Abstract
Pulmonary arterial hypertension (PAH) is characterized by increased proliferation and resistance to apoptosis of pulmonary vascular cells. Increased expression of translationally controlled tumor protein (TCTP), a prosurvival and antiapoptotic mediator, has recently been demonstrated in patients with heritable PAH; however, its role in the pathobiology of PAH remains unclear. Silencing of TCTP in blood outgrowth endothelial cells (BOECs) isolated from control subjects led to significant changes in morphology, cytoskeletal organization, increased apoptosis, and decreased directionality during migration. Because TCTP is also localized in extracellular vesicles, we isolated BOEC-derived extracellular vesicles (exosomes and microparticles) by sequential ultracentrifugation. BOECs isolated from patients harboring BMPR2 mutations released more exosomes than those derived from control subjects in proapoptotic conditions. Furthermore, TCTP expression was significantly higher in exosomes than in microparticles, indicating that TCTP is mainly exported via exosomes. Coculture assays demonstrated that exosomes transferred TCTP from ECs to pulmonary artery smooth muscle cells, suggesting a role for endothelial-derived TCTP in conferring proliferation and apoptotic resistance. In an experimental model of PAH, rats treated with monocrotaline demonstrated increased concentrations of TCTP in the lung and plasma. Consistent with this finding, we observed increased circulating TCTP levels in patients with idiopathic PAH compared with control subjects. Therefore, our data suggest an important role for TCTP in regulating the critical vascular cell phenotypes that have been implicated in the pathobiology of PAH. In addition, this research implicates TCTP as a potential biomarker for the onset and development of PAH.
Keywords: endothelium, exosomes, hypertension, pulmonary, remodeling
Clinical Relevance
We identified that exosome-derived translationally controlled tumor protein (TCTP) uptake from the circulation potentially drives progression of pulmonary arterial hypertension. As a consequence, targeting exosomes and TCTP levels could potentially provide therapeutic benefits for patients with pulmonary arterial hypertension. Furthermore, monitoring circulating TCTP levels could be an indicator of disease onset or clinical outcome.
Pulmonary arterial hypertension (PAH) is a rare disease characterized by a sustained increase in pulmonary artery pressure, often leading to death from right heart failure. The disease is also characterized by severe vascular remodeling that results in occlusion of the small pulmonary arteries (1). Mutations in the gene encoding bone morphogenetic protein receptor type II (BMPR2), a member of the transforming growth factor β (TGF-β) family, are the most common cause of heritable PAH (HPAH) (2–4). The pathobiology of PAH involves diverse molecular mechanisms, including an imbalance of vasodilators and vasoconstrictors, excessive cell proliferation, and impaired apoptosis. These processes lead to the formation of complex plexiform lesions and increased pulmonary vascular resistance. In fact, pulmonary artery smooth muscle cells (PASMCs) in PAH share many common features observed in cancer pathobiology, including expression of prosurvival proteins such as Pim-1 (5) and survivin (6), and a metabolic switch to glycolysis even in normoxic conditions (7).
We recently reported that translationally controlled tumor protein (TCTP) was significantly elevated in blood outgrowth endothelial cells (BOECs) isolated from patients with HPAH harboring a BMPR2 mutation compared with healthy control subjects (8). Although TCTP is found in all eukaryotic organisms, its expression is cell- and tissue-type dependent. Synthesis and degradation of TCTP are regulated in response to a wide range of extracellular signals, including stress conditions, cytokines, and proapoptotic signals. Originally described as a histamine-releasing factor (9), TCTP has been implicated in multiple biological processes, including microtubule stabilization (10, 11) and secretion of proteins through exosomes under the control of TSAP6 and p53 (12). Moreover, TCTP is known to play a role in proproliferation and antiapoptosis due to its specific association with various proteins, especially in the mitochondria. For example, TCTP anchored within the mitochondria inhibits the dimerization of Bax (13) and modulates the antiapoptotic activity of Mcl-1 by its stabilization (14). Furthermore, TCTP is considered an oncogene due to its overexpression in several types of cancer (15, 16). Recent studies have shown that gene silencing of TCTP can revert cancer cells to a normal phenotype (17–20) and inhibit liver and melanoma metastasis (21, 22). TCTP was recently reported to be a positive regulator of epithelial-to-mesenchymal transition (22), a crucial step during tumor invasiveness, metastasis, and fibrosis. In PAH, the process of endothelial-to-mesenchymal transition has been reported to contribute to vascular remodeling (23, 24). Under normal conditions, TCTP circulates in the blood of healthy subjects, but in response to cancer therapy its levels increase in the serum of patients. These changes are detectable before other standard serum biomarkers of apoptosis (25), suggesting TCTP as a candidate marker of early apoptosis. Moreover, loss of tctp expression in mice leads to increased spontaneous apoptosis during embryogenesis and causes lethality between E6.5 and E9.5, indicating a recessive lethal phenotype (13).
There is increasing interest in extracellular vesicles (EVs) in the context of cardiovascular disease (26–31). Several distinct subpopulations of EVs exist, ranging in size from microns to nanometers, and have been further classified according to their origin. Apoptotic bodies (1–5 μm) are larger in size and contain DNA fragments, microparticles or microvesicles (100 nm to 1 μm) originate from the budding of the extracellular membrane, and exosomes (50–100 nm), which are stored within the multivesicular bodies, are released upon activation. Both exosomes and microparticles are potential mediators of signaling between different cell types. EVs act as vehicles for the intracellular transmission of RNA and protein species, which directly impact recipient cell gene expression and cellular phenotype. Unlike cytochrome C, fragmented cytokeratin-18, and lactate dehydrogenase, the release of TCTP into the extracellular space does not require plasma membrane damage (32). Therefore, a rapid increase in circulating levels of TCTP in patients with cancer after treatment could be a paracrine signal from early apoptotic cells activating antiapoptotic mechanisms in neighboring cells. In this context, Sirois and colleagues (33) reported the presence of TCTP in exosomes derived from ECs, pointing to a pivotal role for caspase-3 in the regulation and release of nanovesicles.
We hypothesized that in PAH, the upregulation of TCTP in patients harboring a BMPR2 mutation would exacerbate the proproliferative and apoptotic resistance phenotype observed in pulmonary vascular cells. Also, in the presence of stress conditions, we investigated whether EVs containing TCTP released by activated ECs could be incorporated into PASMCs, transferring the proproliferative and antiapoptotic phenotype and potentially promoting vascular remodeling.
Methods
Migration Assay
BOECs were isolated from the peripheral blood of patients with PAH harboring a BMPR2 mutation and healthy control subjects as previously described (34–36). BOECs were seeded in 2-well culture inserts (Ibidi) for 24 hours at 80% confluence. One hour before image analysis, the culture inserts were removed and endothelial cell basal medium-2 (EGM-2) (Lonza) containing HEPES (20 mM; Thermo Fisher Scientific) and hydroxyurea (2 μM; Sigma Aldrich) was added. Images were taken every 5 minutes during 18 hours at 37°C.
Isolation of EVs
BOECs were cultured until they reached 80% confluence in EGM-2 with 10% FBS. Sequential centrifugations were performed to isolate the different EV subtypes. Microparticles were isolated from the pellet after centrifugation at 20,500 × g for 1 hour at 4°C. Supernatants were then centrifuged at 100,000 × g in a Beckman Optima L-90K ultracentrifuge (Beckman Coulter) for 1 hour. Pelleted exosomes were then washed, resuspended in 100 μl of PBS, and stored at −80°C for further experiments.
Nanoparticle Tracking Analysis
Samples were analyzed using a NanoSight NS500 instrument (NanoSight Ltd.) as previously described (37). Exosomes were diluted in filtered PBS to a concentration of 2 × 108 to 8 × 108 per milliliter. Each video was analyzed to give the mode, mean size, and estimated EV concentration.
Immunofluorescence
BOECs and PASMCs were permeabilized with Triton X-100 (0.5% in PBS++) and blocked with 0.5% BSA. Primary antibodies for CD81 (mouse antihuman; 1.50) and TCTP (rabbit antihuman; 1:100) were detected with the secondary antibodies Alexa Fluor 594 (donkey antimouse, 1:250) and Alexa Fluor 488 (mouse antirabbit, 1:250) (both from Molecular Probes), respectively. Cells were washed and counterstained with phalloidin Alexa Fluor 594 (Molecular Probes) for 20 minutes at room temperature. Slides were washed in PBS and mounted in Vectashield Antifade mounting medium with DAPI (Vector Laboratories).
EV Transfer
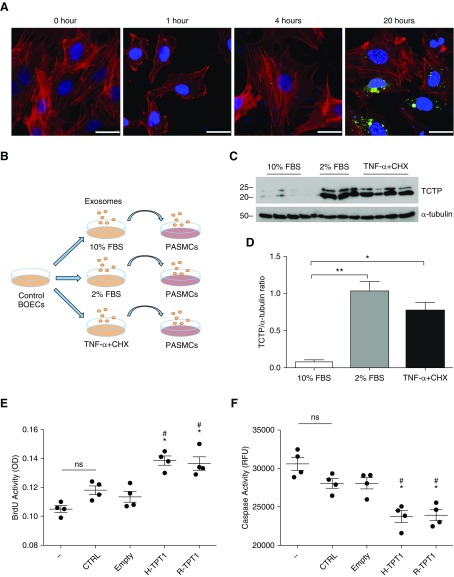
Monolayers of BOECs at 90% confluence were cultured for 24 hours in EGM-2 plus 10% particle-free FBS, endothelial cell basal medium-2 (EBM-2) (Lonza) plus 2% particle-free FBS, or EBM-2 plus 2% particle-free FBS with TNF-α (3 ng/ml) for the last 6 hours. Exosomes in the supernatant were isolated as described above and resuspended in 100 μl of filtered PBS. PKH67 (Sigma Aldrich) staining was performed according to the manufacturer’s instructions. Exosomes were washed and added to confluent PASMCs for 1, 4, and 24 hours. Counterstaining with phalloidin Alexa Fluor 594 and Vectashield Antifade mounting medium with DAPI was performed.
ELISA
Blood samples from patients with idiopathic PAH (IPAH; n = 11) and control subjects (n = 10) were collected in EDTA tubes for plasma isolation (Table E1 in the data supplement). Samples were stored at −80°C. For TCTP quantification by ELISA (Human TCTP ELISA kit; Antibodies-Online GmbH), 100 μl of plasma sample was used. Assays were performed according to the manufacturer’s instructions.
Animal Model
Male Sprague-Dawley rats weighing ∼200 g received a single subcutaneous injection of PBS (control, n = 16) or monocrotaline (MCT, 40 mg/kg; Sigma Aldrich; n = 19) at Day 0 to induce PAH. Right ventricular pressures and volumes were recorded using a Millar SPR-869 pressure-volume catheter (Millar Instruments) in at least two rats from each group at weeks 1, 2, 3, and 4 to identify the onset of the disease.
Statistics
Data from the different groups were compared using Student’s t-test or one-way ANOVA followed by post hoc analysis (whichever was appropriate). P values ≤ 0.05 were set to be statistically significant.
Results
TCTP Plays an Important Role in Apoptosis, Migration, and Cell Morphology in BOECs
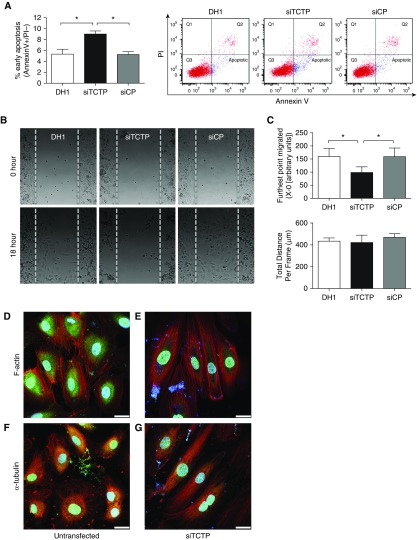
The role of TCTP in vascular cell function was evaluated after siRNA gene silencing. Knockdown of TCTP expression was confirmed in BOECs isolated from control subjects (Figure E1 in the data supplement). Using flow cytometry, AnnexinV+/PI− cells were identified as early apoptotic and quantified as a percentage of the total cell number. After TCTP silencing, BOECs were significantly more sensitive to apoptosis compared with a nontargeting siRNA control or transfection reagent alone (Figure 1A).
Figure 1.
Silencing of translationally controlled tumor protein (TCTP) affected the apoptosis, migration, and morphology of blood outgrowth endothelial cells (BOECs). BOECs were transfected with DharmaFECT1 (DH1) alone, siTCTP, or nontargeting siRNA control (siCP) for 48 hours. (A) Apoptosis was assessed by flow cytometry using AnnexinV+ (FITC)/PI− (PE-Cy7-A) staining (n = 3; one-way ANOVA). (B) Cell migration was assessed using Ibidi culture inserts. After 24 hours, the inserts were removed and cells were treated with EBM-2 (2% FBS, 20 mM HEPES, and 2 μM hydroxyurea). Time-lapse microscopy was undertaken using a Leica SPE imaging system, with images taken every 5 minutes for 18 hours at 37°C. Representative images of BOECs at time 0 and 18 hours after wound healing are shown. (C) Quantification of the farthest point migrated and total distance migrated (n = 4; one-way ANOVA). (D and E) Representative confocal images of BOECs (D) untransfected or (E) transfected with siTCTP and stained with an antibody for TCTP (green) before counterstaining for F-actin (phalloidin; red) and nuclei (DAPI; blue). (F and G) Representative confocal images of BOECs (F) untransfected or (G) transfected with siTCTP and stained with an antibody for TCTP (green) before staining for α-tubulin (red) and counterstaining the nuclei (DAPI; blue). Scale bars: 25 μm. *P < 0.05. Error bars represent mean ± SEM.
To further assess the functional consequences of TCTP silencing, we evaluated migration of BOECs. Knockdown of TCTP led to reduced wound closure at 18 hours compared with transfection reagent alone or a nontargeting siRNA (siControl)-treated cells (Figure 1B). Tracking of individual cells revealed that although the total distance migrated by BOECs treated with a siRNA targeting TCTP (siTCTP) BOECs was unaffected, the farthest point migrated in the wound was significantly reduced. This finding suggests impaired directionality in response to TCTP knockdown (Figures 1C and E2).
We noted that cell morphology was also affected after TCTP silencing. Staining for both F-actin and α-tubulin was performed in nontransfected and siTCTP-treated BOECs. Representative images show the characteristic cobblestone appearance of ECs in BOECs treated with transfection reagent alone (Figures 1D and 1F) and the elongated cell morphology after TCTP knockdown (Figures 1E and 1G). In agreement with previous reports of a specific binding site for tubulin on TCTP, these proteins were found to colocalize, as depicted by increased yellow staining in the untransfected cells (Figures 1F and 1G). Neither transfection reagent alone nor transfection with a nontargeting siRNA control had any effect on cell morphology (Figure E3).
BOECs Harboring BMPR2 Mutations Generate and Release More EVs
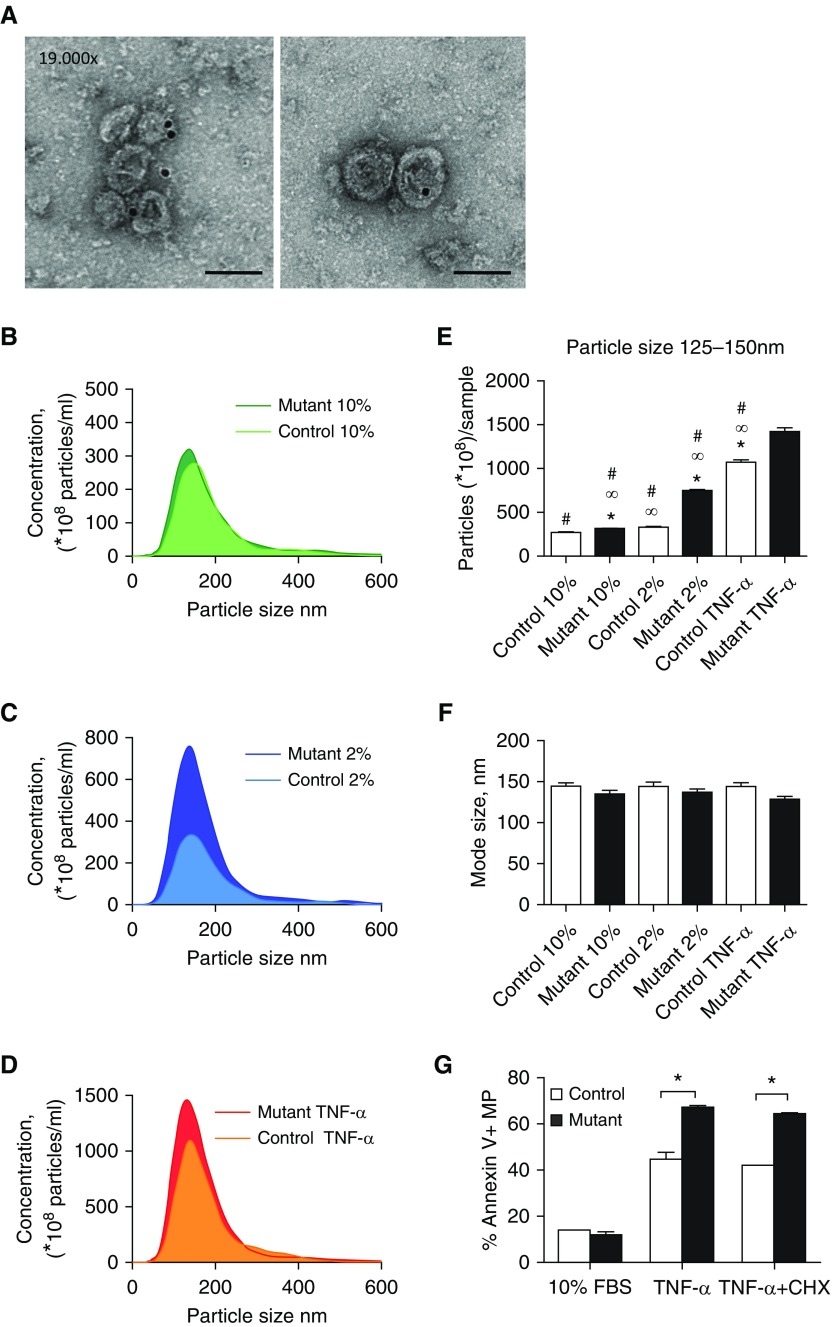
Because increased TCTP expression has been observed in ECs of patients with PAH harboring BMPR2 mutations, we investigated the effect on EV production and release in supernatants from control and patient-derived BOECs. First, we characterized the isolated exosomes by transmission electron microscopy and immunogold staining of a specific exosome marker, CD81. Cup-shaped vesicles (100–150 nm in diameter) with positive staining for CD81 were identified in the ultracentrifuged pellets (Figure 2A). Next, isolated fractions containing exosomes from control and patient-derived BOECs were examined using nanoparticle tracking analysis (NTA). In optimal growth conditions (growth factors plus 10% exosome-free FBS) the concentrations of exosomes released by control and patient-derived BOECs were similar (Figure 2B). However, in 2% exosome-free FBS alone, cells derived from patients with PAH released higher numbers of exosomes compared with those derived from healthy control subjects (Figure 2C). In addition, TNF-α treatment resulted in an increase in the concentration of exosomes in both control and patient BOECs, with the response being more evident in cells harboring a BMPR2 mutation (Figure 2D). Compared with optimal growth conditions, the response of mutant cells to activation conditions (2% FBS) resulted in a twofold increase in exosome production (particle size 125–150 nm), whereas control cells remained unchanged (Figure 2E). TNF-α treatment induced a fourfold increase of exosome (125–150 nm) concentrations in both control and PAH-derived cells compared with optimal growth conditions (Figure 2E). The size of the exosomes remained unchanged in patients and control subjects in all conditions (mode size ranged from 125–150 nm) (Figure 2F). We further characterized other types of EVs released after induction by these stimuli. Control and patient BOEC-derived microparticles (<1 μm) were isolated by centrifugation at 20,000 × g for further quantification. After AnnexinV staining, microparticles were analyzed by flow cytometry. BOECs harboring a BMPR2 mutation released significantly higher levels of microparticles after TNF-α treatment alone or apoptotic stimulation (TNF-α and cycloheximide [CHX]) (Figure 2G). Thus, the greater release of both types of EVs (exosomes and microparticles) by patient-derived BOECs occurred more readily in conditions of serum deprivation or in the presence of the inflammatory cytokine TNF-α.
Figure 2.
BOECs bearing BMPR2 mutations produce and release more extracellular vesicles. (A) Electron microscopy images of exosomes isolated by ultracentrifugation and positive immunogold staining with anti-CD81 antibody. Scale bars: 100 nm. (B–D) Nanoparticle tracking analysis (NTA) assessment of exosomes from human control (light color) and heritable pulmonary arterial hypertension (HPAH)-derived (dark color) BOECs (three replicates for each group; n = 4). (B) Basal conditions: 10% exosome-free FBS plus growth factors for 24 hours (10%). (C) Activation conditions: 2% exosome-free FBS for 24 hours (2%). (D) Stress conditions: 2% exosome-free FBS for 24 hours with TNF-α (3 ng/ml) for the final 6 hours. (E) Particle concentration evaluation of exosomes ranging from 125–150 nm by NTA from control and HPAH-derived BOECs in the above conditions (n = 4 in triplicates; one-way ANOVA, ∞P < 0.05 versus control 10% FBS, *P < 0.05 versus control 2% FBS, #P < 0.05 versus mutant TNF-α). (F) Mode size of nanoparticles in all groups corresponding to the range of exosome size (125–150 nm) (n = 4; one-way ANOVA). (G) Microparticles (<1 μm) were identified and quantified by flow cytometry by positive staining for AnnexinV. Microparticles were derived from control and HPAH-derived BOECs under the following conditions: basal conditions (10% exosome-free FBS plus growth factors for 24 hours [10% FBS; n = 4]; stress conditions (2% exosome-free FBS for 24 hours with TNF-α [3 ng/ml] [TNF-α; n = 4]; and apoptosis/stress conditions (2% exosome-free FBS for 24 hours with TNF-α [3 ng/ml] and cycloheximide [CHX; 20 μg/ml] for the final 6 hours [TNF-α + CHX; n = 4]). One-way ANOVA; *P < 0.05. Error bars represent mean ± SEM.
TCTP Is Exported Mainly via Exosomes from BOECs
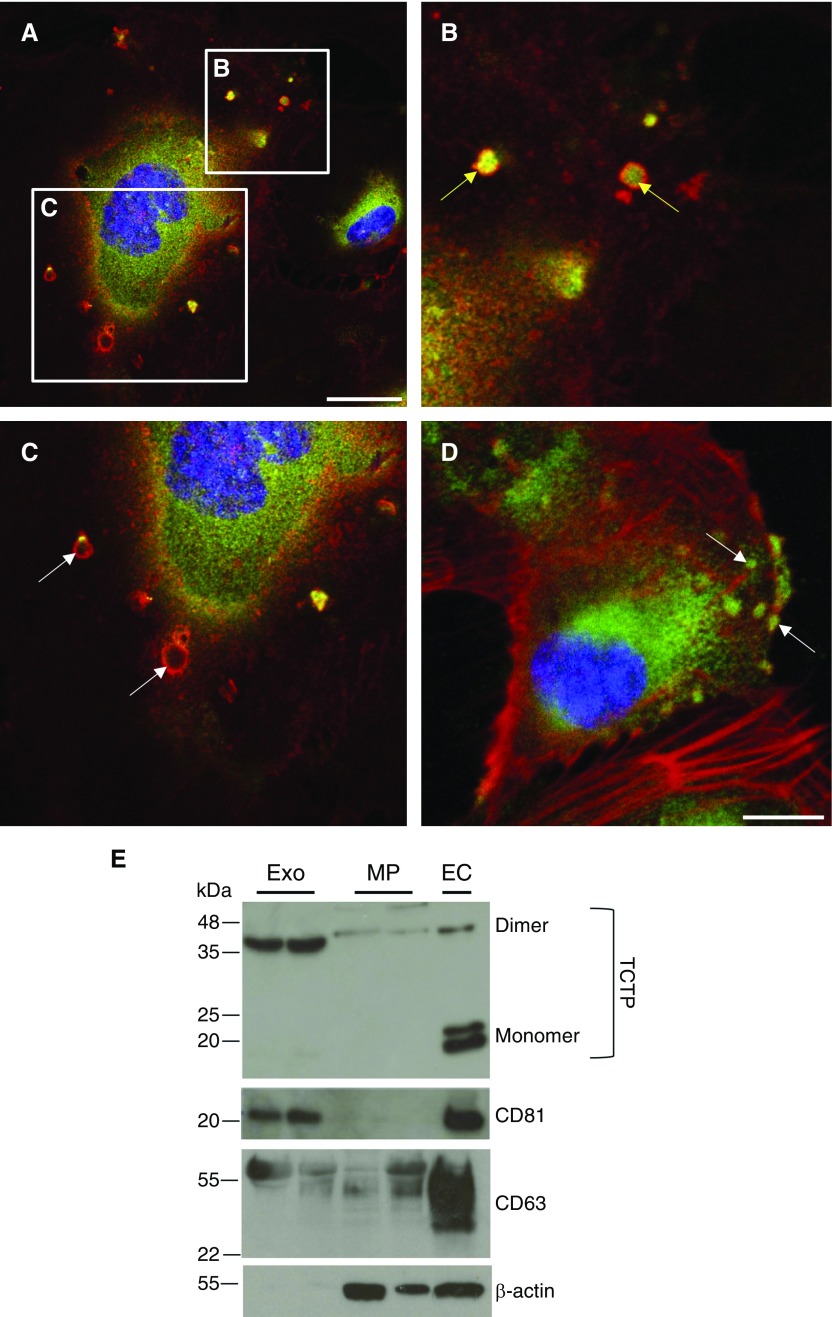
To identify the main EV subtypes involved in TCTP transportation, immunofluorescence staining for TCTP was performed in control BOECs under activation conditions (2% exosome-free FBS). The presence of TCTP was identified in the cytoplasm of the cells (Figure 3A, green), and the presence of vesicles was confirmed using the exosome marker CD81 (Figures 3A–3C, red). Coexpression of TCTP and CD81 was observed in some exosomes (Figure 3B, yellow arrows) but was absent in others (Figure 3C, white arrows). TCTP was also observed in the cytoplasm and at the cell membrane, suggesting potential intracellular trafficking (Figure 3D).
Figure 3.
TCTP is exported via exosomes. (A–C) Representative confocal images of control BOECs stained using a TCTP antibody (green) and C81 (red). (A) BOECs secreting small particles (exosomes) positively stained for CD81 at the membrane. Scale bar: 25 μm. (B) Inset area in image A. Yellow arrows indicate exosomes positive for CD81 surface expression (red) and TCTP (green) intravesicle expression, with colocalization in yellow. (C) Inset area in image A. White arrows indicate TCTP-empty exosomes. (D) Representative confocal image of control BOECs stained using a TCTP antibody (green) and counterstained for F-actin (phalloidin; red). Scale bar: 25 μm. Localization of TCTP-positive intracellular vesicles (green) is indicated by white arrows. (E) Representative immunoblots for TCTP, CD81, CD63, and β-actin protein expression in exosomes (Exo), microparticles (MP), and BOECs (EC).
Immunoblotting revealed that TCTP was mainly expressed in exosomes (CD81 positive, CD63 positive and β-actin negative) and to a lesser extent in microparticles (CD81 negative) (Figure 3E). BOEC lysates were used as positive control, showing the presence of TCTP mainly as a monomer. Amino acids 11–172 of TCTP act as a protein transduction domain (38), which is oxidized and dimerized by disulfide linkages in oxidant-filled cellular fluids. Thus, extracellular TCTP in both exosomes and microparticles is observed as a dimer, whereas intracellular TCTP is seen as a monomer (39, 40).
TCTP Overexpression by Lentiviral Transduction Induces Proliferation and Reduces Apoptosis
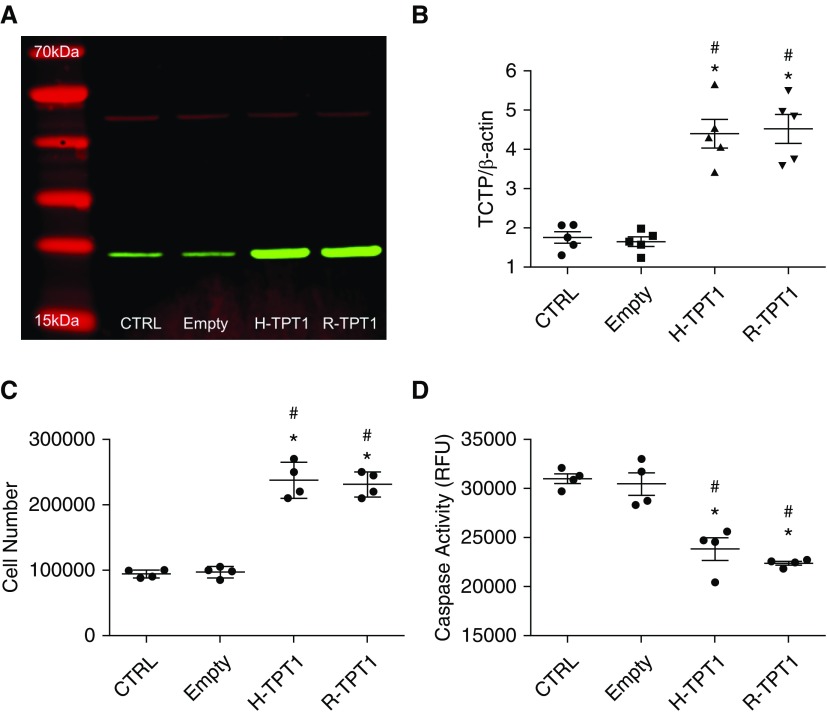
We next assessed the functional consequences of TCTP overexpression on SMCs and ECs. First, PASMCs and human umbilical vein ECs (HUVECs) were transduced with either the gene-encoding human or rat TCTP (H-TPT1 or R-TPT1, respectively) using lentiviral vectors. Transduced PASMCs and HUVECs expressed markedly higher levels of human or rat TCTP when compared with nontransduced or empty lentiviral vector (Figures 4A, 4B, E4A, and E4B). Proliferation and apoptosis was assessed in cells expressing either human or rat TCTP. After 48 hours, cell numbers were significantly increased in both PASMCs and HUVECs expressing human or rat TCTP (Figures 4C and E4C). Conversely, both cell types expressing TCTP had significantly reduced apoptosis as assessed by caspase-3 activity (Figures 4D and E4D).
Figure 4.
TCTP overexpression by lentiviral transduction prevents apoptosis and induces proliferation in pulmonary artery smooth muscle cells (PASMCs). (A) Western blot showing the expression of TCTP (green) and β-actin (red) in nontransduced (CTRL) PASMCs, using lentivirus cells that were transduced with an empty lentiviral vector (Empty) or human TCTP (H-TPT1) or rat Tctp (R-TPT1). (B) Summary data showing the expression of TCTP in transduced and nontransduced PASMCs. Densitometry of TCTP expression levels relative to β-actin. (C) Proliferation assessment of PASMCs cultured in complete media with growth factors and 10% FBS with or without transduction with H-TPT1 or R-TPT1. Cells were counted when 80–90% confluence was reached. (D) Caspase activity measurements of nontransduced cells (CTRL) and transduced PASMCs with vector alone (Empty), H-TPT1, or R-TPT1. One-way ANOVA; #P < 0.001 versus CTRL; *P < 0.001 versus Empty. Error bars represent mean ± SEM.
Exosomes Transfer TCTP to PASMCs
We next investigated whether TCTP could be transferred to PASMCs via exosomes released by BOECs. PASMCs from control subjects were incubated with PKH67-labeled exosomes. After 20 hours of incubation, PKH67-labeled exosomes were observed in the PASMCs’ cytoplasm (Figure 5A). To further confirm the mode of TCTP transfer, exosomes were derived from BOECs exposed to the following stimuli: 10% exosome-free FBS plus growth factors, 2% exosome-free FBS, and 2% exosome-free FBS plus TNF-α and CHX. PASMCs were incubated with the isolated exosomes for 24 hours (Figure 5B). Immunoblotting assessment revealed that TCTP expression was significantly increased in PASMCs incubated with exosomes from BOECs cultured in 2% FBS ± TNF-α and CHX, suggesting that exosomes derived from BOECs under proapoptotic conditions were able to transfer TCTP into the PASMCs (Figures 5C and 5D). The functional consequences of exosome and TCTP transfer were investigated by treating PASMCs with conditioned media from ECs overexpressing TCTP. HUVECs were transduced with either human or rat TCTP (H-TPT1 or R-TPT1, respectively) using lentivirus, and conditioned media were collected after 48 hours. PASMCs were then treated for 4 hours and proliferation or apoptosis was assessed. Proliferation was significantly increased in PASMCs exposed to conditioned media from HUVECs expressing human or rat TCTP when compared with nontransduced or empty vector controls (Figure 5E). In addition, caspase-3 activity was significantly reduced in PAMSCs (Figure 5F).
Figure 5.
PASMCs incorporate activated BOEC-derived exosomes, leading to an increase in TCTP expression. (A) PASMCs were incubated with exosomes labeled with PKH67 (green) for 1, 4, and 20 hours. Representative confocal images of PASMCs counterstained for F-actin (phalloidin; red) and DAPI (blue) are shown. Scale bars: 25 μm. (B) Experimental schematic. Briefly, exosomes were derived from control BOECs under the following conditions: basal conditions (10% exosome-free FBS plus growth factors for 24 hours [10% FBS]), activation conditions (2% exosome-free FBS for 24 hours [2% FBS]), and apoptosis/stress conditions (2% exosome-free FBS for 24 hours with TNF-α [3 ng/ml] and CHX [20 μg/ml] for the final 6 hours [2% FBS TNF-α + CHX]). PASMCs were then incubated with exosomes for 24 hours. (C) Representative immunoblot of TCTP expression in PASMCs incubated with exosomes from the above treatments. Blots were reprobed with α-tubulin to ensure equal loading. (D) Densitometry of TCTP expression levels relative to α-tubulin (n = 4; Student’s t-test). *P < 0.05, **P < 0.005. Error bars represent mean ± SD. (E and F) Proliferation and apoptosis assessments of PASMCs exposed to conditioned media from human umbilical vein endothelial cells overexpressing TCTP after lentiviral transduction. EC-conditioned media were harvested from nontransduced cells (CTRL) or from cells transduced with an empty vector (Empty), human TCTP (H-TPT1), or rat Tctp (R-TPT1). PASMCs were then exposed to fresh media (−) or conditioned media from serum-starved ECs (EC-CM) for 4 hours. (E) For proliferation, bromodeoxyuridine (BrdU) incorporation was assessed. (F) For apoptosis, caspase activity was measured. One-way ANOVA; #P < 0.001 versus CTRL; *P < 0.001 versus Empty. Error bars represent mean ± SEM. ns = not significant; OD = optical density; RFU = relative fluorescence units.
Circulating and Lung Levels of TCTP in an MCT Rat Model of Pulmonary Hypertension
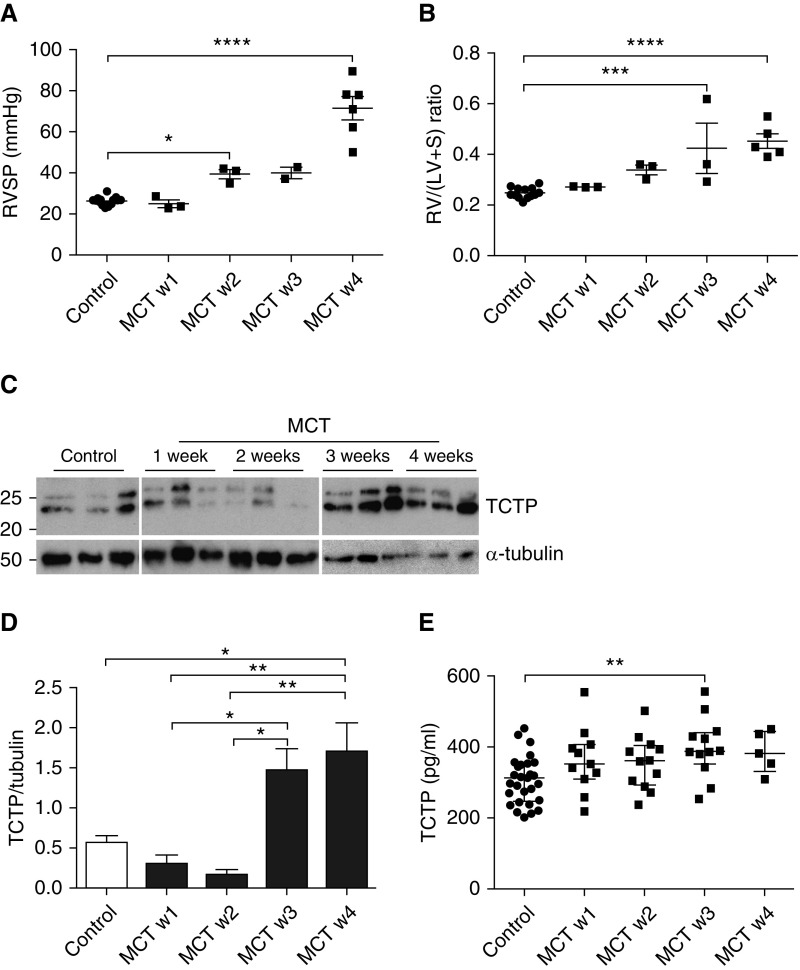
Overall, our in vitro data suggest that inflammatory and proapoptotic conditions, such as those present in PAH, promote increased endothelial exosome release and the enhanced transfer of endothelial-derived TCTP into PASMCs. Given these findings, we used an experimental model of pulmonary hypertension to investigate the circulating and lung levels of TCTP. After MCT administration, right ventricular systolic pressure (RVSP) was measured in groups of control and MCT-treated rats (n = 2/3/group/week). RVSP was significantly increased in MCT-challenged rats by 2 weeks, rising to a level of 71.5 (±5.6 mm Hg), compared with 26.1 (±0.6 mm Hg) in controls, by week 4 (Figure 6A). Furthermore, a significant elevation in right ventricular hypertrophy (Fulton index) was observed after a 3-week MCT treatment (Figure 6B). We assessed the expression of TCTP in the rat lung. TCTP levels in the lung were downregulated at 2 weeks after MCT, but dramatically increased at weeks 3 and 4 in concordance with PAH severity (Figures 6C and 6D). We next investigated the circulating levels of TCTP after exposure to MCT. Similar to the observations in the rat lung, a significant increase was observed after 3 weeks MCT exposure (Figure 6E).
Figure 6.
Increased lung and circulating TCTP levels could contribute to the onset of experimental pulmonary arterial hypertension (PAH). Rats were administered vehicle or monocrotaline (MCT; 40 mg/kg). (A) Right ventricular systolic pressure (RVSP) and (B) right ventricular hypertrophy (RV/(LV+S)) were assessed in control (circles) and MCT-treated rats (squares) at weeks 1, 2, 3, and 4. One-way ANOVA; *P < 0.05, ***P < 0.001, ****P < 0.0001. Error bars represent mean ± SEM. (C) Representative immunoblot of TCTP expression in lungs isolated from controls at 1, 2, 3, and 4 weeks after MCT administration. Blots were reprobed for α-tubulin to ensure equal loading. (D) Densitometry of TCTP expression levels relative to α-tubulin. One-way ANOVA; *P < 0.05, **P < 0.01. Error bars represent mean ± SEM. (E) Plasma levels of TCTP measured by ELISA in control rats (circles) and at 1, 2, 3, and 4 weeks after MCT administration (squares). One-way ANOVA; **P < 0.01. Error bars represent median ± interquartile range.
TCTP Levels in Plasma of Patients with PAH
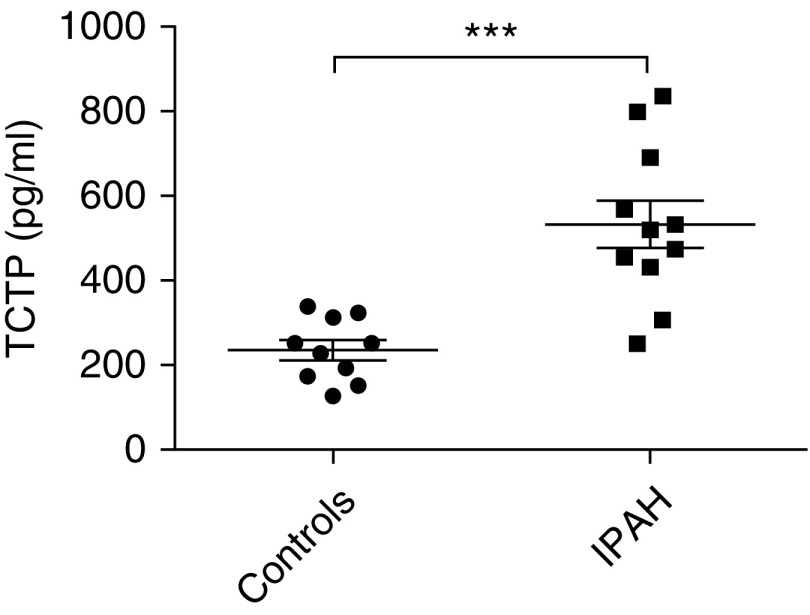
Having observed that rats with experimental PAH exhibit increased TCTP in their lungs, coupled with elevated levels of TCTP in their circulation, we next sought to determine whether this was also the case for patients with IPAH, who also exhibit increased levels of TCTP in their lung tissue (8). Circulating levels of TCTP in plasma were significantly increased in patients with IPAH compared with control subjects (Figure 7). These observations in both human patients with PAH and an experimental animal model of PAH suggest a possible role for TCTP as a biomarker for onset of disease and therapeutic benefits. However, further investigations are required to establish the feasibility of this notion.
Figure 7.
Circulating TCTP levels are altered in PAH. Circulating levels of TCTP were measured in plasma from control subjects and patients with idiopathic PAH (IPAH) by ELISA (control subjects, n = 10; patients with IPAH, n = 11; Student’s t-test). ***P < 0.001. Error bars represent median ± interquartile range.
Discussion
Reduced expression or function of BMPR2 is implicated in nongenetic forms of PAH in humans as well as in animal models (41, 42), but the mechanisms that mediate the hyperproliferation of ECs and SMCs remain unclear. Using proteomic analysis, we previously reported overexpression of TCTP in BOECs derived from patients with PAH, as well as increased TCTP expression in lung tissue sections from patients with PAH (8). Moreover, we identified a plausible link between BMPR2 and TCTP, as knockdown of the receptor showed a dramatic reduction in miR-27b, a known regulator of TCTP expression (25). Similar results were obtained in BOECs derived from patients with PAH harboring a BMPR2 mutation (8). Altogether, these results suggest a potential role for TCTP in the hyperproliferation observed in PAH. In this study, we report a mechanism of cell-to-cell communication between ECs and SMCs that may contribute to the vascular remodeling observed in PAH.
An imbalance between cell proliferation and apoptosis is a common feature shared by PAH and cancer, and leads to vascular remodeling and tumor growth, respectively. It has been reported that TCTP overexpression promotes degradation of p53, negatively regulating apoptosis in lung cancer, whereas knockdown of TCTP expression in lung cancer cells increases apoptosis (43). Our previous data identified that compared with controls, the BOECs harboring BMPR2 mutations expressed higher levels of TCTP and were hyperproliferative. TCTP silencing inhibited proliferation in these HPAH-derived BOECs as well increasing apoptosis (8). Interestingly, in this study, inhibition of TCTP led to uncoordinated migration, such that BOECs lacking TCTP were able to migrate but unable to heal the wound. This finding suggests that TCTP has a role in directing migration, potentially leading to a more metastatic phenotype in BOECs derived from patients with HPAH. In support of this theory, a recent study suggested a role for TCTP in cell invasion and metastasis in cancer through Cdc42/JNK/MMP9 signaling (44). It was previously reported that both Cdc42 and TCTP play pivotal roles in cell morphology regulation, which is also important for metastasis (45). Cell morphological changes are the critical first step in cells undergoing endothelial-to-mesenchymal transition. ECs lose their cobblestone-like monolayer and apical–basal polarity by becoming spindle-shaped mesenchymal cells with migratory pseudopodia or filopodia structures (46). Our results revealed changes in BOEC morphology after siTCTP treatment, supporting previous findings (47) and suggesting that TCTP has the ability to regulate the EC cytoskeleton.
A previous study reported that TCTP can be secreted independently of the endoplasmic reticulum/Golgi pathway (48). However, the mechanisms that stimulate TCTP export from within BOEC-derived exosomes are unclear. Using NTA, we identified differential exosome production in BOECs harboring BMPR-II mutations compared with control cells. Interestingly, in basal conditions (10% FBS with growth factors), exosome production did not differ between control and mutant cells, whereas under low-serum conditions (2% FBS) the number of exosomes increased more than twofold in HPAH-derived BOECs, indicating a more sensitive response of mutant BOECs to changes in the cell environment. However, activation of cells with TNF-α resulted in a similar increase in exosome release in both groups. Accordingly, and as reported previously (8), levels of cleaved caspase-3 also tended to be increased in BOECs from patients with HPAH compared with healthy control subjects in apoptosis-inducing conditions (TNF-α and CHX). We observed exosome transfer to PASMCs and increased TCTP levels in PASMCs after incubation with exosomes derived from activated or stressed BOECs. It is therefore plausible to speculate that in the setting of PAH, the incorporation of exosomes expressing increased levels of TCTP into PASMCs could confer antiapoptotic and proproliferative characteristics. This would contribute to the progression of disease through uncontrolled growth and vascular remodeling. To confirm this hypothesis, further experiments will be necessary. For example, in vitro assays of PASMC proliferation and apoptosis would need to be conducted after exposure to exosomes isolated from control BOECs and BOECs harboring BMPR2 mutations. It would also be of interest to measure the levels of TCTP in exosomes from patients with HPAH versus control subjects.
We previously reported that increased TCTP was observed in the lungs of patients with HPAH and IPAH (8). TCTP expression was largely localized to the endothelial and intimal layers, particularly in areas of complex arterial remodeling associated with end-stage PAH disease, whereas TCTP immunostaining was not present in control lung samples. To further characterize the mechanism of TCTP transportation and paracrine effects, we assessed TCTP expression in an experimental model of pulmonary hypertension. MCT-treated rats developed PAH 2 weeks after treatment, and disease severity increased for the duration of the time course. TCTP lung protein levels increased over time in parallel with the development of disease after MCT. We also observed a significant increase in circulating TCTP levels during disease progression in the MCT model of PAH. Of note, circulating TCTP levels were increased in the plasma samples of patients with IPAH to a much greater degree than in the MCT model. In fact, circulating TCTP was previously suggested to be a promising serum biomarker of apoptosis in patients with lung cancer (49). We therefore propose that the measurement of TCTP might be a potential indicator of EC apoptosis in PAH, and could be used in the diagnosis or assessment of severity of this disease.
TCTP is highly expressed in cells bearing the BMPR2 mutation, and, as in tumors, regulation of cell proliferation is the therapeutic goal (19, 20). In research for new therapies, TCTP has been a particularly interesting target in cancer. In the context of colorectal cancer, sertraline, a selective serotonin reuptake inhibitor, has been shown to have anticancer activity both in cancer cell lines and in colorectal cancer xenografts (50). Interestingly, both sertraline and fluoxetine were shown to inhibit MCT-induced pulmonary hypertension and vascular remodeling in rats by inhibiting serotonin internalization via the serotonin transporter (51, 52). It has also been proposed that sertraline prevents the binding of TCTP to mouse double minute 2 (MDM2), preventing the destabilization of p53 (53, 54). Whether one pathway is prevalent over the other, or whether the two have a synergistic effect remains unclear. Recent studies have shown the efficacy of antihistaminics in the reduction of TCTP (also known as histamine-releasing factor) (19, 55). Treatment with levomepromazine and buclizine downregulated TCTP expression and inhibited cancer cell growth by directly binding TCTP, downregulating G1 of the cell cycle in metastatic carcinoma cell lines (55). Dihydroartemisinin, a common antimalarial therapy, exhibits anticancer activity (56) by decreasing cell proliferation and increasing cell death by targeting the phosphorylated form of TCTP (57).
Here we provide evidence for TCTP export via exosomes and its capacity to be transferred to neighboring cells. Potentially, the level of TCTP determines cell morphology as well as cell fate, defining whether a cell becomes proliferative or differentiated. In the context of PAH, we believe the data shown here will contribute to our understanding of the proliferative and antiapoptotic phenotype of BMPR2 mutant cells, and suggest a potential target for new therapies. Increased circulating levels of TCTP in both IPAH and MCT-treated rats suggest TCTP as a potential indicator of the onset of disease and a circulating biomarker in response to PAH antiremodeling therapy.
Acknowledgments
Acknowledgment
The authors thank Dr. Ian L. Sargent and Dr. Rebecca Dragovic at the Nuffield Department of Obstetrics & Gynaecology, University of Oxford, for allowing us to perform the NTA. They also thank Jeremy Skepper for processing the samples for epithelial-to-mesenchymal transition, and Dr. Mark Toshner, Dr. Michael Newman, and Ms. Carmen Treacy at Papworth Hospital, Cambridgeshire, for their assistance with the patient plasma sample collection.
Footnotes
E.F. was supported by the British Heart Foundation and the Societat Catalana de Pneumologia. B.J.D. and M.L.O. were funded by the British Heart Foundation. This work was funded by awards to N.W.M. (British Heart Foundation Program Grant and the Fondation Leducq) and D.J.S. (Canadian Institutes for Health Foundation Award). Infrastructure support was provided by the Cambridge National Institute for Health Research Biomedical Research Center.
Author Contributions: E.F. designed, performed, and analyzed the majority of the in vitro and in vivo experiments, and wrote the manuscript. B.J.D. designed, performed, and analyzed a number of in vitro experiments, and wrote the manuscript. D.H. designed, performed, and analyzed a number of in vitro experiments. M.L.O. contributed to the conception of the study and the design of experiments, and contributed to writing the manuscript. S.M. performed and analyzed in vivo experiments. J.D. optimized the human and rat TCTP ELISAs. L.L. and X.D.Y. provided tissue from in vivo experiments. D.J.S. contributed to the conception of the study and the design of some experiments, and contributed to writing the manuscript. N.W.M. contributed to the conception of the study and the design of multiple experiments, and wrote the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2017-0129OC on April 20, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43(12) Suppl S:13S–24S. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Deng Z, Haghighi F, Helleby L, Vanterpool K, Horn EM, Barst RJ, et al. Fine mapping of PPH1, a gene for familial primary pulmonary hypertension, to a 3-cM region on chromosome 2q33. Am J Respir Crit Care Med. 2000;161:1055–1059. doi: 10.1164/ajrccm.161.3.9906051. [DOI] [PubMed] [Google Scholar]
- 3.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA, III, Loyd JE, et al. International PPH Consortium. Heterozygous germline mutations in BMPR2, encoding a TGF-β receptor, cause familial primary pulmonary hypertension. Nat Genet. 2000;26:81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 4.Thomson JR, Machado RD, Pauciulo MW, Morgan NV, Humbert M, Elliott GC, et al. Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding BMPR-II, a receptor member of the TGF-β family. J Med Genet. 2000;37:741–745. doi: 10.1136/jmg.37.10.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paulin R, Courboulin A, Meloche J, Mainguy V, Dumas de la Roque E, Saksouk N, et al. Signal transducers and activators of transcription-3/Pim1 axis plays a critical role in the pathogenesis of human pulmonary arterial hypertension. Circulation. 2011;123:1205–1215. doi: 10.1161/CIRCULATIONAHA.110.963314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McMurtry MS, Archer SL, Altieri DC, Bonnet S, Haromy A, Harry G, et al. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J Clin Invest. 2005;115:1479–1491. doi: 10.1172/JCI23203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lavoie JR, Ormiston ML, Perez-Iratxeta C, Courtman DW, Jiang B, Ferrer E, et al. Proteomic analysis implicates translationally controlled tumor protein as a novel mediator of occlusive vascular remodeling in pulmonary arterial hypertension. Circulation. 2014;129:2125–2135. doi: 10.1161/CIRCULATIONAHA.114.008777. [DOI] [PubMed] [Google Scholar]
- 9.MacDonald SM, Rafnar T, Langdon J, Lichtenstein LM. Molecular identification of an IgE-dependent histamine-releasing factor. Science. 1995;269:688–690. doi: 10.1126/science.7542803. [DOI] [PubMed] [Google Scholar]
- 10.Gachet Y, Tournier S, Lee M, Lazaris-Karatzas A, Poulton T, Bommer UA. The growth-related, translationally controlled protein P23 has properties of a tubulin binding protein and associates transiently with microtubules during the cell cycle. J Cell Sci. 1999;112:1257–1271. doi: 10.1242/jcs.112.8.1257. [DOI] [PubMed] [Google Scholar]
- 11.Jeon HJ, You SY, Park YS, Chang JW, Kim JS, Oh JS. TCTP regulates spindle microtubule dynamics by stabilizing polar microtubules during mouse oocyte meiosis. Biochim Biophys Acta. 2016;1863:630–637. doi: 10.1016/j.bbamcr.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Lespagnol A, Duflaut D, Beekman C, Blanc L, Fiucci G, Marine JC, et al. Exosome secretion, including the DNA damage-induced p53-dependent secretory pathway, is severely compromised in TSAP6/Steap3-null mice. Cell Death Differ. 2008;15:1723–1733. doi: 10.1038/cdd.2008.104. [DOI] [PubMed] [Google Scholar]
- 13.Susini L, Besse S, Duflaut D, Lespagnol A, Beekman C, Fiucci G, et al. TCTP protects from apoptotic cell death by antagonizing Bax function. Cell Death Differ. 2008;15:1211–1220. doi: 10.1038/cdd.2008.18. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Peng HW, Cheng YS, Yuan HS, Yang-Yen HF. Stabilization and enhancement of the antiapoptotic activity of Mcl-1 by TCTP. Mol Cell Biol. 2005;25:3117–3126. doi: 10.1128/MCB.25.8.3117-3126.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acunzo J, Baylot V, So A, Rocchi P. TCTP as therapeutic target in cancers. Cancer Treat Rev. 2014;40:760–769. doi: 10.1016/j.ctrv.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Miao X, Chen YB, Xu SL, Zhao T, Liu JY, Li YR, et al. TCTP overexpression is associated with the development and progression of glioma. Tumour Biol. 2013;34:3357–3361. doi: 10.1007/s13277-013-0906-9. [DOI] [PubMed] [Google Scholar]
- 17.Tuynder M, Fiucci G, Prieur S, Lespagnol A, Géant A, Beaucourt S, et al. Translationally controlled tumor protein is a target of tumor reversion. Proc Natl Acad Sci USA. 2004;101:15364–15369. doi: 10.1073/pnas.0406776101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arcuri F, Papa S, Carducci A, Romagnoli R, Liberatori S, Riparbelli MG, et al. Translationally controlled tumor protein (TCTP) in the human prostate and prostate cancer cells: expression, distribution, and calcium binding activity. Prostate. 2004;60:130–140. doi: 10.1002/pros.20054. [DOI] [PubMed] [Google Scholar]
- 19.Tuynder M, Susini L, Prieur S, Besse S, Fiucci G, Amson R, et al. Biological models and genes of tumor reversion: cellular reprogramming through tpt1/TCTP and SIAH-1. Proc Natl Acad Sci USA. 2002;99:14976–14981. doi: 10.1073/pnas.222470799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li F, Zhang D, Fujise K. Characterization of fortilin, a novel antiapoptotic protein. J Biol Chem. 2001;276:47542–47549. doi: 10.1074/jbc.M108954200. [DOI] [PubMed] [Google Scholar]
- 21.Ma Q, Geng Y, Xu W, Wu Y, He F, Shu W, et al. The role of translationally controlled tumor protein in tumor growth and metastasis of colon adenocarcinoma cells. J Proteome Res. 2010;9:40–49. doi: 10.1021/pr9001367. [DOI] [PubMed] [Google Scholar]
- 22.Bae SY, Kim HJ, Lee KJ, Lee K. Translationally controlled tumor protein induces epithelial to mesenchymal transition and promotes cell migration, invasion and metastasis. Sci Rep. 2015;5:8061. doi: 10.1038/srep08061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arciniegas E, Frid MG, Douglas IS, Stenmark KR. Perspectives on endothelial-to-mesenchymal transition: potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1–L8. doi: 10.1152/ajplung.00378.2006. [DOI] [PubMed] [Google Scholar]
- 24.Ranchoux B, Antigny F, Rucker-Martin C, Hautefort A, Péchoux C, Bogaard HJ, et al. Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation. 2015;131:1006–1018. doi: 10.1161/CIRCULATIONAHA.114.008750. [DOI] [PubMed] [Google Scholar]
- 25.Lo WY, Wang HJ, Chiu CW, Chen SF. miR-27b-regulated TCTP as a novel plasma biomarker for oral cancer: from quantitative proteomics to post-transcriptional study. J Proteomics. 2012;77:154–166. doi: 10.1016/j.jprot.2012.07.039. [DOI] [PubMed] [Google Scholar]
- 26.Rohde E, Bartmann C, Schallmoser K, Reinisch A, Lanzer G, Linkesch W, et al. Immune cells mimic the morphology of endothelial progenitor colonies in vitro. Stem Cells. 2007;25:1746–1752. doi: 10.1634/stemcells.2006-0833. [DOI] [PubMed] [Google Scholar]
- 27.de Jong OG, Verhaar MC, Chen Y, Vader P, Gremmels H, Posthuma G, et al. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J Extracell Vesicles. 2012;1 doi: 10.3402/jev.v1i0.18396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baer C, Squadrito ML, Iruela-Arispe ML, De Palma M. Reciprocal interactions between endothelial cells and macrophages in angiogenic vascular niches. Exp Cell Res. 2013;319:1626–1634. doi: 10.1016/j.yexcr.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 30.Sheldon H, Heikamp E, Turley H, Dragovic R, Thomas P, Oon CE, et al. New mechanism for Notch signaling to endothelium at a distance by Delta-like 4 incorporation into exosomes. Blood. 2010;116:2385–2394. doi: 10.1182/blood-2009-08-239228. [DOI] [PubMed] [Google Scholar]
- 31.Salomon C, Ryan J, Sobrevia L, Kobayashi M, Ashman K, Mitchell M, et al. Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS One. 2013;8:e68451. doi: 10.1371/journal.pone.0068451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinthujaroen P, Wanachottrakul N, Pinkaew D, Petersen JR, Phongdara A, Sheffield-Moore M, et al. Elevation of serum fortilin levels is specific for apoptosis and signifies cell death in vivo. BBA Clin. 2014;2:103–111. doi: 10.1016/j.bbacli.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sirois I, Raymond MA, Brassard N, Cailhier JF, Fedjaev M, Hamelin K, et al. Caspase-3-dependent export of TCTP: a novel pathway for antiapoptotic intercellular communication. Cell Death Differ. 2011;18:549–562. doi: 10.1038/cdd.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ormiston ML, Deng Y, Stewart DJ, Courtman DW. Innate immunity in the therapeutic actions of endothelial progenitor cells in pulmonary hypertension. Am J Respir Cell Mol Biol. 2010;43:546–554. doi: 10.1165/rcmb.2009-0152OC. [DOI] [PubMed] [Google Scholar]
- 35.Ormiston ML, Toshner MR, Kiskin FN, Huang CJ, Groves E, Morrell NW, et al. Generation and culture of blood outgrowth endothelial cells from human peripheral blood. J Vis Exp. 2015;106:e53384. doi: 10.3791/53384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geti I, Ormiston ML, Rouhani F, Toshner M, Movassagh M, Nichols J, et al. A practical and efficient cellular substrate for the generation of induced pluripotent stem cells from adults: blood-derived endothelial progenitor cells. Stem Cells Transl Med. 2012;1:855–865. doi: 10.5966/sctm.2012-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gardiner C, Ferreira YJ, Dragovic RA, Redman CW, Sargent IL. Extracellular vesicle sizing and enumeration by nanoparticle tracking analysis. J Extracell Vesicles. 2013;2 doi: 10.3402/jev.v2i0.19671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim M, Kim M, Kim HY, Kim S, Jung J, Maeng J, et al. A protein transduction domain located at the NH2-terminus of human translationally controlled tumor protein for delivery of active molecules to cells. Biomaterials. 2011;32:222–230. doi: 10.1016/j.biomaterials.2010.08.077. [DOI] [PubMed] [Google Scholar]
- 39.Kim M, Min HJ, Won HY, Park H, Lee JC, Park HW, et al. Dimerization of translationally controlled tumor protein is essential for its cytokine-like activity. PLoS One. 2009;4:e6464. doi: 10.1371/journal.pone.0006464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim M, Maeng J, Lee K. Dimerization of TCTP and its clinical implications for allergy. Biochimie. 2013;95:659–666. doi: 10.1016/j.biochi.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 41.Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, Trembath RC, et al. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation. 2002;105:1672–1678. doi: 10.1161/01.cir.0000012754.72951.3d. [DOI] [PubMed] [Google Scholar]
- 42.Long L, Crosby A, Yang X, Southwood M, Upton PD, Kim DK, et al. Altered bone morphogenetic protein and transforming growth factor-β signaling in rat models of pulmonary hypertension: potential for activin receptor-like kinase-5 inhibition in prevention and progression of disease. Circulation. 2009;119:566–576. doi: 10.1161/CIRCULATIONAHA.108.821504. [DOI] [PubMed] [Google Scholar]
- 43.Rho SB, Lee JH, Park MS, Byun HJ, Kang S, Seo SS, et al. Anti-apoptotic protein TCTP controls the stability of the tumor suppressor p53. FEBS Lett. 2011;585:29–35. doi: 10.1016/j.febslet.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 44.Xiao B, Chen D, Luo S, Hao W, Jing F, Liu T, et al. Extracellular translationally controlled tumor protein promotes colorectal cancer invasion and metastasis through Cdc42/JNK/ MMP9 signaling. Oncotarget. 2016;7:50057–50073. doi: 10.18632/oncotarget.10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qadir MI, Parveen A, Ali M. Cdc42: role in cancer management. Chem Biol Drug Des. 2015;86:432–439. doi: 10.1111/cbdd.12556. [DOI] [PubMed] [Google Scholar]
- 46.Frid MG, Kale VA, Stenmark KR. Mature vascular endothelium can give rise to smooth muscle cells via endothelial-mesenchymal transdifferentiation: in vitro analysis. Circ Res. 2002;90:1189–1196. doi: 10.1161/01.res.0000021432.70309.28. [DOI] [PubMed] [Google Scholar]
- 47.Bazile F, Pascal A, Arnal I, Le Clainche C, Chesnel F, Kubiak JZ. Complex relationship between TCTP, microtubules and actin microfilaments regulates cell shape in normal and cancer cells. Carcinogenesis. 2009;30:555–565. doi: 10.1093/carcin/bgp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amzallag N, Passer BJ, Allanic D, Segura E, Théry C, Goud B, et al. TSAP6 facilitates the secretion of translationally controlled tumor protein/histamine-releasing factor via a nonclassical pathway. J Biol Chem. 2004;279:46104–46112. doi: 10.1074/jbc.M404850200. [DOI] [PubMed] [Google Scholar]
- 49.Kim JE, Koo KH, Kim YH, Sohn J, Park YG. Identification of potential lung cancer biomarkers using an in vitro carcinogenesis model. Exp Mol Med. 2008;40:709–720. doi: 10.3858/emm.2008.40.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gil-Ad I, Zolokov A, Lomnitski L, Taler M, Bar M, Luria D, et al. Evaluation of the potential anti-cancer activity of the antidepressant sertraline in human colon cancer cell lines and in colorectal cancer-xenografted mice. Int J Oncol. 2008;33:277–286. [PubMed] [Google Scholar]
- 51.Zhai FG, Zhang XH, Wang HL. Fluoxetine protects against monocrotaline-induced pulmonary arterial hypertension: potential roles of induction of apoptosis and upregulation of Kv1.5 channels in rats. Clin Exp Pharmacol Physiol. 2009;36:850–856. doi: 10.1111/j.1440-1681.2009.05168.x. [DOI] [PubMed] [Google Scholar]
- 52.Li XQ, Hong Y, Wang Y, Zhang XH, Wang HL. Sertraline protects against monocrotaline-induced pulmonary hypertension in rats. Clin Exp Pharmacol Physiol. 2006;33:1047–1051. doi: 10.1111/j.1440-1681.2006.04485.x. [DOI] [PubMed] [Google Scholar]
- 53.Amson R, Pece S, Lespagnol A, Vyas R, Mazzarol G, Tosoni D, et al. Reciprocal repression between P53 and TCTP. Nat Med. 2011;18:91–99. doi: 10.1038/nm.2546. [DOI] [PubMed] [Google Scholar]
- 54.Bommer UA, Vine KL, Puri P, Engel M, Belfiore L, Fildes K, et al. Translationally controlled tumour protein TCTP is induced early in human colorectal tumours and contributes to the resistance of HCT116 colon cancer cells to 5-FU and oxaliplatin. Cell Commun Signal. 2017;15:9. doi: 10.1186/s12964-017-0164-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seo EJ, Efferth T. Interaction of antihistaminic drugs with human translationally controlled tumor protein (TCTP) as novel approach for differentiation therapy. Oncotarget. 2016;7:16818–16839. doi: 10.18632/oncotarget.7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crespo-Ortiz MP, Wei MQ. Antitumor activity of artemisinin and its derivatives: from a well-known antimalarial agent to a potential anticancer drug. J Biomed Biotechnol. 2012;2012:247597. doi: 10.1155/2012/247597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lucibello M, Adanti S, Antelmi E, Dezi D, Ciafrè S, Carcangiu ML, et al. Phospho-TCTP as a therapeutic target of dihydroartemisinin for aggressive breast cancer cells. Oncotarget. 2015;6:5275–5291. doi: 10.18632/oncotarget.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]