Abstract
Urinary tract infections (UTIs) caused by Escherichia coli (E. coli) are the most common types of infections in women. The antibiotic resistance of E. coli is increasing rapidly, causing physicians to hesitate when selecting oral antibiotics. In this review, our objective is to ensure that clinicians understand the current seriousness of antibiotic-resistant E. coli, the mechanisms by which resistance is selected for, and methods that can be used to prevent antibiotic resistance.
1. Introduction
Community-acquired uncomplicated urinary tract infections (UTIs) account for a large proportion of infectious diseases in females [1], and a substantial amount of oral antibiotics is prescribed on a daily basis to treat UTIs among females in community-based outpatient clinics. Because E. coli accounts for up to 80% of community-acquired uncomplicated UTIs, these bacteria should be targeted when choosing empirical antibiotics [2]. In 2011, the Infectious Diseases Society of America (IDSA) recommended that trimethoprim-sulfamethoxazole (cotrimoxazole), nitrofurantoin, fosfomycin, or pivmecillinam be used if local resistance rates of uropathogens causing acute uncomplicated UTIs do not exceed 20% or if the infecting strain is known to be susceptible to these drugs [3]. Fluoroquinolones or beta-lactams such as cephalosporins are recommended as alternatives. Therefore, an awareness of regional susceptibility data regarding E. coli (antibiograms) is very important for selecting appropriate empirical antibiotics. However, the rate at which E. coli strains are becoming resistant to the vast majority of antibiotics is increasing worldwide. In addition, Enterobacteriaceae harbor gene(s) conferring resistance to almost all antibiotics [4] and plasmids harboring these resistance determinants can be transferred between bacteria, even between species, such that the acquisition of resistance to new antibiotics may only be a matter of time. Therefore, it is much more important to recognize practical rationales, including prescribing antibiotics when there is evidence of an infection, promoting appropriate use of antibiotics and increasing efforts for preventing UTIs. Following such strategies is essential because the abuse or misuse of antibiotics can lead to resistance via the emergence of mutant strains [5], and unresolved, relapsed UTIs tend to be resistant to previously used antibiotics [6].
Herein, we searched for an antibiotic resistance patterns for the past decade, especially with regard to oral antibiotics, reviewed the mechanisms of antibiotic resistance in E. coli, and suggest several strategies to overcome the challenges associated with these important issues.
2. Methods
We searched several databases, including PubMed, ISI Web of Science, Scopus, and Google Scholar using the following keywords: “Escherichia coli” or “E. coli”, “resistant” or “resistance”, “urinary tract infections” or “UTI”, “epidemiology”, “community”, and “acquired”. A key word was added when searching for regional antimicrobial susceptibilities, such as “Europe” or countries in Europe, such as “England”, “UK”, “France”, “Germany”, “Russia”, “Italy”, or “Spain”; “Asia” or countries in Asia, such as “China”, “Korea”, “Japan”, “Taiwan”, “Hong Kong”, “India”, or “Pakistan”; “America” or countries in North or South America, such as “US”, “Canada”, “Mexico”, or “Brazil”; “Mediterranean”, or countries in the Mediterranean region, such as “Greece” or “Turkey”; “Middle East” or countries in the Middle East region, such as “Egypt”, “Saudi Arabia”, or “Iran”; or “Australia”. When searching for the mechanisms of antibiotics resistance, “mechanism” was searched for with a key word, such as “co-trimoxazole” or “trimethoprim sulfamethoxazole”, “fluoroquinolone”, “beta-lactams” or “beta-lactamase”, “inhibitor”, “fosfomycin”, “nitrofurantoin”, or “carbapenemase”. When searching for antibiotic treatment or prevention, a word such as “strategy”, “treatment”, “management”, “preventive”, or “prevention” was added. During the review of articles, related articles on this subject were also reviewed.
3. Antimicrobial Susceptibility Pattern of E. coli in Community-Acquired Urinary Tract Infections for Oral Antibiotics in Recent Decades
There are limited oral options for the treatment of ESBL-producing bacteria associated with lower urinary tract infections (acute cystitis). Cotrimoxazole was a typical antibiotic used to treat UTIs, but the resistance of E. coli to this drug has markedly increased. According to the literature published in the past decade, in Asia, a 10~15% resistance rate to this drug was reported in Japan [7], with approximately 30% resistance rates observed in China and south Korea [8, 9]. In Europe and the Mediterranean region, the resistance rates of E. coli to cotrimoxazole varied but were usually over 15% [10–13]. However, there was an interesting report wherein the authors emphasized the role of cotrimoxazole in empirical antibiotics because of the recent decrease in the resistance rate to cotrimoxazole in several European countries due to its low prescription rate [14]. However, it may be not possible to reuse the drug worldwide within the next several years, and close observation of surveillance data will be required.
With respect to fluoroquinolones, in Japan and Australia, the susceptibility of E. coli to these drugs was approximately 90% [7, 15] and varied between 70~88% in the US [16] and 74~84% in China [8]. Middle and North European countries showed a fluoroquinolone susceptibility of 80% or greater [10, 11], while other European or some Mediterranean regions showed approximately a 60% susceptibility [12, 13]. Similar to cotrimoxazole, there was evidence that escape from exposure to this antibiotic will increase antimicrobial susceptibility in UTIs. According to Lee et al., the susceptibility of gram-negative bacteria to ciprofloxacin was much higher in patients less than 20 years old than in patients more than 20 years old. The reason for this observation may be the lower exposure to fluoroquinolones in young individuals because these drugs are not recommended for use in those under 20 years old [17].
One recent issue of importance is the increasing prevalence of extended spectrum beta lactamase- (ESBL-) producing E. coli. The prevalence of ESBL-producing E. coli has been increasing globally, as shown in Table 1 [12, 18–36]. Before 2010, the vast majority of countries showed less than a 5~10% prevalence of ESBL-producing E. coli, whereas the prevalence exceeded 10% in the local communities of many countries. Therefore, the increase in ESBL-producing E. coli is no different than that of cotrimoxazole-resistant E. coli or fluoroquinolone-resistant E. coli, and the prevalence of ESBL-producing E. coli is likely to increase soon.
Table 1.
The prevalence of extended spectrum beta lactamase-producing E. coli in community acquired urinary tract infections before and after 2010.
| Before 2010 | After 2010 | References | |
|---|---|---|---|
| Europe | |||
| UKa | 4.6% | 6.6% | [18] |
| France | 1.1% | 3.3% | [19, 20] |
| Spain | 2.4~18.2% | 8.9~23.6% | [21–23] |
| Mediterranean region | |||
| Italy | 3.5% | 6.7% | [24, 25] |
| Turkey | 8~13.1% | 24% | [12, 26] |
| South Asia | 21.7% | 33.2% | [27] |
| Far east Asia | 4.8~7.5%b | 7.6~10.7% | [28–30] |
| Latin America | 1.7%c | 7.1~12.5% | [31–33] |
| US and Canada | 7.4 | 1.8~8% | [34–36] |
a: Possibly contaminated by a nosocomial source.
b: The source of the specimens maybe from community acquired UTIs but was not described precisely.
c: The data were collected from multinational sources.
Fosfomycin is an oral antibiotic agent that has broad activity against multidrug resistant (MDR), pathogens including ESBL-producing E. coli. Fosfomycin inhibits the synthesis of peptidoglycan at an earlier step than beta-lactam or glycopeptide antibiotics and has a broad spectrum of activity against various gram-positive and gram-negative bacteria, including methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus spp. Fosfomycin has been shown to have advantages in the treatment of UTIs due to its high concentration in the urinary tract, which exceeds 2,000 mg/L after the initial administration and remains at high levels for a prolonged period, over 24 hours. However, fosfomycin should not be used for pyelonephritis or in patients with bacteremia due to inadequate concentrations within the bloodstream [37, 38]. Fosfomycin susceptibility in uropathogens, including E. coli, is currently greater than 90%, even in ESBL-producing E. coli [39–41].
Another oral antimicrobial agent that can be considered for the treatment of ESBL-producing E. coli cystitis is nitrofurantoin. Nitrofurantoin is a drug that has been used since 1950s to treat uncomplicated UTIs and works by damaging bacterial DNA in its highly active reduced form. Now, and even in earlier eras of widespread use, the baseline resistance to nitrofurantoin was low (0–5%) [42, 43]. Nitrofurantoin should only be used for lower UTIs, and its use should be avoided in patients with a creatinine clearance of less than 60 mL/minute, as reduced renal function results in decreased active drug within the urine [44].
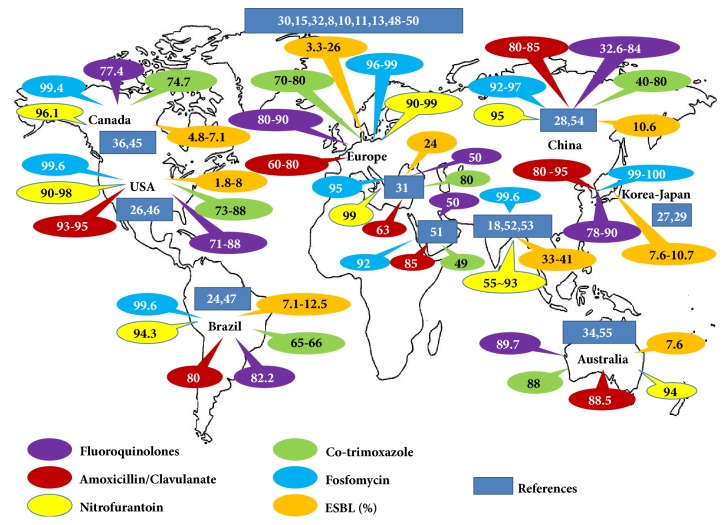
Figure 1 [7–13, 15, 18, 20, 21, 23, 27, 33, 35, 36, 45–55] shows the efficacies of several oral antibiotics against E. coli in community-acquired uncomplicated UTIs. Because fosfomycin and nitrofurantoin have not been included in the antimicrobial formularies of many institutes, it is difficult to achieve previous susceptibility data. Furthermore, because of the history of disappointing in vitro results at the beginning of fosfomycin-susceptibility testing, the use of the drug has been limited in the US and in many other countries [56]. Interestingly, these older medicines have become more important because of the high sensitivity of E. coli to these drugs in the era of antibiotic resistance. Recent studies have shown that these antibiotics have over 90~95% efficacies in almost all areas studied (Figure 1), although there may be no way to predict another decrease in the use of these drugs for UTI treatment.
Figure 1.
Worldwide susceptibilities of E. coli to oral antibiotics in community-acquired urinary tract infections in the last decade.
4. Plasmid-Mediated Dissemination of Antibiotic Resistance Determinants
Plasmid is a generic term for DNA molecules other than chromosomes that can independently replicate in bacterial cells [57]. Although not necessary under most circumstances, plasmids can encode genes that promote bacterial survival and can be transferred to their descendants. Antimicrobial resistance determinants are some of the most important elements carried by plasmids. Although plasmids can be transmitted to other species via conjugation without becoming integrated into DNA [58], sometimes plasmids can integrate into chromosomal DNA for replication. Types of plasmids associated with ESBL-producing E. coli include IncFII, IncN, and IncI1, among which IncI1 resistance plasmids are known to contribute to CTX-M type ESBL dissemination in E. coli [59]. Resistance determinants against most antimicrobial agents can be conferred from species to species via plasmids, which is why systematic monitoring for antibiotic resistance in communities is important, as is the education of staff concerning infection control and prevention, especially in intensive care units. The next section will describe the details of epidemiological studies of plasmid-mediated resistance in E. coli infections.
5. Mechanisms of Action and Resistance to Anti-E. coli Drugs and Its Microbiological Epidemiology
Because tetrahydrofolate is required to make both purines and pyrimidines, its synthesis is important for understanding the mechanism of cotrimoxazole, which is a combination of trimethoprim and sulfamethoxazole. Trimethoprim is a structural analog of dihydrofolic acid that competitively inhibits the synthesis of tetrahydrofolic acid. Sulfamethoxazole, which has a sulfonyl group instead of a carbonyl group, is an analog of para-aminobenzoic acid that competitively inhibits the synthesis of dihydrofolic acid. Over two decades after its first use in 1974 [60], this drug has remained the first-line treatment for uncomplicated UTIs in adults [61]. Because of the widespread resistance to the drug, cotrimoxazole has been gradually replaced by fluoroquinolones since approximately the year 2000 [62]. The mechanism of bacterial resistance to cotrimoxazole is due to (1) drug efflux pumps, (2) the degradation of the antibiotics by enzymes, (3) the alteration of antibiotic binding targets, and (4) the loss of drug entry points, all of which can occur via chromosomal mutations or the acquisition of plasmids [63].
Fluoroquinolones have a keto acetic acid group where fluoroquinolone−topoisomerase binding is facilitated through a water−metal ion bridge [64]. Eventually, fluoroquinolone−topoisomerase complex inhibits topoisomerase activity, and subsequently DNA replication is blocked. Acquisition of resistance to fluoroquinolones is from both chromosome and plasmid. Chromosomal-mediated resistance decrease in fluoroquinolone uptake and the expression of efflux pumps. Plasmid-encoded proteins which are associated with fluoroquinolones resistance are (1) Qnr proteins which decrease topoisomerase-DNA binding and protects enzyme-DNA complexes from quinolones, (2) Aac(6′)-lb-cr which acetylate the free nitrogen of the C7 ring of the quinolones, and (3) plasmid-encoded efflux pumps such as QepA1 and QepA2 [64, 65].
As shown in Figure 1, β-lactams, which are typically prescribed clinically in medical offices, are losing their efficacy in many areas because of the loss of their activity against E. coli. The mechanism of resistance of E. coli to β-lactams is essentially due to plasmid-mediated transmission of genes encoding β-lactamases. In contrast, in Klebsiella species, another species that causes uncomplicated urinary tract infection encodes a β-lactamase (e.g., SHV) on its chromosome [66]. It is not easy for general physicians to understand the classification of β-lactamases. In brief, according to the preferences of researchers, two classifications have been used to describe β-lactamase, including molecular classification from A to D and functional classification with regard to activity against β-lactamase inhibitors [67]. In general, class C corresponds to functional group 1, classes A and D correspond to group 2, and class B corresponds to group 3. ESBLs comprise group 2be (molecular class A) and can hydrolyze penicillin and first generation cephalosporins but can be inhibited by clavulanic acid and tazobactam. ESBLs can hydrolyze at least one of the following antibiotics at a 10% increased rate over that of benzylpenicillin: cefotaxime, ceftazidime, and/or aztreonam [67, 68]. TEM, a common genotype of β-lactamase, is named by a patient whose name was Temoneira, although the origin of TEM has not been identified precisely [69]. TEM-1 and TEM-2 belong to the group 2b (class A) β-lactamase genotype and have the ability to hydrolyze penicillin and/or first-generation cephalosporin, whereas CTX-M-15, which has recently become the most well-known genotype among β-lactamases, is common in ESBL-producing E. coli and is affiliated with group 2be (class A). The origin of CTX-M β-lactamases is believed to be from chromosomal ESBL genes in Kluyvera spp. [70]. A large-scale investigation (72 hospitals) in the US conducted in 2012 revealed that CTX-M-15 was the most common genotype in ESBL-producing E. coli [71]. Meanwhile, many recent studies have emphasized that the relative proportion of sequence type 131 E. coli (E. coli ST131) is predominant among all ESBL-producing E. coli [72, 73] and have shown that CTX-M-15 was encoded in plasmids and was transferred horizontally [74, 75]. When we compared research on non-ESBL-producing and fluoroquinolone-resistant ST131 E. coli isolates [76] to another study in which ST131 E. coli produced CTX-M-15 beta-lactamase and was resistant to fluoroquinolone [77], it appears that CTX-M-15 may be a β-lactamase that is acquired from plasmids in fluoroquinolone-resistant E. coli [78]. AmpC-type β-lactamases (group 1, class C) are encoded in chromosomes and are inducible by antibiotic pressure, such as amoxicillin. Beta-lactam antibiotics such as cefoxitin induce AmpC expression by binding to transpeptidases (penicillin-binding proteins), which results in a balance shift to murein degradation that subsequently activates the transcriptional regulator AmpR and increases its promoter activity [79]. These β-lactamases are generally resistant to clavulanic acid, cephalosporins, and cephamycin and can be expressed by Citrobacter spp., Serratia spp., and Enterobacter spp. but are rarely observed in E. coli [80, 81]. Dissemination of AmpC can occur via the mobilization of chromosomal AmpC genes from different enteric bacteria, such as C. freundii and E. cloacae, and their subsequent horizontal transfer to other species [82]. The first plasmid-borne AmpC gene identified was CMY-1 [83], followed by MIR-1 and CMY-2. Currently, CMY-2-type genes have been suggested to be one of the most common plasmid-borne AmpC enzymes [84]. According to a previous study by Sidjabat et al., a single IncI1 plasmid carrying blaCMY-2 was predominant among different clones of E. coli, suggesting the occurrence of horizontal transfer of this IncI1, blaCMY-2-carrying plasmid [85].
OXA family β-lactamases (group 2d, class D) hydrolyze oxacillin at a faster rate (> 50%) than that observed for benzylpenicillin. OXA-related β-lactamases have recently been identified in plasmids from E. coli [86] that exhibit low-level resistance to imipenem and resistance to ertapenem. Plasmid-mediated dissemination of OXA-48-like carbapenemases in E. coli has been observed in many European countries [87].
Besides OXA family β-lactamases, K. pneumoniae carbapenemase (KPC: group 2f, Class A) and metallo-beta-lactamases (MLBs: group 3, class B) are important types of carbapenemases. KPC enzyme in clinical isolate was first identified in 1996 [88]. In recent decade, the KPC determinants are identified world-widely and the incidence rate is rapidly increasing. KPC producers have been reported mostly from hospital-acquired K. pneumoniae isolates, but KPC-producing E. coli and other enterobacterial species have also been described [89]. In a report from Italian nationwide surveillance from outpatients, 93.2% of carbapenemases were associated with blaKPC type carbapenemase where the majority came from K. pneumoniae and 4.2% from E. coli [90]. Especially with respect to E. coli, Kalyan et al. emphasized that spread of blaKPC producing E. coli is mainly caused by horizontal transfer of blaKPC harboring plasmids such as IncFIA, IncFIIK1, IncFIIK2, or IncN [91].
Although MBLs were originally known as chromosomally encoded enzymes, the most frequently identified MBLs (IMPs and VIMs) have been observed to be encoded in plasmids from the family Enterobacteriaceae [92]. MBLs of the IMP-type appear to be primarily restricted to the Asian continent and are only rarely identified in Europe among enterobacterial isolates. In contrast, VIM-type MBLs have been identified worldwide in enterobacterial isolates responsible for large hospital outbreaks [93, 94]. NDM-1 (New Delhi metallo-ß-lactamase) is highly prevalent in the Indian subcontinent but has also been identified in many countries worldwide, demonstrating its rapid dissemination. The blaNDM-1 gene is primarily plasmid-associated.
Fosfomycin, which was discovered in 1969 [95], inhibits bacterial wall (peptidoglycan) biosynthesis by acting as an analog of phosphoenolpyruvate and binding UDP-GlcNAc enopyruvyl transferase, inactivating the enzyme [96]. Fosfomycin resistance has been identified in some bacteria that resulted from the mutation of UDP-GlcNAc enopyruvyl transferase [97]. However, in E. coli, both mutation-induced resistance and acquired resistance can occur. Several fosfomycin modifying enzymes, including FosA, encoded by plasmid-borne genes can confer fosfomycin resistance in E. coli.
Nitrofurantoin is one of the few drugs that can be used during pregnancy [98]. By oxygen-insensitive nitrofuran reductase, active intermediates of the drug can transferred into the bacteria where they act upon ribosimes and DNA [99], although the precise mechanisms of action of these intermediates have not yet been identified. The nfsA and nfsB genes, encoding nitroreductases, are encoded in E. coli [100]. Mutation in these genes in bacteria can lead to resistance to this drug. According to recent report, bacterial resistance to nitrofurantoin was shown be mediated by OqxAB efflux pumps [101], and the authors described that mutation in the resistant determinant genes nfsA and nfsB could be transmitted by plasmid.
Colistin is sensitive to most of gram negative bacteria, even to blaNDM-1 producing Enterobacteriaceae [102]. The action of colistin is known that cationic structure of the drug binds with anionic lipopolysaccharides causing displacement of cationic (calcium and magnesium) peptides from the outer cell membrane of gram negative bacteria, leading to disruption of the outer membrane and permeability change [103]. The mechanism of resistance has not been clearly understood, but several mechanisms such as outer membrane modification, over-expression of efflux pump, and overproduction of capsule polysaccharide have been suggested [104]. Recent data shows that plasmid mediated gene such as MCR-1 plays a great role on horizontal dissemination of colistin resistance in community [105]. In addition, Mao et al. emphasized that exposure to antibiotics elicited the emergence of MDR E. coli harboring MCR-1 [106].
6. Risk Factors for Acquisition of Antimicrobial Resistance in E. coli
Antibiotic exposure is the most important factor for the selection of antimicrobial resistance. Lee et al. described that increased exposure to fluoroquinolones/cephalosporins made bacteria more resistant to fluoroquinolone/cephalosporins [30]. Although it is not fully understood in detail how antibiotic resistance arises in microorganisms after their exposure to antibiotics, Baquero suggested that exposure to very low antibiotic concentrations can select for low-level resistant mutants, which serve as stepping stones to the strains with high-level resistance [5]. Similarly, Cantón et al. suggested that the use of an antibiotic at a concentration capable of preventing the generation of mutants, above the minimal inhibitory concentration, would restrict the emergence of such first-step mutants within a susceptible population [107]. Undesirable exposure to antibiotics typically occurs due to the abuse or misuse of antibiotics. In many countries, antibiotics can be obtained over the counter and are as easy to obtain as aspirin and cough medicine [108], which is a major contributing factor to antibiotics abuse. Recently, a study from India, where resistant rate of antibiotics has been relatively high, investigated antibiotics misuse where participants with limited access to an allopathic doctor, either for logistical or economic reasons, were observed to be more likely to purchase medications directly from a pharmacy without a prescription [109]. In the United States 20 years ago, experts estimated that at least half of the human therapeutic use of antibiotics in the United States was unnecessary or inappropriate [4].
Colonization has also been suggested to be risk factor for the selection of antimicrobial resistance. Most clinical factors associated with colonization and infection by ESBL-producing organisms involve healthcare exposure, such as hospitalization, residence in a long-term care facility, hemodialysis use, and the presence of an intravascular catheter [110, 111]. In a study of Dutch individuals who had no ESBL colonization prior to international travel, 34 percent overall and 75 percent of individuals who travelled to southern Asia became colonized by ESBL-producing strains following their travels [112]. Another report showed an ESBL prevalence of 49.0-64.0% for residents and 5.2-14.5% for staff [113]. Thus, travelers to endemic areas, hospitalized patients, care-givers in health care unit, guardians of in-patients, and hospital workers, including residents, are at an increased risk of colonization by antimicrobial resistant bacteria, showing the importance of environmental hygiene and taking precautions against contact with MDR bacteria. Once a cluster of resistant bacteria colonizes any part of the human body, it is possible that the bacteria will grow and horizontally transfer plasmid-encoded resistance genes to other susceptible bacteria or to different species [58].
Another important route of slow encroachment by resistant bacteria is the dispensing of antibiotics into ecosystems. Harrison et al. demonstrated that human ingestion of animal and plant food products carries a strong potential for the spread of antibiotic resistance genes via the consumption of antibiotic residues and antibiotic-resistant bacteria [4]. The authors concluded that the continued use of antibiotics in livestock and other agricultural endeavors may soon make these drugs ineffective for human therapeutic use.
Finally, indwelling catheters, which lead to complicated UTIs, are a known a risk factor for the acquisition of MDR bacteria [114].
7. Complicated UTIs and MDR Gram-Negative Bacteria
Classically, UTIs with functional or anatomical abnormalities of the urinary tract are named complicated UTIs [115]. With respect to complicated UTIs, treatment of asymptomatic bacteriuria has not been shown to be beneficial; it could increase the risk of the development of antimicrobial-resistant uropathogens [116]. Antimicrobial resistance is more common in complicated UTIs [42], which may be because patients with complications are more vulnerable to UTIs and are more likely to be exposed to antibiotics, catheterization, and hospital sources.
Meanwhile, a major point in complicated UTIs is the concept of drainage of infected materials to reduce treatment periods and prevent infections from ascending to upper tract. Lee et al. emphasized that drainage of prostatic abscesses would reduce the period of antibiotic administration [117]. A report of long-term bladder management in spinal cord injury showed that condom catheter use (passive drainage) increases the vulnerability of patients to severe infection rather than intermittent catheterization (active drainage) [118].
Catheter-associated UTIs have multiple confounding factors associated with emerging antimicrobial resistance, that is, hospital factors due to frequent hospitalization, foreign body bridges between the urinary bladder and the outside of the body, and frequent antibiotic exposure. A previous study conducted with outpatient UTIs showed that catheter-associated UTIs were more closely associated to exposure to antibiotics and exhibited a higher occurrence of infection caused by an atypical organism, such as Citrobacter species, Proteus mirabilis, Morganella morganii, Enterobacter species, and Pseudomonas aeruginosa rather than E. coli [17]. Those atypical organisms can harbor MDR determinant and can transfer the resistance determinants (e.g., AmpC gene) to E. coli (see Section 5). Therefore, regarding plasmid-mediated resistance determinants, catheter associated UTIs obviously contribute to the emergence of resistant strains. Therefore, in managing neurogenic bladder with high bladder volume or high bladder pressure, avoidance of unnecessary catheterization and/or a change in the catheterization method used, from indwelling catheters to clean intermittent catheterization, must be considered [42].
A recent study recommended the use of amoxicillin/clavulanate (or amoxicillin plus aminoglycoside), cefixime, ceftibuten, levofloxacin, ciprofloxacin, and fosfomycin as empirical antibiotics against catheter-associated UTIs, whereas recommended regimens for empiric treatment of uncomplicated UTIs were fosfomycin, nitrofurantoin and pivmecillinam [119]. However, cultivation should be performed prior to the use of empirical antibiotics, especially in complicated UTIs because atypical and/or MDR microorganisms are more likely to be isolated.
8. Antibiotic Treatment of UTIs by MDR Gram-Negative Bacteria
In regard to laboratory cut-off values of microbial load (103 cfu/ml, 104 cfu/ml, or 105 cfu/ml), it is very difficult to determine treatment initiation in certain cut-off value. However, bacterial count is usually 102 ~ 104 cfu/ml in many patients with UTIs, and half of women with symptomatic cystitis have bacteriuria lower than 105 cfu/ml [120]. Franz et al. also suggested antibiotic treatment in symptomatic patients with microbial load between 102 and 105 cfu/ml [121]. Furthermore, when severe infection such as urinary sepsis was suspected, physicians should start antibiotic therapy before the cultivation report (no time to wait laboratory microbial count) because of its high mortality. Therefore, symptoms (or signs) may be more important factor than the cut-off values of laboratory microbial load to initiate the treatment of UTIs.
Clinical studies have indicated that delayed treatment with inappropriate antibiotics for MDR bacteremia would exert a negative influence on patient mortality [122, 123]. Therefore, when a MDR bacterial infection is suspected and previous cultivation reports are not available, or when a patient has a systemic illness, physicians may be obligated to choose parenteral agents such as piperacillin/tazobactam or carbapenems empirically. In these circumstances, patients require hospitalization.
Currently, the emergence of many types of carbapenemases has caused a sense of crisis for physicians. However, several new drugs have been developed that have allowed physicians to save the use of carbapenems. Ceftazidime/avibactam is a new cephalosporin β-lactamase inhibitor combination targeting to Enterobacteriaceae and Pseudomonas aeruginosa, which can be used as an alternative to carbapenems for infections caused by ESBL- or AmpC-producing gram-negative bacteria [124, 125]. Ceftolozane/tazobactam can also be used as an alternative to carbapenems to treat ESBL-producing gram negative infections [126].
A recent study suggested a treatment algorithm for MDR gram-negative bacterial infections [127]. If an MDR bacterial infection is suspected, and there was no past carbapenem resistance, it is recommended to use parenteral amoxicillin/clavulanate or piperacillin/tazobactam followed by oral fosfomycin, nitrofurantoin or pivmecillinam plus amoxicillin/clavulanate, unless patients have systemic illness, whereas if there was no susceptibility data or patients have systemic illness, it is recommended to use carbapenems, temocillin, or ceftolozane/tazobactam. If carbapenem resistance had been noted, specific antibiotics should be considered according to the local policy of avoiding the development of antibiotic resistance. Therefore, investigations concerning the types of carbapenemases and their surveillance are very important. For example, if a cultivation assay result identifies a strain as being resistant to carbapenemase and metallo-β-lactamases is known as a prevalent type in the local area, physicians can use colistin or tigecycline [128].
9. Contradiction between the Use of and Resistance of Antibiotics, Focusing Acute Cystitis
The fact that exposure to antibiotics increases resistance is a problem for clinicians who need to continue to treat infected patients. It is true that the use of antibiotics should be reduced to decrease the development of strains that are resistant to antibiotics. However, excessive limitation of antibiotics for treating symptomatic UTI or for prophylaxis may lead to another cost increase due to recurrence. Therefore, we need to develop a strategy to adequately control urinary tract infections while minimizing the increase in antibiotic resistance.
First, a management strategy should be developed for systemic and localized infections. In the case of pyelonephritis that causes systemic infections, including UTI sepsis, broad spectrum antibiotics should be used intensively. When a course of antibiotics is started empirically, the choice of agent should be reevaluated once culture results are available. Continuous surveillance of antibiotic resistance patterns by region is essential for the appropriate selection of antibiotics for empirical treatment.
Reducing the total amount of antibiotic use is important for resistance control. The use of cephalosporins and quinolones and the long-term use of antibiotics have been identified as risk factors for infections caused by extended-spectrum ESBL E. coli and Klebsiella species [129, 130]. The use of broad spectrum antibiotics to treat systemic infection may be inevitable. However, total antibiotic usage is higher in non-febrile uncomplicated UTIs (in other words, acute cystitis). Finally, an effective strategy for controlling antibiotic resistance is to use antibiotics appropriately for cystitis treatment and to prevent recurrent cystitis using available means.
Furthermore, this strategy is more important and necessary because of the occurrence of collateral damage, which describes increased colonization or infection by MDR organisms with the use of broad-spectrum antimicrobials, including fluoroquinolones and cephalosporins [131, 132]. Thus, fluoroquinolones and cephalosporins are no longer recommended as the first-line treatment for acute uncomplicated cystitis in the EAU and IDSA guidelines. Instead, drugs that cause minimal resistance and have a propensity for collateral damage are recommended as the first-line treatment, such as nitrofurantoin, fosfomycin, and pivmecillinam [3, 133].
Obviously, the best way to treat UTIs should be to use optimal antibiotics instead of empirical treatment, if possible, based on culture findings [134]. This principle is the same whether it is simple cystitis or febrile UTI. Of course, it is well known that worldwide socio-economic status varies from region to region, and a full laboratory examination cannot be performed for all patients [135, 136]. Therefore, it is not possible to implement consistent guidelines with respect to laboratory diagnostics. However, we should be aware of the high incidence of infectious diseases, even if they cause low mobility or mortality, such as cystitis. It is also important to strengthen the natural defense mechanism of the human body for helping recovery from the infected condition and to prevent recurrence of the same disease, rather than blind antibiotic prescription.
10. Prevention of Infectious Disease Is the Best Option for Antibiotic Resistance Control, Especially in Recurrent UTI
Recurrent UTI is defined as recurrence of uncomplicated and/or complicated UTIs, with a frequency of at least three UTIs/year or two UTIs in the last six months. Generally, UTIs differ from other infectious diseases in which pathognomonic sources are transmitted from the outside, such as sexually transmitted infections or respiratory tract infections. The bacteria that cause UTIs have characteristics that are typically symbiotic with the human body, and when an imbalance arises for some reason, it can result in infections of the urinary tract [137]. Even individuals who do not have a urinary system abnormality are at risk to become infected, and some individuals suffer from repeated UTIs without apparent cause. Moreover, a person with a structural or functional abnormality of the urinary tract is at a higher risk for urinary tract infections. For this reason, efforts to manage the exposure of humans to infectious agents can reduce the incidence of urinary tract infections, and these efforts may help to slow the development of antibiotic resistance [58].
11. Proper Bladder Emptying
All physicians treating UTIs should have awareness of urogenital anomalies and the need for proper bladder emptying. Congenital urinary tract abnormalities should be investigated for pediatric UTIs. For adults, close history taking can identify functional factors, such as bladder outlet obstruction or underactive bladder caused by spinal cord lesions. Physicians should also pay attention to noticeable problems, such as spinal cord injury and congenital anomalies in the urogenital system, as well as inconspicuous problems, such as BPH or DM polyneuropathy. Urogenital imaging studies are performed when evaluating recurrent UTIs, but additional measurements such as postvoid residual urine volume should be considered [138, 139]. If there is a problem in bladder function, proper bladder emptying methods, such as clean intermittent catheterization (CIC), the use of urethral catheters, or the administration of some medications, such as alpha-blockers for sphincter relaxation, should be actively explored. Proper emptying of the bladder is the most important factor in recurrent UTI control.
12. Nonantimicrobial Prophylaxis
The active use of nonantimicrobial prophylaxis is often indicated and does not result in an increase in antimicrobial resistance of the commensal flora, as nonantimicrobial prophylaxis, immunoactive agents, probiotics (Lactobacillus spp.), cranberry-based products, D-mannose, hormonal replacement (in postmenopausal women), and others have been studied [140–144]. Among these modalities, the urinary immunopotentiator is now well documented and strongly recommended in the guidelines [145]. The oral immunostimulant OM-89 (Uro-Vaxom®), an extract of 18 different serotypes of heat-killed uropathogenic E. coli, stimulates innate immunity by increasing non-specific and specific humoral and cellular immune responses by stimulating the production of interferon-γ and tumor necrosis factor-γ, as well as the activities of lymphocytes and macrophages [146–148]. Uro-Vaxom® is a safe and effective medicine that can reduce recurrent UTI episodes [140, 149–151] and can effectively reduce the repeated use of antibiotics [152]. Physicians need to actively use immunoactive agents that have been proven effective rather than letting patients find a solution or take medication on their own. However, a weakness of these agents is that they cannot completely prevent the recurrence of an infection and require relatively long-term use. Therefore, to increase compliance, it is important to provide sufficient information to the patient and to establish a good doctor-patient relationship.
13. Awareness of Asymptomatic Bacteriuria (ABU)
ABU should be distinguished from symptomatic UTI. ABU occurs in an estimated 1-5% of healthy pre-menopausal females, increasing to 4-19% in otherwise healthy elderly females and men, also occurring in 0.7-27% of patients with diabetes, 2-10% of pregnant women, 15-50% of institutionalized elderly patients, and 23-89% of patients with spinal cord injuries [153, 154]. ABU does not cause systemic influences, such as renal damage [155]. Thus, treatment of ABU is not recommended in patients without risk factors [153]. Furthermore, ABU should not be overtreated without the awareness of the physician [156]. Even in catheterized patients (also see Section 7), antibiotics should be considered only when patients with indwelling catheters present symptoms or they have any complications during placement or exchanges of catheters [157]. In contrast, considering ABU in pregnancy, many researches recommended treating the UTIs because not to treat UTIs in pregnant women can increase the possibilities of preterm labor or low birth-weight [158].
The major risk factor for ABU is diabetes mellitus. DM, even when well regulated, is reported to correlate to a higher frequency of ABU [159]. Considering the high morbidity and mortality of symptomatic UTI in DM, patients should be sufficiently treated by nonantibiotic methods. In particular, bladder dysfunction, such as diabetic cytopathy, should be carefully considered, and proper bladder emptying must be encouraged with diverse methods [160]. Even in these cases, immunopotentiators can be a good option for preventing symptomatic UTIs.
14. Pain Control for Cystitis Patients
For patients with nonfebrile uncomplicated cystitis, active pain control, and minimal use of antibiotics should be prioritized. Uncomplicated cystitis can be a self-limited disease in many cases. One study even showed that only symptomatic care using NSAID could be as effective as antibiotics in acute cystitis [161]. Pain in acute cystitis is a natural consequence of the inflammatory response, and pain-mediated urinary frequency or urgency is the chief complaint of patients. Therefore, for this self-limited disease, pain killers, including NSAIDs, may be a good option for symptomatic care as well as reducing the consumption of antibiotics. Delayed treatment is also a good strategy for antimicrobial-sparing [162]. Another caution is the abuse or misuse of overactive bladder (OAB) medicine with anticholinergic effects for acute urgency or urge incontinence in UTIs. Overuse of anticholinergic medicine can interfere with proper bladder emptying, and adverse effects with respect to UTI control may occur.
15. Antimicrobial Stewardship
Antibiotics are overused across the world through their prescription, self-medication, or over-the-counter (OTC) availability. With the quantity of antibiotic use linked to antibiotic resistance, society should seek to preserve the use of this irreplaceable resource through education and regulation [163].
Antimicrobial stewardship programs aim to optimize the outcomes of prevention and treatment of infection while curbing the overuse and misuse of antimicrobial agents [145, 164, 165]. Antimicrobial stewardship has a positive clinical impact on UTIs caused by ESBL-producing E. coli [166]. To this end, antimicrobial therapy should be tailored to each patient, taking into consideration the severity of disease, individual and local patterns of antimicrobial resistance and the potential for collateral damage associated with antimicrobial use. Selecting the correct drug, dose, as well the shortest clinically effective duration of therapy when possible, is key to optimal antimicrobial stewardship [134]. Some prescription strategies should be considered carefully, including the following [167]:
Precise indication for antibiotic treatment
Choice of the appropriate compound
Appropriate dosage
Adequate route of administration
Administration timing and treatment length
All physicians who treat UTIs should take on the responsibility of antimicrobial stewardship.
16. Conclusion
Even if new antibiotics are introduced and appear on the market, the development of resistance to these antibiotics by E. coli will begin immediately. The mechanisms by which E. coli becomes resistant to antibiotics vary greatly with the antibiotic, but genes conferring resistance can be transmitted via plasmids among species. Therefore, the implementation of antibiotic stewardship programs is crucial to minimize the chance of selecting for resistant resistance. Such programs should be founded on the following principles: (1) antibiotics should be used when there is evidence of a bacterial infection to minimize the unnecessary exposure of patients to antibiotics; (2) ABU should not be treated (if there is no risk factor) to minimize unnecessary exposure to antibiotics; (3) cultivation before using antibiotics and using appropriate antibiotics (if possible, considering using nitrofurantoin, fosfomycin, or pivmecillinam as first-line antibiotics) should be performed according to regional susceptibility data to decrease the chance of “collateral damage”; (4) the use of appropriate antibiotic doses, not underdoses, to potentially reduce mutant formation; and (5) the use of antibiotics for appropriate durations to reduce recurrence (appropriate de-escalation with repeated culture). In addition, to prevent overuse of antibiotics, self-medication, or over-the-counter (OTC) availability should be limited by education or regulation. Furthermore, paying attention to hygiene, especially individuals who travel to endemic areas or who are frequently in circumstances with a high risk of exposure to antibiotic resistant bacteria (e.g., healthcare units), could reduce the chance of colonization by resistant micro-organisms. Finally, the use of nonantimicrobial prophylaxis could effectively reduce the total amount of antibiotic consumption.
In general, cotrimoxazole has under 80% sensitivity and fluoroquinolones have approximately 80% sensitivity, but the latter drug shows under 60% sensitivity in some parts of Asia, the Middle-East, and the Mediterranean region. Beta-lactam with inhibitor, amoxicillin/clavulanate, shows approximately 80% sensitivity, except for some European countries and the Mediterranean region. South Asia is an endemic region of ESBL producing E. coli. Yet, nitrofurantoin and fosfomycin show over 90% of sensitivity in the most of countries of the world.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Gupta K., Hooton T. M., Stamm W. E. Increasing antimicrobial resistance and the management of uncomplicated community-acquired urinary tract infections. Annals of Internal Medicine. 2001;135(1):41–50. doi: 10.7326/0003-4819-135-1-200107030-00012. [DOI] [PubMed] [Google Scholar]
- 2.Kang C., Kim J., Park D. W., et al. Clinical Practice Guidelines for the Antibiotic Treatment of Community-Acquired Urinary Tract Infections. Infection & Chemotherapy. 2018;50(1):67–100. doi: 10.3947/ic.2018.50.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta K., Hooton T. M., Naber K. G., et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clinical Infectious Diseases. 2011;52(5):e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 4.Harrison J. W., Svec T. A. The beginning of the end of the antibiotic era? Part I. The problem: Abuse of the “miracle drugs”. Quintessence International. 1998;29(3):151–162. [PubMed] [Google Scholar]
- 5.Baquero F. Low-level antibacterial resistance: A gateway to clinical resistance. Drug Resistance Updates. 2001;4(2):93–105. doi: 10.1054/drup.2001.0196. [DOI] [PubMed] [Google Scholar]
- 6.Kim H. Y., Lee S.-J., Lee D. S., Yoo J. M., Choe H.-S. Microbiological Characteristics of Unresolved Acute Uncomplicated Cystitis. Microbial Drug Resistance. 2016;22(5):387–391. doi: 10.1089/mdr.2015.0241. [DOI] [PubMed] [Google Scholar]
- 7.Hayami H., Takahashi S., Ishikawa K., et al. Nationwide surveillance of bacterial pathogens from patients with acute uncomplicated cystitis conducted by the Japanese surveillance committee during 2009 and 2010: antimicrobial susceptibility of Escherichia coli and Staphylococcus saprophyticus. Journal of Infection and Chemotherapy. 2013;19(3):393–403. doi: 10.1007/s10156-013-0606-9. [DOI] [PubMed] [Google Scholar]
- 8.Yang B., Yang F., Wang S., et al. Analysis of the spectrum and antibiotic resistance of uropathogens in outpatients at a tertiary hospital. Journal of Chemotherapy. 2018;30(3):145–149. doi: 10.1080/1120009X.2017.1418646. [DOI] [PubMed] [Google Scholar]
- 9.Seo M.-R., Kim S.-J., Kim Y., et al. Susceptibility of Escherichia coli from community-acquired urinary tract infection to fosfomycin, nitrofurantoin, and temocillin in Korea. Journal of Korean Medical Science. 2014;29(8):1178–1181. doi: 10.3346/jkms.2014.29.8.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chervet D., Lortholary O., Zahar J.-R., Dufougeray A., Pilmis B., Partouche H. Antimicrobial resistance in community-acquired urinary tract infections in Paris in 2015. Médecine et Maladies Infectieuses. 2017 doi: 10.1016/j.medmal.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Seitz M., Stief C., Waidelich R. Local epidemiology and resistance profiles in acute uncomplicated cystitis (AUC) in women: A prospective cohort study in an urban urological ambulatory setting. BMC Infectious Diseases. 2017;17(1):p. 685. doi: 10.1186/s12879-017-2789-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yilmaz N., Agus N., Bayram A., et al. Antimicrobial susceptibilities of Escherichia coli isolates as agents of community-acquired urinary tract infection (2008-2014) Turkish Journal of Urology. 2016;42(1):32–36. doi: 10.5152/tud.2016.90836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trevino M., Losada I., Fernandez-Perez B., et al. Surveillance of antimicrobial susceptibility of Escherichia coli producing urinary tract infections in Galicia (Spain) Revista Espanola De Quimioterapia. 2016;29(2):86–90. [PubMed] [Google Scholar]
- 14.Caron F., Wehrle V., Etienne M. The comeback of trimethoprim in France. Médecine et Maladies Infectieuses. 2017;47(4):253–260. doi: 10.1016/j.medmal.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Australian Commission on Safety and Quality in Health Care. Preliminary Report on Antimicrobial Use and Resistance in Australia (AURA) Sydney, Australia: ACSQHC; 2014. [Google Scholar]
- 16.Sanchez G. V., Adams S. J. E., Baird A. M. G., Master R. N., Clark R. B., Bordon J. M. Escherichia coli antimicrobial resistance increased faster among geriatric outpatients compared with adult outpatients in the USA, 2000-10. Journal of Antimicrobial Chemotherapy. 2013;68(8):1838–1841. doi: 10.1093/jac/dkt110.dkt110 [DOI] [PubMed] [Google Scholar]
- 17.Lee D. S., Choe H.-S., Kim H. Y., et al. Role of age and sex in determining antibiotic resistance in febrile urinary tract infections. International Journal of Infectious Diseases. 2016;51:89–96. doi: 10.1016/j.ijid.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Toner L., Papa N., Aliyu S. H., Dev H., Lawrentschuk N., Al-Hayek S. Extended-spectrum beta-lactamase-producing Enterobacteriaceae in hospital urinary tract infections: incidence and antibiotic susceptibility profile over 9 years. World Journal of Urology. 2016;34(7):1031–1037. doi: 10.1007/s00345-015-1718-x. [DOI] [PubMed] [Google Scholar]
- 19.Arpin C., Quentin C., Grobost F., et al. Nationwide survey of extended-spectrum β-lactamase-producing Enterobacteriaceae in the French community setting. Journal of Antimicrobial Chemotherapy. 2009;63(6):1205–1214. doi: 10.1093/jac/dkp108. [DOI] [PubMed] [Google Scholar]
- 20.Martin D., Fougnot S., Grobost F., et al. Prevalence of extended-spectrum beta-lactamase producing Escherichia coli in community-onset urinary tract infections in France in 2013. Journal of Infection. 2016;72(2):201–206. doi: 10.1016/j.jinf.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Artero A., Esparcia A., Alberola J., Madrazo M., Nogueira J. M., Eiros J. M. Prospective cohort study of risk factors for extended-spectrum ß-lactamase-producing Escherichia coli urinary tract infections in elderly patients admitted to hospital. International Journal of Clinical Practice. 2017;71(9) doi: 10.1111/ijcp.13001. [DOI] [PubMed] [Google Scholar]
- 22.Cuevas O., Cercenado E., Gimeno M., et al. Comparative in vitro activity of cefditoren and other antimicrobials against Enterobacteriaceae causing community-acquired uncomplicated urinary tract infections in women: A Spanish nationwide multicenter study. Diagnostic Microbiology and Infectious Disease. 2010;67(3):251–260. doi: 10.1016/j.diagmicrobio.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Arana D. M., Rubio M., Alós J. Evolution of antibiotic multiresistance in Escherichia coli and Klebsiella pneumoniae isolates from urinary tract infections: A 12-year analysis (2003–2014) Enfermedades Infecciosas y Microbiología Clínica. 2017;35(5):293–298. doi: 10.1016/j.eimce.2017.03.020. [DOI] [PubMed] [Google Scholar]
- 24.Luzzaro F., Mezzatesta M., Mugnaioli C., et al. Trends in production of extended-spectrum β-lactamases among enterobacteria of medical interest: report of the second Italian nationwide survey. Journal of Clinical Microbiology. 2006;44(5):1659–1664. doi: 10.1128/jcm.44.5.1659-1664.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Picozzi S., Ricci C., Gaeta M., et al. Do we really know the prevalence of multi-drug resistant Escherichia coli in the territorial and nosocomial population? Urology Annals. 2013;5(1):25–29. doi: 10.4103/0974-7796.106962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tasbakan M., Pullukcu H., Sipahi O., Yamazhan T., Arda B., Ulusoy S. Pooled analysis of resistance patterns of Escherichia coli strains isolated from urine cultures in turkey: comparison of 1997–2001 and 2002–2007 periods. International Journal of Infectious Diseases. 2008;12(1):e112–e113. doi: 10.1016/j.ijid.2008.05.282. [DOI] [Google Scholar]
- 27.Patwardhan V., Kumar D., Goel V., Singh S. Changing prevalence and antibiotic drug resistance pattern of pathogens seen in community-acquired pediatric urinary tract infections at a tertiary care hospital of North India. Journal of Laboratory Physicians. 2017;9(4):264–268. doi: 10.4103/JLP.JLP_149_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pai H. The characteristics of extended-spectrum β-lactamases in Korean isolates of Enterobacteriaceae. Yonsei Medical Journal. 1998;39(6):514–519. doi: 10.3349/ymj.1998.39.6.514. [DOI] [PubMed] [Google Scholar]
- 29.Kim B., Kim J., Seo M.-R., et al. Clinical characteristics of community-acquired acute pyelonephritis caused by ESBL-producing pathogens in South Korea. Infection. 2013;41(3):603–612. doi: 10.1007/s15010-013-0441-z. [DOI] [PubMed] [Google Scholar]
- 30.Lee D. S., Choe H.-S., Lee S. J., et al. Antimicrobial susceptibility pattern and epidemiology of female urinary tract infections in South Korea, 2010-2011. Antimicrobial Agents and Chemotherapy. 2013;57(11):5384–5393. doi: 10.1128/AAC.00065-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sader H. S., Gales A. C., Pfaller M. A., et al. Pathogen frequency and resistance patterns in Brazilian hospitals: summary of results from three years of the SENTRY Antimicrobial Surveillance Program. Brazilian Journal of Infectious Diseases. 2001;5(4):200–214. doi: 10.1590/s1413-86702001000400006. [DOI] [PubMed] [Google Scholar]
- 32.Blanco V. M., Maya J. J., Correa A., et al. Prevalence and risk factors for extended-spectrum β-lactamase-producing Escherichia coli causing community-onset urinary tract infections in Colombia. Enfermedades Infecciosas y Microbiología Clínica. 2016;34(9):559–565. doi: 10.1016/j.eimc.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonçalves L. F., de Oliveira Martins-Júnior P., de Melo A. B., et al. Multidrug resistance dissemination by extended-spectrum β-lactamase-producing Escherichia coli causing community-acquired urinary tract infection in the Central-Western Region, Brazil. Journal of Global Antimicrobial Resistance. 2016;6:1–4. doi: 10.1016/j.jgar.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Hoban D. J., Nicolle L. E., Hawser S., Bouchillon S., Badal R. Antimicrobial susceptibility of global inpatient urinary tract isolates of Escherichia coli: Results from the Study for Monitoring Antimicrobial Resistance Trends (SMART) program: 2009-2010. Diagnostic Microbiology and Infectious Disease. 2011;70(4):507–511. doi: 10.1016/j.diagmicrobio.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 35.Doi Y., Park Y. S., Rivera J. I., et al. Community-associated extended-spectrum β-lactamase-producing Escherichia coli infection in the United States. Clinical Infectious Diseases. 2013;56(5):641–648. doi: 10.1093/cid/cis942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karlowsky J. A., Denisuik A. J., Lagacé-Wiens P. R. S., et al. In vitro activity of fosfomycin against Escherichia coli isolated from patients with urinary tract infections in Canada as part of the CANWARD surveillance study. Antimicrobial Agents and Chemotherapy. 2014;58(2):1252–1256. doi: 10.1128/aac.02399-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Raz R. Fosfomycin: An old-new antibiotic. Clinical Microbiology and Infection. 2012;18(1):4–7. doi: 10.1111/j.1469-0691.2011.03636.x. [DOI] [PubMed] [Google Scholar]
- 38.Naber K. G., Thyroff-Friesinger U. Fosfomycin trometamol versus ofloxacin/Co-trimoxazole as single dose therapy of acute uncomplicated urinary tract infection in females: a multicentre study. Infection. 1990;18, supplement 2:S70–S76. doi: 10.1007/bf01643431. [DOI] [PubMed] [Google Scholar]
- 39.Oteo J., Orden B., Bautista V., et al. CTX-M-15-producing urinary Escherichia coli O25b-ST131-phylogroup B2 has acquired resistance to fosfomycin. Journal of Antimicrobial Chemotherapy. 2009;64(4):712–717. doi: 10.1093/jac/dkp288. [DOI] [PubMed] [Google Scholar]
- 40.Honderlick P., Cahen P., Gravisse J., Vignon D. Uncomplicated urinary tract infections, what about fosfomycin and nitrofurantoin in 2006? Pathologie Biologie. 2006;54(8-9):462–466. doi: 10.1016/j.patbio.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 41.Cho Y. H., Jung S. I., Chung H. S., et al. Antimicrobial susceptibilities of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in health care-associated urinary tract infection: focus on susceptibility to fosfomycin. International Urology and Nephrology. 2015;47(7):1059–1066. doi: 10.1007/s11255-015-1018-9. [DOI] [PubMed] [Google Scholar]
- 42.Melekos M. D., Naber K. G. Complicated urinary tract infections. International Journal of Antimicrobial Agents. 2000;15(4):247–256. doi: 10.1016/S0924-8579(00)00168-0. [DOI] [PubMed] [Google Scholar]
- 43.Huttner A., Verhaegh E. M., Harbarth S., Muller A. E., Theuretzbacher U., Mouton J. W. Nitrofurantoin revisited: A systematic review and meta-analysis of controlled trials. Journal of Antimicrobial Chemotherapy. 2015;70(9):2456–2464. doi: 10.1093/jac/dkv147. [DOI] [PubMed] [Google Scholar]
- 44.Oplinger M., Andrews C. O. Nitrofurantoin contraindication in patients with a creatinine clearance below 60 mL/min: Looking for the evidence. Annals of Pharmacotherapy. 2013;47(1):106–111. doi: 10.1345/aph.1R352. [DOI] [PubMed] [Google Scholar]
- 45.Lagacé-Wiens P. R. S., Adam H. J., Low D. E., et al. Trends in antibiotic resistance over time among pathogens from Canadian hospitals: results of the CANWARD study 2007-11. Journal of Antimicrobial Chemotherapy. 2013;68(1):i23–i29. doi: 10.1093/jac/dkt023. [DOI] [PubMed] [Google Scholar]
- 46.Keepers T. R., Gomez M., Celeri C., Krause K. M., Biek D., Critchley I. Fosfomycin and comparator activity against select Enterobacteriaceae, Pseudomonas, and Enterococcus urinary tract infection isolates from the United States in 2012. Infectious Diseases and Therapy. 2017;6(2):233–243. doi: 10.1007/s40121-017-0150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naber K. G., Schito G., Botto H., Palou J., Mazzei T. Surveillance study in europe and brazil on clinical aspects and Antimicrobial Resistance Epidemiology in Females with Cystitis (ARESC): implications for empiric therapy. European Urology. 2008;54(5):1164–1178. doi: 10.1016/j.eururo.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 48.Rossignol L., Vaux S., Maugat S., et al. Incidence of urinary tract infections and antibiotic resistance in the outpatient setting: a cross-sectional study. Infection. 2017;45(1):33–40. doi: 10.1007/s15010-016-0910-2. [DOI] [PubMed] [Google Scholar]
- 49.Khanna N., Boyes J., Lansdell P. M., Hamouda A., Amyes S. G. B. Molecular epidemiology and antimicrobial resistance pattern of extended-spectrum-β-lactamase-producing Enterobacteriaceae in Glasgow, Scotland. Journal of Antimicrobial Chemotherapy. 2012;67(3):573–577. doi: 10.1093/jac/dkr523. [DOI] [PubMed] [Google Scholar]
- 50.Pobiega M., Wojkowska-Mach J., Chmielarczyk A., et al. Molecular characterization and drug resistance of Escherichia coli strains isolated from urine from long-term care facility residents in Cracow, Poland. Medical Science Monitor. 2013;19(1):317–326. doi: 10.12659/msm.883898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.H A. L., M A. E., R A. A., et al. Antimicrobial Sensitivities of Pathogens Causing Community Acquired Urinary Tract Infection in Adult Patients. J Basic Appl Sci Res. 2016;6(1):31–35. [Google Scholar]
- 52.Tulara N. K. Nitrofurantoin and Fosfomycin for Extended Spectrum Beta-lactamases Producing Escherichia coli and Klebsiella pneumoniae. Journal of Global Infectious Diseases. 2018;10(1):19–21. doi: 10.4103/jgid.jgid_72_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jena J., Sahoo R. K., Debata N. K., Subudhi E. Prevalence of TEM, SHV, and CTX-M genes of extended-spectrum β-lactamase-producing Escherichia coli strains isolated from urinary tract infections in adults. 3 Biotech. 2017;7(4):p. 244. doi: 10.1007/s13205-017-0879-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma K. L., Wang C. X. Analysis of the spectrum and antibiotic resistance of uropathogens in vitro: Results based on a retrospective study from a tertiary hospital. American Journal of Infection Control. 2013;41(7):601–606. doi: 10.1016/j.ajic.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 55.Fasugba O., Mitchell B. G., Mnatzaganian G., Das A., Collignon P., Gardner A. Five-year antimicrobial resistance patterns of urinary Escherichia coli at an Australian tertiary hospital: Time series analyses of prevalence data. Plos One. 2016;11(10):p. e0164306. doi: 10.1371/journal.pone.0164306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Popovic M., Steinort D., Pillai S., Joukhadar C. Fosfomycin: an old, new friend? European Journal of Clinical Microbiology & Infectious Diseases. 2010;29(2):127–142. doi: 10.1007/s10096-009-0833-2. [DOI] [PubMed] [Google Scholar]
- 57.Lederberg J. Cell Genetics and Hereditary Symbiosis. Physiological Reviews. 1952;32(4):403–430. doi: 10.1152/physrev.1952.32.4.403. [DOI] [PubMed] [Google Scholar]
- 58.Smillie C., Garcillan-Barcia M. P., Francia M. V., Rocha E. P., de la Cruz F. Mobility of Plasmids. Microbiology and Molecular Biology Reviews. 2010;74(3):434–452. doi: 10.1128/MMBR.00020-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carattoli A. Plasmids and the spread of resistance. International Journal of Medical Microbiology. 2013;303(6-7):298–304. doi: 10.1016/j.ijmm.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 60.Torok E., Moran E., Cooke F. Oxford Handbook of Infectious Diseases and Microbiology . 2nd. Chapter 2. Oxford University Press; 2009. [DOI] [Google Scholar]
- 61.Naber K. G. Treatment options for acute uncomplicated cystitis in adults. Journal of Antimicrobial Chemotherapy. 2000;46(1):23–27. doi: 10.1093/jac/46.suppl_1.23. [DOI] [PubMed] [Google Scholar]
- 62.Garrison J., Hooton T. M. Fluoroquinolones in the treatment of acute uncomplicated urinary tract infections in adult women. Expert Opinion on Pharmacotherapy. 2001;2(8):1227–1237. doi: 10.1517/14656566.2.8.1227. [DOI] [PubMed] [Google Scholar]
- 63.Huovinen P. Resistance to trimethoprim-sulfamethoxazole. Clinical Infectious Diseases. 2001;32(11):1608–1614. doi: 10.1086/320532. [DOI] [PubMed] [Google Scholar]
- 64.Aldred K. J., Kerns R. J., Osheroff N. Mechanism of quinolone action and resistance. Biochemistry. 2014;53(10):1565–1574. doi: 10.1021/bi5000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Strahilevitz J., Jacoby G. A., Hooper D. C., Robicsek A. Plasmid-mediated quinolone resistance: a multifaceted threat. Clinical Microbiology Reviews. 2009;22(4):664–689. doi: 10.1128/cmr.00016-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gniadkowski M. Evolution of extended-spectrum beta-lactamases by mutation. Clinical Microbiology and Infection. 2008;14:11–32. doi: 10.1111/j.1469-0691.2007.01854.x. [DOI] [PubMed] [Google Scholar]
- 67.Bush K., Jacoby G. A. Updated functional classification of β-lactamases. Antimicrobial Agents and Chemotherapy. 2010;54(3):969–976. doi: 10.1128/aac.01009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roy C., Foz A., Segura C., Tirado M., Foster C., Reig R. Plasmid-determined β-lactamases identified in a group of 204 ampicillin-resistant Enterobacteriaceae. Journal of Antimicrobial Chemotherapy. 1983;12(5):507–510. doi: 10.1093/jac/12.5.507. [DOI] [PubMed] [Google Scholar]
- 69.Bradford P. A. Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clinical Microbiology Reviews. 2001;14(4):933–951. doi: 10.1128/CMR.14.4.933-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Olson A. B., Silverman M., Boyd D. A., et al. Identification of a progenitor of the CTX-M-9 group of extended-spectrum β-lactamases from Kluyvera georgiana isolated in Guyana. Antimicrobial Agents and Chemotherapy. 2005;49(5):2112–2115. doi: 10.1128/AAC.49.5.2112-2115.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Castanheira M., Farrell S. E., Krause K. M., Jones R. N., Sader H. S. Contemporary diversity of β-lactamases among Enterobacteriaceae in the nine U.S. census regions and ceftazidime-avibactam activity tested against isolates producing the most prevalent β-lactamase groups. Antimicrobial Agents and Chemotherapy. 2014;58(2):833–838. doi: 10.1128/aac.01896-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Banerjee R., Strahilevitz J., Johnson J. R., et al. Predictors and molecular epidemiology of community-onset extended-spectrum β-lactamase-producing Escherichia coli infection in a Midwestern Community. Infection Control and Hospital Epidemiology. 2013;34(9):947–953. doi: 10.1086/671725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gibreel T. M., Dodgson A. R., Cheesbrough J., Fox A. J., Bolton F. J., Upton M. Population structure, virulence potential and antibiotic susceptibility of uropathogenic Escherichia coli from Northwest England. Journal of Antimicrobial Chemotherapy. 2012;67(2):346–356. doi: 10.1093/jac/dkr451.dkr451 [DOI] [PubMed] [Google Scholar]
- 74.Woodford N., Carattoli A., Karisik E., Underwood A., Ellington M. J., Livermore D. M. Complete nucleotide sequences of plasmids pEK204, pEK499, and pEK516, encoding CTX-M enzymes in three major Escherichia coli lineages from the United Kingdom, all belonging to the international O25:H4-ST131 clone. Antimicrobial Agents and Chemotherapy. 2009;53(10):4472–4482. doi: 10.1128/AAC.00688-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Novais Â., Viana D., Baquero F., Martínez-Botas J., Cantón R., Coque T. M. Contribution of IncFII and broad-host IncA/C and IncN plasmids to the local expansion and diversification of phylogroup B2 Escherichia coli ST131 clones carrying bla CTX-M-15and qnrS1 genes. Antimicrobial Agents and Chemotherapy. 2012;56(5):2763–2766. doi: 10.1128/AAC.06001-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cagnacci S., Gualco L., Debbia E., Schito G. C., Marchese A. European emergence of ciprofloxacin-resistant Escherichia coli clonal groups O25:H4-ST 131 and O15:K52:H1 causing community-acquired uncomplicated cystitis. Journal of Clinical Microbiology. 2008;46(8):2605–2612. doi: 10.1128/JCM.00640-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nicolas-Chanoine M.-H., Blanco J., Leflon-Guibout V., et al. Intercontinental emergence of Escherichia coli clone O25:H4-ST131 producing CTX-M-15. Journal of Antimicrobial Chemotherapy. 2008;61(2):273–281. doi: 10.1093/jac/dkm464. [DOI] [PubMed] [Google Scholar]
- 78.Nicolas-Chanoine M.-H., Bertrand X., Madec J.-Y. Escherichia coli ST131, an intriguing clonal group. Clinical Microbiology Reviews. 2014;27(3):543–574. doi: 10.1128/CMR.00125-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jacobs C., Frère J.-M., Normark S. Cytosolic intermediates for cell wall biosynthesis and degradation control inducible β-lactam resistance in gram-negative bacteria. Cell. 1997;88(6):823–832. doi: 10.1016/S0092-8674(00)81928-5. [DOI] [PubMed] [Google Scholar]
- 80.Bush K., Tanaka S. K., Bonner D. P., Sykes R. B. Resistance caused by decreased penetration of beta-lactam antibiotics into Enterobacter cloacae. Antimicrobial Agents and Chemotherapy. 1985;27(4):555–560. doi: 10.1128/AAC.27.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Livermore D. M. Clinical significance of beta-lactamase induction and stable derepression in gram-negative rods. European Journal of Clinical Microbiology & Infectious Diseases. 1987;6(4):439–445. doi: 10.1007/BF02013107. [DOI] [PubMed] [Google Scholar]
- 82.Philippon A., Arlet G., Jacoby G. A. Plasmid-determined AmpC-type β-lactamases. Antimicrobial Agents and Chemotherapy. 2002;46(1):1–11. doi: 10.1128/aac.46.1.1-11.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bauernfeind A., Schweighart S., Chong Y. Extended broad spectrum β-lactamase in Klebsiella pneumoniae including resistance to cephamycins. Infection. 1989;17(5):316–321. doi: 10.1007/BF01650718. [DOI] [PubMed] [Google Scholar]
- 84.Denisuik A. J., Lagacé-Wiens P. R. S., Pitout J. D., et al. Molecular epidemiology of extended-spectrum β-lactamase-, AmpC β-lactamase- and carbapenemase-producing Escherichia coli and Klebsiella pneumoniae isolated from Canadian hospitals over a 5 year period: CANWARD 2007–11. Journal of Antimicrobial Chemotherapy. 2013;68(1):i57–i65. doi: 10.1093/jac/dkt027. [DOI] [PubMed] [Google Scholar]
- 85.Sidjabat H. E., Seah K. Y. O., Coleman L., et al. Expansive spread of IncI1 plasmids carrying blaCMY-2 amongst Escherichia coli. International Journal of Antimicrobial Agents. 2014;44(3):203–208. doi: 10.1016/j.ijantimicag.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 86.Fernańdez J., Montero I., Fleites A., Rodicio M. R. Cluster of Escherichia coli isolates producing a plasmid-mediated OXA-48 β-lactamase in a Spanish hospital in 2012. Journal of Clinical Microbiology. 2014;52(9):3414–3417. doi: 10.1128/jcm.01271-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tängdén T., Giske C. G. Global dissemination of extensively drug-resistant carbapenemase-producing Enterobacteriaceae: clinical perspectives on detection, treatment and infection control. Journal of Internal Medicine. 2015;277(5):501–512. doi: 10.1111/joim.12342. [DOI] [PubMed] [Google Scholar]
- 88.Yigit H., Queenan A. M., Anderson G. J., et al. Novel carbapenem-hydrolyzing β-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrobial Agents and Chemotherapy. 2001;45(4):1151–1161. doi: 10.1128/AAC.01445-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Naas T., Dortet L., Iorga B. I. Structural and functional aspects of class a carbapenemases. Current Drug Targets. 2016;17(9):1006–1028. doi: 10.2174/1389450117666160310144501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Giani T., Antonelli A., Caltagirone M., et al. Evolving beta-lactamase epidemiology in Enterobacteriaceae from Italian nationwide surveillance, October 2013: KPC-carbapenemase spreading among outpatients. Eurosurveillance. 2017;22(31) doi: 10.2807/1560-7917.ES.2017.22.31.30583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chavda K. D., Chen L., Jacobs M. R., Bonomo R. A., Kreiswirth B. N. Molecular diversity and plasmid analysis of KPC-producing Escherichia coli. Antimicrobial Agents and Chemotherapy. 2016;60(7):4073–4081. doi: 10.1128/AAC.00452-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Queenan A. M., Bush K. Carbapenemases: the versatile β-lactamases. Clinical Microbiology Reviews. 2007;20(3):440–458. doi: 10.1128/CMR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cantón R., Akóva M., Carmeli Y., et al. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clinical Microbiology and Infection. 2012;18(5):413–431. doi: 10.1111/j.1469-0691.2012.03821.x. [DOI] [PubMed] [Google Scholar]
- 94.Kazmierczak K. M., Rabine S., Hackel M., et al. Multiyear, multinational survey of the incidence and global distribution of metallo-β-lactamase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrobial Agents and Chemotherapy. 2016;60(2):1067–1078. doi: 10.1128/AAC.02379-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hendlin D., Stapley E. O., Jackson M., et al. Phosphonomycin, a new antibiotic produced by strains of streptomyces. Science. 1969;166(3901):122–123. doi: 10.1126/science.166.3901.122. [DOI] [PubMed] [Google Scholar]
- 96.Dijkmans A. C., Zacarías N. V. O., Burggraaf J., et al. Fosfomycin: pharmacological, clinical and future perspectives. Antibiotics. 2017;6(4) doi: 10.3390/antibiotics6040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Falagas M. E., Vouloumanou E. K., Samonis G., Vardakasa K. Z. Fosfomycin. Clinical Microbiology Reviews. 2016;29(2):321–347. doi: 10.1128/CMR.00068-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee M., Bozzo P., Einarson A., Koren G. Urinary tract infections in pregnancy. Canadian Family Physician. 2008;54(6):853–854. [PMC free article] [PubMed] [Google Scholar]
- 99.Tu Y., McCalla D. R. Effect of activated nitrofurans on DNA. BBA Section Nucleic Acids and Protein Synthesis. 1975;402(2):142–149. doi: 10.1016/0005-2787(75)90032-5. [DOI] [PubMed] [Google Scholar]
- 100.Whiteway J., Koziarz P., Veall J., et al. Oxygen-insensitive nitroreductases: Analysis of the roles of nfsA and nfsB in development of resistance to 5-nitrofuran derivatives in Escherichia coli. Journal of Bacteriology. 1998;180(21):5529–5539. doi: 10.1128/jb.180.21.5529-5539.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ho P.-L., Ng K.-Y., Lo W.-U., et al. Plasmid-mediated OqxAB is an important mechanism for nitrofurantoin resistance in Escherichia coli. Antimicrobial Agents and Chemotherapy. 2016;60(1):537–543. doi: 10.1128/AAC.02156-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kumarasamy K. K., Toleman M. A., Walsh T. R., et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. The Lancet Infectious Diseases. 2010;10(9):597–602. doi: 10.1016/S1473-3099(10)70143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bialvaei A. Z., Samadi Kafil H. Colistin, mechanisms and prevalence of resistance. Current Medical Research and Opinion. 2015;31(4):707–721. doi: 10.1185/03007995.2015.1018989. [DOI] [PubMed] [Google Scholar]
- 104.Kim Y., Bae I. K., Lee H., Jeong S. H., Yong D., Lee K. In vivo emergence of colistin resistance in Acinetobacter baumannii clinical isolates of sequence type 357 during colistin treatment. Diagnostic Microbiology and Infectious Disease. 2014;79(3):362–366. doi: 10.1016/j.diagmicrobio.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 105.Liu Y.-Y., Wang Y., Walsh T. R., et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. The Lancet Infectious Diseases. 2016;16(2):161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 106.Mao J., Liu W., Wang W., Sun J., Lei S., Feng Y. Antibiotic exposure elicits the emergence of colistin- and carbapenem-resistant Escherichia coli coharboring MCR-1 and NDM-5 in a patient. Virulence. 2018;9(1):1001–1007. doi: 10.1080/21505594.2018.1486140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cantón R., Lode H., Graninger W., Milkovich G. Respiratory tract infections: at-risk patients, who are they? Implications for their management with levofloxacin. International Journal of Antimicrobial Agents. 2006;28(2):S115–S127. doi: 10.1016/j.ijantimicag.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 108.Simon H. J., Folb P. I., Rocha H. Policies, laws, and regulations pertaining to antibiotics: report of task force 3. Clinical Infectious Diseases. 1987;9(3):p. S231. doi: 10.1093/clinids/9.Supplement_3.S261. [DOI] [PubMed] [Google Scholar]
- 109.Barker A. K., Brown K., Ahsan M., Sengupta S., Safdar N. Social determinants of antibiotic misuse: a qualitative study of community members in Haryana, India. BMC Public Health. 2017;17(1, article no. 333) doi: 10.1186/s12889-017-4261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jacoby G. A., Munoz-Price L. S. The new ß-lactamases. The New England Journal of Medicine. 2005;352(4):380–391. doi: 10.1056/NEJMra041359. [DOI] [PubMed] [Google Scholar]
- 111.Paterson D. L., Ko W.-C., Von Gottberg A., et al. International Prospective Study of Klebsiella pneumoniae Bacteremia: Implications of Extended-Spectrum β-Lactamase Production in Nosocomial Infections. Annals of Internal Medicine. 2004;140(1):26–32. doi: 10.7326/0003-4819-140-1-200401060-00008. [DOI] [PubMed] [Google Scholar]
- 112.Arcilla M. S., van Hattem J. M., Haverkate M. R., et al. Import and spread of extended-spectrum β-lactamase-producing Enterobacteriaceae by international travellers (COMBAT study): a prospective, multicentre cohort study. The Lancet Infectious Diseases. 2017;17(1):78–85. doi: 10.1016/S1473-3099(16)30319-X. [DOI] [PubMed] [Google Scholar]
- 113.Aschbacher R., Pagani E., Confalonieri M., et al. Review on colonization of residents and staff in Italian long-term care facilities by multidrug-resistant bacteria compared with other European countries. Antimicrobial Resistance and Infection Control. 2016;5:p. 33. doi: 10.1186/s13756-016-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chenoweth C. E., Gould C. V., Saint S. Diagnosis, management, and prevention of catheter-associated urinary tract infections. Infectious Disease Clinics of North America. 2014;28(1):105–119. doi: 10.1016/j.idc.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ronald A. R., Pattullo A. L. S. The natural history of urinary infection in adults. Medical Clinics of North America. 1991;75(2):299–312. doi: 10.1016/S0025-7125(16)30455-2. [DOI] [PubMed] [Google Scholar]
- 116.Cardenas D. D., Hooton T. M. Urinary tract infection in persons with spinal cord injury. Archives of Physical Medicine and Rehabilitation. 1995;76(3):272–280. doi: 10.1016/S0003-9993(95)80615-6. [DOI] [PubMed] [Google Scholar]
- 117.Lee D. S., Choe H., Kim H. Y., et al. Acute bacterial prostatitis and abscess formation. BMC Urology. 2016;16(1):p. 38. doi: 10.1186/s12894-016-0153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gao Y., Danforth T., Ginsberg D. A. Urologic Management and Complications in Spinal Cord Injury Patients: A 40- to 50-year Follow-up Study. Urology. 2017;104:52–58. doi: 10.1016/j.urology.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 119.Concia E., Bragantini D., Mazzaferri F. Clinical evaluation of guidelines and therapeutic approaches in multi drug-resistant urinary tract infections. Journal of Chemotherapy. 2017;29(1):19–28. doi: 10.1080/1120009X.2017.1380397. [DOI] [PubMed] [Google Scholar]
- 120.Kunin C. M., White L. V., Tong Hua Hua A reassessment of the importance of 'low-count' bacteriuria in young women with acute urinary symptoms. Annals of Internal Medicine. 1993;119(6):454–460. doi: 10.7326/0003-4819-119-6-199309150-00002. [DOI] [PubMed] [Google Scholar]
- 121.Franz M., Hörl W. H. Common errors in diagnosis and management of urinary tract infection. II: Clinical management. Nephrology Dialysis Transplantation . 1999;14(11):2754–2762. doi: 10.1093/ndt/14.11.2754. [DOI] [PubMed] [Google Scholar]
- 122.Melzer M., Petersen I. Mortality following bacteraemic infection caused by extended spectrum beta-lactamase (ESBL) producing E. coli compared to non-ESBL producing E. coli. Infection. 2007;55(3):254–259. doi: 10.1016/j.jinf.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 123.Esparcia A., Artero A., Eiros J. M., et al. Influence of inadequate antimicrobial therapy on prognosis in elderly patients with severe urinary tract infections. European Journal of Internal Medicine. 2014;25(6):523–527. doi: 10.1016/j.ejim.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 124.Chalhoub H., Tunney M., Stuart Elborn J., et al. Avibactam confers susceptibility to a large proportion of ceftazidimeresistant Pseudomonas aeruginosa isolates recovered from cystic fibrosis patients. Journal of Antimicrobial Chemotherapy. 2014;70(5):1596–1598. doi: 10.1093/jac/dku551.dku551 [DOI] [PubMed] [Google Scholar]
- 125.Sader H. S., Castanheira M., Mendes R. E., Flamm R. K., Farrell D. J., Jones R. N. Ceftazidime-avibactam activity against multidrug-resistant Pseudomonas aeruginosa isolated in U.S. Medical Centers in 2012 and 2013. Antimicrobial Agents and Chemotherapy. 2015;59(6):3656–3659. doi: 10.1128/AAC.05024-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sader H. S., Farrell D. J., Flamm R. K., Jones R. N. Ceftolozane/tazobactam activity tested against aerobic Gram-negative organisms isolated from intra-abdominal and urinary tract infections in European and United States hospitals (2012) Infection. 2014;69(3):266–277. doi: 10.1016/j.jinf.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 127.Hawkey P. M., Warren R. E., Livermore D. M., et al. Treatment of infections caused by multidrug-resistant gram-negative bacteria: Report of the British society for antimicrobial chemotherapy/healthcare infection society/british infection association joint working party. Journal of Antimicrobial Chemotherapy. 2018;73(3):iii2–iii78. doi: 10.1093/jac/dky027. [DOI] [PubMed] [Google Scholar]
- 128.Jean S.-S., Lee W.-S., Lam C., Hsu C.-W., Chen R.-J., Hsueh P.-R. Carbapenemase-producing Gram-negative bacteria: Current epidemics, antimicrobial susceptibility and treatment options. Future Microbiology. 2015;10(3):407–425. doi: 10.2217/FMB.14.135. doi: 10.2217/FMB.14.135. [DOI] [PubMed] [Google Scholar]
- 129.Skippen I., Shemko M., Turton J., Kaufmann M. E., Palmer C., Shetty N. Epidemiology of infections caused by extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella spp.: a nested case-control study from a tertiary hospital in London. Journal of Hospital Infection. 2006;64(2):115–123. doi: 10.1016/j.jhin.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 130.Stapleton P. J., Lundon D. J., McWade R., et al. Antibiotic resistance patterns of Escherichia coli urinary isolates and comparison with antibiotic consumption data over 10 years, 2005–2014. Irish Journal of Medical Science. 2017;186(3):733–741. doi: 10.1007/s11845-016-1538-z. [DOI] [PubMed] [Google Scholar]
- 131.Paterson D. L. “Collateral damage” from cephalosporin or quinolone antibiotic therapy. Clinical Infectious Diseases. 2004;38(4):S341–S345. doi: 10.1086/382690. [DOI] [PubMed] [Google Scholar]
- 132.Cassier P., Lallechère S., Aho S., et al. Cephalosporin and fluoroquinolone combinations are highly associated with CTX-M β-lactamase-producing Escherichia coli: A case-control study in a French teaching hospital. Clinical Microbiology and Infection. 2011;17(11):1746–1751. doi: 10.1111/j.1469-0691.2010.03349.x. [DOI] [PubMed] [Google Scholar]
- 133.Grabe M., Bartoletti R., Bjerklund-Johansen T. E., et al. European association of urology (EAU) guidelines office. Guidelines on Urological Infections. 2015:15–16. [Google Scholar]
- 134.Abbo L. M., Hooton T. M. Antimicrobial stewardship and urinary tract infections. Antibiotics. 2014;3(2):174–192. doi: 10.3390/antibiotics3020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sammon J. D., Sharma P., Rahbar H., et al. Predictors of admission in patients presenting to the emergency department with urinary tract infection. World Journal of Urology. 2014;32(3):813–819. doi: 10.1007/s00345-013-1167-3. [DOI] [PubMed] [Google Scholar]
- 136.Brown P., Ki M., Foxman B. Acute pyelonephritis among adults: Cost of illness and considerations for the economic evaluation of therapy. PharmacoEconomics. 2005;23(11):1123–1142. doi: 10.2165/00019053-200523110-00005. [DOI] [PubMed] [Google Scholar]
- 137.Svanborg C., Godaly G. Bacterial virulence in urinary tract infection. Infectious Disease Clinics of North America. 1997;11(3):513–529. doi: 10.1016/S0891-5520(05)70371-8. [DOI] [PubMed] [Google Scholar]
- 138.Biering-Sørensen F., Bagi P., Hoiby N. Urinary tract infections in patients with spinal cord lesions: Treatment and prevention. Drugs. 2001;61(9):1275–1287. doi: 10.2165/00003495-200161090-00004. [DOI] [PubMed] [Google Scholar]
- 139.Stamm W. E., Raz R. Factors contributing to susceptibility of postmenopausal women to recurrent urinary tract infections. Clinical Infectious Diseases. 1999;28(4):723–725. doi: 10.1086/515209. [DOI] [PubMed] [Google Scholar]
- 140.Beerepoot M. A. J., Geerlings S. E., Van Haarst E. P., Mensing Van Charante N., Ter Riet G. Nonantibiotic prophylaxis for recurrent urinary tract infections: A systematic review and meta-analysis of randomized controlled trials. The Journal of Urology. 2013;190(6):1981–1989. doi: 10.1016/j.juro.2013.04.142. [DOI] [PubMed] [Google Scholar]
- 141.Schwenger E. M., Tejani A. M., Loewen P. S. Probiotics for preventing urinary tract infections in adults and children. Cochrane Database of Systematic Reviews. 2015;(12):p. CD008772. doi: 10.1002/14651858.CD008772.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kontiokari T., Sundqvist K., Nuutinen M., Pokka T., Koskela M., Uhari M. Randomised trial of cranberry-lingonberry juice and Lactobacillus GG drink for the prevention of urinary tract infections in women. BMJ. 2001;322(7302):p. 1571. doi: 10.1136/bmj.322.7302.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kranjčec B., Papeš D., Altarac S. D-mannose powder for prophylaxis of recurrent urinary tract infections in women: A randomized clinical trial. World Journal of Urology. 2014;32(1):79–84. doi: 10.1007/s00345-013-1091-6. [DOI] [PubMed] [Google Scholar]
- 144.Raz R., Stamm W. E. A Controlled Trial of Intravaginal Estriol in Postmenopausal Women with Recurrent Urinary Tract Infections. The New England Journal of Medicine. 1993;329(11):753–756. doi: 10.1056/NEJM199309093291102. [DOI] [PubMed] [Google Scholar]
- 145.Bonkat G., Pickard R., Bartoletti R., et al. European association of urology (EAU) guidelines office. Guidelines on Urological Infections. 2017:15–16. [Google Scholar]
- 146.Wybran J., Libin M., Schandene L. Activation of natural killer cells and cytokine production in man by bacterial extracts. Immunopharmacology and Immunotoxicology. 1989;11(1):17–32. doi: 10.3109/08923978909082140. [DOI] [PubMed] [Google Scholar]
- 147.Van Pham T., Kreis B., Corradin-Betz S., Bauer J., Mauel J. Metabolic and functional stimulation of lymphocytes and macrophages by an Escherichia coli extract (OM-89): in vitro studies. Journal of Biological Response Modifiers. 1990;9(2):231–240. [PubMed] [Google Scholar]
- 148.Bosch A., Benedi V. J., Pares R., Jofre J. Enhancement of the humoral immune response and resistance to bacterial infection in mice by the oral administration of a bacterial immunomodulator (OM-89) Immunopharmacology and Immunotoxicology. 1988;10(3):333–343. doi: 10.3109/08923978809041425. [DOI] [PubMed] [Google Scholar]
- 149.Bauer H. W., Rahlfs V. W., Lauener P. A., Bleßmann G. S. S. Prevention of recurrent urinary tract infections with immuno-active E. coli fractions: A meta-analysis of five placebo-controlled double-blind studies. International Journal of Antimicrobial Agents. 2002;19(6):451–456. doi: 10.1016/S0924-8579(02)00106-1. [DOI] [PubMed] [Google Scholar]
- 150.Naber K. G., Cho Y.-H., Matsumoto T., Schaeffer A. J. Immunoactive prophylaxis of recurrent urinary tract infections: a meta-analysis. International Journal of Antimicrobial Agents. 2009;33(2):111–119. doi: 10.1016/j.ijantimicag.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 151.Bauer H. W., Alloussi S., Egger G., Blümlein H., Cozma G., Schulman C. C. A Long-Term, multicenter, double-blind study of an Escherichia Coli extract (OM-89) in female patients with recurrent urinary tract infections. European Urology. 2005;47(4):542–548. doi: 10.1016/j.eururo.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 152.Muzzi-Bjornson L., Macera L. Preventing infection in elders with long-term indwelling urinary catheters. Journal of the American Association of Nurse Practitioners. 2011;23(3):127–134. doi: 10.1111/j.1745-7599.2010.00588.x. [DOI] [PubMed] [Google Scholar]
- 153.Bonkat G., Pickard R., Bartoletti R., et al. European association of urology (EAU) guidelines office. Guidelines on Urological Infections. 2017:8–9. [Google Scholar]
- 154.Nicolle L. E., Bradley S., Colgan R., Rice J. C., Schaeffer A., Hooton T. M. Infectious diseases society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clinical Infectious Diseases. 2005;40(5):643–654. doi: 10.1086/427507. [DOI] [PubMed] [Google Scholar]
- 155.Tencer J. Asymptomatic bacteriuria - A long-term study. Scandinavian Journal of Urology. 1988;22(1):31–34. doi: 10.1080/00365599.1988.11690380. [DOI] [PubMed] [Google Scholar]
- 156.Lee M. J., Kim M., Kim N.-H., et al. Why is asymptomatic bacteriuria overtreated?: A tertiary care institutional survey of resident physicians. BMC Infectious Diseases. 2015;15:p. 289. doi: 10.1186/s12879-015-1044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cooper F. P., Alexander C. E., Sinha S., Omar M. I. Policies for replacing long-term indwelling urinary catheters in adults. Cochrane Database of Systematic Reviews. 2016;7 doi: 10.1002/14651858.CD011115.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kazemier B. M., Koningstein F. N., Schneeberger C., et al. Maternal and neonatal consequences of treated and untreated asymptomatic bacteriuria in pregnancy: A prospective cohort study with an embedded randomised controlled trial. The Lancet Infectious Diseases. 2015;15(11):1324–1333. doi: 10.1016/S1473-3099(15)00070-5. [DOI] [PubMed] [Google Scholar]
- 159.Zhanel G. G., Harding G. K. M., Nicolle L. E. Asymptomatic bacteriuria in patients with diabetes mellitus. Reviews of Infectious Diseases. 1990;13(1):150–154. doi: 10.1093/clinids/12.5.150. [DOI] [PubMed] [Google Scholar]
- 160.Fünfstück R., Nicolle L. E., Hanefeld M., Naber K. G. Urinary tract infection in patients with diabetes mellitus. Clinical Nephrology. 2012;77(1):40–48. doi: 10.5414/CN107216. [DOI] [PubMed] [Google Scholar]
- 161.Vik I., Bollestad M., Grude N., et al. Ibuprofen versus mecillinam for uncomplicated cystitis - a randomized controlled trial study protocol. BMC Infectious Diseases. 2014;14:p. 693. doi: 10.1186/s12879-014-0693-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Little P., Moore M. V., Turner S., et al. Effectiveness of five different approaches in management of urinary tract infection: Randomised controlled trial. BMJ. 2010;340:p. c199. doi: 10.1136/bmj.c199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Dryden M. S., Cooke J., Davey P. Antibiotic stewardship—more education and regulation not more availability? Journal of Antimicrobial Chemotherapy. 2009;64(5):885–888. doi: 10.1093/jac/dkp305. [DOI] [PubMed] [Google Scholar]
- 164.Allerberger F., Gareis R., Jindrák V., Struelens M. J. Antibiotic stewardship implementation in the EU: The way forward. Expert Review of Anti-infective Therapy. 2009;7(10):1175–1183. doi: 10.1586/ERI.09.96. [DOI] [PubMed] [Google Scholar]
- 165.Cefai C., Charani E., Craven L., et al. Guideline: Antimicrobial stewardship: systems and processes for effective antimicrobial medicine use, 2015.
- 166.Esteve-Palau E., Grau S., Herrera S., et al. Impact of an antimicrobial stewardship program on urinary tract infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Revista Espanola De Quimioterapia. 2018 [PMC free article] [PubMed] [Google Scholar]
- 167.Bartoletti R., Cai T., Wagenlehner F. M., Naber K., Bjerklund Johansen T. E. Treatment of Urinary Tract Infections and Antibiotic Stewardship. European Urology Supplements. 2016;15(4):81–87. doi: 10.1016/j.eursup.2016.04.003. [DOI] [Google Scholar]