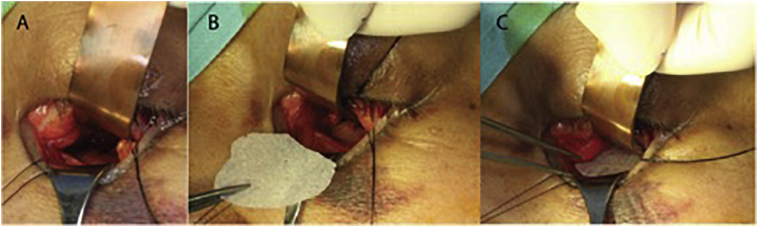
Both procedures were performed under general anaesthesia via a transconjunctival approach. An incision was made 5 mm below lid margin and dissection made along pre-septal plane to inferior orbital rim. The orbital floor was then exposed at sub-periosteal plane with defect identified and orbital contents reduced. A porous polyethylene sheet (0.85 mm thick) was trimmed and molded to fit the size of defect according to the sterilized 3D printed model (Figs. 2-D, 3-E) and inserted to reconstitute the orbital floor (Fig. 4). Forced duction test was performed immediately after the procedure to confirm there was no entrapment of extraocular muscles. The surgical wounds were repaired and patients instructed to freely mobilize their ocular muscles after surgery.
Fig. 4.
Intraoperative photograph of (A) the transconjunctival approach. (B) and (C) Sequence of inserting the reshaped Medpor sheet over the orbital floor bone defect.
Results
The time required to prepare the digital 3D models at the computer workstation was within 1 h for each patient and the 3DP turnover time was within three working days. The first patient received operation 18 days after injury and the second 11 days after injury.
The operative duration was 1 h 7 min and 1 h 10 min respectively. On reviewing the previous 15 consecutive orbital floor reconstructions with implants in our centre, the operative time ranged from 46 min to 2 h 10 min (median 1 h 36 min), hence the operative was reduced by nearly 30 min with aid of the 3DP bone model.
Both patients were followed up in out-patient setting for over 2 months with satisfactory results. They recovered uneventfully after the operation with normal extraocular eye moments and resolution of diplopia. None of them suffered from enophthalmos or orbital dystopia.
Discussion
3D printing technology has a wide variety of application in the medical field [6]. Novel surgical applications driven by 3DP are increasingly common in plastic reconstructive surgery [8], dental surgery, orthopaedics [9], and cardiovascular surgery. With evolving technology, 3D printers are more compact and accessible and the costs of printing are substantially reduced.
Our hospital has recently purchased an industrial grade FDM printer that is shared across clinical specialties and specifically used for preparing bone models and surgical guides. As such, 3D printing is readily accessible in our centre at relatively low cost and high efficiency. Our orbital floor fracture model was estimated at USD $20–30 per print and printing time was 8–10 h. Including a post-processing time for removal of supporting material and quarantine after sterilization, the total turnover time is around 3 days. Logistically, this enables 3DP models to be used for semi-elective situations where surgery may be performed in 1–2 weeks.
In our centre, we have applied 3DP in elective scenarios such as free fibula mandibular reconstructions, microtia reconstructions and limb deformity corrections by anatomical models and surgical guides. Both the first and second authors are surgeons experienced in preparing 3D models for various applications in plastic surgery and orthopaedic surgery.
Before the availability of patient specific 3DP models, size and shape of defect of orbital floor blow out fracture was estimated from the two-dimensional CT scan images. And with the small trans-conjunctival incision, the bony defect especially the posterior landing zone was difficult to be visualized clearly during the operation. Sometimes the implant had to be repeatedly retrieved from the subperiosteal plane after insertion for trimming and molding for more precise placement which could theoretically increase implant fatigue, increase soft tissue handling, worsen tissue edema and prolong operative time. In the two cases presented above, porous polyethylene implants were precisely shaped with ease using a 3DP orbital floor bone model under direct visualization. The printed defect corresponded well with the actual orbital floor defect seen via transconjunctival incision. And both implants were successfully inserted subperiosteally at the first attempt and steadily seated over the defect. We felt that the technique has considerably shortened the operative time and the duration of anaesthesia and could potentially decrease implant fatigue.
Apart from surgical planning, 3DP models are effectively being used in patient education and communication where surgeons can better explain the pathoanatomy and the surgical procedure. Patients benefit from a deeper understanding via visual and tactile feedback using their own anatomical model.
We are aware of recently available 3DP patient specific titanium implants as an alternative in craniofacial bone defect reconstruction. The optimal material for customizable orbital floor implants should be cheap while providing permanent stability via fibrovascular in-growth with low complication rates. We favor the current technique of 3DP template shaped polymer implant over metal due to following reasons. Firstly, its porosity allows for fibrovascular tissue in-growth [10] and very low extrusion rate in our experience. Secondly, the current FDM printed ABS polymer model remains far more economical than 3DP titanium implants. Thirdly, metallic implants placed critically near the eye are unnecessary hazards that preclude any future MRI studies.
The current study is a limited report of two cases. We aim to in detail document further success and difficulties encountered though a larger clinical case series.
Conclusion
A 3DP orbital floor bone model as an intraoperative template is useful in orbital floor fracture reconstruction using porous polyethylene. The implant can be easily and accurately shaped to reconstitute the defect. Production of the 3DP bone model is straightforward. Patient care is enhanced by effectively decreasing the operative time and the duration of anaesthesia.
Acknowledgement and conflict of interest statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The authors report no conflicts of interest.
References
- 1.Crafts T.D., Ellsperman S.E., Wannemuehler T.J., Bellicchi T.D., Shipchandler T.Z., Mantravadi A.V. Three-dimensional printing and its applications in otorhinolaryngology-head and neck surgery. Otolaryngol. Head Neck Surg. Jun 2017;156(6):999–1010. doi: 10.1177/0194599816678372. [DOI] [PubMed] [Google Scholar]
- 2.Sun M.T., Wu W., Watanabe A. Orbital blowout fracture location in Japanese and Chinese patients. Jpn. J. Ophthalmol. Jan 2015;59(1):65–69. doi: 10.1007/s10384-014-0357-x. [DOI] [PubMed] [Google Scholar]
- 3.de Silva D.J.R.G. Orbital blowout fractures and race. Ophthalmology. Aug 2011;118(8):4. doi: 10.1016/j.ophtha.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Avashia Y.J., Sastry A., Fan K.L., Mir H.S., Thaller S.R. Materials used for reconstruction after orbital floor fracture. J. Craniofac. Surg. Nov 2012;23(7 Suppl 1):1991–1997. doi: 10.1097/SCS.0b013e31825aada1. [DOI] [PubMed] [Google Scholar]
- 5.Fedorov A., Beichel R., Kalpathy-Cramer J. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging. Nov 2012;30(9):1323–1341. doi: 10.1016/j.mri.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chae M.P., Rozen W.M., McMenamin P.G., Findlay M.W., Spychal R.T., Hunter-Smith D.J. Emerging applications of bedside 3D printing in plastic surgery. Front Surg. 2015;2:25. doi: 10.3389/fsurg.2015.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez M., Block M., Espalin D. Aug 2012. Sterilization of FDM-manufactured Parts. Paper Presented at: Proceedings of the 2012 Annual International Solid Freeform Fabrication Symposium, Austin, TX. [Google Scholar]
- 8.AlAli A.B., Griffin M.F., Butler P.E. Three-dimensional printing surgical applications. Eplasty. 08/14/2015;15:e37. [PMC free article] [PubMed] [Google Scholar]
- 9.Wong T.M., Jin J., Lau T.W. The use of three-dimensional printing technology in orthopaedic surgery. J. Orthop. Surg. Jan 2017;25(1) doi: 10.1177/2309499016684077. (2309499016684077) [DOI] [PubMed] [Google Scholar]
- 10.Lee H., Baek S. Comparison of early fibrovascular proliferation according to orbital implant in orbital floor fracture reconstruction. J. Craniofac. Surg. Sep 2012;23(5):1518–1523. doi: 10.1097/SCS.0b013e31825a61de. [DOI] [PubMed] [Google Scholar]