Abstract
Type 2 diabetes mellitus (T2D) is a major risk factor for cardiovascular disease (CVD), the most common cause of death in T2D. Despite improved risk factor control, however, adults with T2D continue to experience substantial excess CVD risk. Until recently, however, improved glycemic control has not been associated with robust macrovascular benefit. The advent of two new classes of anti-hyperglycemic agents, the sodium-glucose cotransporter-2 inhibitors (SGLT2-i) and the glucagon-like peptide-1 receptor agonists (GLP-1 RA), and their respective large cardiovascular outcome trials, has led to a paradigm shift in how cardiologists and heath care practitioners conceptualize T2D treatment. Herein we will review the recent trial evidence, the potential mechanisms of action of SGLT2-i and GLP-1 RA, safety concerns, and their use for the primary prevention of CVD as well as in diabetics with impaired renal function and heart failure.
Keywords: Cardiovascular disease, anti-diabetic therapy, type 2 diabetes, primary prevention, secondary prevention
Condensed abstract
Until recently, glycemic control has not been associated with macrovascular benefit in type 2 diabetes mellitus (T2D). The advent of two new classes of glucose lowering agents, the sodium-glucose cotransporter-2 inhibitors (SGLT2-i) and the glucagon-like peptide-1 receptor agonists (GLP-1 RA), has led to a paradigm shift in how cardiologists and heath care practitioners conceptualize T2D treatment. Herein we will review the recent trial evidence, the potential mechanisms of action of SGLT2-i and GLP-1 RA, safety concerns, and their use for the primary prevention of CVD as well as in diabetics with impaired renal function and heart failure.
Graphical Abstract
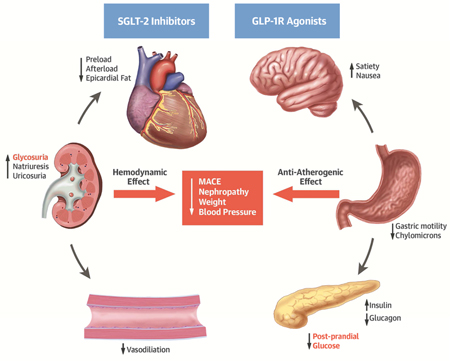
Central Illustration: Potential Pathways of Cardiovascular Benefit from Use of SGLT2 Inhibitors and GLP1 Receptor Antagonists for Patients with T2D. GLP-1 RA, glucagon-like peptide receptor agonist; SGLT2-i, sodium-glucose cotransporter 2 inhibitor. Potential mechanisms of action of SGTL2-i and GLP-1 RA to mediate glycemic control and cardiovascular benefit. The cardiovascular benefit of SGLT2-i may occur through glycosuria and favorable hemodynamic effects. Conversely, the benefit of the GLP-1 RA may occur via post-prandial pancreatic insulin secretion and favorable anti-atherogenic effects.
Introduction
Type 2 diabetes mellitus (T2D) is a major risk factor for cardiovascular disease (CVD), the most common cause of death in T2D (1). Traditional CVD risk factor management for patients with T2D who have or are at elevated risk for CVD includes a multifactorial lifestyle intervention along with intensive interventions to control blood pressure, lipids, antiplatelet therapy and glycemic therapy, as reviewed previously (2). A focus on traditional risk factor control has led to substantial reductions in the burden of CVD for adults with T2D (3, 4). Despite improved risk factor control, however, adults with T2D continue to experience substantial excess CVD risk. Historically, many physicians have dichotomized management of patients with diabetes into two categories; (1) to improve glycemic control to reduce microvascular complications, and (2) controlling established CVD risk factors, such as tobacco use, hyperlipidemia and hypertension physicians sought to reduce risk of macrovascular disease, the biggest driver of morbidity and mortality for patients with T2D. In this setting, antidiabetic agents were used primarily for glucose lowering, requiring titration and monitoring of therapy even though glycemic control had not been associated with reduced cardiovascular risk. Cardiologists and other providers caring for the diabetic patient deferred diabetes management to experts in endocrinology or diabetes care. Over the last several years, trials designed first to demonstrate safety of newer antidiabetic agents demonstrated superiority for CVD risk reduction among adults with T2D with a history of or at high risk for recurrent CVD events. These findings have implications for cardiologists and healthcare providers who commonly care for adults with T2D and elevated CVD risk.
Herein we will review and integrate these recent data into updated management pathways for adults with T2D at high risk for CVD. The focus will be upon reviewing recent trial evidence for agents in the two major new classes with demonstrated efficacy for CVD risk reduction: the sodium-glucose cotransporter 2 inhibitors (SGLT2-i) and the glucagon-like peptide-1 receptor agonists (GLP-1 RA). Recent reviews have included most (5–7), but not all (8, 9) recent cardiovascular outcome trials with relevance for care of adults with T2D and heightened CVD risk. We will add to recent reviews by including an examination of the use of SGLT2-i and GLP-1 RA for cardiorenal protection in the high-risk diabetic patient, and also focus on the use of these agents in the setting of comorbid heart failure (HF) risk. We will also examine the role of background cardiovascular and antidiabetic medical therapy in these recent trials. Finally, we will examine emerging evidence for use of these agents for primary as well as secondary CVD prevention. A discussion of other agents, such as dipeptidyl peptidase-4 inhibitors, with less well established CVD risk reduction profiles is beyond the scope of this manuscript, and we refer the interested reader to prior reviews for an examination of other antidiabetic drug classes for CVD risk reduction in the high-risk adult with T2D (10,11).
The Development of Cardiovascular (CV) Safety and Outcome Trials for the High-Risk Diabetic Patient
The rationale for the development of cardiovascular outcome studies has been reviewed in detail previously (11,12). In brief, partly due to signals of adverse cardiovascular safety with earlier glucose-lowering medications (13), the U.S. Food and Drug Administration and European Medicines Agency subsequently required new glucose-lowering therapies to demonstrate CV safety in prospective, randomized controlled outcome trials (12). Designed for detection of risk signals, some of these CV outcome trials have not only demonstrated CV safety, but have also shown robust reductions in CV events and all-cause mortality (5–8). As recommended (12), these CV outcome trials have focused primarily on high-risk diabetic patients, such as patients with preexisting vascular disease, renal impairment, advanced age or multiple risk factors for CVD. These patients are commonly referred to cardiology practices, and an in-depth review of the results from recent major CV outcome trials will assist the cardiologist and other healthcare practitioners in caring for the high-risk patient with T2D. We will begin by reviewing the mechanism and major trial outcomes and safety for the SGLT2-i, followed by a discussion of the GLP-1 RA. We will then discuss issues germane to both classes of agents in recent CV outcomes trials, including issues related to concomitant cardiovascular medical therapy and insulin use in these recent CV outcomes trials, and the application of these newer agents for the primary prevention of CVD in adults with T2D. A summary of the major trial results is presented in Table 1.
Table 1.
Summary of the GLP-1 RA and SGLT2-i cardiovascular outcome trials.
| Trial | EMPA-REG | CANVAS | LEADER | SUSTAIN-6 |
|---|---|---|---|---|
| Agent | Empagliflozin | Canagliflozin | Liraglutide | Semaglutide |
| N | 7020 | 10142 | 9340 | 3297 |
|
Follow-up (median) |
3.1 years | 2.4 years | 3.8 years | 2.1 years |
|
Baseline HbA1c (mean) |
8.1% | 8.2% | 8.7% | 8.7% |
|
Primary Outcome |
CV Death Nonfatal MI Nonfatal Stroke |
CV Death Nonfatal MI Nonfatal Stroke |
CV Death Nonfatal MI Nonfatal Stroke |
CV Death Nonfatal MI Nonfatal Stroke |
| HR (95% CI) |
0.86 (0.74– 0.99), p=0.04 |
0.86 (0.75–0.97), p=0.02 |
0.87 (0.78–0.97) p=0.01 |
0.74 (0.58–0.95) p=0.02 |
|
Adverse Events |
Genital infections (male and female) |
Amputations, fractures, male genital infections, female mycotic infections, volume depletion |
Acute gallstone disease, injection site-reactions, and adverse events leading to drug discontinuation (nausea, vomiting, diarrhea, abdominal pain/discomfort, anorexia) |
Retinopathy, gastrointestinal disorders, any adverse leading to drug discontinuation (nausea, vomiting, diarrhea in a dose- dependent response) |
Abbreviations: HbA1c, Hemoglobin A1c; HR, Hazard Ratio
Bolded outcome was statistically significant (p<0.05)
The SGLT2 Inhibitors
The SGLT2-i have demonstrated impressive reductions in CV risk in two major CV outcomes trials, EMPA-REG OUTCOME and the CANVAS Program (5,8), with other trials in this drug class ongoing (14). The potential mechanisms of effect have been described in detail (15), and will be summarized here and in the Central Illustration.
Potential Mechanisms of Benefit for the SGLT2 Inhibitors
Metabolic effects
SGLT2-i work by inhibiting the high-capacity, low-affinity SGLT2 receptor in the proximal tubule of the kidney, which is responsible for reabsorbing approximately 90% of filtered glucose (16). Paradoxically, in hyperglycemic states such as diabetes, SGLT2 activity is increased and leads to greater reabsorption of both glucose and salt (17). Importantly for safety, the glucose lowering effects of SGLT2-i decrease at lower plasma glucose levels, thereby accounting for the reduced risk of hypoglycemia seen with this class of anti-diabetic agents (15). As seen in EMPA-REG OUTCOME and the CANVAS Program, treatment with SGLT2-i improves cardiovascular and microvascular endpoints in patients with T2D (5, 8). Notably, the difference in magnitude reduction in glycated hemoglobin (HbA1c) between the active treatment and placebo arms in these trials was modest (0.3% and 0.6% in EMPA-REG and CANVAS, respectively) and is unlikely to account for the reduction in CV events with SGLT2-i (5, 8). Although still speculative, non-glycemic effects of SGLT2-i likely drive the observed weight loss, reduction in blood pressure, and preservation of renal function. Improvements in these pathogenic risk factors may reduce CV events, heart failure, and progression of nephropathy (15). Interestingly, both empagliflozin and canagliflozin demonstrated small increases (≈ 3–4 mg/dL increases in LDL cholesterol) over the trial duration (5, 8, 18). Some SGLT2-i such as canagliflozin have also been shown to reduce epicardial adipose tissue, which may be linked to coronary atherogenesis and impaired myocardial function, possibly providing an additional mechanism of CV benefit for SGLT2-i (19); a clinical trial examining dapagliflozin and epicardial adipose tissue is ongoing [NCT02235298].
SGLT2-i induced glucosuria can promote uric acid excretion, with animal models suggesting a possible inhibitory effect of glucosuria on uric acid reabsorption, mediated by the GLUT9 isoform 2 transporter (20). High uric acid levels have been associated with increased cardiovascular and renal disease (21). Glucosuria also leads to ongoing caloric loss and a persistent catabolic state and increased ketogenesis (15). The resulting mild ketonemia caused by SGLT2-i may be an efficient fuel substrate for the heart, and may mitigate some of the metabolic effects associated with incipient heart failure (18).
Hemodynamic Effects
The very early reduction in CV mortality observed in the EMPA-REG OUTCOME and early reduction in heart failure in CANVAS, along with heterogeneity of the HRs for the atherosclerotic components of the three-point MACE, suggest that the early cardioprotective mechanism of benefit from SGLT2-i may be related to improved hemodynamic status (5, 8, 22). This reasoning is supported by a recent post-hoc mediation analysis of EMPA-REG OUTCOME which demonstrated that plasma volume, as measured by hemodynamic markers (e.g. hematocrit), appeared to have a larger impact on the reduction of CV mortality than measures of glycemia (23).
SGLT2-i may also derive hemodynamic benefit through a reduction in blood pressure, but this is unlikely to explain the rapid reduction in CV mortality observed in the SGLT2-i CV outcome trials. A meta-analysis of 27 SGLT2-i trials demonstrated a systolic blood pressure reduction of approximately 4 mmHg among patients with T2D, likely driven by natriuresis osmotic diuretic effects (24). Animal studies have suggested that SGLT2-i have the potential to restore nocturnal dipping and have an additive effect when combined with use of a renin-angiotensin-aldosterone system (RAAS) inhibitor, possibly due to effects of RAAS in the volume contracted state (15,25). The natriuretic and diuretic effects of SGLT2-i may also improve arterial stiffness (15), an independent subclinical predictor of CV risk and mortality (26), although the exact mechanism remains unclear. Moreover, a reduction in blood pressure can mitigate heart failure risk by reducing cardiac afterload and improving coronary flow and cardiac contractility. A reduction in plasma volume via natriuresis and osmotic diuresis can also reduce cardiac preload and myocardial stretch, thus protecting against the progression of heart failure and arrhythmogenesis, respectively (27).
The effects of SGLT2-i on renal hemodynamics and glomerular function may be a primary mechanism through which CV benefit from this class of agents is derived. The cardiorenal benefits of SGLT2-i include lowering intraglomerular pressure and reducing diabetic hyperfiltration (28), a process characterized by diminished distal salt delivery and maladaptive tubuloglomerular feedback, resulting in afferent arteriole vasodilatation and hyperfiltration (29). SGLT2-i counteract this process and lower intraglomerular pressure leading to cardiorenal protective effects for patients with diabetes. A reduction in intraglomerular pressure may also suppress renal inflammation and fibrosis, further protecting against nephropathy and albuminuria (15). Current evidence from cardiovascular outcome trials with SGLT2-i supports this possibility (Figure 4). The ongoing CREDENCE trial [NCT02065791] evaluating primary renal endpoints will further define the cardiorenal protective effects of canaglaflozin in approximately 4,200 adults with T2D and diabetic nephropathy (defined as stage 2 or 3 chronic kidney disease with macroalbuminuria) on a maximal tolerated angiotensin-converting enzyme inhibitor or angiotensin receptor blocker. (30). The primary endpoint of this important trial includes a composite of end-stage renal disease, doubling of serum creatinine, and renal or cardiovascular death.
Figure 4: Summary of Renal Benefits in Major Recent Trials of SGTL2-i and GLP-1 RA.
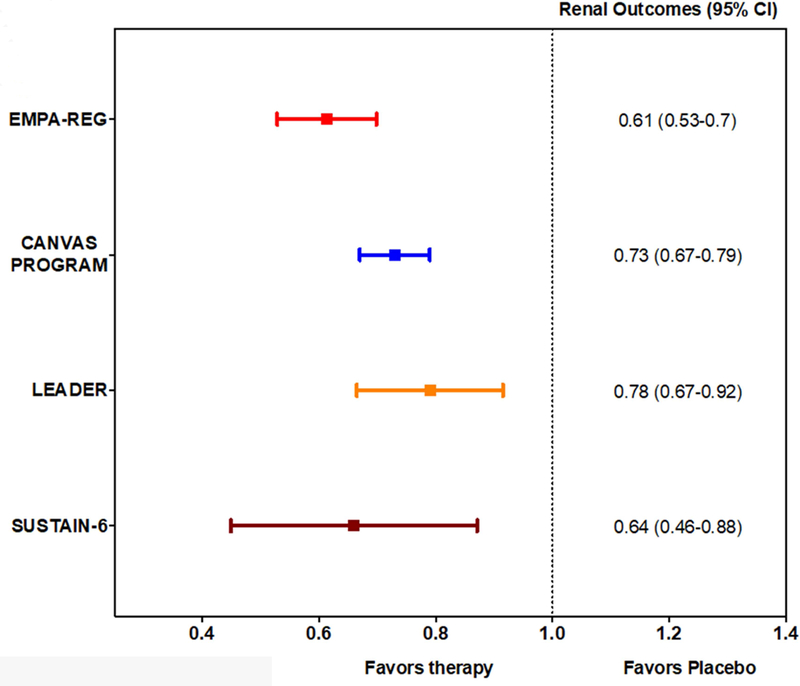
Renal outcomes were all favorably reduced by therapy in EMPA-REG, CANVAS Program, LEADER, and SUSTAIN-6. All trials used a roughly similar composite for adverse renal outcomes including progression of albuminuria.
Clinical Trial Evidence supporting SGLT2-i use for the reduction of CVD
Major cardiovascular outcome trials have been completed for two agents in this class: empagliflozin (EMPA-REG OUTCOME) and canagliflozin (CANVAS Program [Canagliflozin Cardiovascular Assessment Study] (5,8), with results from trials of other agents expected in 2019 (15,30,31). In both EMPA-REG OUTCOME and the CANVAS Program, SGLT2-i led to reductions in the 3-component major adverse cardiovascular endpoint (MACE-3; cardiovascular death, nonfatal myocardial infarction (MI); or non-fatal stroke) (Figure 1). Reduced heart failure hospitalizations (Figure 1) and renal outcomes (Figure 4) were also demonstrated but were not formally tested in the CANVAS program because of the hierarchical testing plan (8). One difference between EMPA-REG OUTCOME and the CANVAS Program is the significant reduction in CV and all-cause mortality with empagliflozin, both of which were not observed in the CANVAS Program (5,8).
Figure 1: Summary of Reductions in Major Adverse Cardiac Events in recent SGLT2-i Trials.
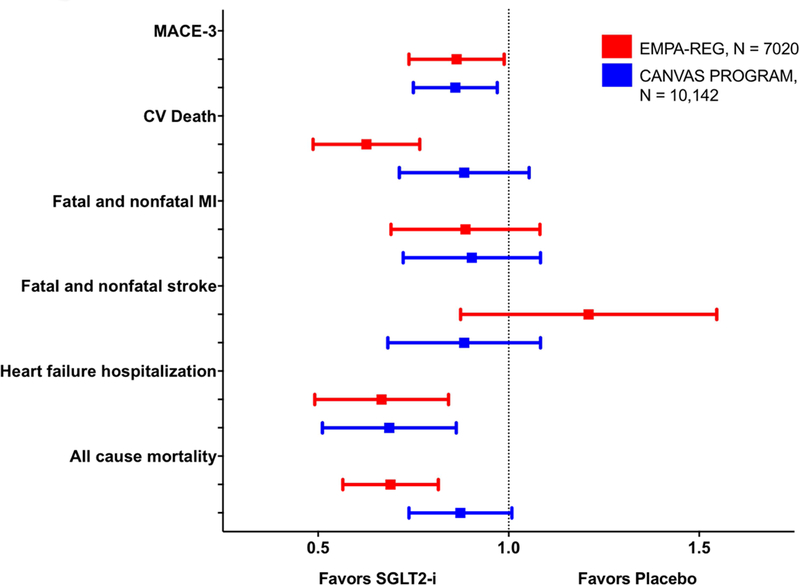
MACE, major adverse cardiac event; MI, myocardial infarction; SGLT2-i, sodium-glucose cotransporter 2 inhibitor. Both empagliflozin and canagliflozin significantly reduced MACE-3 and heart failure hospitalization in EMPA-REG and CANVAS Program, respectively. A reduction in cardiovascular death and all-cause mortality was observed with use of empagliflozin.
The main reason for the difference in study outcomes between these two trials may be attributable to differences in the enrolled study populations and differential follow-up duration. Participants in EMPA-REG OUTCOME were followed for a median of 3.1 years and all were required to have a history of cardiovascular disease (coronary artery disease, stroke or PAD). Participants in the CANVAS Program were followed for a shorter duration (median of 2.4 years) and could have either CV risk factors alone (34% of participants) or established CVD (66%). Reflecting the higher-risk population enrolled in EMPA-REG (secondary prevention), the MACE-3 composite and all-cause mortality were substantially higher in placebo group of EMPA-REG OUTCOME compared to the CANVAS Program (43.9 vs. 31.5 per 1000 patient-years, respectively) (5, 8, 14). The CANVAS Program is combination of 2 separate studies: although both had identical entry criteria (8), follow-up duration differed substantially: mean follow-up duration in CANVAS was 5.7 years, vs. 2.1 years in the CANVAS-R study (8). As noted previously (14), the combination of ≈ 1/3 primary prevention patients in the CANVAS program and shorter-term treatment in roughly half the population (CANVAS-R) may partially explain a smaller effect of canagliflozin compared with empagliflozin.
Cardiorenal Protection and SGLT2-i
Type 2 diabetes is a major risk factor for macrovascular and microvascular disease (32). Kidney disease develops in nearly 35% of patients with T2D and is associated with increased mortality (33). Both EMPA-REG OUTCOME and the CANVAS program demonstrated cardiorenal protective effects of SGLT2-i with empagliflozin or canagliflozin, respectively (Figure 4). The renal benefits of empagliflozin were reported as a prespecified secondary analysis from EMPA-REG OUTCOME (34). Participants in EMPA-REG had an estimated glomerular filtration rate ≥ 30 ml/min per 1.73 m2 of body surface area (5). The prespecified renal outcomes included incident or worsening nephropathy (progression to macroalbuminuria, doubling of the serum creatinine level, initiation of renal-replacement therapy, or death from renal disease) and incident albuminuria (34). Overall, there was nearly a 40% reduction (HR 0.61; 95% CI 0.53–0.70) in the primary renal outcome (absolute risk reduction 6.1%) for participants receiving empagliflozin compared to placebo, Figure 4 (34). Although the CANVAS Program analysis plan precluded formal assessments of statistical significance, the point estimates for reduction in renal events with empagliflozin and canagliflozin suggest a consistency of benefit for reduction in renal events with SGTL2-i (5,8). A post-hoc analysis of the CANVAS Program evaluating participants with an estimated glomerular filtration rate (eGFR) down to 30 mL/min/1.73 m2 found a similar reduction in cardiovascular events and progression of kidney disease among those with impaired renal function at baseline, despite a progressive attenuation of HbA1C lowering with SGLT2-i at lower eGFR (35).
Heart Failure Benefits and SGLT2-i
Similar to the relationship between diabetes and renal dysfunction, heart failure (HF) is also highly prevalent in patients with T2D (36). Patients with T2D and comorbid HF have an extremely poor prognosis, with a median survival of approximately 4 years (37). As shown in Figure 1, the magnitude of reduction in the heart failure composite endpoint is similar for empagliflozin and canagliflozin (22, 38). It has been suggested that the rapid benefit observed with the SGLT2-i is unlikely due to reductions in atherothrombotic events via improved control of classical CV risk factors, but rather related to the hemodynamic and diuretic effects of SGLT2-i given the rapidity of benefit (38–40). Limitations of these trials include the absence of systematically collected baseline biomarkers of heart failure or echocardiography data (22, 38). It is also important to note that EMPA-REG OUTCOME and the CANVAS Program were not designed as heart failure trials and had few patients with investigator-reported heart failure at baseline. Moreover, the safety and efficacy of SGLT2-i in patients with clinical symptoms of heart failure is unknown. However, given the baseline characteristics of patients included in these studies (older, long duration of T2D, comorbid CAD and hypertension), and the high usage of drugs to treat heart failure, it is reasonable to assume that the burden of comorbid left ventricular dysfunction and/or HF in this population was substantial (22). Recent analyses have also suggested the reduction in HF and mortality may be a class effect applicable to a broad population of patients with T2D in real-word practice settings (41, 42). The ongoing heart failure trials with empagliflozin (EMPEROR) and dapagliflozin (DAPA HF) will provide further insight into SGLT-2i and heart failure in patients with and without diabetes, as well as heart failure patients with both preserved and reduced ejection fraction.
Reported Side effects of SGLT2-i use in Cardiovascular Outcome Trials
Figure 3A presents the a summary of the side effects observed in the major SGLT2-i cardiovascular outcome trials (5,8). In general, empagliflozin and canagliflozin were well tolerated. Approximately 23% and 29% of participants in EMPA-REG OUTCOME and CANVAS discontinued active study drug, compared to 29% and 30% for placebo, respectively (5,8). The percent discontinuation of active drug is similar to other major placebo-controlled cardiovascular outcome trials (43). Genital infections were more common with SGLT2-i vs. placebo in both EMPA-REG OUTCOME and the CANVAS Program. However, these infections infrequently resulted in study drug discontinuation. Additionally, there were differences in the definition and gender distribution of genital infections between the two trials, but these differences also did not appear to influence rates of drug discontinuation (5, 8). Importantly, there was no difference in the occurrence of complicated urinary tract infections between participants receiving SGLT2-i compared to placebo in these trials. The risk of volume depletion appears similar with both empagliflozin and canagliflozin, though volume depletion was statistically more common only in the CANVAS Program (5, 8). The CANVAS Program identified new safety concerns for amputations which were collected as an adverse event of special interest and fractures (Figure 3A). Increased risk of fractures or amputations have not been demonstrated with empagliflozin (44, 45), although lower limb amputations were not prespecified events of concern in EMPA-REG OUTCOME. While increased amputations with canagliflozin have also been reported from the U.S. Food and Drug Administration Adverse Event Reporting System (46) and in a propensity matched cohort from the U.S. Department of Defense Military Health System (42), a plausible mechanism for effects of SGLT2-i on bone or vascular biology has not been determined (14).
Figure 3: Summary of Side Effects in Major Recent Trials of SGLT2-i and GLP-1 RA.
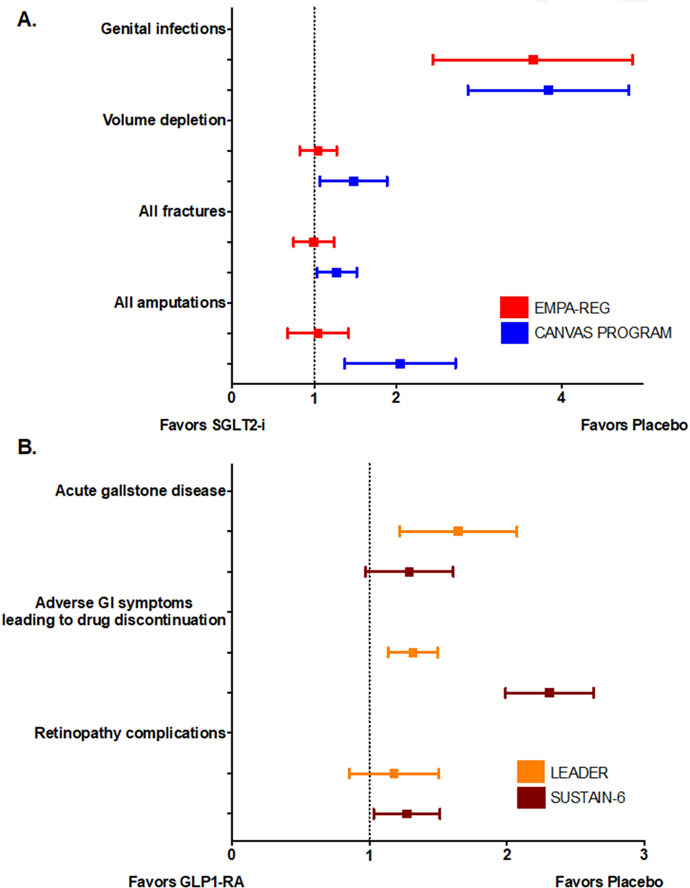
GI, gastrointestinal; GLP-1RA, glucagon-like peptide receptor agonist; SGLT2-i, sodium-glucose cotransporter 2 inhibitor. Panel A. Side effects of SGLT2-i. Genital infections were significantly increased with SGLT-2i use in EMPA-REG OUTCOME and the CANVAS Program. Amputation risk was significantly increased with canagliflozin use. Panel B. Side effects of GLP-1 RA. Liraglutide and semaglutide were significantly associated with higher drug discontinuation rates due to adverse GI symptoms. Acute gallstone disease was significantly increased with liraglutide use and retinopathy complication was significantly increased with semaglutide use.
The GLP-1 Receptor Agonists
In comparison to the CV outcomes trials with SGLT2-i, trial results with the GLP-1 RA have been more heterogeneous (6, 7, 9, 47); there are additional trials ongoing using albiglutide (HARMONY outcomes, NCT02465515) and dulaglutide (REWIND NCT01394952). The potential mechanisms of effect have been described in detail (48, 49), and are summarized here and in the Central Illustration.
Potential Mechanisms of Benefit for the GLP-1 Receptor Agonists
Oral glucose ingestion results in higher serum insulin levels than an equivalent parenteral glucose load, likely mediated by the incretin pathway (50). Glucagon-like peptide-1 (GLP-1), a type of incretin polypeptide, is secreted by the distal intestinal L-cells in response to oral nutrient ingestion and has several downstream effects prior to its rapid degradation by DPP4. (51). Endogenous GLP-1 acts primarily to stimulate pancreatic beta-cells to release insulin and inhibit glucagon secretion, thereby providing a glucose-dependent mechanism to reduce post-prandial hyperglycemia without resulting in significant hypoglycemia (52). In vivo and animal studies have demonstrated a GLP-1 mediated increase in pancreatic islet and beta-cell mass, highlighting a potential mechanism to retard T2D progression (53). The incretin pathway may be impaired in T2D secondary to reduced GLP-1 secretion and resistance, providing a target for pharmacological intervention (54).
Synthetic GLP-1 RA are resistant to DPP4 degradation and accentuate the pleotropic effects associated with GLP-1 polypeptides (55). To date, there are six FDA approved GLP-1-RA, which differ in structure and duration of effect. These include: exenatide (both short-acting and long-acting formulations), liraglutide, semaglutide, dulaglutide, lixisenatide, and albiglutide. Both exenatide and lixisenatide are derived from exogenous Gila monster venom while the others are modifications of endogenous GLP-1 (56). The longer-acting formulations are associated with dose-dependent reductions in HbA1c and lowering of fasting (vs. post-prandial) reductions in glucose. The shorter acting formulations are associated with greater post-prandial glucose lowering, likely mediated more by slowing of gastric motility as opposed to insulin release, as discussed in detail elsewhere (56,57). Large CV outcome trials have been completed for 4 of the GLP-1 RA, all of which demonstrated non-inferiority compared to placebo in T2D (6,7,9,47). However, only liraglutide (LEADER) and semaglutide (SUSTAIN-6) showed superiority in reducing MACE (6, 7), as summarized in Figure 2. The CV benefit with liraglutide and semaglutide compared to placebo is unlikely to be driven by the modest glycemic difference between treatment and placebo groups (0.4% and 0.8% for liraglutide and semaglutide compared to placebo, respectively) (6, 7), but rather a collection of favorable non-glycemic effects on CV risk factors including weight, blood pressure, lipids, and renal protection.
Figure 2: Summary of Reductions in Major Adverse Cardiac Events in recent GLP-1 RA trials.
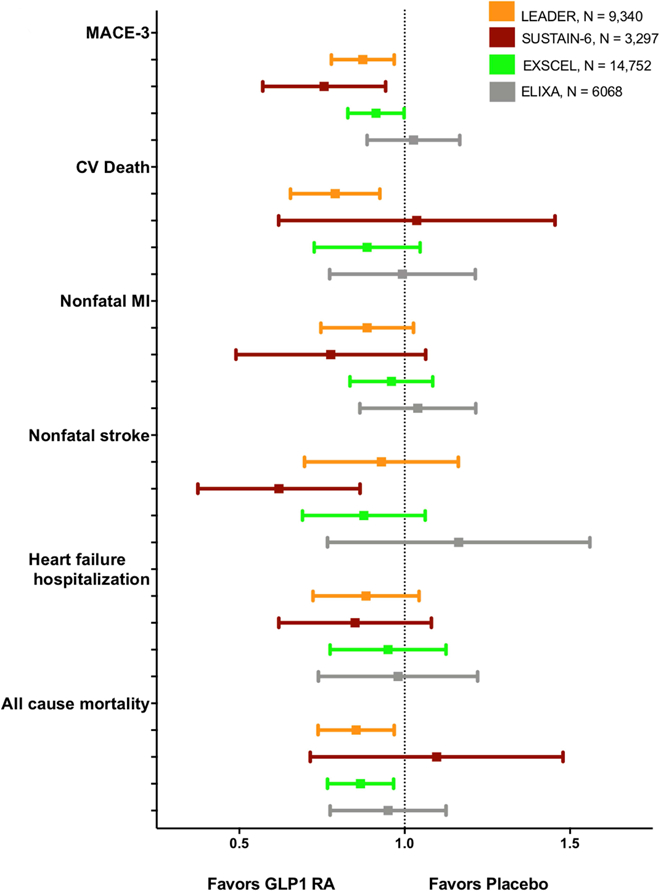
CV, cardiovascular; GLP-1 RA, glucagon-like peptide receptor agonist; MACE, major adverse cardiac events; MI, myocardial infarction. Liraglutide and semaglutide significantly reduced MACE-3 in LEADER and SUSTAIN-6, respectively. Liraglutide significantly reduced CV death, heart-failure hospitalization, and all-cause mortality. Semaglutide significantly reduced non-fatal stroke.
Weight
Liraglutide and semaglutide lowered weight by 2.3 kg and 3.6 kg more than placebo, respectively, in their CV outcome trials (6,7). This is slightly more weight than the ≈1.6–2.0 kg weight loss observed in the major SGLT2-i trials (5,8). The weight loss associated with GLP-1 RA is likely multifactorial and due to mechanisms leading to reduced caloric intake, as opposed to the glycosuric caloric loss associated with SGLT2-i (15). GLP-1 receptors in the hypothalamus and intestine have been implicated in the promotion of satiety, appetite suppression, delayed gastric emptying, along with several adverse gastrointestinal side effects (e.g. nausea, vomiting, and diarrhea). Collectively these effects result in consistent weight loss with GLP-1 RA use. A randomized controlled trial of 3,731 overweight to obese non-diabetic participants treated with once daily high-dose liraglutide (3.0 mg) demonstrated a mean 5.6 kg weight loss greater than the placebo group (58). In this trial rates of progression from normoglycemia to prediabetes or prediabetes to T2D were also lower in patients treated with liraglutide. Importantly, there has been no significant hypoglycemia with GLP-1 RA use in trials enrolling diabetic (6, 7) or non-diabetic participants (58).
Blood Pressure
LEADER and SUSTAIN-6 demonstrated modest systolic blood pressure reductions of 1.2 mmHg and 1.9 mmHg more than placebo, respectively. The antihypertensive benefit could be related to GLP-1 induced atrial natriuretic peptide release by cardiomyocytes, resulting in improved endothelial function, vasodilatation, and natriuresis (48, 59). Emerging evidence in animals also links GLP-1 agonism with improvement in cardiac efficiency in obesity and after myocardial infarction (60).
Lipids
GLP-1 RA agents can also exert favorable effects on lipid profiles (61), possibly through reduced post-prandial enterocyte chylomicron production. The increase in post-prandial insulin and reduction in glucagon can also lower non-esterified fatty acids and inhibit adipose tissue lipolysis (48, 49), further improving lipid profiles. A large meta-analysis of 35 GLP-1 RA trials demonstrated modest reductions in total cholesterol, low-density lipoprotein cholesterol, and triglycerides (61). The more intensive dose of semaglutide in SUSTAIN-6 also lowered serum triglycerides (≈8 mg/dL) and free fatty acids ≈0.05 mmol/L) compared to placebo (7). This contrasts with the small increases in HDL (≈2–3 mg/dL) and LDL (≈ 3–4 mg/dL) observed in SGT2-i outcomes trials compared to placebo (5,8).
Improvements in Renal Function
The mechanism underlying the renal benefits in LEADER and SUSTAIN-6 is not well understood. Treatment with liraglutide and semaglutide was associated with a 22% and 36% reduction in nephropathy (defined as the new-onset of macroalbuminuria or a doubling of the serum creatinine level and an eGFR under 45 mL/ min / 1.73 m2 in both trials as well as the need for continuous renal-replacement therapy in SUSTAIN-6), respectively, driven by a reduction in the incidence of new-onset persistent macroalbuminuria (6), (Figure 4) . It is unclear if the renal benefit is due to direct GLP-1 RA effects on the kidney, or indirect effects on risk factors for nephrotoxicity (e.g. improved blood pressure and glycemic control). In-vivo, animal, and human studies have also demonstrated other non-glycemic effects of GLP-1 RA, including anti-inflammatory properties, improved vascular endothelial function, ischemic conditioning, and anti-thrombotic effects via inhibition of platelet aggregation (48,62,63).
Clinical Trial Evidence supporting GLP-1 RA use for the reduction of CVD
The LEADER trial randomized 9,340 patients with T2D to either the once-daily injectable 1.8 mg (or max tolerated dose) of liraglutide or placebo for a median follow-up of 3.8 years. The primary endpoint, a three-point MACE (MACE-3) including nonfatal myocardial infarction (MI), nonfatal stroke, and cardiovascular death, was significantly reduced in patients treated with liraglutide (HR, 0.87; 95% CI, 0.78–0.97), driven by a 22% significant reduction in CV death (95% CI, 0.66–0.93) and nonsignificant reductions in nonfatal MI (HR, 0.88; 95% CI, 0.75–1.03) and nonfatal stroke (HR, 0.89; 95% CI, 0.72–1.11) (6), Figure 2. There was also a significant 15% reduction in all-cause mortality (95% CI,0.75–0.97) along with a non-significant reduction in HF hospitalizations (6). The SUSTAIN-6 trial randomized 3,297 T2D patients to two doses of once-weekly injectable semaglutide (0.5mg or 1.0 mg) or placebo for a median 2.1 years (7). Using the same MACE-3 composite, semaglutide was associated with a significant 26% reduction in the primary endpoint (95% CI, 0.58–0.95) (7), Figure 2. In contrast to LEADER, semaglutide did not reduce CV death (HR, 0.98; 95% CI, 0.65–1.48) or all-cause mortality (HR, 1.05; 95% CI, 0.74–1.50), but did demonstrate a significant reduction in nonfatal strokes (HR, 0.61; 95% CI, 0.38–0.99) and a non-significant trend toward fewer non-fatal MI (HR, 0.74; 95% CI, 0.51–1.08) (6, 7), Figure 2.
The trial participants in LEADER and SUSTAIN-6 were similar at baseline. Roughly 80% of participants in both trials had established stable cardiovascular disease and an average HbA1c of 8.7% (6, 7). Potential explanations for the lack of CV or all-cause mortality benefit in SUSTAIN-6 include the smaller trial population and follow-up duration nearly 2 years shorter than LEADER (6, 7). Subgroup analyses of LEADER revealed heterogeneity for liraglutide treatment among patients with established cardiovascular disease at baseline and those with impaired renal function (<60 mL/min/1.73 m2), although the distribution of benefit with liraglutide was not consistent among LEADER participants with impaired renal function (6, 7).
Notably, a third GLP-1 RA agent, lixisenatide, did not demonstrate superiority in cardiovascular outcomes reduction in the ELIXA trial (47). In comparison to LEADER and SUSTAIN-6, enrollment in the ELIXA trial was restricted to T2D patients with a recent acute coronary syndrome (<180 days prior), randomizing 6,068 participants to once-daily injectable lixisenatide or placebo for a median follow-up of 2.1 years (47). ELIXA also added unstable angina hospitalization to the triple endpoint but found no difference in the primary endpoint (HR, 0.94; 95% CI, 0.78–1.13); in any individual component; or in all-cause mortality (47). Compared to the other GLP-1 RA agents, lixinesatide has a shorter half-life (≈3 hours), and demonstrated smaller effects on glycemic control (0.27% reduction in HbA1c), systolic blood pressure, and weight reduction than either liraglutide or semaglutide (6, 7, 47). Although ELIXA participants had a recent acute coronary syndrome, they were younger, with lower systolic blood pressure, diabetes duration, and better glycemic control (entry HbA1c 7.7%) compared to the other GLP1-RA cardiovascular outcome trials. The rate of the primary endpoint (including unstable angina) in ELIXA (6.4 per 100 patient-years) was greater than that observed in LEADER, SUSTAIN-6 and the ELIXA trial (3.9, 4.4 and 4.0 per 100 patient-years, respectively). (6, 7, 9, 47)
Finally, a fourth GLP-1 RA agent, exenatide, in the largest GLP-1 RA cardiovascular outcomes trial to date, did not reach superiority for a reduction in cardiovascular outcomes compared with placebo (9). The EXSCEL trial randomized 14,752 participants with T2D and established CVD to either the once-weekly injectable exenatide or placebo for a median follow-up of 3.2 years (9) for a MACE-3 composite outcome. The EXSCEL trial demonstrated a non-significant trend towards benefit with exenatide (HR, 0.91; 95% CI, 0.83–1.00, P = 0.06 for superiority), Figure 2. All-cause mortality was reduced by 14%, although due to hierarchical testing this result is not considered statistically significant (9). Several trial design factors and baseline patient selection may also explain the lack of superiority in EXSCEL. In comparison to LEADER and SUSTAIN-6, EXSCEL had a smaller proportion of participants with established cardiovascular disease (≈80% vs. 73%, respectively) with better baseline glycemic control (HbA1c 8.7% vs. 8.0%) (9). Furthermore, discontinuation of study drug (43.0%) and placebo (45.2%) may have limited power in the EXSCEL trial to detect a significant reduction in the primary endpoint.
Results from the long-acting GLP-1 RA trials with albiglutide (HARMONY Outcome, NCT02465515) and dulaglutide (REWIND, NCT01394952) will help clarify whether the cardiovascular benefit demonstrated by GLP-1 RA agents is a class-effect (namely with the longer-acting, more potent, agents) or a drug-specific effect only seen with liraglutide and semaglutide.
Safety Concerns with the GLP-1 Receptor Agonists
Both LEADER and SUSTAIN-6 have illuminated potential deleterious effects associated with GLP-1 RA use, including the risk for retinopathy, acute gallstone disease (Figure 3B) and increased heart rate.
In SUSTAIN-6, semaglutide was associated with a significantly increased risk of retinopathy (3.0%) (7). In the LEADER trial, retinopathy was numerically more common among participants randomized to liraglutide (2.3%) than to placebo (2.0%), but this difference was non-significant (6) (Figure 3B). Reassuringly, a large meta-analysis of 37 GLP-1 RA trials of 21,782 participants with diabetes treated with GLP-1 RA did not find a meaningful increase in retinopathy events (64), suggesting that retinopathy may be a semaglutide-specific drug effect or a type I error, rather than a class effect. The pathologic mechanism behind the retinopathy complications is unclear. A previous study noted that rapid glucose lowering with insulin among patients with type 1 diabetes was associated with worsening of retinopathy (65), but the applicability of this finding is unclear.
LEADER demonstrated a greater incidence of acute gallbladder disease with liraglutide (3.1%) compared to placebo (1.9%), a finding that was also numerically greater with semaglutide (3.6%) in SUSTAIN-6, Figure 3B. A similar observation was made in a large population-based cohort study in patients treated with exenatide or liraglutide with or without other glucose-lowering agents (66). The mechanism of this side effect is likely multifaceted, possibly related to rapid weight loss leading to supersaturation of bile acid cholesterol, impaired gallbladder emptying, and cholangiocyte proliferation (66). Despite previous concerns regarding pancreatitis and medullary thyroid cancer risk associated with GLP-1 RA use (67), this was not substantiated in LEADER, SUSTAIN-6, nor in a recent meta-analysis of all four CV outcome trials combined (68).
GLP-1 RA use is also associated with increased heart rate, especially with the long-acting formulations, though the mechanism has yet to be elucidated (69). In the FIGHT trial, a blinded, randomized phase 2 study of 300 hospitalized patients with systolic heart failure (approximately 60% of whom had concomitant T2D) there was a 30% non-significant increased risk of re-hospitalization for heart failure in participants treated with liraglutide (70). While LEADER demonstrated numerically, albeit non-significantly, fewer heart failure hospitalizations, only 18% of the trial population had chronic heart failure (NYHA Class II or III) at baseline (6) . In SUSTAIN-6, nearly 24% of participants had chronic heart failure (NYHA Class II or III) at trial entry, and randomization to semaglutide was associated with a numerically greater but statistically non-significant increase in the risk of heart failure hospitalization (7). Hence , though GLP1-RA are not contraindicated in patients with heart failure, therapeutic alternatives such as the SGLT2-i appear to have more demonstrable benefit among diabetic patients with a history of heart failure.
Background Medical Therapy and Risk Factor Control in Cardiovascular Outcome Trials
CV risk factors, such as smoking, hypertension, and dyslipidemia independently contribute to CVD risk in T2D patients (2, 71). Several cardiovascular risk factors contribute to the development of T2D, and patients with T2D have a higher prevalence of cardiovascular risk factors (72). Treatment of cardiovascular risk factors in T2D prevents and slows CVD progression, especially when multiple risk factors are addressed simultaneously (2, 73–75). However, almost half of U.S. adults with T2D still do not achieve goals for cardiovascular risk reduction (4). When evaluating the efficacy of the newer glucose lowering agents it is important to consider background medical therapy and risk factor control in these trials.
Online Figure 1 presents the mean baseline systolic blood pressure (SBP), body mass index and low-density lipoprotein cholesterol ( LDL-C) for participants in the 4 major cardiovascular outcome trials reviewed (5–8). At trial entry, participants had a mean SBP greater than 135 mmHg, a BMI greater than 30 kg/m2, and an LDL-C greater than 80 mg/dL. As shown in Online Figure 2, roughly 25–30% of patients in these trials were not on a statin at baseline despite guideline recommendations to treat T2DM with moderate to high-intensity statins (71, 76). Subgroup analyses from EMPA-REG, the CANVAS Program, LEADER, and SUSTAIN-6 showed that active treatment reduced cardiovascular events regardless of baseline risk factor control. Overall, background medical therapy for CVD prevention in these trials was similar to or exceeds that seen in current clinical practice (4). Recent analyses of SGT2-i use in clinical practice suggests a magnitude of benefit comparable to that observed in recent clinical trials (41, 42).
eFigure 1. Baseline Cardiovascular Risk Factor Control in Recent Trials of New Antidiabetic Agents.
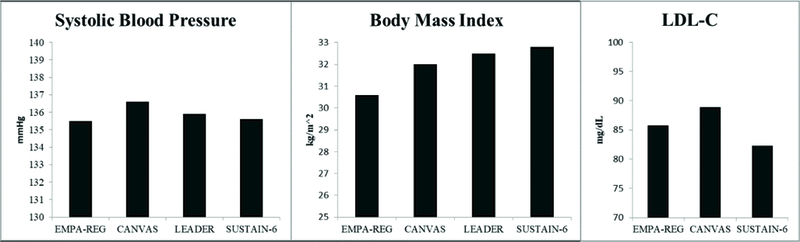
LDL-C, low-density lipoprotein cholesterol
Baseline systolic blood pressure, body mass index, and LDL cholesterol.
eFigure 2a. Baseline Medication Use Reported in Recent trials of New Antidiabetic Agents.
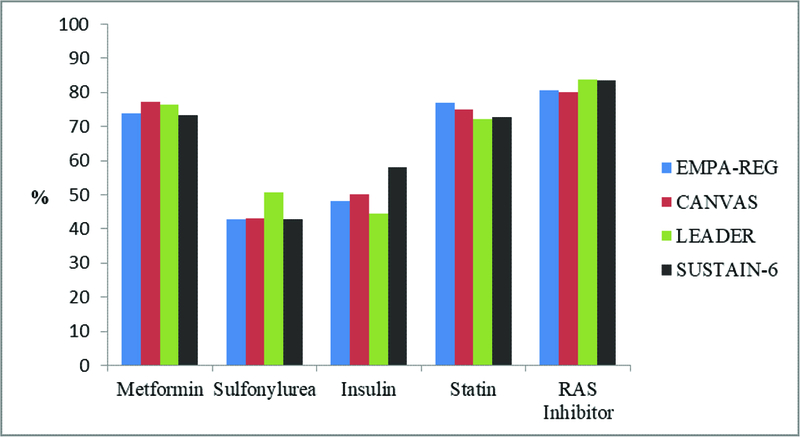
RAS, renin-angiotensin system
Baseline anti-glycemic agent, statin, and RAS inhibitor use.
Baseline metformin, sulfonylurea, and insulin use was similar for participants in EMPA-REG, CANVAS Program, LEADER, and SUSTAIN-6, Online Figure 2A. Among trials that reported post randomization changes in glucose lowering therapies (EMPA-REG, LEADER and SUSTAIN-6) insulin use increased significantly in the placebo arm vs. active comparator (5–8), Online Figure 2B. Sulfonylureas and insulin can lead to weight gain and potentiate hypoglycemia, both of which are associated with increased CVD morbidity and mortality (77, 78). While participants randomized to SGLT2-i or GLP-1 RA lost more weight than those receiving placebo, weight for the placebo arm did not increase. Moreover, there was no significant difference in episodes of severe hypoglycemia between active treatment and placebo in these four trials (8). These findings suggest that post-randomization confounding by increased use of glucose lowering therapies with adverse side effect profiles is unlikely to explain the magnitude of outcome benefit observed (79).
Application of New Therapies to Primary or Secondary Prevention
A key question moving forward will be whether the reduction in CVD events observed with SGLT2-i and GLP-1 RA among T2D patients with established CVD is applicable to the primary prevention of CVD in T2D patients with multiple CVD risk factors. On the one hand, as reviewed, one potential explanation for the lack of mortality benefit in the CANVAS Program and failure of the EXSCEL trial to demonstrate superiority for CV outcomes may be the larger populations of lower risk T2D patients enrolled compared to trials that enrolled populations at higher CVD risk (8, 9). Heterogeneity of treatment effect was also observed with liraglutide, favoring its use in T2D patients with established CVD (6). On the other hand, a recent secondary analysis from the CANVAS Program did not demonstrate heterogeneity of treatment effect with canagliflozin across the primary and secondary prevention groups (80). The ongoing DECLARE-TIMI-58 trial with dapagliflozin will be the largest SGLT2-i trial to date with nearly 60% of the study population a primary prevention cohort (31). DECLARE-TIMI 58 will provide crucial data about expanding the use of these glucose lowering agents to a lower risk, larger population of T2D patients.
Since the glucose lowering effects of SGTL2-i and GLP-1 RA appear to be in part dependent on glucose concentration, and the risk of hypoglycemia is very low, the potential that these agents could be used to lower elevated CVD risk among patients with metabolic syndrome or prediabetes is appealing. In fact, GLP-1 RA have demonstrated efficacy for weight loss and improvement in CV risk factors among obese non-diabetic and prediabetic patients (58). High-dose liraglutide was also demonstrated in this trial to delay the onset of T2D (58).
A New Treatment Paradigm and Future Directions
We have reviewed the remarkable advances the recent CV outcomes trials with SGLT2-i and GLP-1 RA represent for patients with diabetes and healthcare providers, including cardiologists. Figure 5 presents a treatment algorithm encapsulating these recent studies and indicates potential future directions. There are a few issues worth highlighting from the figure and directions forward.
Figure 5: A New Algorithm for CVD Risk Reduction in T2D.
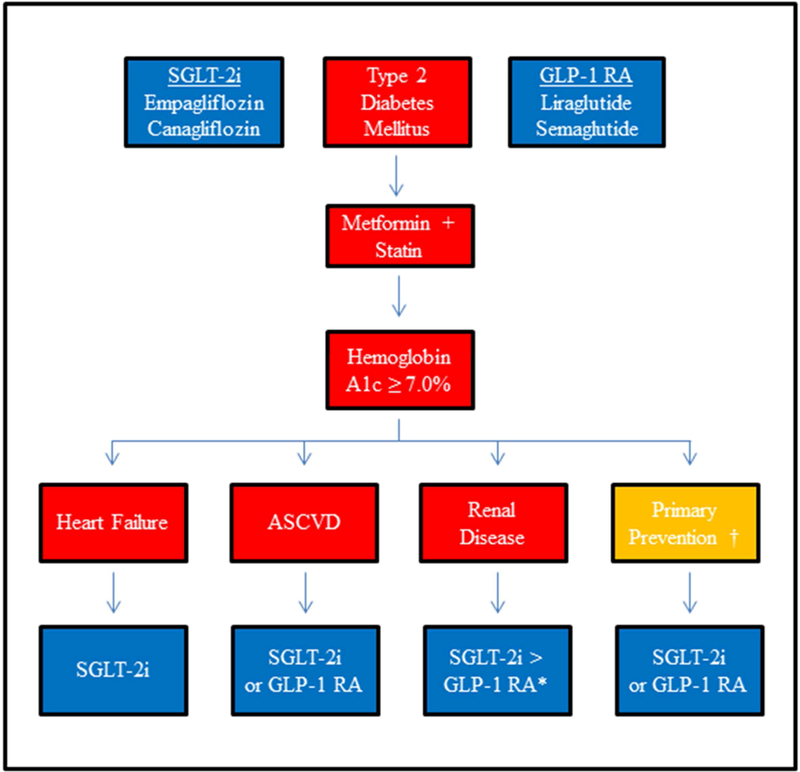
ASCVD, atherosclerotic cardiovascular disease; GLP-1 RA, glucagon-like peptide 1 receptor agonist; SGLT2-i, sodium-glucose cotransporter 2 inhibitor. Proposed algorithm to reduce cardiovascular risk with glucose lowering agents in type 2 diabetics. After the addition of metformin and a moderate to high-intensity statin, clinicians can consider adding an SGLT2-i or GLP-1 RA if the hemoglobin A1c remains above goal of 7.0%. The preference for adding an SGLT2-i or GLP-1 RA can be individualized based on a history of heart failure, ASCVD, or renal disease. In renal disease, SGLT2-i may be favored over GLP-1 RA given the more comprehensive renal benefits associated with SGLT2-i. There is less robust evidence to support the use of these agents for primary prevention.
First, all recent CV outcome trials reviewed were completed on a background of metformin therapy, along with other glucose lowering agents (eFigure 2a). Until the completion of these recent trials, metformin was the only drug with evidence for CV benefit, albeit in very modest numbers of patients with small number of events and relatively low event rates (81). As noted (11), metformin does not cause weight gain or increase risk of hypoglycemia, has many years of safety evidence, and is inexpensive. Therefore, metformin is justifiably used as a first-line therapy for T2D patients with and without established CVD. However, the thousands of patients enrolled in these recent CV outcomes trials dwarf the number included in the landmark UK Prospective Diabetes Study (81). Whether or not metformin background therapy is warranted with concomitant use of SGLT2-i or GLP-1 RA is not entirely certain, and is worthy of future investigation. It is also worth exploring the outcome benefits of SGLT2-i and GLP-1 RA use among patients with diabetes under better glucose control at baseline; participants in the trials reviewed had an average baseline HbA1c over 8%.
Second, very limited data exist on the use of SGLT2-i and GLP-1 RA in combination. Recent data with the combination of 10 mg daily oral dapagliflozin with once-weekly subcutaneous 2 mg exenatide among patients with poor glycemic control on metformin monotherapy demonstrated improved glycemic control, weight loss and lower SBP (82). However, data is needed on the combination of these agents for reduction CV events. A recent network meta-analysis indicates that compared to GLP-1 RA and DPP4 inhibitors, SGLT2-i were most likely to rank best for all-cause and CV mortality, along with heart failure and MI outcomes (83). GLP-1 RA ranked best for stroke reduction, but had increased side effects leading to study drug termination compared to SGLT2-i (83). A comparative effectiveness trial of these two agents, with or without background metformin therapy, may be highly informative.
Third, the use of SGLT2-i and GLP-1 RA warrant dedicated randomized trial level investigation of their effects among T2D patients with reduced eGFR and objectively determined heart failure at baseline. As indicated in this review, there are several ongoing trials in this area that will hopefully shed light on the use of these new agents in critically important patient populations at elevated risk of cardiovascular morbidity and mortality.
Finally, we need to examine the potential benefit of these agents for patients with disordered glucose metabolism, including prediabetes or metabolic syndrome. It has been shown that the vascular effects of disordered glucose metabolism begin significantly before the diagnostic threshold for diabetes has been reached (84). The trials reviewed overall show reductions in CV outcomes likely independent of their effects on glycemic control (5–8). Moreover, the glucose lowering effects of SGLT2-i and GLP-1 RA are glucose-dependent, with very low if any risk of hypoglycemia. Therefore, the study of these agents among patients with prediabetes or metabolic syndrome, with established CVD or multiple CVD risk factors, may be a transformative use of these medications and a method towards primordial CV risk reduction among patients with abnormal glucose metabolism. The experience of statins for the secondary prevention of CVD regardless of baseline LDL (85), or for the primary prevention of CVD (86), may provide a useful paradigm to guide future studies of these agents in the growing population of individuals at elevated risk for T2D.
eFigure 2b. Post Randomization Insulin Use in EMPA-REG, LEADER and SUSTAIN-6.
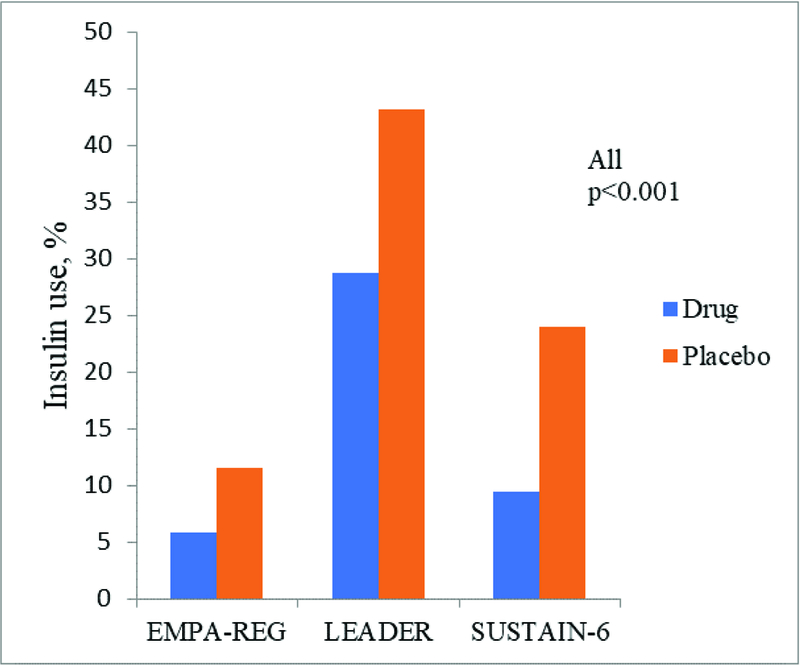
Post-randomization use of insulin in the SGLT2-i or GLP-1 RA group compared to the placebo group.
Acknowledgments
Funding: Dr. Newman was partially funded by the National Heart, Lung, and Blood Institute (NHLBI) of the NIH (K23HL125991). Dr. Berger was partially funded by the NHLBI of the NIH (HL114978). Dr. Aleman has been partially funded by the American Heart Association. Drs. Vani, Weintraub and Schwartzbard have no funding to disclose. Funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the article.
Dr. Weintraub has received honoraria from Amgen, Sanofi and Gilead for consulting; has served on the speakers bureau for Amgen; has received research funding from Amarin, Sanofi and Ionis. Dr. Berger has received research funding from AstraZeneca and Janssen. Dr. Schwartzbard has received research funding to New York University from Merck/Pfizer, Amarin, Sanofi and Ionis.
Abbreviations:
- SGLT2-I
sodium-glucose cotransporter-2 inhibitors
- GLP1-RA
glucagon-like peptide-1 receptor agonists
- T2D
type 2 diabetes mellitus
- CVD
cardiovascular disease
- BMI
body mass index
- HR
hazard ratio
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
low-density lipoprotein cholesterol
- HbA1c
hemoglobin a1C
- MI
myocardial infarction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Schwartzbard serves as a consultant to the formulary committee for Optum Rx. Drs. Newman, Vani and Aleman have no disclosures.
Tweet: The Changing Landscape of Diabetes Therapy for CV Risk Reduction – a paradigm shift to reduce CVD risk in diabetes
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics−−2014 update: a report from the American Heart Association. Circulation 2014;129:e28–e292. Available at: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=24352519&retmode=ref&cmd=prlinks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newman JD, Schwartzbard AZ, Weintraub HS, Goldberg IJ, Berger JS. Primary Prevention of Cardiovascular Disease in Diabetes Mellitus. J. Am. Coll. Cardiol 2017;70:883–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gregg EW, Li Y, Wang J, et al. Changes in Diabetes-Related Complications in the United States, 1990–2010. N. Engl. J. Med 2014;370:1514–1523. [DOI] [PubMed] [Google Scholar]
- 4.Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999–2010. N. Engl. J. Med 2013;368:1613–1624. [DOI] [PubMed] [Google Scholar]
- 5.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 6.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med 2016;375:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marso SP, Bain SC, Consoli A, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med 2016;375:1834–1844. [DOI] [PubMed] [Google Scholar]
- 8.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med 2017:NEJMoa1611925–14. [DOI] [PubMed]
- 9.Holman RR, Bethel MA, Mentz RJ, et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med 2017;377:1228–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Standl E, Schnell O, McGuire DK, Ceriello A, Rydén L. Integration of recent evidence into management of patients with atherosclerotic cardiovascular disease and type 2 diabetes. Lancet Diabetes Endocrinol 2017;5:391–402. [DOI] [PubMed] [Google Scholar]
- 11.Sattar N, Petrie MC, Zinman B, Januzzi JL Jr. Novel Diabetes Drugs and the Cardiovascular Specialist. J. Am. Coll. Cardiol 2017;69:2646–2656. [DOI] [PubMed] [Google Scholar]
- 12.Hirshberg B, Katz A. Cardiovascular outcome studies with novel antidiabetes agents: scientific and operational considerations. Diabetes Care 2013;36 Suppl 2:S253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nissen SE, Wolski K. Effect of Rosiglitazone on the Risk of Myocardial Infarction and Death from Cardiovascular Causes. N. Engl. J. Med 2007;356:2457–2471. [DOI] [PubMed] [Google Scholar]
- 14.Bethel MA, McMurray JJV. Class Effect for Sodium Glucose-Cotransporter-2 Inhibitors in Cardiovascular Outcomes. Circulation 2018;137:1218–1220. [DOI] [PubMed] [Google Scholar]
- 15.Heerspink HJL, Perkins BA, Fitchett DH, Husain M, Cherney DZI. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus. Circulation 2016;134:752–772. [DOI] [PubMed] [Google Scholar]
- 16.Gallo LA, Wright EM, Vallon V. Probing SGLT2 as a therapeutic target for diabetes: basic physiology and consequences. Diab Vasc Dis Res 2015;12:78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes 2005;54:3427–3434. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez V, Weiss MC, Weintraub H, Goldberg IJ, Schwartzbard A. Cardiovascular disease leads to a new algorithm for diabetes treatment. Journal of Clinical Lipidology 2017;11:1126–1133. [DOI] [PubMed] [Google Scholar]
- 19.Yagi S, Hirata Y, Ise T, et al. Canagliflozin reduces epicardial fat in patients with type 2 diabetes mellitus. Diabetol Metab Syndr 2017;9:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chino Y, Samukawa Y, Sakai S, et al. SGLT2 inhibitor lowers serum uric acid through alteration of uric acid transport activity in renal tubule by increased glycosuria. Biopharm Drug Dispos 2014;35:391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borghi C, Rosei EA, Bardin T, et al. Serum uric acid and the risk of cardiovascular and renal disease. J. Hypertens 2015;33:1729–41–discussion 1741. [DOI] [PubMed] [Google Scholar]
- 22.Fitchett D, Butler J, van de Borne P, et al. Effects of empagliflozin on risk for cardiovascular death and heart failure hospitalization across the spectrum of heart failure risk in the EMPA-REG OUTCOME® trial. Eur. Heart J 2017;39:363–370. [DOI] [PubMed] [Google Scholar]
- 23.Inzucchi SE, Zinman B, Fitchett D, et al. How Does Empagliflozin Reduce Cardiovascular Mortality? Insights From a Mediation Analysis of the EMPA-REG OUTCOME Trial. Diabetes Care 2018;41:356–363. [DOI] [PubMed] [Google Scholar]
- 24.Baker WL, Smyth LR, Riche DM, Bourret EM, Chamberlin KW, White WB. Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. J Am Soc Hypertens 2014;8:262–75.e9. [DOI] [PubMed] [Google Scholar]
- 25.Takeshige Y, Fujisawa Y, Rahman A, et al. A sodium-glucose co-transporter 2 inhibitor empagliflozin prevents abnormality of circadian rhythm of blood pressure in salt-treated obese rats. Hypertens. Res 2016;39:415–422. [DOI] [PubMed] [Google Scholar]
- 26.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. JACC 2010;55:1318–1327. [DOI] [PubMed] [Google Scholar]
- 27.Rajasekeran H, Lytvyn Y, Cherney DZI. Sodium-glucose cotransporter 2 inhibition and cardiovascular risk reduction in patients with type 2 diabetes: the emerging role of natriuresis. Kidney International 2016;89:524–526. [DOI] [PubMed] [Google Scholar]
- 28.Skrtic M, Cherney DZI. Sodium-glucose cotransporter-2 inhibition and the potential for renal protection in diabetic nephropathy. Curr Opin Nephrol Hypertens 2015;24:96–103. [DOI] [PubMed] [Google Scholar]
- 29.Vallon V, Richter K, Blantz RC, Thomson S, Osswald H. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J Am Soc Nephrol 1999;10:2569–2576. [DOI] [PubMed] [Google Scholar]
- 30.Jardine MJ, Mahaffey KW, Neal B, et al. The Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) Study Rationale, Design, and Baseline Characteristics. American journal of nephrology 2018;46:462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raz I, Mosenzon O, Bonaca MP, et al. DECLARE-TIMI 58: Participants’ baseline characteristics. Diabetes Obes Metab 2018;32:515. [DOI] [PubMed] [Google Scholar]
- 32.Emerging Risk Factors Collaboration, Seshasai SRK, Kaptoge S, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N. Engl. J. Med 2011;364:829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011;305:2532–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N. Engl. J. Med 2016;375:323–334. [DOI] [PubMed] [Google Scholar]
- 35.Neuen BL, Ohkuma T, Neal B, et al. Cardiovascular and Renal Outcomes With Canagliflozin According to Baseline Kidney Function: Data from the CANVAS Program. Circulation 2018:CIRCULATIONAHA.118.035901. [DOI] [PMC free article] [PubMed]
- 36.Shah AD, Langenberg C, Rapsomaniki E, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1·9 million people. Lancet Diabetes Endocrinol 2015;3:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cubbon RM, Adams B, Rajwani A, et al. Diabetes mellitus is associated with adverse prognosis in chronic heart failure of ischaemic and non-ischaemic aetiology. Diab Vasc Dis Res 2013;10:330–336. [DOI] [PubMed] [Google Scholar]
- 38.Rådholm K, Figtree G, Perkovic V, et al. Canagliflozin and Heart Failure in Type 2 Diabetes Mellitus: Results From the CANVAS Program (Canagliflozin Cardiovascular Assessment Study). Circulation 2018:CIRCULATIONAHA.118.034222. [DOI] [PMC free article] [PubMed]
- 39.Abdul-Ghani M, Del Prato S, Chilton R, DeFronzo RA. SGLT2 Inhibitors and Cardiovascular Risk: Lessons Learned From the EMPA-REG OUTCOME Study. Diabetes Care 2016;39:717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fitchett DH, Udell JA, Inzucchi SE. Heart failure outcomes in clinical trials of glucose-lowering agents in patients with diabetes. Eur J Heart Fail 2017;19:43–53. [DOI] [PubMed] [Google Scholar]
- 41.Kosiborod M, Cavender MA, Fu AZ, et al. Lower Risk of Heart Failure and Death in Patients Initiated on Sodium-Glucose Cotransporter-2 Inhibitors Versus Other Glucose-Lowering Drugs: The CVD-REAL Study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors). Circulation 2017;136:249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Udell JA, Yuan Z, Rush T, Sicignano NM, Galitz M, Rosenthal N. Cardiovascular Outcomes and Risks After Initiation of a Sodium Glucose Cotransporter 2 Inhibitor: Results From the EASEL Population-Based Cohort Study (Evidence for Cardiovascular Outcomes With Sodium Glucose Cotransporter 2 Inhibitors in the Real World). Circulation 2018;137:1450–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ayabe K, Goto S, Goto S. Persistence and Discontinuation of Oral Anticoagulant: Remaining Issues Not Addressed by Phase III Clinical Trials. J Am Heart Assoc 2016;5:e003258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kohler S, Zeller C, Iliev H, Kaspers S. Safety and Tolerability of Empagliflozin in Patients with Type 2 Diabetes: Pooled Analysis of Phase I–III Clinical Trials. Advances in Therapy 2017;34:1707–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inzucchi SE, Iliev H, Pfarr E, Zinman B. Empagliflozin and Assessment of Lower-Limb Amputations in the EMPA-REG OUTCOME Trial. Diabetes Care 2017;41:e4–e5. [DOI] [PubMed] [Google Scholar]
- 46.Fadini GP, Avogaro A. SGLT2 inhibitors and amputations in the US FDA Adverse Event Reporting System. Lancet Diabetes Endocrinol 2017;5:680–681. [DOI] [PubMed] [Google Scholar]
- 47.Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome. N. Engl. J. Med 2015;373:2247–2257. [DOI] [PubMed] [Google Scholar]
- 48.Drucker DJ. The biology of incretin hormones. Cell Metabolism 2006;3:153–165. [DOI] [PubMed] [Google Scholar]
- 49.Nauck MA, Meier JJ, Cavender MA, Abd El Aziz M, Drucker DJ. Cardiovascular Actions and Clinical Outcomes With Glucagon-Like Peptide-1 Receptor Agonists and Dipeptidyl Peptidase-4 Inhibitors. Circulation 2017;136:849–870. [DOI] [PubMed] [Google Scholar]
- 50.Perley MJ, Kipnis DM. Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic sujbjects. J. Clin. Invest 1967;46:1954–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deacon CF. Circulation and degradation of GIP and GLP-1. Horm Metab Res 2004;36:761– 765. [DOI] [PubMed] [Google Scholar]
- 52.Lim GE, Brubaker PL. Glucagon-Like Peptide 1 Secretion by the L-Cell: The View From Within. Diabetes 2006;55:S70–S77. [Google Scholar]
- 53.MacDonald PE, El-Kholy W, Riedel MJ, Salapatek AMF, Light PE, Wheeler MB. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes 2002;51 Suppl 3:S434–42. [DOI] [PubMed] [Google Scholar]
- 54.Smilowitz NR, Donnino R, Schwartzbard A. Glucagon-like peptide-1 receptor agonists for diabetes mellitus: a role in cardiovascular disease. Circulation 2014;129:2305–2312. [DOI] [PubMed] [Google Scholar]
- 55.Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care 2011;34 Suppl 2:S279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nature Publishing Group 2012;8:728–742. [DOI] [PubMed] [Google Scholar]
- 57.Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab 2015;6:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pi-Sunyer X, Astrup A, Fujioka K, et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. N. Engl. J. Med 2015;373:11–22. [DOI] [PubMed] [Google Scholar]
- 59.Kim M, Platt MJ, Shibasaki T, et al. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nature Medicine 2013;19:567–575. [DOI] [PubMed] [Google Scholar]
- 60.Sassoon DJ, Tune JD, Mather KJ, et al. Glucagon-Like Peptide 1 Receptor Activation Augments Cardiac Output and Improves Cardiac Efficiency in Obese Swine After Myocardial Infarction. Diabetes 2017;66:2230–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun F, Wu S, Wang J, et al. Effect of Glucagon-like Peptide-1 Receptor Agonists on Lipid Profiles Among Type 2 Diabetes: A Systematic Review and Network Meta-analysis. Clinical Therapeutics 2015;37:225–241.e8. [DOI] [PubMed] [Google Scholar]
- 62.Timmers L, Henriques JPS, de Kleijn DPV, et al. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. JACC 2009;53:501–510. [DOI] [PubMed] [Google Scholar]
- 63.Nystrom T, Gutniak MK, Zhang Q, et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab 2004;287:E1209–15. [DOI] [PubMed] [Google Scholar]
- 64.Dicembrini I, Nreu B, Scatena A, et al. Microvascular effects of glucagon-like peptide-1 receptor agonists in type 2 diabetes: a meta-analysis of randomized controlled trials. Acta Diabetologica 2017;54:933–941. [DOI] [PubMed] [Google Scholar]
- 65.Dahl-Jorgensen K, Brinchmann-Hansen O, Hanssen KF, Sandvik L, Aagenaes O. Rapid tightening of blood glucose control leads to transient deterioration of retinopathy in insulin dependent diabetes mellitus: the Oslo study. Br Med J (Clin Res Ed) 1985;290:811–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Faillie J- L, Yu OH, Yin H, Hillaire-Buys D, Barkun A, Azoulay L. Association of Bile Duct and Gallbladder Diseases With the Use of Incretin-Based Drugs in Patients With Type 2 Diabetes Mellitus. JAMA Intern Med 2016;176:1474–1481. [DOI] [PubMed] [Google Scholar]
- 67.Nauck MA, Friedrich N. Do GLP-1-based therapies increase cancer risk? Diabetes Care 2013;36 Suppl 2:S245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bethel MA, Patel RA, Merrill P, et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol 2018;6:105–113. [DOI] [PubMed] [Google Scholar]
- 69.Lorenz M, Lawson F, Owens D, et al. Differential effects of glucagon-like peptide-1 receptor agonists on heart rate. Cardiovasc Diabetol 2017;16:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Margulies KB, Hernandez AF, Redfield MM, et al. Effects of Liraglutide on Clinical Stability Among Patients With Advanced Heart Failure and Reduced Ejection Fraction. JAMA 2016;316:500–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.American Diabetes Association. Standards of Medical Care in Diabetes - 2016. Diabetes Care 2016;39:S1–S93.26696671 [Google Scholar]
- 72.Leon BM, Maddox TM. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes 2015;6:1246– 1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gaede P, Vedel P, Larsen N, Jensen GVH, Parving H-H, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N. Engl. J. Med 2003;348:383–393. [DOI] [PubMed] [Google Scholar]
- 74.Gaede P, Vedel P, Parving HH, Pedersen O. Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet 1999;353:617–622. [DOI] [PubMed] [Google Scholar]
- 75.Farkouh ME, Boden WE, Bittner V, et al. Risk factor control for coronary artery disease secondary prevention in large randomized trials. J. Am. Coll. Cardiol 2013;61:1607–1615. [DOI] [PubMed] [Google Scholar]
- 76.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63(25 Pt B):2889–934. [DOI] [PubMed] [Google Scholar]
- 77.Desouza CV, Bolli GB, Fonseca V. Hypoglycemia, Diabetes, and Cardiovascular Events. Diabetes Care 2010;33:1389–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Udell JA, Cavender MA, Bhatt DL, Chatterjee S, Farkouh ME, Scirica BM. Glucose-lowering drugs or strategies and cardiovascular outcomes in patients with or at risk for type 2 diabetes: a meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol 2015;3:356–366. [DOI] [PubMed] [Google Scholar]
- 79.Manson JE, Shufelt CL, Robins JM. The Potential for Postrandomization Confounding in Randomized Clinical Trials. JAMA 2016;315:2273–2274. [DOI] [PubMed] [Google Scholar]
- 80.Mahaffey KW, Neal B, Perkovic V, et al. Canagliflozin for Primary and Secondary Prevention of Cardiovascular Events: Results From the CANVAS Program (Canagliflozin Cardiovascular Assessment Study). Circulation 2018;137:323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. The Lancet 1998;352:854–865. [PubMed] [Google Scholar]
- 82.Frías JP, Guja C, Hardy E, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol 2016;4:1004–1016. [DOI] [PubMed] [Google Scholar]
- 83.Zheng SL, Roddick AJ, Aghar-Jaffar R, et al. Association Between Use of Sodium-Glucose Cotransporter 2 Inhibitors, Glucagon-like Peptide 1 Agonists, and Dipeptidyl Peptidase 4 Inhibitors With All-Cause Mortality in Patients With Type 2 Diabetes. JAMA 2018;319:1580–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wasserman DH, Wang TJ, Brown NJ. The Vasculature in Prediabetes. Circ. Res 2018;122:1135–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7–22.12114036 [Google Scholar]
- 86.Ridker PM, Danielson E, Fonseca FAH, et al. Rosuvastatin to Prevent Vascular Events in Men and Women with Elevated C-Reactive Protein. N. Engl. J. Med 2008;359:2195–2207. [DOI] [PubMed] [Google Scholar]


