Abstract
Resolution of leishmaniasis depends upon parasite control and limiting inflammation. CD4+ Th1 cells are required to control parasites, while CD8+ T cells play a dual role: they promote Th1 cell differentiation, but can also increase inflammation at the site of infection as a consequence of cytolysis. While CD8+ T cells taken from leishmanial lesions are cytolytic, here we showed that only a few CD8+ T cells produced IFN-γ. Correspondingly, only low levels of IL-12 and/or IL-12 mRNA levels were present in lesions from infected mice, as well as patients. Addition of IL-12 increased IFN-γ production by CD8+ T cells isolated from leishmanial lesions, suggesting that a lack of IL-12 at the site of infection limits IFN-γ production by CD8+ T cells. To determine if CD8+ T cells could promote resistance in vivo if IL-12 was present, we administered IL-12 to leishmania-infected RAG mice reconstituted with CD8+ T cells. IL-12 treatment increased the ability of CD8+ T cells to make IFN-γ, but CD8+ T cells still failed to control the parasites. Furthermore, despite the ability of CD8+ T cells to promote immunity to secondary infections, we also found that CD8+ T cells from immune mice were unable to control leishmania in RAG mice. Taken together, these results indicate that lesional CD8+ T cells fail to make IFN-γ due to a deficit in IL-12, but that even with IL-12 CD8+ T cells are unable to control leishmania in the absence of CD4+ T cells.
Introduction
Cutaneous leishmaniasis is a major public health problem with an estimate of one million new cases each year (1). Disease develops after the infection with parasites from the genus Leishmania and both the parasite species and the immune response of the infected host determine disease severity (2). Therefore, dissecting the role the immune response plays in controlling disease or promoting inflammation is essential for designing vaccines and therapies for leishmaniasis patients.
Upon leishmania infection, dendritic cells release the cytokine IL-12 and induce the differentiation of CD4+ T cells into T helper 1 (Th1) cells, a critical step for IFN-γ production (3, 4). The production of IFN-γ is essential to control leishmania parasites through the generation of nitric oxide and superoxide anion, as both can effectively kill leishmania parasites (5, 6). Besides CD4+ T cells, CD8+ T cells are also capable of making IFN-γ in leishmaniasis (7–10). In fact, IFN-γ produced by CD8+ T cells contributes to CD4+ T cell-differentiation into protective Th1 cells after infection (7). Conversely, CD8+ T cells present in the skin can contribute to inflammation thereby promoting disease severity in murine and human cutaneous leishmaniasis (11–17). The inability of CD8+ T cells alone to play a protective role can be experimentally demonstrated by adoptively transferring CD8+ T cells into RAG mice, which leads to severe pathology and no parasite control (10, 13). Once recruited into lesions, CD8+ T cells exhibit a cytotoxic profile, which results in killing of infected and uninfected cells, inflammasome activation and IL-1β release (12). This cascade of events promotes severe inflammation, parasite dissemination and is associated with grave disease manifestations in patients. Therefore, CD8+ T cells have been shown to play distinctive functions in disease: they can play a protective role by producing IFN-γ that promotes Th1 cell development or they can be pathogenic in the skin by being cytotoxic.
Since CD8+ T cells have been associated with promoting protection in low dose primary infections (7, 10), as well as in resistance to secondary infections (8, 9), they have long been considered a target for a leishmanial vaccine (18–21). However, given their potential pathologic role, an important question to address is whether their cytolytic (and consequently pathologic) activity can be limited, thus generating CD8+ T cells that only play a protective role. To address this we adoptively transferred perforin deficient CD8+ T cells into RAG mice, which blocked the immunopathologic activity of the CD8+ T cells. However, CD8+ T cells were still unable to control the parasites (13).
Here we have investigated whether the inability of CD8+ T cells to provide protection in the absence of CD4+ T cells might be due to a deficit in IFN-γ production by CD8+ T cells at the infection site. We found that CD8+ T cells do not make IFN-γ within lesions and that the inability of CD8+ T cells to produce IFN-γ in the skin can be explained by the lack of local IL-12 production. This led us to test if CD8+ T cells could provide protection in the absence of CD4+ T cells if they made IFN-γ. Exogenous administration of IL-12 induced IFN-γ producing CD8+ T cells in the skin; however, CD8+ T cells were unable to provide protection in the absence of CD4+ T cells. Immune CD8+ T cells also could not prevent parasite replication and therefore we conclude that CD8+ T cells are pathogenic in the skin after leishmania infection and cannot be rendered protective even when signals to induce IFN-γ are provided.
Materials and Methods
Mice.
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee, University of Pennsylvania Animal Welfare. BALB/c and C57BL/6 CD45.2 or CD45.1 mice (6 weeks old) were purchased from Charles River, and RAG−/− (B6.129S7-RAG1tm1Mom) and C57BL/6 IL-12p40 YFP reporter mice were purchased from The Jackson Laboratory. Ifng/Thy1.1 knock-in mice were provided by C. Weaver (University of Alabama). Both males and females were used for experiments. All mice were maintained in a specific pathogen-free environment at the University of Pennsylvania Animal Care Facilities.
Parasites.
L. braziliensis parasites (strain MHOM/BR/01/BA788) and L. major Friedlin strain (MHOM/IL/80/FN) were grown in Schneider’s insect medium (GIBCO) supplemented with 20% heat-inactivated FBS, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Metacyclic enriched promastigotes were used for infection (22). Mice were infected with either 105 or 106 metacyclic promastigotes in the left ear, and the course of lesion progression was monitored weekly by measuring the diameter of ear induration with digital calipers (Fisher Scientific).
DNA and recombinant IL-12 treatment.
The DNA adjuvant construct encoding IL-12 has been described previously (23) and was provided and supplied by Inovio Pharmaceuticals. BALB/c mice were injected with 60 μg of IL-12 or empty plasmid in the ear together with leishmania parasites. RAG mice were infected and treated with 0.5 μg intraperitoneally of recombinant IL-12 everyday until day 9 post infection.
Cell purification and adoptive transfer.
Splenocytes were collected from CD45.2 Thy1.1 IFN-γ reporter mice and labeled with 1.25 μM of Carboxyfluorescein succinimidyl ester (CFSE) for 10 minutes at room temperature. The reaction was quenched by the addition of newborn calf serum and cells were washed by centrifugation three times. Cell suspension was then transferred intravenously into CD45.1 recipients that were immediately infected with L. major. For experiments with RAG mice, splenocytes from C57BL/6 mice were collected, red blood cells lysed with ACK lysing buffer (LONZA) and CD8+ T cells were purified using a magnetic bead separation kit (Miltenyi Biotec). Three million CD8+ T cells were transferred into RAG mice that were subsequently infected with L. braziliensis. Mice reconstituted with CD8+ T cells received 4 injections of 250 μg of anti-CD4 within the first 2 weeks in order to ensure that no CD4+ T cells were present.
Skin preparation.
Infected and uninfected ears were harvested, the dorsal and ventral layers of the ear separated, and the ears incubated in RPMI (Gibco) with 250 μg/mL of Liberase TM (Roche) for 90 mins at 37oC/5% CO2. Following incubation, the enzyme reaction was stopped using 1mL of RPMI media containing 10% FBS. Ears were dissociated using a cell strainer (40 μm, BD Pharmingen) and an aliquot of the cell suspension was used for parasite titration.
In vitro stimulation of skin cells with cytokines.
Skin cell suspension was incubated overnight with RPMI supplemented with 10% heat-inactivated FBS, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were incubated with media only or cytokines were added at the following concentrations: IL-12 at 20 ng/mL and IL-18 at 50 ng/mL.
Parasite titration.
The parasite burden in the ears was quantified as described previously (7). Briefly, the homogenate was serially diluted (1:10) in 96-well plates and incubated at 26°C. The number of viable parasites was calculated from the highest dilution at which parasites were observed after 7 days.
Flow cytometric analysis.
Cell suspensions from mice were incubated with PMA (50 ng/mL), ionomycin (500 ng/mL) and Brefeldin A (BFA) (10 μg /mL) (all from SIGMA) or BFA only, as indicated, for IFN-γ intracellular staining. Before surface and intracellular staining, cells were washed and stained with live/dead fixable aqua dead cell stain kit (Molecular Probes), according to manufacturer instructions. All flow cytometry analysis was performed using the FlowJo Software. The antibodies were: anti-CD11b eF450, anti-CD3 eFluor 450, anti-Thy1.1 (CD90.1) PeCy7 and anti-IFN-γ PeCy7 (all from eBioscience), anti-CD4 APC-AF780 (Invitrogen) and anti-CD8β PerCPCy5.5 (Biolegend).
Transcriptional profiling.
For transcriptional profiling, cRNA was generated from 10 normal skin and 25 lesion biopsy samples as described previously (14). Data is deposited on the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/) for public access (GSE number GSE55664).
Statistical analysis.
Data are presented as mean ± standard error or individual samples. Statistical significance was determined using the two-tailed unpaired Student’s t-test. All statistical analysis was calculated using Prism software (GraphPad). Differences were considered significant when p ≤ 0.05 (*), p ≤ 0.01 (**) or p ≤ 0.001 (***).
Results
CD8+ T cells fail to produce IFN-γ in leishmania-infected skin.
IFN-γ is essential for controlling leishmania infection (2), and while IFN-γ from CD8+ T cells facilitates Th1 cell differentiation in the lymph nodes, the inability of CD8+ T cells to provide protection in the absence of CD4+ T cells could be because they fail to produce IFN-γ within lesions. To directly address this issue, CD8+ T cells from the skin and draining LNs (LN) of L. major infected C57BL/6 mice were analyzed for IFN-γ production after PMA and ionomycin stimulation. We found that CD8+ T cells from the LN were capable of producing IFN-γ, while CD8+ T cells from the skin produced little to no IFN-γ (Figure 1A and B). Without PMA and ionomycin stimulation IFN-γ was not detected in the skin or LN at this time-point (data not shown).
Figure 1:
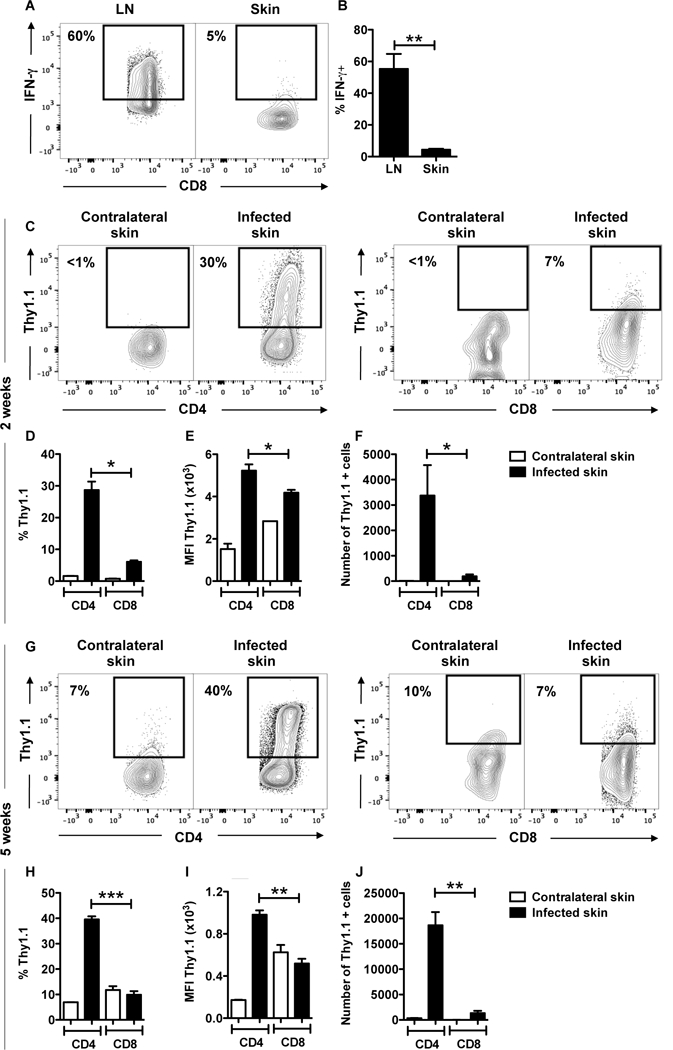
CD8+ T cells do not produce IFN-g in the skin in response to L. major. (A, B) C57BL/6 mice were infected in the skin with 106 L. major and 5 weeks post infection mice were euthanized. The expression of intracellular IFN-g in CD8+ T cells was measured by flow cytometry in the draining LNs (LN) and infected ears. Depicted are (A) representative contour plots and bar graphs showing (B) percentage of IFN-g expressing CD8+ T cells in the draining lymph nodes (LN) and infected skin. (C-J) IFN-g reporter (Thy1.1) mice were infected in the skin with 106 L. major and 2 (C-F) or 4 (G-J) weeks post infection mice were euthanized. The expression of Thy1.1 directly ex vivo in CD4+ and CD8+ T cells was measured by flow cytometry in contralateral and infected ears. Depicted are (C, G) representative contour plots and bar graphs showing (D, H) percentage, (E, I) mean fluorescence intensity (MFI) and (F, J) number of Thy 1.1 expressing CD4+ and CD8+ T cells. Flow plots pregated on live/singlets/CD3/CD8b or CD4. Representative data from 3 or more independent experiments (n = 3 – 5 mice per group) are presented. *p ≤ 0.05, **p ≤ 0.01 or *** p≤ 0.001
We next compared IFN-γ expression in CD4+ and CD8+ T cells from the lesions, and used an IFN-γ reporter mouse that expresses Thy1.1 as a result of IFN-γ transcription, thus allowing us to directly assess IFN-γ without restimulation of the cells (24). At 2 (Figure 1 C-F) and 5 (Figure 1 G-J) weeks post infection, CD4+ T cells produced IFN-γ, while significantly fewer CD8+ T cells present in the infected skin were capable of producing IFN-γ (Figure 1 C, D and G, H). Not only were there few CD8+ T cells that produced IFN-γ, but CD8+ T cells also produced lower amounts of IFN-γ on a per cell basis, as evidenced by lower mean fluorescence intensity (MFI) levels (Figure 1 E and I). An analysis of the number of IFN-γ producing cells indicated that more CD4+ T cells produce IFN-γ in comparison to CD8+ T cells (Figure 1 F and J), and when we phenotyped all the cells within lesions that were making IFN-γ, over 80% were CD4+ T cells (Supplemental Figure 1). To ensure that we were studying T cells that were responding to the infection, we CFSE-labeled splenocytes and transferred them to CD45 congenic mice (Figure 2 A). The majority of donor cells we found in the lesions 2 weeks later were CFSEdim, indicating that they had proliferated in response to the infection. As above, we found that CD4+ T cells were producing IFN-γ in the lesions, but proliferating CD8+ T cells still produced very little IFN-γ (Figure 2 B and C). To determine if this was unique to L. major, we assessed IFN-γ production by CD8+ T cells in lesions from L. braziliensis infected mice. C57BL/6 mice were infected with L. major (Supplemental Figure 2 A and B) or L. braziliensis (Supplemental Figure 2 C and D) or BALB/c mice were infected with L. braziliensis (Supplemental Figure 2 E and F) and intracellular IFN-γ production without stimulation was determined by flow cytometry at 5 weeks post infection. Cells from the skin of all groups of infected mice showed a greater percentage of CD4+ T cells expressing IFN-γ protein than CD8+ T cells (Supplemental Figure 2A-F). Taken together, these results show that CD8+ T cells are not a major source of IFN-γ within leishmanial lesions.
Figure 2:
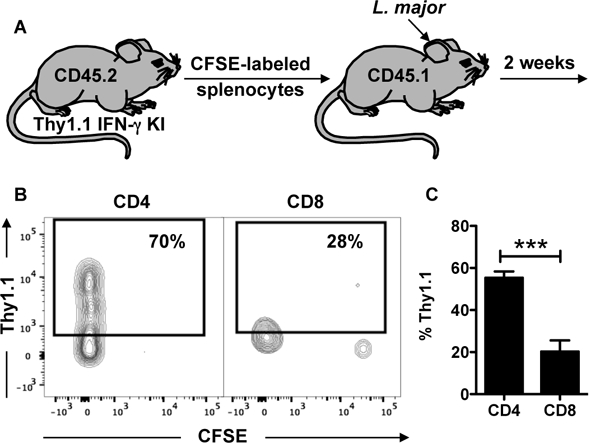
CD8+ T cells that have proliferated in response to L. major infection do not produce IFN-g in the skin. (A) Splenocytes from CD45.2 Thy1.1 IFN-g reporter mice were stained with CFSE and transferred into CD45.1 congenic mice infected with L. major. Two weeks post infection, mice were euthanized and donor cells were analyzed for CFSE dilution and IFN-g production. Depicted are (B) representative contour plots and (C) bar graph of Thy1.1 expressing donor CD4+ and CD8+ T cells. Flow plots pregated on live/singlets/CD3/CD8b or CD4. Representative data from 4 independent experiments (n = 3 mice) are presented. ***p ≤ 0.001
IL-12 expression is deficient in leishmania-infected skin from mice and humans.
IL-12 induces IFN-γ production and is required for resistance to leishmania infection (3, 25). Therefore, we considered the possibility that IL-12 levels in the skin were insufficient to induce IFN-γ production by CD8+ T cells. Therefore, we assessed expression of IL-12 in the lesions of either L. major or L. braziliensis-infected mice, and for that we used an IL-12p40 reporter mouse. We found that IL-12p40 expression was not altered in mice infected with L. major or L. braziliensis in comparison to the contralateral ears (Figure 3 A, B). However, we could detect IL-12p40 expression in the draining LNs of the same groups of mice (Figure 3 C, D). The low level expression of IL-12 mRNA was not only seen in mice. We previously published a genomic profiling comparing human normal skin with L. braziliensis patients’ lesions (14) and here we used this dataset to ask if IL-12 gene expression was differentially expressed between the two groups. We found that both IL12A and IL12B mRNA levels are unchanged between normal skin and lesions (Figure 3 E, F). Importantly, the levels of expression in both groups reach the lower limit of detection of the assay, suggesting that IL12A and IL12B are not expressed in the skin of either normal or lesion biopsies. Together, these results indicate that IL-12 production is not present in the skin of patients or mice infected with leishmania and led us to hypothesize that CD8+ T cells are defective in IFN-γ production due to the lack of IL-12 signaling at the site of infection.
Figure 3:
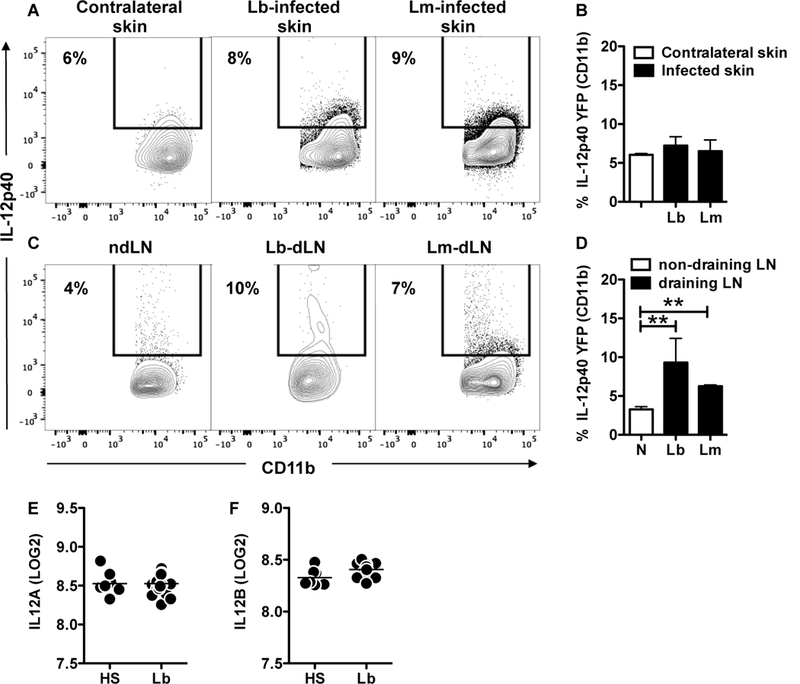
IL-12 is not produced in leishmania lesions from mice and humans. IL-12p40 reporter mice were infected in the skin with L. major or L. braziliensis and 2 weeks post infection mice were euthanized. Cells from the (A, B) contralateral (a combination between contralateral skin from Lb and Lm infected mice) and infected skin or (C, D) non-draining (ndLN, a combination between ndLN from Lb and Lm infected mice) and draining lymph nodes (dLN) were analyzed for IL-12p40 expression directly ex vivo by flow cytometry. Depicted are (A, C) representative contour plots and (B, D) bar graphs showing the percentage of IL-12p40+ CD11b cells. Flow plots pregated on live/singlets/CD11b.Representative data from 2 independent experiments (n = 3 mice per group) with similar results are presented. **p ≤ 0.01. LOG2 expression of (E) IL12A and (F) IL12B in the skin of healthy subjects (HS) and L. braziliensis patients (Lb). Data obtained from 10 HS and 25 Lb.
CD8+ T cells from leishmanial lesions make IFN-γ in the presence of IL-12.
To determine if CD8+ T cells from lesions could make IFN-γ if IL-12 was present, single cell suspensions from the lesions of mice infected with L. major for 2 weeks were incubated overnight with or without IL-12, the cells received brefeldin A for the last 4 hours of culture, and IFN-γ intracellular protein expression was determined by flow cytometry. Cultures without the addition of cytokines (media) showed that CD8+ T cells did not make IFN-γ (Figure 4A) while an average of 60% of the CD4+ T cells present in the skin produced IFN-γ (Figure 4B). While neither IL-12 nor IL-18 alone significantly altered IFN-γ production by either CD4+ or CD8+ T cells (Figure 4A,B), both CD4+ and CD8+ T cells stimulated with IL-12 in the presence of IL-18 increased their IFN-γ production (Figure 4C). Although CD8+ T cells significantly increased their capacity to make IFN-γ in the presence of IL-12+IL-18, only 50% of those cells produced IFN-γ in this ideal scenario. These results indicate that lack of IL-12 in the skin has a dramatic impact in the capacity of CD8+ T cells to produce IFN-γ, whereas it has only a mild effect on IFN-γ production by CD4+ T cells.
Figure 4:
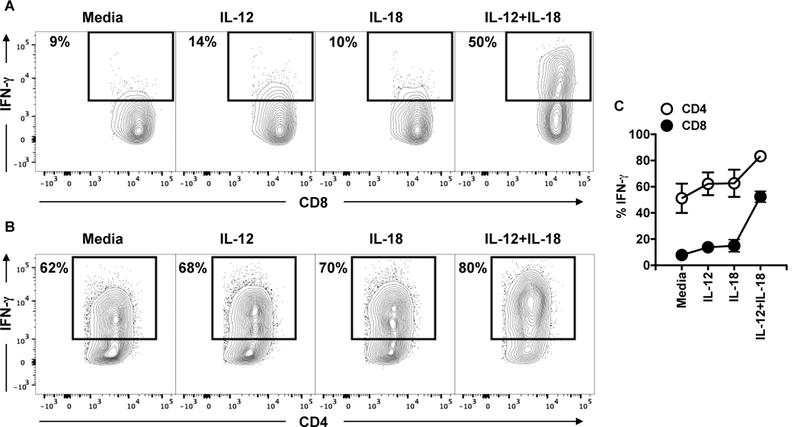
CD4+ and CD8+ T cells have different requirements for IFN-g in leishmania-infected skin. C57BL/6 were infected in the skin with 106 L. major and 2 weeks post infection mice were euthanized. Cells from the infected skin were cultured with media or cytokines overnight and BFA for the last 4 hours; the expression of IFN-g was measured by flow cytometry. CD4+ and CD8+ T cells were analyzed for the expression IFN-g by flow cytometry. Depicted are (A and B) representative contour plots and (C) graph showing expression of IFN-g. Representative data from 4 independent experiments (n = 3 mice per group) with similar results are presented.
IL-12 treatment in vivo enhances IFN-γ production by CD8+ T cells in the skin.
To ask if providing IL-12 in the skin early after infection could enhance IFN-γ production by CD8+ T cells, we infected mice with L. braziliensis in the presence of an IL-12 plasmid or a control plasmid. As expected, the administration of the IL-12 plasmid at the site of infection reduced the lesion development in mice and also significantly decreased the number of parasites in the skin 6 weeks post infection (Figure 5 A, B). To test if IL-12 administration had an impact in IFN-γ production by CD8+ T cells, we took single cell suspensions from the ears of naïve, control or IL-12-treated mice and checked for intracellular IFN-γ production by CD8+ T cells by flow cytometry. We found that mice that received IL-12 had a significantly higher percentage of CD8+ T cells producing IFN-γ in the skin detected after PMA and ionomycin stimulation directly ex vivo (Figure 5 C, D). Hence, IL-12 administration in the skin provides signals to induce IFN-γ producing CD8+ T cells in the skin.
Figure 5:
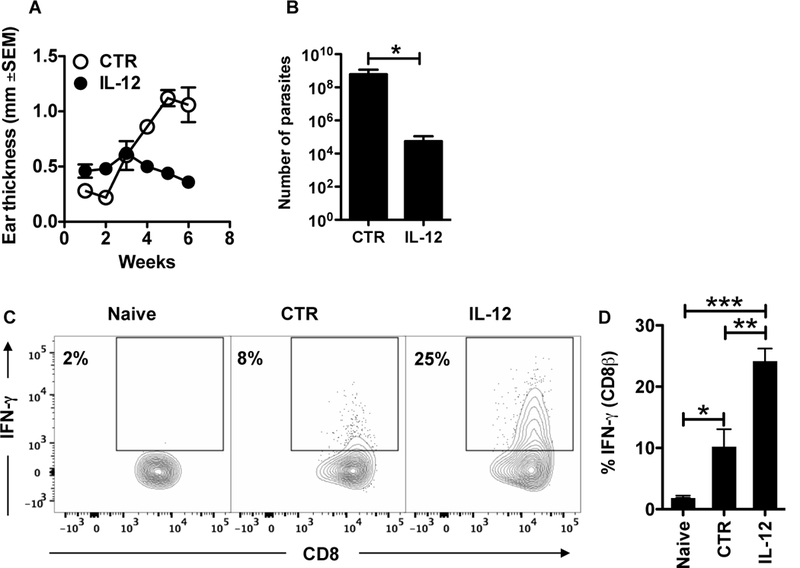
IL-12 treatment enhances IFN-g production by CD8+ T cells in the skin. BALB/c mice were infected in the skin with 105 L. braziliensis in conjunction with a control (CTR) or IL-12 plasmid; (A) ear thickness was assessed weekly. Six weeks post infection mice were euthanized and the (B) number of parasites was determined in the skin and lesions were digested and used for flow cytometric analysis of intracellular IFN-g. Depicted are (C) representative contour plots and (D) bar graph of IFN-g intracelullar staining. Flow plots pregated on live/singlets/CD3/CD8b.Data from one experiment (n = 5 mice per group). *p ≤ 0.05, **p ≤ 0.01 or ***p ≤ 0.001
IL-12 administration is not sufficient to induce protection by CD8+ T cells in vivo.
To determine if IL-12 administration could promote the development of IFN-γ producing CD8+ T cells that could mediate protection in the absence of CD4+ T cells, RAG mice infected with L. braziliensis were reconstituted with CD8+ T cells and received injections of recombinant IL-12 during the first week of infection or were left untreated. RAG mice reconstituted with CD8+ T cells and infected with either L. major or L. braziliensis develop severe non-healing lesions without any evidence of parasite control (10, 13). We hypothesized that IL-12 administration would promote the development of protective CD8+ T cells, and that a reduced parasite burden would limit pathology. Indeed, we found that administration of recombinant IL-12 into mice prevented the development of severe lesions in mice that received WT CD8+ T cells (Figure 6 A, left). Treatment with IL-12 not only prevented the development of severe lesions at the primary site, but also blocked the development of metastatic lesions at other skin sites (Figure 6 A, right). Surprisingly, the abrogated lesion development in mice treated with IL-12 was accompanied by no reduction in the number of parasites in the skin (Figure 6 B), though we could detect an increase in IFN-γ expressing CD8+ T cells in the skin of mice after treatment with IL-12 (Figure 6 C, D). These results indicate that while IL-12 plays a role in regulating the CD8+ T cell response in RAG mice, CD8+ T cells by themselves, even in the presence of IL-12, are unable to provide parasite control.
Figure 6:
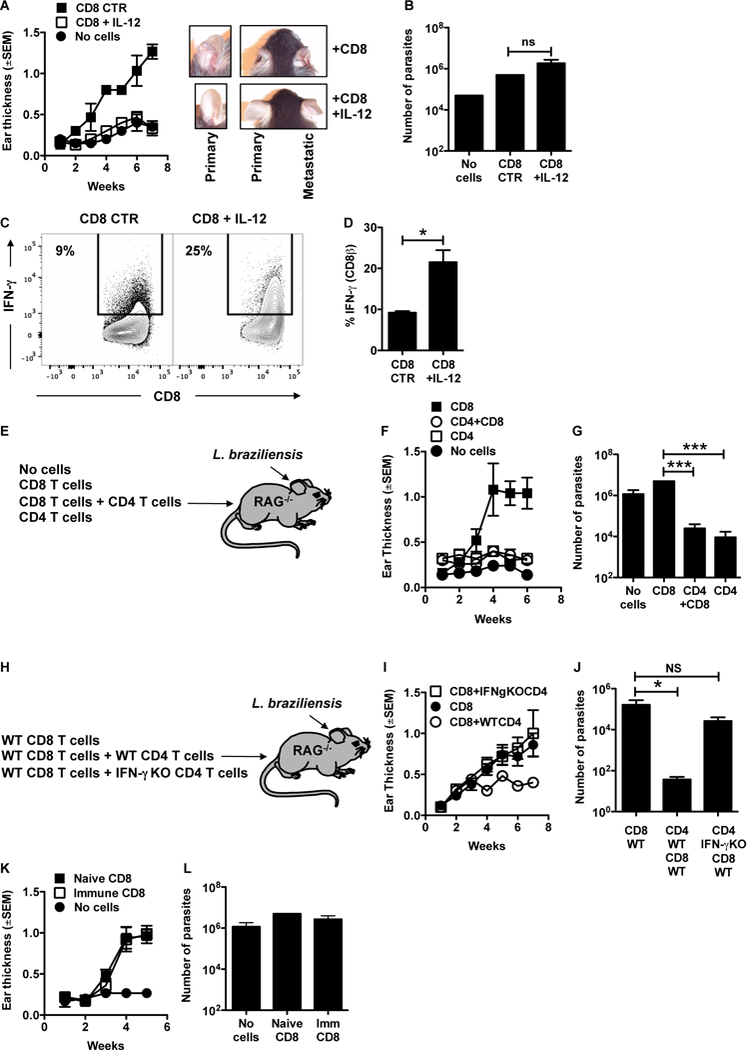
CD8+ T cells are unable to control leishmania infection. (A-D) RAG−/− mice were infected with L. braziliensis and reconstituted with CD8+ T cells or did not receive cells. At days 0 through 9 mice were treated with 0.5 mg/mouse of IL-12 i.p.; (A) course of infection and (B) number of parasites assessed in the skin at 7 weeks post infection. (C) Representative contour plots and (D) bar graphs of IFN-g expression in CD8+ T cells from the skin. Representative data from 4 independent experiments (n = 3 to 5 mice per group) with similar results are presented. Flow plots pregated on live/singlets/CD3/CD8b. (E) RAG−/− mice were infected with L. braziliensis and reconstituted with CD8+ T cells, CD4+ T cells or CD8+ and CD4+ T cells or did not receive cells. (F) Course of infection and (G) number of parasites assessed in the skin at 7 weeks post infection. Representative data from 2 independent experiments (n = 5 mice per group) with similar results are presented. (H) RAG−/− mice were infected with L. braziliensis and reconstituted with WT CD8+ T cells, WT CD8+ and WT CD4+ T cells or WT CD8+ and IFN-g KO CD4+ T cells or did not receive cells. (I) Course of infection and (J) number of parasites assessed in the skin at 7 weeks post infection. (K,L) C57BL/6 mice naive or infected with L. braziliensis for 10–15 weeks were euthanized and splenocytes were used as donors of CD8+ T cells. RAG−/− mice were infected with L. braziliensis and reconstituted with immune or naive CD8+ T cells or did not receive cells and (K) course of infection and (L) number of parasites assessed in the skin at 7 weeks post infection. Representative data from 3 independent experiments for the course of infection with similar results are presented; and one for the parasite titration (n = 5 mice per group) *p ≤ 0.05 or ***p ≤ 0.001; ns, non-significant
IFN-γ derived from CD4+ T cells is sufficient for parasite control in Leishmania infection.
In order to determine if CD4+ T cells alone were capable of controlling parasites, we infected RAG mice with L. braziliensis in the skin and reconstituted mice with either CD8+ T cells alone, CD8+ T cells and CD4+ T cells, CD4+ T cells alone or no cells (Figure 6 E). As previously demonstrated (13), RAG mice with no cells and those that were reconstituted with CD8+ T cells have similar number of parasites in the skin, though lesions are nearly absent in RAG mice with no cells and large and severe in RAG mice with CD8+ T cells (Figure 6 G). In contrast, RAG mice that were reconstituted with CD4+ and CD8+ T cells control lesion development and parasite growth (Figure 6 F,G)(13). Importantly, mice reconstituted with CD4+ T cells alone and the combination of CD4+ CD8+ T control parasites suggesting that CD4+ T cells alone can control L. braziliensis infection (Figure 6 F,G). To test if this is due to IFN-γ production, we reconstituted RAG mice with WT CD8+ T cells together with WT of IFN-γ deficient (IFN-γ KO) CD4+ T cells (Figure 6 H). Our results showed that RAG mice reconstituted with IFN-γ KO CD4+ T cells + WT CD8+ T cells had similar lesion sizes and parasite numbers when compared to mice reconstituted with CD8+ T cells alone and significantly larger lesion and higher parasite numbers than RAG mice reconstituted with WT CD4+ T cells + CD8+ T cells (Figure 6 I,J). Together, these results suggest that IFN-γ derived from CD4+ T cells is not only required, but is also sufficient to control leishmania parasites in the skin.
CD8+ T cells from immune mice fail to provide protection in RAG mice.
CD8+ T cell have long been considered targets for vaccine development in leishmaniasis. Hence, we next asked if immune CD8+ T cells were better able to provide protection than effector CD8+ T cells. RAG mice were infected with L. braziliensis and reconstituted with CD8+ T cells obtained from either naïve or immune mice. The course of infection (Figure 6 K) and parasite numbers in the skin (Figure 6 L) showed that similar to naïve CD8+ T cells, immune CD8+ T cells were unable to protect RAG mice from L. braziliensis infection. These data suggest that even primed CD8+ T cells from immune mice by themselves are unable to control a L. braziliensis infection.
Altogether, our results demonstrate that the skin microenvironment of leishmania-infected mice is deficient in the appropriate signals to promote IFN-γ production by CD8+ T cells. The lack of sufficient IL-12 in the skin prevents CD8+ T cells from becoming IFN-γ producers without affecting CD4+ Th1 cells, suggesting different requirements for CD4+ and CD8+ T cells in IFN-γ production. However, even in optimal conditions, in which IL-12 is provided, CD8+ T cells are still unable to provide protection.
Discussion
Leishmania parasites are controlled by IFN-γ that activates macrophages to kill the intracellular parasites. It is well established that IL-12 dependent generation of IFN-γ producing CD4+ Th1 cells is critical for resistance to these parasites and that CD8+ T cells can promote CD4+ Th1 cell development, as well as enhance resistance to reinfection (3, 4, 7–9, 25, 26). Paradoxically, however, CD8+ T cells have also been shown to mediate excessive inflammation in mice and in patients, promoting the destruction of the skin architecture leading to ulcer development, as well as promoting more severe forms of the disease, such as mucosal and disseminated leishmaniasis (11–17, 27–29). Previous studies found that in the absence of CD4+ T cells, CD8+ T cells were unable to control leishmania infection (10, 13), and here we investigated why this was the case. We found that in contrast to CD8+ T cells in the draining LNs, CD8+ T cells in leishmania lesions fail to make IFN-γ. We discovered that the inability of CD8+ T cells to make IFN-γ in lesions is due to a deficit in IL-12 production. Importantly, however, even when we administered IL-12 and increased the production of IFN-γ by CD8+ T cells, the IFN-γ production was still insufficient to provide protection. Thus, our results indicate that the protective role for CD8+ T cells is dependent upon the presence of CD4+ T cells, and in the absence of CD4+ T cells the primary role of CD8+ T cells is pathologic.
The heterogeneity of CD8+ T cells is most often investigated in the context of the longevity. For example, a large number of studies have described the characteristics of memory CD8+ T cells and the CD8+ T cells that have the potential to develop into memory T cells (30). CD8+ T cells can make cytokines (such as IFN-γ) and can be cytolytic, and it is often assumed that once CD8+ T cells become fully activated they perform both functions. However, it is increasingly clear that this is not the case. Indeed, studies of CD8+ T cell clones from HIV patients found that most CD8+ T cells were either cytolytic or made IFN-γ, and only a few performed both functions (31). The factors that lead to CD8+ T cells exhibiting exclusively one function or the other are not particularly well understood, and this study indicates that one such factor may be the location of the CD8+ T cells. We previously found that CD8+ T cells in leishmanial lesions express granzymes and perforin, and exhibit cytolytic activity that leads to extensive cell death, inflammation, IL-1β secretion and pathology (13, 14). These results are consistent with previous findings showing that CD8+ T cells within lymphoid tissues are defective at killing, while once in the tissues they are armed to kill target cells (32–34). Here we show that not only are lesional CD8+ T cells pathologic, they fail to make IFN-γ in the lesions due to limited IL-12 levels in the lesions.
In contrast to the clear protective role CD8+ T cells play in visceral leishmaniasis, the role of CD8+ T cells in protecting against cutaneous leishmaniasis is still poorly understood (35, 36). While CD8+ T cells were initially found not to be required for protection against L. major in mice (37–39), other studies found that they were required (10). We found that this discrepancy was due to a difference in the dose of parasites used to infect mice (7). CD8+ T cells were not required for protection against a high dose of L. major parasites, but were required when mice were infected with a low dose of parasites (7). The protective role for CD8+ T cells in this model was to provide IFN-γ to promote CD4+ Th1 cell development, since CD8+ deficient mice infected with a low dose developed a dominant CD4+ Th2 response (7). Thus, we hypothesized that one important function for CD8+ T cells in leishmaniasis was to promote CD4+ Th1 cell development in the dLN when there was a low level of antigen stimulation. Our current results indicate that this may be the only situation in which CD8+ T cells produce IFN-γ in cutaneous leishmaniasis.
The limited production of IL-12 within leishmanial lesions was unexpected, since IL-12 is required for the development of a protective immune response to leishmania (3, 25). On the other hand, leishmania is not a strong inducer of IL-12, and several studies have shown that infected dendritic cells are unable to make IL-12 even when stimulated with LPS (40, 41). However, it should be pointed out that the literature on this issue is contradictory, which may be due to studies with different parasites, different host cells, and assuming that IL-12 in a culture with both infected and uninfected cells is coming from the infected cells (42–48). It has been shown that leishmania infection inhibits IL-12 promoter activity, and this inhibition appears to be due to the use of CR3 for entry (41, 49). In fact, instead of the infected cells making IL-12, IL-12 is produced by uninfected bystander dendritic cells, which may be one reason we fail to see high levels of IL-12 in lesions with lots of parasites (40). While the mechanism involved in bystander production of IL-12 is not totally clear, we previously reported that both a parasite product and TNF are required for bystander production of IL-12 (40). Interestingly, the deficit is limited to the lesion site, since IL-12 was present in the draining lymph nodes. This might be anticipated, as the lymph nodes will contain the highest percentage of mature DCs, with a lower number of infected cells.
Our data corroborate results from other groups showing that in leishmania patients CD4+ T cells produce more IFN-γ than CD8+ T cells and that CD4+ T cells are better able to control leishmania infection in vitro compared to CD8+ T cells (15). Here we expanded these findings by demonstrating that not only are CD4+ T cells the major source of IFN-γ - taking into account all other possible sources of IFN-γ − but we show that CD4+ T cells are sufficient to control both parasite replication and lesion development in cutaneous disease. One surprising finding was that CD4+ T cells and CD8+ T cells have distinct requirements for IFN-γ production. We previously reported that CD4+ Th1 cells do not require IL-12 production in order to maintain a Th1 phenotype (50, 51), which is consistent with our current finding that in the absence of IL-12 in the skin, CD4+ T cells still express IFN-γ. In contrast, we found that CD8+ T cells in the skin need constant IL-12 signaling in order to produce IFN-γ during infection. Thus, CD8+ T cells in leishmanial lesions appear to require a constant reminder in the form of IL-12 in order to make IFN-γ.
CD8+ T cells have long been considered a good target for vaccination in leishmania infection, and for visceral leishmaniasis this may be the case (35). However, based upon our results, we hypothesize that the protective role of CD8+ T cells in cutaneous leishmaniasis is primarily an indirect effect and is mediated by their capacity to promote CD4+ Th1 cell development in the draining LN during priming, rather than their ability to control parasites within the skin. Thus, CD8+ T cells may not be a great target for a vaccine in cutaneous leishmaniasis, since it is unclear how one would ensure that they made IFN-γ and were protective, rather than acting as cytolytic cells and promoting pathology.
Supplementary Material
Acknowledgments
The authors wish to thank Ba Nguyen for assistance.
Funding: These studies were funded by NIH/NIAID (RO1 AI 106842).
References
- 1.Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M, and Team WHOLC. 2012. Leishmaniasis worldwide and global estimates of its incidence. PloS one 7: e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott P, and Novais FO. 2016. Cutaneous leishmaniasis: immune responses in protection and pathogenesis. Nature reviews. Immunology 16: 581–592. [DOI] [PubMed] [Google Scholar]
- 3.Heinzel FP, Schoenhaut DS, Rerko RM, Rosser LE, and Gately MK. 1993. Recombinant interleukin 12 cures mice infected with Leishmania major. The Journal of experimental medicine 177: 1505–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott P, Natovitz P, Coffman RL, Pearce E, and Sher A. 1988. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. The Journal of experimental medicine 168: 1675–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liew FY, Millott S, Parkinson C, Palmer RM, and Moncada S. 1990. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from L-arginine. Journal of immunology 144: 4794–4797. [PubMed] [Google Scholar]
- 6.Murray HW 1981. Susceptibility of Leishmania to oxygen intermediates and killing by normal macrophages. The Journal of experimental medicine 153: 1302–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uzonna JE, Joyce KL, and Scott P. 2004. Low dose Leishmania major promotes a transient T helper cell type 2 response that is down-regulated by interferon gamma-producing CD8+ T cells. The Journal of experimental medicine 199: 1559–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller I, Kropf P, Louis JA, and Milon G. 1994. Expansion of gamma interferon-producing CD8+ T cells following secondary infection of mice immune to Leishmania major. Infection and immunity 62: 2575–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller I, Kropf P, Etges RJ, and Louis JA. 1993. Gamma interferon response in secondary Leishmania major infection: role of CD8+ T cells. Infection and immunity 61: 3730–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belkaid Y, Von Stebut E, Mendez S, Lira R, Caler E, Bertholet S, Udey MC, and Sacks D. 2002. CD8+ T cells are required for primary immunity in C57BL/6 mice following low-dose, intradermal challenge with Leishmania major. Journal of immunology 168: 3992–4000. [DOI] [PubMed] [Google Scholar]
- 11.Cardoso TM, Machado A, Costa DL, Carvalho LP, Queiroz A, Machado P, Scott P, Carvalho EM, and Bacellar O. 2015. Protective and pathological functions of CD8+ T cells in Leishmania braziliensis infection. Infection and immunity 83: 898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Novais FO, Carvalho AM, Clark ML, Carvalho LP, Beiting DP, Brodsky IE, Carvalho EM, and Scott P. 2017. CD8+ T cell cytotoxicity mediates pathology in the skin by inflammasome activation and IL-1beta production. PLoS pathogens 13: e1006196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Novais FO, Carvalho LP, Graff JW, Beiting DP, Ruthel G, Roos DS, Betts MR, Goldschmidt MH, Wilson ME, de Oliveira CI, and Scott P. 2013. Cytotoxic T cells mediate pathology and metastasis in cutaneous leishmaniasis. PLoS pathogens 9: e1003504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novais FO, Carvalho LP, Passos S, Roos DS, Carvalho EM, Scott P, and Beiting DP. 2015. Genomic profiling of human Leishmania braziliensis lesions identifies transcriptional modules associated with cutaneous immunopathology. The Journal of investigative dermatology 135: 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santos Cda S, Boaventura V, Ribeiro Cardoso C, Tavares N, Lordelo MJ, Noronha A, Costa J, Borges VM, de Oliveira CI, Van Weyenbergh J, Barral A, Barral-Netto M, and Brodskyn CI. 2013. CD8(+) granzyme B(+)-mediated tissue injury vs. CD4(+)IFNgamma(+)-mediated parasite killing in human cutaneous leishmaniasis. The Journal of investigative dermatology 133: 1533–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brodskyn CI, Barral A, Boaventura V, Carvalho E, and Barral-Netto M. 1997. Parasite-driven in vitro human lymphocyte cytotoxicity against autologous infected macrophages from mucosal leishmaniasis. Journal of immunology 159: 4467–4473. [PubMed] [Google Scholar]
- 17.Faria DR, Souza PE, Duraes FV, Carvalho EM, Gollob KJ, Machado PR, and Dutra WO. 2009. Recruitment of CD8(+) T cells expressing granzyme A is associated with lesion progression in human cutaneous leishmaniasis. Parasite immunology 31: 432–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colmenares M, Kima PE, Samoff E, Soong L, and McMahon-Pratt D. 2003. Perforin and gamma interferon are critical CD8+ T-cell-mediated responses in vaccine-induced immunity against Leishmania amazonensis infection. Infection and immunity 71: 3172–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurunathan S, Sacks DL, Brown DR, Reiner SL, Charest H, Glaichenhaus N, and Seder RA. 1997. Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major. The Journal of experimental medicine 186: 1137–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jayakumar A, Castilho TM, Park E, Goldsmith-Pestana K, Blackwell JM, and McMahon-Pratt D. 2011. TLR1/2 activation during heterologous prime-boost vaccination (DNA-MVA) enhances CD8+ T Cell responses providing protection against Leishmania (Viannia). PLoS neglected tropical diseases 5: e1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhee EG, Mendez S, Shah JA, Wu CY, Kirman JR, Turon TN, Davey DF, Davis H, Klinman DM, Coler RN, Sacks DL, and Seder RA. 2002. Vaccination with heat-killed leishmania antigen or recombinant leishmanial protein and CpG oligodeoxynucleotides induces long-term memory CD4+ and CD8+ T cell responses and protection against leishmania major infection. The Journal of experimental medicine 195: 1565–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spath GF, and Beverley SM. 2001. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Experimental parasitology 99: 97–103. [DOI] [PubMed] [Google Scholar]
- 23.Morrow MP, Pankhong P, Laddy DJ, Schoenly KA, Yan J, Cisper N, and Weiner DB. 2009. Comparative ability of IL-12 and IL-28B to regulate Treg populations and enhance adaptive cellular immunity. Blood 113: 5868–5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrington LE, Janowski KM, Oliver JR, Zajac AJ, and Weaver CT. 2008. Memory CD4 T cells emerge from effector T-cell progenitors. Nature 452: 356–360. [DOI] [PubMed] [Google Scholar]
- 25.Sypek JP, Chung CL, Mayor SE, Subramanyam JM, Goldman SJ, Sieburth DS, Wolf SF, and Schaub RG. 1993. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. The Journal of experimental medicine 177: 1797–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, and Locksley RM. 1989. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. The Journal of experimental medicine 169: 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crosby EJ, Clark M, Novais FO, Wherry EJ, and Scott P. 2015. Lymphocytic Choriomeningitis Virus Expands a Population of NKG2D+CD8+ T Cells That Exacerbates Disease in Mice Coinfected with Leishmania major. Journal of immunology 195: 3301–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crosby EJ, Goldschmidt MH, Wherry EJ, and Scott P. 2014. Engagement of NKG2D on bystander memory CD8 T cells promotes increased immunopathology following Leishmania major infection. PLoS pathogens 10: e1003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faria DR, Gollob KJ, Barbosa J Jr., Schriefer A, Machado PR, Lessa H, Carvalho LP, Romano-Silva MA, de Jesus AR, Carvalho EM, and Dutra WO. 2005. Decreased in situ expression of interleukin-10 receptor is correlated with the exacerbated inflammatory and cytotoxic responses observed in mucosal leishmaniasis. Infection and immunity 73: 7853–7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaech SM, and Cui W. 2012. Transcriptional control of effector and memory CD8+ T cell differentiation. Nature reviews. Immunology 12: 749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varadarajan N, Julg B, Yamanaka YJ, Chen H, Ogunniyi AO, McAndrew E, Porter LC, Piechocka-Trocha A, Hill BJ, Douek DC, Pereyra F, Walker BD, and Love JC. 2011. A high-throughput single-cell analysis of human CD8(+) T cell functions reveals discordance for cytokine secretion and cytolysis. The Journal of clinical investigation 121: 4322–4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marzo AL, Yagita H, and Lefrancois L. 2007. Cutting edge: migration to nonlymphoid tissues results in functional conversion of central to effector memory CD8 T cells. Journal of immunology 179: 36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masopust D, Vezys V, Marzo AL, and Lefrancois L. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291: 2413–2417. [DOI] [PubMed] [Google Scholar]
- 34.Michonneau D, Sagoo P, Breart B, Garcia Z, Celli S, and Bousso P. 2016. The PD-1 Axis Enforces an Anatomical Segregation of CTL Activity that Creates Tumor Niches after Allogeneic Hematopoietic Stem Cell Transplantation. Immunity 44: 143–154. [DOI] [PubMed] [Google Scholar]
- 35.Stager S, and Rafati S. 2012. CD8(+) T cells in leishmania infections: friends or foes? Frontiers in immunology 3: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Novais FO, and Scott P. 2015. CD8+ T cells in cutaneous leishmaniasis: the good, the bad, and the ugly. Seminars in immunopathology 37: 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huber M, Timms E, Mak TW, Rollinghoff M, and Lohoff M. 1998. Effective and long-lasting immunity against the parasite Leishmania major in CD8-deficient mice. Infection and immunity 66: 3968–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Overath P, and Harbecke D. 1993. Course of Leishmania infection in beta 2-microglobulin-deficient mice. Immunology letters 37: 13–17. [DOI] [PubMed] [Google Scholar]
- 39.Wang ZE, Reiner SL, Hatam F, Heinzel FP, Bouvier J, Turck CW, and Locksley RM. 1993. Targeted activation of CD8 cells and infection of beta 2-microglobulin-deficient mice fail to confirm a primary protective role for CD8 cells in experimental leishmaniasis. Journal of immunology 151: 2077–2086. [PubMed] [Google Scholar]
- 40.Carvalho LP, Pearce EJ, and Scott P. 2008. Functional dichotomy of dendritic cells following interaction with Leishmania braziliensis: infected cells produce high levels of TNF-alpha, whereas bystander dendritic cells are activated to promote T cell responses. Journal of immunology 181: 6473–6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jayakumar A, Donovan MJ, Tripathi V, Ramalho-Ortigao M, and McDowell MA. 2008. Leishmania major infection activates NF-kappaB and interferon regulatory factors 1 and 8 in human dendritic cells. Infection and immunity 76: 2138–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bennett CL, Misslitz A, Colledge L, Aebischer T, and Blackburn CC. 2001. Silent infection of bone marrow-derived dendritic cells by Leishmania mexicana amastigotes. European journal of immunology 31: 876–883. [DOI] [PubMed] [Google Scholar]
- 43.Gorak PM, Engwerda CR, and Kaye PM. 1998. Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovani infection. European journal of immunology 28: 687–695. [DOI] [PubMed] [Google Scholar]
- 44.Jayakumar A, Widenmaier R, Ma X, and McDowell MA. 2008. Transcriptional inhibition of interleukin-12 promoter activity in Leishmania spp.-infected macrophages. The Journal of parasitology 94: 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Konecny P, Stagg AJ, Jebbari H, English N, Davidson RN, and Knight SC. 1999. Murine dendritic cells internalize Leishmania major promastigotes, produce IL-12 p40 and stimulate primary T cell proliferation in vitro. European journal of immunology 29: 1803–1811. [DOI] [PubMed] [Google Scholar]
- 46.Vasquez RE, Xin L, and Soong L. 2008. Effects of CXCL10 on dendritic cell and CD4+ T-cell functions during Leishmania amazonensis infection. Infection and immunity 76: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Stebut E, Belkaid Y, Jakob T, Sacks DL, and Udey MC. 1998. Uptake of Leishmania major amastigotes results in activation and interleukin 12 release from murine skin-derived dendritic cells: implications for the initiation of anti-Leishmania immunity. The Journal of experimental medicine 188: 1547–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Stebut E, Belkaid Y, Nguyen BV, Cushing M, Sacks DL, and Udey MC. 2000. Leishmania major-infected murine langerhans cell-like dendritic cells from susceptible mice release IL-12 after infection and vaccinate against experimental cutaneous Leishmaniasis. European journal of immunology 30: 3498–3506. [DOI] [PubMed] [Google Scholar]
- 49.Ricardo-Carter C, Favila M, Polando RE, Cotton RN, Bogard Horner K, Condon D, Ballhorn W, Whitcomb JP, Yadav M, Geister RL, Schorey JS, and McDowell MA. 2013. Leishmania major inhibits IL-12 in macrophages by signalling through CR3 (CD11b/CD18) and down-regulation of ETS-mediated transcription. Parasite immunology 35: 409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pakpour N, Zaph C, and Scott P. 2008. The central memory CD4+ T cell population generated during Leishmania major infection requires IL-12 to produce IFN-gamma. Journal of immunology 180: 8299–8305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Constantinescu CS, Hondowicz BD, Elloso MM, Wysocka M, Trinchieri G, and Scott P. 1998. The role of IL-12 in the maintenance of an established Th1 immune response in experimental leishmaniasis. European journal of immunology 28: 2227–2233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


