Abstract
Barth Syndrome (BTHS) is an X-linked recessive disorder characterized by cardiomyopathy and muscle weakness. The underlying cause of BTHS is a mutation in the tafazzin (TAZ) gene, a key enzyme of cardiolipin biosynthesis. The lack of CL arising from loss of TAZ function results in destabilization of the electron transport system, promoting oxidative stress that is thought to contribute to development of cardioskeletal myopathy. Indeed, in vitro studies demonstrate that mitochondria-targeted antioxidants improve contractile capacity in TAZ-deficient cardiomyocytes. The purpose of the present study was to determine if resolving mitochondrial oxidative stress would be sufficient to prevent cardiomyopathy and skeletal myopathy in vivo using a mouse model of BTHS. To this end we crossed mice that overexpress catalase in the mitochondria (MCAT mice) with TAZ-deficient mice (TAZKD) to produce TAZKD mice that selectively overexpress catalase in the mitochondria (TAZKD+MCAT mice). TAZKD+MCAT mice exhibited decreased mitochondrial H2O2 emission and lipid peroxidation compared to TAZKD littermates, indicating decreased oxidative stress. Despite the improvements in oxidative stress, TAZKD+MCAT mice developed cardiomyopathy and mild muscle weakness similar to TAZKD littermates. These findings indicate that resolving oxidative stress is not sufficient to suppress cardioskeletal myopathy associated with BTHS.
Keywords: Barth Syndrome, cardiomyopathy, oxidative stress, mitochondria, reactive oxygen species
1. Introduction
Barth Syndrome (BTHS) is a devastating human disease caused by heritable mutations in the tafazzin gene (TAZ) [1, 2]. Two major hallmarks of BTHS are cardiomyopathy and skeletal myopathy [3–5]. Currently, there is no treatment specific to BTHS making abbreviated lifespan and low quality of life inevitable for those afflicted. [6] TAZ is a phospholipid transacylase that catalyzes the final step in the biosynthesis of the mitochondrial phospholipid cardiolipin [7, 8]. CL is vital to the stability of the inner mitochondrial membrane and the complexes of the electron transport system (ETS) [9–11]. The lack of TAZ causes a loss of CL and accumulation of monolysocardiolipin (MLCL), which is unable to bind ETS complexes [12, 13]. The resulting CL deficiency impairs oxidative phosphorylation and increases mitochondrial reactive oxygen species (ROS) production [14]. The elevated ROS and subsequent oxidative stress are implicated in cardioskeletalmyopathy observed in BTHS [15, 16]. Published studies have demonstrated that treating TAZ-deficient cardiomyocytes with mitochondria-targeted antioxidants is sufficient to improve contractile qualities and sarcomere organization [17, 18]. This suggests that decreasing mitochondrial induced oxidative damage could potentially ameliorate phenotypes associated with BTHS. The purpose of the present study was to assess whether resolving oxidative stress could prevent cardiomyopathy and skeletal myopathy in a murine model of BTHS. To test this hypothesis TAZ-deficient mice (TAZKD) were crossed with mice overexpressing mitochondrial-targeted catalase (MCAT) to produce a TAZKD model with elevated mitochondrial antioxidant activity (TAZKD+MCAT). TAZKD mice mimic the phenotypes observed in BTHS, including higher levels of mitochondrial ROS production [19, 20]. The MCAT mouse is a well-validated model that exhibits decreased mitochondria-induced oxidative stress in conditions such as pathological aging, obesity, and others [21–24]. Here we show that TAZKD+MCAT mice have decreased H2O2 emission and lipid peroxidation in cardiac and skeletal muscle, but this is not sufficient to prevent cardiomyopathy or skeletal muscle weakness.
2. Materials and Methods
2.1. Animals
Heterozygous TAZKD mice were obtained from the Jackson Laboratory (Stock number 014648). Heterozygous MCAT mice were kindly donated for this study by the laboratory of Dr. Peter Rabinovitch. TAZ knockdown was induced in utero by supplying 625 mg/kg doxycycline chow (Envigo, TD.09628) as previously described [25]. Briefly, female TAZKD or MCAT mice were maintained on doxycycline chow (625 mg/kg) at least 5 days before being mated with male TAZKD or MCAT mice. The doxycycline diet was removed during the mating period, and following copulation, males were removed and the doxycycline diet was reintroduced for the duration of gestation. Following weaning all offspring were maintained on the doxycycline diet for the remainder of the study. Male mice were used in experiments as BTHS almost exclusively affects males. Mice were maintained in a temperature controlled room on a 12-hour light/dark cycle. For terminal experiments mice were given intraperitoneal injection of 80 mg/kg ketamine and 10 mg/kg xylazine, after which tissues were harvested. All animal procedures used in this study were approved by Institutional Animal Care and Use Committee at East Carolina University and University of Utah.
2.2. Metabolic Phenotyping
Body composition in mice was measured using an EchoMRI™ 700 Analyzer. Whole-body VO2, RER, activity levels were assessed using a 16-cage TSE-Systems PhenoMaster/LabMaster rodent calorimetry system.
2.3. High-resolution Respirometry and Fluorometry
Mitochondrial O2 utilization was measured using the Oroboros O2K Oxygraphs. Cardiac or skeletal muscle were minced in mitochondrial isolation medium (300 mM sucrose, 10 mM HEPES, 1 mM EGTA) and subsequently homogenized using a Teflon-glass system. Homogenates were then centrifuged at 800 x g for 10 minutes, after which the supernatant was taken and centrifuged at 12,000 x g for 10 minutes. The resulting pellet was carefully resuspended in mitochondrial isolation medium. Isolated mitochondria were then added to the oxygraph chambers containing assay buffer (MES potassium salt 105 mM, KCl 30 mM, KH2PO4 10 mM, MgCl2 5 mM, BSA 0.5 mg/ml). Respiration was measured in response to the following substrates: 0.5 mM malate, 5 mM pyruvate, 5 mM glutamate, 10 mM succinate, 1.5 μM FCCP, 0.02 mM palmitoyl-carnitine, 5 mM L-carnitine. ATP production was measured fluorometrically using a Horiba Fluoromax 3 (Horiba Scientific), by enzymatically coupling ATP production to NADPH synthesis as previously described [26]. Respiration and ATP production were measured in the presence of 2 mM ADP.
2.4. MALDI-TOF Mass Spectrometry
Levels of CL and MLCL were detected by adapting a previously described technique using MALDI-TOF mass spectrometry [27]. Lipids were extracted from isolated mitochondria by adding 200 μg of mitochondrial protein to a mixture of methanol and chloroform and then vortexed for 45 seconds. 0.88% KCl was then added to the sample mixture, and samples were centrifuged at 800 x g for 10 minutes to separate the aqueous and organic phases. The organic phase was removed and subsequently dried under a steady stream of N2. The dried lipids were subsequently reconstituted in chloroform. 10mg/ml 9-aminoacridine in 60:40 isopropanol: acetonitrile was used as the matrix. The matrix was added to the reconstituted samples in a 1:1 ratio. Matrix by itself was added to an MTP AnchorChip 384 well plate, and the sample matrix mixture was then added onto a layer of dried matrix. After drying, an additional layer of matrix was added to the wells. The sample plate was then analyzed using a Bruker Autoflex Speed MALDITOF system in negative ionization reflectron mode with 5,000 spectra collected per sample.
2.5. H2O2 Measurements
Mitochondrial H2O2 emission was determined in isolated mitochondria from cardiac and skeletal muscle. Briefly, isolated mitochondria were added to 1 ml of assay buffer containing Amplex Ultra Red, which produces a fluorescent product when oxidized by H2O2. H2O2 emission was measured following the addition of 0.5 M succinate for a final concentration of 10 mM. The appearance of the fluorescent product was measured by a Horiba Fluoromax 3 fluorometer (excitation/emission 565/600).
2.6. Echocardiography
Echocardiographic measurements were made following a standard protocol [28]. Briefly, mice were anesthetized with a 0.5–2% isoflurane in an oxygen mix and kept on a heated plate to maintain body temperature. Heart rate was kept between 400–500 bpm for all measurements taken to ensure physiological relevance. The Vevo 2100™ High-Resolution In Vivo Imaging System (VisualSonics) was used with a 30-MHz transducer to take echocardiographic recordings in both B-mode and M-mode. M-mode recordings from transthoracic short-axis view were used to measure the thickness of left ventricular walls, and also to measure left ventricular interior diameter. From these measures fractional shortening (FS) was calculated. B-mode recordings from transthoracic long-axis view were used to measure left ventricular volume during diastole and systole. These measures were used to calculate ejection fraction (EF), stroke volume and cardiac output.
2.7. Cardiac Histology
A transverse section of frozen hearts was taken and fixed in 10% neutral formalin for 24 hours at room temperature. After 24 hours hearts were moved to tubes containing fresh fixative and fixed for an additional 72 hours at room temperature. Following fixation, hearts were suspended in 70% ethanol for 24 hours at 4˚ C. Tissues were then embedded in paraffin, and 5 μm transverse sections were taken and stained with Masson’s trichrome stain to detect collagen as an assessment of fibrosis. Stained sections were imaged using an Axio Scan Z.1 (Zeiss).
2.8. Assessments of Muscle Contractility
Maximal force production was assessed in soleus muscles using an ex vivo small animal muscle contraction apparatus (Aurora Scientific). We modified an existing protocol for ex vivo muscle contractions to acquire force frequency curves for the soleus [29]. Briefly, soleus muscles were carefully excised from live unconscious mice and attached to the anchor and force transducer of the apparatus and submerged in Krebs-Henseliet buffer (118 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.25 mM CaCl2, 1.2 mM KH2PO4, 25 mM NaHCO3, 11 mM glucose). Muscles were then set at an optimal length for maximal contraction and then stimulated at increasing frequencies until maximal force production was achieved.
2.9. Western Blotting
Frozen muscles or cardiac tissue were homogenized in lysis buffer and subsequently centrifuged at 12,000 x g for 10 minutes. Mitochondria isolated for previous experiments was used for Western blotting. Sample protein content was determined using the BCA assay from Pierce Scientific. Equal amounts of protein were then mixed with Laemmeli Sample Buffer and loaded onto 4–15% gradient gels (Bio-Rad). Proteins were then transferred onto nitrocellulose membranes and blocked (5% BSA in TBST) and subsequently treated with primary antibodies for catalase (ABCAM ab1877), 4-HNE (ABCAM ab48506), cleaved caspase 3 (Cell Signaling D175), cleaved caspase 6 (Cell Signaling D162), citrate synthase (ABCAM ab96600), or Total OXPHOS Rodent Cocktail (ABCAM ab110413). Following incubation, blots were washed in TBST, incubated in appropriate secondary antibodies and washed in TBST before imaging using a FluorChem E imager (ProteinSimple).
2.10. RNA/DNA isolation and quantitative PCR
RNA was isolated from frozen cardiac or skeletal muscle tissue using the TRIzol reagent. Frozen tissue was homogenized in TRIzol, after which 200 μl of chloroform was added. Following centrifugation at 12,000 rpm, the supernatant was taken and added to 500 μl of isopropanol to precipitate RNA. RNA was then pelleted by centrifugation at 12,000 rpm and subsequently washed with 75% ethanol. RNA pellets were resuspended in nuclease free Tris-EDTA buffer and quantified via spectrophotometry. cDNA was synthesized using an iSCRIPT cDNA Synthesis Kit (Bio-Rad). Genomic DNA for assessments of mitochondrial DNA (mtDNA) was isolated using a commercially available kit according to the manufacturer’s instructions (Qiagen 69504). Genomic DNA or cDNA was added to a mixture of SYBR Green (Thermo Fisher Scientific) and primers. The sample mixtures were pipetted onto a 384 plate and analyzed with a QuantStudio 12K Flex (Life Technologies). The following primers were used in this study: TAZ fwd, CCC-TCC-ATG-TGA-AGT-GGC-CAT-TCC; TAZ rev, GGT-GGT-TGG-AGA-CGGTGA-TAA-GG; NDUFA12 fwd, AGG-ATT-GGT-ACA-CTG-GTG-GG; NDUFA12 rev, CGG-TGGTGT-AGA-TGA-CCC-AT; SDHD fwd, CAT-GGC-GGT-TCT-CTT-AAA-GC; SDHD rev, TGA-CACATA-AGC-GGG-TCG-A; COXIV fwd, GAG-CCT-GAT-TGG-CAA-GAG-AG; COXIV rev, GATCAG-CGT-AAG-TGG-GGA-AA. mtDNA fwd, TTAAGA-CAC-CTT-GCC-TAG-CCA-CAC; mtDNA rev, CGG-TGG-CTG-GCA-CGA-AAT-T; nucDNA fwd, ATG-ACG-ATA-TCG-CTG-CGC-TG; nucDNA rev, TCA-CTT-ACC-TGGTGC-CTA-GGG-C.
2.11. Statistical Analysis
Statistical analysis was performed using Prism 7 software (GraphPad). Two-way ANOVAs followed by Sidak’s multiple comparisons were used to test statistical significance between the groups.
3. Results
3.1. Mouse Phenotyping
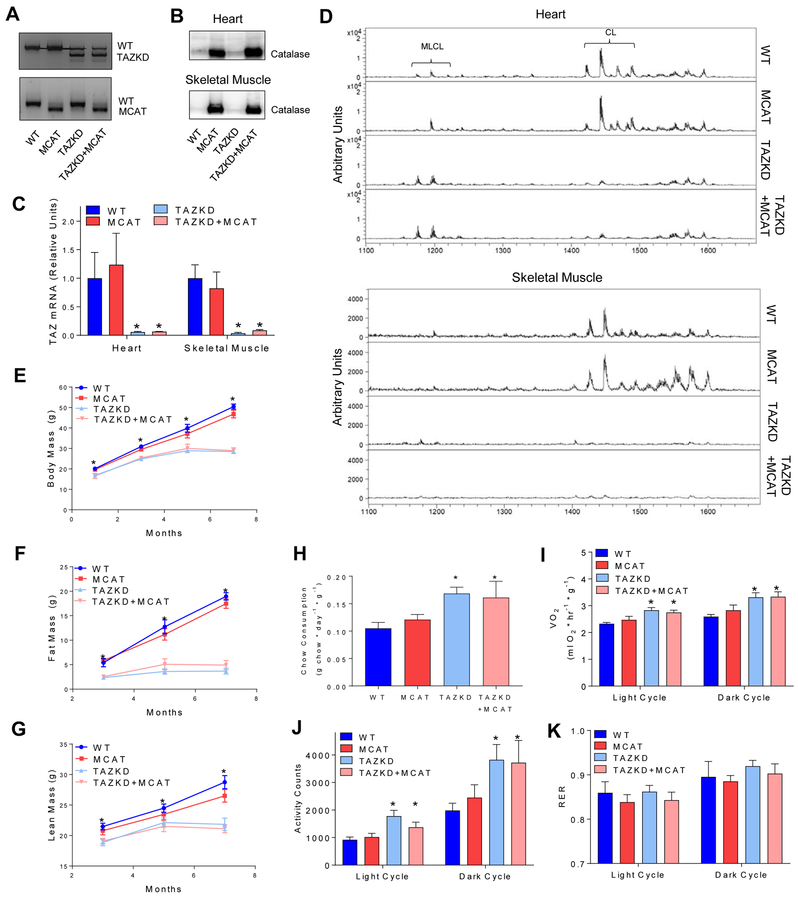
Experimental groups: C57BL6/J (WT), MCAT, TAZKD, and TAZKD+MCAT. Knockdown of TAZ was achieved as previously described using 625 mg/kg doxycycline chow [25]. Genotypes were confirmed using genomic PCR (Figure 1A), western blotting for catalase in the mitochondria (Figure 1B), and TAZ gene expression (Figure 1C). TAZ knockdown was sufficient to dramatically reduce CL levels and increase MLCL in heart and skeletal muscle in both TAZKD and TAZKD+MCAT mice (Figure 1D). Abundance of CL species was not affected by the transgenic expression of catalase in TAZKD mice. WT and MCAT mice both gained weight which was likely due to hyperphagic response to doxycycline [30] (Figure 1E). Consistent with previous reports, both TAZKD and TAZKD+MCAT mice had lower fat and lean mass compared to WT and MCAT controls (Figure 1F, G), even though they consumed more food than WT and MCAT mice [19] (Figure 1H). The differences in body weight likely come from elevated energy expenditure and activity levels in TAZKD mice (Figure 1I–K). Importantly, MCAT overexpression did not affect these parameters with or without TAZKD.
Figure 1:
A) PCR results confirming the genotype of experimental mice. B) Western blot confirming the oxerexpression of catalase in mitochondria isolated from cardiac and skeletal muscle. n = 4 per group. C) TAZ mRNA levels in heart and skeletal muscle as assessed by quantitative real-time PCR (qPCR). n = 4–5 per group. D) MALDI-TOF mass spectrum of CL and MLCL in lipids extracted from isolated cardiac or skeletal muscle mitochondria. n = 4 per group. E) Body mass (F), Fat mass (G), and Lean mass were assessed throughout the lifespan of the mice. n = 5–7 per group. H) Daily chow consumption. n = 4–8 per group. I) Whole-body VO2 measured via metabolic cages for 48 hours J) Activity counts measured over 48 hours in metabolic cages. K) Respiratory Exchange Ratio (RER) measured over 48 hours in metabolic cages. n = 4–8 per group. *: Main effect of TAZKD, p < 0.05.
3.2. Overexpression of Catalase Targeted to the Mitochondria Decreases Oxidative Stress in TAZKD Mice
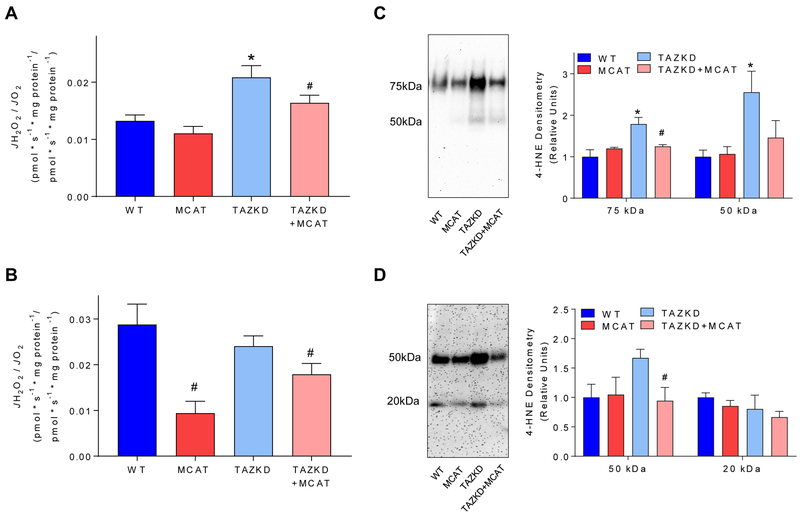
We sought to determine if MCAT overexpression was sufficient to reduce levels of oxidative stress in TAZ-deficient cardiac and skeletal muscles. Indeed, MCAT overexpression suppressed the rate H2O2 production relative to O2 consumption in both cardiac and skeletal muscle mitochondria (Figure 2A, B). Reduction in oxidative stress was also confirmed by a robust normalization of 4-hydroxynonenal (4-HNE) staining (Figure 2C, D), suggesting that lipid peroxidation induced by TAZKD was completely suppressed in the presence of MCAT.
Figure 2:
A) Mitochondrial H2O2 production relative to O2 consumption in mitochondria isolated from cardiac tissue in response to 10 mM succinate. n = 4–7 per group. B) Mitochondrial H2O2 production relative to O2 consumption in mitochondria isolated from skeletal muscle in response to 10 mM succinate. n = 4 per group. C) Western blot of 4-hydroxynonenal (4-HNE), an aldehyde product of lipid peroxidation, in cardiac tissue. n = 5 per group. D) 4-HNE western blot in skeletal muscle. n = 4 per group. H2O2 measurements and assessments of 4-HNE were made in 7–8 month old mice. *: Effect of TAZKD by multiple comparisons test. #: Effect of MCAT by multiple comparisons test. p < 0.05.
3.3. TAZKD+MCAT Mice are not Protected from Cardioskeletal Myopathy
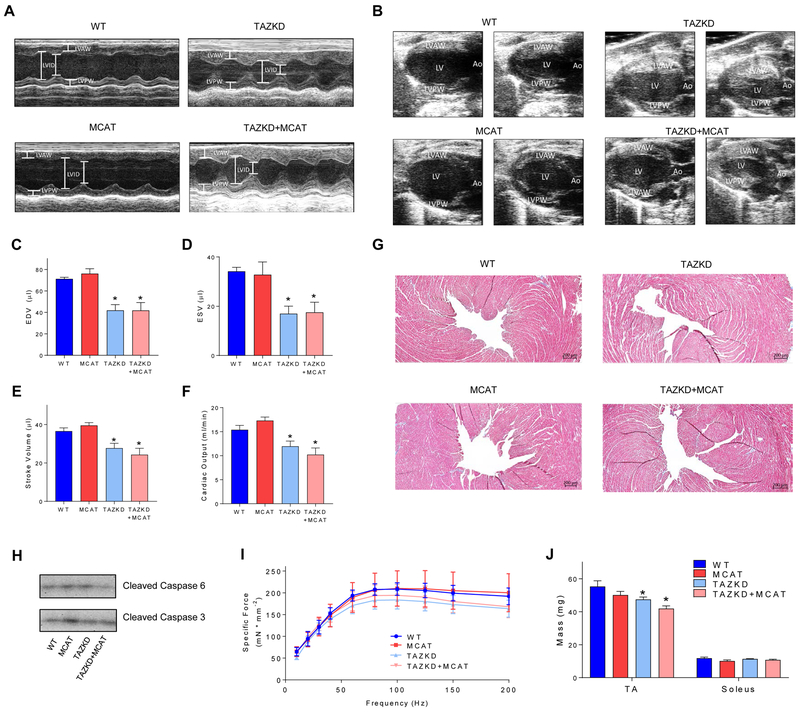
TAZKD mice develop cardiomyopathy by 7–8 months of age [25, 31], we therefore made all cardiac assessments in 7–8 month old mice. Heart weight normalized to body weight was greater by 30% and 26% in TAZKD and TAZKD+MCAT mice, respectively, compared with WT mice (Table 1). Cardiac function was assessed via echocardiography. Heart rate (HR) was not different among the groups. TAZKD and TAZKD+MCAT mice both developed hypertrophic cardiomyopathy, represented by thicker left ventricular anterior wall (LVAW) and left ventricular posterior wall (LVPW) in TAZKD or TAZKD+MCAT mice compared to WT or MCAT mice (Table 1, Figure 3A). EF and FS [20] were both greater in TAZKD or TAZKD+MCAT mice compared with WT or MCAT mice. Left ventricular volume was lower by nearly 50% during both systole and diastole in TAZKD or TAZKD+MCAT mice compared to WT or MCAT mice (Figure 3B–D). These defects led to 23% and 25% lower stroke volume and cardiac output in TAZKD mice, with similar defects present in TAZKD+MCAT mice (Figure 3E&F). These findings are consistent with previously published models of hypertrophic cardiomyopathy [32]. Neither TAZKD or TAZKD+MCAT hearts showed evidence of fibrosis or cell-death (Figure 3G and H).
Table 1. Echocardiographic assessments were made in 7–8 month old mice. Measurements of the cardiac parameters were measured in short axis view using M-mode.
All values are mean ± standard error. Asterisks indicate a significant main effect of TAZ knockdown. HW, heart weight; HW/BW, heart weight/body weight; HR, heart rate; LVID;d, left ventricular interior diameter at diastole; LVID;s, left ventricular interior diameter at systole; LVAW;d, left ventricular anterior wall thickness at diastole; LVAW;s, left ventricular anterior wall thickness at systole; LVPW;d, left ventricular posterior wall thickness at diastole; LVPW;s, left ventricular posterior wall thickness at diastole; EF, ejection fraction; FS, fractional shortening. n = 4–7 per group.
| WT | MCAT | TAZKD | TAZKD+MCAT | |
|---|---|---|---|---|
| HW | 127.2 ±3.53 | 132.95 ±7.17 | 116.41 ±4.30* | 108.42 ±2.72* |
| HW/BW (mg/g) | 2.86 ±0.20 | 2.95 ±0.12 | 4.06 ±0.17* | 3.88 ±0.21* |
| HR (bpm) | 438.51 ± 11.10 | 423.33 ± 10.60 | 431.80 ± 12.63 | 420.27 ±4.66 |
| LVID;d (mm) | 4.30 ±0.08 | 4.25 ±0.12 | 3.41 ±0.14* | 3.11 ±0.21* |
| LVID;s (mm) | 3.01 ±0.19 | 3.05 ±0.12 | 2.04 ±0.20* | 1.70 ±0.21* |
| LVAW;d (mm) | 1.18 ±0.08 | 1.14±0.05 | 1.34 ±0.05* | 1.53 ±0.17* |
| LVAW;s (mm) | 1.54±0.11 | 1.53 ±0.11 | 1.89 ±0.06* | 2.14±0.13* |
| LVPW;d (mm) | 0.84 ±0.07 | 0.84 ±0.04 | 1.21 ±0.08* | 1.48 ±0.12* |
| LVPW;s (mm) | 1.23 ± 0.08 | 1.21 ±0.08 | 1.74 ±0.10* | 1.85 ±0.15* |
| EF (%) | 51.16± 1.63 | 54.52 ±3.17 | 72.06 ±4.42* | 76.96 ±6.13* |
| FS (%) | 25.91 ± 1.02 | 28.18 ±2.08 | 41.10±3.41* | 45.45 ±5.34* |
indicates a main effect of genotype, p < 0.05.
Figure 3:
A) Echocardiographic images of the left ventricle viewed in short-axis view using M-mode. B) Echocardiographic images of the left ventricle viewed in long-axis view using B-mode. C) End diastolic volume (EDV). D) End systolic volume (ESV). E) Stroke volume. F) Cardiac output. Echocardiograms were performed on anesthetized 7–8 month old mice. Anesthetized heart rate was maintained above 400 bpm for physiological relevance. n = 4–7 per group. G) Histological analysis of myocardium surrounding the left ventricle. 5 μm sections were stained with Masson’s trichrome stain to detect fibrosis. Collagen is stained blue, while nuclei are stained dark purple and cytoplasm pink/red. H) Western blot for apoptotic proteins cleaved caspase 3 and 6. n = 4 per group. I) Force frequency curves for soleus muscles contracted ex vivo using commercially available small animal contractile apparatus (Aurora Scientific). n = 5 per group. J) Muscle mass of the soleus and tibialis anterior (TA). *: Main effect of TAZKD, p < 0.05.
To assess skeletal muscle function, we stimulated soleus muscles at increasing frequencies until maximal force was produced. Soleus muscles from TAZKD mice tended to have decreased force production at higher frequencies, which was not rescued by MCAT overexpression (Figure 3I). Consistent with body composition data, muscle mass for tibialis anterior (TA) was lower in TAZKD or TAZKD+MCAT mice compared with WT or MCAT mice, though this difference was not observed in soleus muscles (Figure 3J).
3.4. Mitochondrial Respiration and Protein Abundance of ETS Complexes is Decreased in TAZKD and TAZKD+MCAT Mice
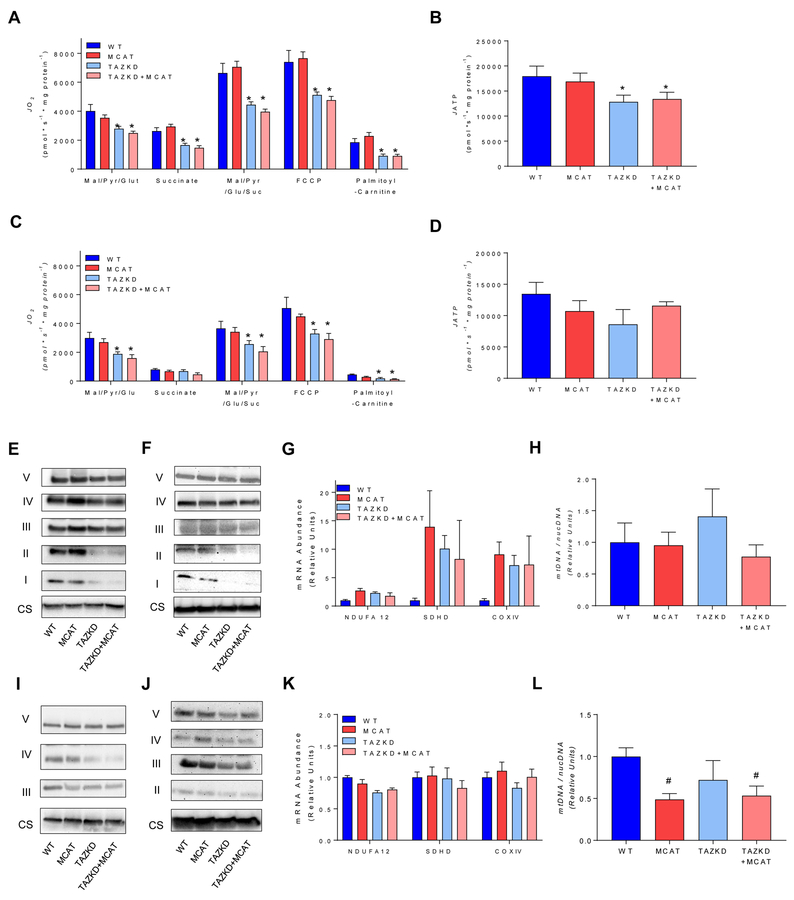
To determine if MCAT overexpression improved mitochondrial function associated with TAZ deficiency, we assessed mitochondrial oxidative phosphorylation in isolated mitochondria from cardiac and skeletal muscle using a variety of substrates. In cardiac mitochondria, respiratory rates were substantially reduced in TAZKD mice which was not rescued in TAZKD+MCAT mice (Figure 4A). Notably, maximal capacity of oxidative phosphorylation, assessed by measuring O2 consumption in the presence of malate, pyruvate, glutamate and succinate was lower in cardiac mitochondria from TAZKD or TAZKD+MCAT mice compared to WT or MCAT mice. Maximal ETS capacity, measured by the addition of carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP) was also lower in TAZKD or TAZKD+MCAT mice compared with WT or MCAT mice. The rate of ATP production in the presence of malate, pyruvate, glutamate and succinate was lower in hearts from TAZKD or TAKZD+MCAT compared with healthy controls (Figure 4B). In skeletal muscle mitochondria, respiratory rates were reduced with TAZ deficiency and not rescued with MCAT overexpression (Figure 4C). In contrast, TAZ deficiency did not significantly reduce the rate of ATP production (Figure 4D) which likely contributed to the lack of a robust skeletal myopathy phenotype in TAZKD and TAZKD+MCAT mice.
Figure 4:
A) Oxygen utilization measured in isolated cardiac mitochondria via high-resolution respirometry. Oxygen consumption was measured in response to the following substrates in the presence of 2 mM ADP: 0.5 mM malate, 5 mM pyruvate, 5 mM glutamate, 10 mM succinate, 1.5 μM carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP) and 0.02 mM palmitoyl-carnitine. n = 4–7 per group. B) ATP production in isolated cardiac mitochondria in response to 0.5 mM malate, 5 mM pyruvate, 5 mM glutamate, 5 mM succinate in the presence of 2 mM ADP. n = 3–6 per group. C) Oxygen consumption measured in isolated skeletal muscle mitochondria using the same substrates described for cardiac mitochondria. n = 4–6 per group. D) ATP production in skeletal muscle mitochondria using the same conditions described for cardiac mitochondria. E) Western blot of protein in ETS complexes I-V and citrate synthase (CS) in isolated cardiac mitochondria. n = 4 per group. F) Western blot of ETS protein complexes and CS in cardiac tissue homogenates. n = 4 per group. G) mRNA abundance of components of the ETS in cardiac tissue. H) Ratio of mitochondrial to nuclear DNA in cardiac tissue. n = 4–8 per group. I) Western blot of ETS protein complex and CS in isolated skeletal muscle mitochondria. n = 4 per group. Bands for complex I and II were too faint to visualize. J) Western blot of ETS protein complexes and CS in skeletal muscle homogenates. n = 4 per group. Bands for complex I were too faint to visualize. K) mRNA abundance of components of the ETS in soleus muscle. L) Ratio of mitochondrial to nuclear DNA in soleus muscle. n = 5 per group Mitochondrial assessments were made in 7–8 month old mice. *: Main effect of TAZKD. #: Main effect of MCAT. p < 0.05.
Next, we examined the effect of TAZ deficiency on the abundance of ETS enzymes. We reasoned that a reduction in mitochondrial CL may negatively affect ETS proteins. Indeed, TAZ deficiency reduced content of ETS enzymes per unit of mitochondria, likely contributing to the lower capacity for oxidative phosphorylation in TAZKD and TAZKD+MCAT hearts (Figure 4E). Lower ETS abundance was also reflected in western blotting in whole heart tissue (Figure 4F), suggesting that TAZ deficiency did not promote a compensatory proliferation of mitochondria. TAZ deficiency did not lower ETS mRNA levels (Figure 4G), indicating that reduced ETS proteins or altered mitochondrial function is likely independent of transcriptional mechanisms, and perhaps due to instability of ETS proteins in altered mitochondrial membrane environment. Similarly, mtDNA/nucDNA levels were not different in hearts from the four groups (Figure 4H), confirming that TAZ knockdown did not induce compensatory mitochondrial biogenesis in heart. Findings in skeletal muscle were similar to those in heart. Protein abundance for ETS enzymes were lower in TAZKD or TAZKD mice compared with WT or MCAT mice in isolated mitochondria or in whole muscle homogenates (Figure 4I and J), These differences were not reflected in mRNA levels for ETS enzymes or mtDNA/nucDNA levels in skeletal muscle (Figure 4K and L). Overexpression of MCAT decreased mtDNA (Figure 4L) in skeletal muscle. Several studies have demonstrated that mtDNA content can coincide with oxidative stress in skeletal muscle as well as other cell types [33–35]. Indeed, the effect of MCAT on mtDNA/nucDNA was reminiscent of its effects on the rate of H2O2 production (Figure 2B).
4. Discussion
BTHS is a rare but fatal X-linked disorder resulting in cardioskeletal myopathy caused by mutations in the TAZ gene, a transacylase which catalyzes the final reaction in CL biosynthesis [2, 3, 8]. The lack of CL destabilizes the components of the ETS resulting in blunted oxidative phosphorylation and elevated ROS production [15]. Excess mitochondrial ROS and the resultant oxidative stress have been implicated in the pathology of BTHS. Indeed, independent groups have demonstrated that contractile qualities of TAZ deficient cardiomyocytes are improved following treatment with mitochondria-targeted antioxidants [17, 18]. The purpose of the present study was to determine whether suppressing mitochondrial ROS and the subsequent oxidative stress would be sufficient to prevent BTHS-induced cardioskeletal myopathy in vivo. MCAT mice overexpress catalase selectively in the mitochondria and therefore reduce mitochondrial H2O2 emission and oxidative stress. Crossing MCAT and TAZKD mice yielded TAZKD+MCAT mice that exhibited decreased levels of oxidative stress compared with TAZKD littermates, however this did not prevent cardiomyopathy or mild muscle weakness. Other than exhibiting decreased oxidative stress, TAZKD+MCAT mice were phenotypically identical to TAZKD mice in all parameters assessed including respiratory capacity.
Previous studies examining the TAZKD mouse have reported increased mitochondrial H2O2 emission and oxidative stress in both cardiac and skeletal muscle [19, 20]. Here we show that the development of cardioskeletal myopathy in TAZKD mice is not dependent on mitochondria-induced oxidative stress. In our study TAZKD mice exhibited greater levels of mitochondrial H2O2 emission and whole tissue lipid peroxidation. Comparatively, TAZKD+MCAT mice exhibited decreased H2O2 emission as well as markedly reduced levels of lipid peroxidation. These findings indicate that suppressing mitochondrial ROS production is sufficient to ameliorate whole tissue levels of oxidative stress in TAZKD mice. However, although TAZKD+MCAT mice exhibited decreased oxidative stress, they still developed cardioskeletal myopathy. As there were no differences in cardiac or skeletal muscle function between TAZKD and TAZKD+MCAT mice, our findings suggest that mitochondrial ROS does not have a causative role in the etiology of cardioskeletal myopathy in BTHS.
Both TAZKD and TAZKD+MCAT mice exhibited defects in mitochondrial oxidative phosphorylation, suggesting that TAZ deficient cardiomyopathy may develop as a result of defects in oxidative phosphorylation. Indeed, the heart relies heavily on oxidative metabolism to meet the incredible energy demand of constant contraction. Fatty acid oxidation in particular can account for up to 90% of the heart’s ATP production [36, 37]. The defect in mitochondrial respiration observed in TAZKD and TAZKD+MCAT mice was most drastic when fatty acid substrates were used (a greater than 50% reduction). These defects in oxidative metabolism were accompanied by reductions in protein abundance of ETS complexes in both cardiac and skeletal muscle. Decreased protein content of ETS enzymes did not coincide with reductions in mRNA abundance, suggesting that the loss of ETS proteins is likely due to alterations in the inner mitochondrial membrane. Furthermore, the loss of respiratory capacity and ETS protein did not prompt an increase in mtDNA content. Together with data that TAZ deficiency reduced ETS protein content in isolated mitochondria and in whole tissue, these findings suggest that reduced mitochondrial function was not met with a compensatory increase in mitochondrial density. The defects in oxidative metabolism are likely major contributors to the development of cardiomyopathy in TAZKD mice. Indeed, a recent study demonstrated that cardiomyopathy in TAZKD mice can be prevented by inducing mitochondrial biogenesis, and increasing electron transfer capacity of the ETS in cardiac tissue [38]. Although decreased CL content leads to disruption of the ETS that results in increased ROS production, this may merely be a symptom and not a cause in BTHS.
In addition to detriments in cardiac and skeletal muscle performance, TAZKD mice had lower lean mass, and decreased TA mass compared to healthy controls. This is consistent with reports of growth delay in individuals with BTHS [5]. Although the mechanisms of growth delay in BTHS have not been thoroughly explored, excess mitochondrial ROS is implicated in other pathologies of muscle loss including sarcopenia and cancer cachexia [39–41]. Like TAZKD mice, TAZKD+MCAT mice exhibited lower lean mass as well as lower TA mass indicating that suppressing mitochondrial ROS in heart and skeletal muscle is not sufficient to prevent growth delay in BTHS. The lower lean mass may be due to alterations in cellular energetics resulting from mitochondrial defects.
Previous studies using the TAZKD mice have reported the development of dilated cardiomyopathy at 7–8 months of age, characterized by thinner ventricular walls and dilated ventricular chamber [25, 31]. In our study TAZKD mice clearly developed hypertrophic cardiomyopathy, with thicker ventricular walls and reduced ventricular volume resulting in decreased stroke volume and cardiac output. Our data are consistent with previously published models of cardiac hypertrophy [32]; however it is unclear why TAZKD mice developed hypertrophic cardiomyopathy instead of the reported dilated cardiomyopathy. A difference in knockdown efficiency likely does not explain the development of hypertrophic over dilated cardiomyopathy, as we achieved a similar level of TAZ knockdown and CL depletion compared with previous studies [25]. However, one potential difference is the lack of a change in mtDNA content. Acehan et al. reported an approximately 8-fold increase in mtDNA/nucDNA in hearts from 8 month-old TAZKD hearts compared to wildtype control [25]. We did not observe such a difference. This difference may represent how cardiac mitochondria may have adapted differently in our studies. Regardless, while the majority of BTHS patients present with dilated cardiomyopathy [5, 6], some develop hypertrophic cardiomyopathy. Thus, even though we do not have a full explanation for why the cardiomyopathic phenotype for this study was different from published reports, they still represent a valid model for BTHS in humans. While fibrosis and apoptosis can be characteristic of either hypertrophic or dilated cardiomyopathy, neither appear to occur in TAZ-deficient cardiomyopathy [42]. Individuals with BTHS exhibit large variability in terms of physiological phenotype for unknown reasons [5, 43, 44]. Similarly, an observation that TAZKD mice were presented with hypertrophic cardiomyopathy may be a product of complex interaction with the environment such as housing condition, air and water contaminants, microbiota, etc.
In conclusion, suppressing mitochondrial ROS by overexpressing mitochondrial-targeted catalase was not sufficient to prevent cardioskeletal myopathy in the mouse model of BTHS. Our findings indicate that excess mitochondrial ROS and oxidative stress do not have causative roles in classical features of BTHS such as cardioskeletal myopathy and growth delay. Consistent with previous reports, we demonstrate defects in mitochondrial respiration in heart and skeletal muscle [45] which were not rescued by selectively overexpressing catalase in the mitochondria. Thus, BTHS-induced cardioskeletal myopathy is promoted by defects in other functions of mitochondria, that may include blunted oxidative phosphorylation.
Acknowledgements
We would like to thank Dr. Peter Rabinovitch for kindly donating MCAT mice for this study.
Funding
This work was funded by National Institutes of Health grants DK095774, DK107397, DK109888, to K.F., DK110656 to P.D.N., and American Heart Association grant 18PRE33960461 to A.R.P.V.
Abbreviations:
- 4-HNE
4-hydroxynonenal
- BTHS
Barth Syndrome
- BW
Body weight
- CL
Cardiolipin
- CS
Citrate synthase
- EF
Ejection fraction
- ETS
Electron transport system
- FCCP
Carbonyl cyanide-p-trifluoromethoxyphenylhydrazone
- FS
Fractional shortening
- HR
Heart rate
- HW
Heart weight
- LVAW
Left ventricular anterior wall
- LVAW;d
Left ventricular anterior wall at diastole
- LVAW;s
Left ventricular anterior wall at systole
- LVID;d
Left ventricular interior diameter at diastole
- LVID;s
Left ventricular interior diameter at systole
- LVPW;d
Left ventricular posterior wall at diastole
- LVPW
Left ventricular posterior wall
- LVPW;s
Left ventricular posterior wall at systole
- MCAT
Mitochondrial-targeted catalase
- MLCL
monolysocardiolipin
- mtDNA
Mitochondrial DNA
- nucDNA
nuclear DNA
- ROS
Reactive oxygen species
- TAZ
Tafazzin
- TAZKD
Tafazzin-deficient mice
- TAZKD+MCAT
Tafazzin-deficient mice with overexpression of mitochondrial-targeted catalase
- WT
Wild-type
Footnotes
Disclosures
None.
References
- [1].Ades LC, Gedeon AK, Wilson MJ, Latham M, Partington MW, Mulley JC, Nelson J, Lui K, Sillence DO, Barth syndrome: clinical features and confirmation of gene localisation to distal Xq28, Am J Med Genet 45(3) (1993) 327–34. [DOI] [PubMed] [Google Scholar]
- [2].Bione S, D’Adamo P, Maestrini E, Gedeon AK, Bolhuis PA, Toniolo D, A novel X-linked gene, G4.5. is responsible for Barth syndrome, Nat Genet 12(4) (1996) 385–9. [DOI] [PubMed] [Google Scholar]
- [3].Barth PG, Valianpour F, Bowen VM, Lam J, Duran M, Vaz FM, Wanders RJ, X-linked cardioskeletal myopathy and neutropenia (Barth syndrome): an update, Am J Med Genet A 126A(4) (2004) 349–54. [DOI] [PubMed] [Google Scholar]
- [4].Dudek J, Maack C, Barth syndrome cardiomyopathy, Cardiovasc Res (2017). [DOI] [PubMed] [Google Scholar]
- [5].Spencer CT, Bryant RM, Day J, Gonzalez IL, Colan SD, Thompson WR, Berthy J, Redfearn SP, Byrne BJ, Cardiac and clinical phenotype in Barth syndrome, Pediatrics 118(2) (2006) e337–46. [DOI] [PubMed] [Google Scholar]
- [6].Jefferies JL, Barth syndrome, Am J Med Genet C Semin Med Genet 163C(3) (2013) 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Xu Y, Kelley RI, Blanck TJ, Schlame M, Remodeling of cardiolipin by phospholipid transacylation, J Biol Chem 278(51) (2003) 51380–5. [DOI] [PubMed] [Google Scholar]
- [8].Xu Y, Malhotra A, Ren M, Schlame M, The enzymatic function of tafazzin, J Biol Chem 281(51) (2006) 39217–24. [DOI] [PubMed] [Google Scholar]
- [9].Fry M, Blondin GA, Green DE, The localization of tightly bound cardiolipin in cytochrome oxidase, J Biol Chem 255(20) (1980) 9967–70. [PubMed] [Google Scholar]
- [10].Fry M, Green DE, Cardiolipin requirement for electron transfer in complex I and III of the mitochondrial respiratory chain, J Biol Chem 256(4) (1981) 1874–80. [PubMed] [Google Scholar]
- [11].Zhang M, Mileykovskaya E, Dowhan W, Gluing the respiratory chain together. Cardiolipin is required for supercomplex formation in the inner mitochondrial membrane, J Biol Chem 277(46) (2002) 43553–6. [DOI] [PubMed] [Google Scholar]
- [12].Valianpour F, Mitsakos V, Schlemmer D, Towbin JA, Taylor JM, Ekert PG, Thorburn DR, Munnich A, Wanders RJ, Barth PG, Vaz FM, Monolysocardiolipins accumulate in Barth syndrome but do not lead to enhanced apoptosis, J Lipid Res 46(6) (2005) 1182–95. [DOI] [PubMed] [Google Scholar]
- [13].Xu Y, Phoon CK, Berno B, D’Souza K, Hoedt E, Zhang G, Neubert TA, Epand RM, Ren M, Schlame M, Loss of protein association causes cardiolipin degradation in Barth syndrome, Nat Chem Biol 12(8) (2016) 641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Xu Y, Sutachan JJ, Plesken H, Kelley RI, Schlame M, Characterization of lymphoblast mitochondria from patients with Barth syndrome, Lab Invest 85(6) (2005) 823–30. [DOI] [PubMed] [Google Scholar]
- [15].Dudek J, Cheng IF, Balleininger M, Vaz FM, Streckfuss-Bomeke K, Hubscher D, Vukotic M, Wanders RJ, Rehling P, Guan K, Cardiolipin deficiency affects respiratory chain function and organization in an induced pluripotent stem cell model of Barth syndrome, Stem Cell Res 11(2) (2013) 806–19. [DOI] [PubMed] [Google Scholar]
- [16].Saric A, Andreau K, Armand AS, Moller IM, Petit PX, Barth Syndrome: From Mitochondrial Dysfunctions Associated with Aberrant Production of Reactive Oxygen Species to Pluripotent Stem Cell Studies, Front Genet 6 (2015) 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].He Q, Harris N, Ren J, Han X, Mitochondria-targeted antioxidant prevents cardiac dysfunction induced by tafazzin gene knockdown in cardiac myocytes, Oxid Med Cell Longev 2014 (2014) 654198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang G, McCain ML, Yang L, He A, Pasqualini FS, Agarwal A, Yuan H, Jiang D, Zhang D, Zangi L, Geva J, Roberts AE, Ma Q, Ding J, Chen J, Wang DZ, Li K, Wang J, Wanders RJ, Kulik W, Vaz FM, Laflamme MA, Murry CE, Chien KR, Kelley RI, Church GM, Parker KK, Pu WT, Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies, Nat Med 20(6) (2014) 616–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cole LK, Mejia EM, Vandel M, Sparagna GC, Claypool SM, Dyck-Chan L, Klein J, Hatch GM, Erratum. Impaired Cardiolipin Biosynthesis Prevents Hepatic Steatosis and Diet-Induced Obesity. Diabetes 2016;65:3289–3300, Diabetes 66(1) (2017) 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Szczepanek K, Allegood J, Aluri H, Hu Y, Chen Q, Lesnefsky EJ, Acquired deficiency of tafazzin in the adult heart: Impact on mitochondrial function and response to cardiac injury, Biochim Biophys Acta 1861(4) (2016) 294–300. [DOI] [PubMed] [Google Scholar]
- [21].Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW 3rd, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD, Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans, J Clin Invest 119(3) (2009) 573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K, Martin GM, Loeb LA, Ladiges WC, Rabinovitch PS, Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging, Circulation 119(21) (2009) 2789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lee HY, Choi CS, Birkenfeld AL, Alves TC, Jornayvaz FR, Jurczak MJ, Zhang D, Woo DK, Shadel GS, Ladiges W, Rabinovitch PS, Santos JH, Petersen KF, Samuel VT, Shulman GI, Targeted expression of catalase to mitochondria prevents age-associated reductions in mitochondrial function and insulin resistance, Cell Metab 12(6) (2010) 668–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Schriner SE, Linford NJ, Extension of mouse lifespan by overexpression of catalase, Age (Dordr) 28(2) (2006) 209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Acehan D, Vaz F, Houtkooper RH, James J, Moore V, Tokunaga C, Kulik W, Wansapura J, Toth MJ, Strauss A, Khuchua Z, Cardiac and skeletal muscle defects in a mouse model of human Barth syndrome, J Biol Chem 286(2) (2011) 899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lark DS, Torres MJ, Lin CT, Ryan TE, Anderson EJ, Neufer PD, Direct real-time quantification of mitochondrial oxidative phosphorylation efficiency in permeabilized skeletal muscle myofibers, Am J Physiol Cell Physiol 311(2) (2016) C239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Angelini R, Lobasso S, Gorgoglione R, Bowron A, Steward CG, Corcelli A, Cardiolipin fingerprinting of leukocytes by MALDI-TOF/MS as a screening tool for Barth syndrome, J Lipid Res 56(9) (2015) 1787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lindsey ML, Iyer RP, Zamilpa R, Yabluchanskiy A, DeLeon-Pennell KY, Hall ME, Kaplan A, Zouein FA, Bratton D, Flynn ER, Cannon PL, Tian Y, Jin YF, Lange RA, Tokmina-Roszyk D, Fields GB, de Castro Bras LE, A Novel Collagen Matricryptin Reduces Left Ventricular Dilation Post-Myocardial Infarction by Promoting Scar Formation and Angiogenesis, J Am Coll Cardiol 66(12) (2015) 1364–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].O’Connell G, Guo G, Stricker J, Quinn LS, Ma A, Pistilli EE, Muscle-specific deletion of exons 2 and 3 of the IL15RA gene in mice: effects on contractile properties of fast and slow muscles, J Appl Physiol (1985) 118(4) (2015) 437–48. [DOI] [PubMed] [Google Scholar]
- [30].Angelakis E, Million M, Kankoe S, Lagier JC, Armougom F, Giorgi R, Raoult D, Abnormal weight gain and gut microbiota modifications are side effects of long-term doxycycline and hydroxychloroquine treatment, Antimicrob Agents Chemother 58(6) (2014) 3342–7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [31].Soustek MS, Falk DJ, Mah CS, Toth MJ, Schlame M, Lewin AS, Byrne BJ, Characterization of a transgenic short hairpin RNA-induced murine model of Tafazzin deficiency, Hum Gene Ther 22(7) (2011) 865–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Leatherbury L, Yu Q, Chatterjee B, Walker DL, Yu Z, Tian X, Lo CW, A novel mouse model of X-linked cardiac hypertrophy, Am J Physiol Heart Circ Physiol 294(6) (2008) H2701–11. [DOI] [PubMed] [Google Scholar]
- [33].Barrientos A, Casademont J, Cardellach F, Ardite E, Estivill X, Urbano-Marquez A, Fernandez-Checa JC, Nunes V, Qualitative and quantitative changes in skeletal muscle mtDNA and expression of mitochondrial-encoded genes in the human aging process, Biochem Mol Med 62(2) (1997) 165–71. [DOI] [PubMed] [Google Scholar]
- [34].Lee HC, Yin PH, Lu CY, Chi CW, Wei YH, Increase of mitochondria and mitochondrial DNA in response to oxidative stress in human cells, Biochem J 348 Pt 2 (2000) 425–32. [PMC free article] [PubMed] [Google Scholar]
- [35].Lee HC, Wei YH, Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress, Int J Biochem Cell Biol 37(4) (2005) 822–34. [DOI] [PubMed] [Google Scholar]
- [36].Bing RJ, Cardiac Metabolism, Physiol Rev 45 (1965) 171–213. [DOI] [PubMed] [Google Scholar]
- [37].Opie LH, Metabolism of the heart in health and disease. I, Am Heart J 76(5) (1968) 685–98. [DOI] [PubMed] [Google Scholar]
- [38].Huang Y, Powers C, Moore V, Schafer C, Ren M, Phoon CK, James JF, Glukhov AV, Javadov S, Vaz FM, Jefferies JL, Strauss AW, Khuchua Z, The PPAR panagonist bezafibrate ameliorates cardiomyopathy in a mouse model of Barth syndrome, Orphanet J Rare Dis 12(1) (2017) 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Brioche T, Lemoine-Morel S, Oxidative Stress, Sarcopenia, Antioxidant Strategies and Exercise: Molecular Aspects, Curr Pharm Des 22(18) (2016) 2664–78. [DOI] [PubMed] [Google Scholar]
- [40].Laviano A, Meguid MM, Preziosa I, Rossi Fanelli F, Oxidative stress and wasting in cancer, Curr Opin Clin Nutr Metab Care 10(4) (2007) 449–56. [DOI] [PubMed] [Google Scholar]
- [41].Meng SJ, Yu LJ, Oxidative stress, molecular inflammation and sarcopenia, Int J Mol Sci 11(4) (2010) 1509–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Phoon CK, Acehan D, Schlame M, Stokes DL, Edelman-Novemsky I, Yu D, Xu Y, Viswanathan N, Ren M, Tafazzin knockdown in mice leads to a developmental cardiomyopathy with early diastolic dysfunction preceding myocardial noncompaction, J Am Heart Assoc 1(2) (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Johnston J, Kelley RI, Feigenbaum A, Cox GF, Iyer GS, Funanage VL, Proujansky R, Mutation characterization and genotype-phenotype correlation in Barth syndrome, Am J Hum Genet 61(5) (1997) 1053–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ronvelia D, Greenwood J, Platt J, Hakim S, Zaragoza MV, Intrafamilial variability for novel TAZ gene mutation: Barth syndrome with dilated cardiomyopathy and heart failure in an infant and left ventricular noncompaction in his great-uncle, Mol Genet Metab 107(3) (2012) 428–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Powers C, Huang Y, Strauss A, Khuchua Z, Diminished Exercise Capacity and Mitochondrial bc1 Complex Deficiency in Tafazzin-Knockdown Mice, Front Physiol 4 (2013) 74. [DOI] [PMC free article] [PubMed] [Google Scholar]