Abstract
Cognitive Neuropsychology (CN) has had an immense impact on the understanding of the normal cognitive processes underlying reading, spelling, spoken language comprehension and production, spatial attention, memory, visual perception, and orchestration of actions, through detailed analysis of behavioral performance by neurologically impaired individuals. However, there are other domains of cognition and communication that have rarely been investigated with this approach. Many cognitive neuropsychologists have extended their work in language, perception, or attention by turning to functional neuroimaging or lesion-symptom mapping to identify the neural mechanisms underlying the cognitive mechanisms they have identified. Another approach to extending one’s research in CN is to apply the methodology to other cognitive functions. We briefly review the domains evaluated using methods of CN to develop cognitive architectures and computational models and the domains that have used functional neuroimaging and other brain mapping approaches in healthy controls to identify the neural substrates involved in cognitive tasks, over the past 20 years. We argue that in some domains, neuroimaging studies have preceded the careful analysis of the cognitive processes underlying tasks that are studied, with the consequence that results are difficult to interpret. We use this analysis as the basis for discussing opportunities for expanding the field.
Keywords: cognitive neuropsychology, brain mapping, fMRI
Early functional imaging studies using O-15 Positron Emission Tomography (PET) demonstrated that it is possible to identify areas of the brain where blood flow correlates with performance of some task. Some of the earliest studies of language, which did consider cognitive architectures of tasks like reading and naming, revealed areas of the brain that were activated in some tasks but not others (Petersen, Fox, Posner, Mintun, & Raichle, 1989; Petersen, Fox, Snyder, & Raichle, 1990; Posner, Petersen, Fox, & Raichle, 1988). These studies indicated that prefrontal regions were preferentially activated during a variety of “semantic” tasks. While the studies may not have been accurate in identifying the role of prefrontal regions during these tasks, they were groundbreaking in showing that areas of the brain are differentially activated during certain types of cognitive processing. Since that time, there has been an explosion of studies evaluating brain regions where measures of activation (e.g. the BOLD effect in fMRI) are correlated with performance on a task (compared to another task or baseline). However, in some cases, studies have evaluated areas of activation correlated with task performance without careful consideration of what cognitive processes are involved in the task. We propose that it is premature to identify brain regions that are engaged during a task without understanding the nature of the processing responsible for the observed activation. That is, investigations of the neural mechanisms of a task with functional neuroimaging without consideration of the cognitive architecture of the task is like putting the cart before the horse. However, the lure of neuroimaging studies has drawn many investigators to develop more sophisticated and fine-tuned imaging of activation associated with cognitive tasks in place of the arguably more difficult task of developing architectures and computational models of the cognitive processes underlying the task being investigated.
Consider the overwhelming number of functional neuroimaging studies that have investigated the neural processes underlying empathy. For example, one meta-analysis of the brain regions involved in empathy (Fan, Duncan, de Greck, & Northoff, 2011) included 40 fMRI investigations of the following tasks:
Tasks requiring participants to observe the sensory state or emotional state of others, with data analysis focused on a “specifically empathy-related context.”
Tasks requiring participants to share the emotional states of others and make inferences about the feelings of others.
Tasks requiring participants to imagine others’ feelings or evaluate emotions from the perspective of another.
Tasks requiring perception of emotional or sensory states of others, if activation correlated with measures of the participants’ empathy.
The meta-analysis identified a “core network” of brain regions where activation correlated with performance across all tasks and stimuli. The network included dorsal anterior cingulate cortex (ACC), anterior middle cingulate cortex (aMCC), supplementary motor area (SMA) and bilateral anterior insula (AI). Other meta-analyses have also yielded evidence that AI and ACC are engaged during tasks involving empathy (Gu et al., 2012; Lamm, Decety, & Singer, 2011). However, these same regions are engaged in a large number of other emotional and social tasks (Bush, Luu, & Posner, 2000), coding of task relevance (Downar, Crawley, Mikulis, & Davis, 2001), conflict monitoring (Botvinick, Nystrom, Fissell, Carter, & Cohen, 1999), and error detection (Carter et al., 1998). What sort of processing is taking place in these regions? Most studies have not attempted to isolate distinct cognitive processes underlying empathy, beyond two complex stages or components – emotional contagion and cognitive perspective-taking (Shamay-Tsoory, Aharon-Peretz, & Perry, 2009). Each of these functions entails a number of separable processes. For example, emotional contagion requires recognizing facial expressions or affective prosody in another, as well as experiencing the emotion oneself. Cognitive perspective-taking requires attention and working memory, abstract reasoning, belief attribution, and suppression of one’s own perspective, at least some of which can be selectively impaired by brain damage (Rankin, Kramer, & Miller, 2005; Rankin et al., 2006; Samson, Apperly, Kathirgamanathan, & Humphreys, 2005; Samson, Apperly, & Humphreys, 2007). Do all of these processes engage the anterior insula and dorsal anterior cingulate? Few studies have identified areas of activation associated with separate component processes underlying emotional contagion or cognitive perspective-taking. Likewise very few investigators have attempted to identify neurologically impaired individuals with selective damage to a particular process underlying empathy ((Hillis, 2014), but see (Samson et al., 2007).
Here we identify the range of domains in which there has been an attempt to develop or refine a cognitive architecture through the methodology of cognitive neuropsychology (CN) – which we define as the study of neurologically impaired individuals to identify cognitive processes that can be selectively impaired. Because we have narrowly defined the approach of CN to identifying selective impairment of particular cognitive processes, we examine studies that have used the single case or case series approach. In parallel we evaluate the range of domains in which there has been an attempt to identify the neural regions or neural circuitry underlying specific tasks, using functional neuroimaging or other brain mapping techniques (e.g. electrophysiology) in healthy participants (what we will refer to as the methods of cognitive neuroscience, or CNS).1 The domains were roughly based on those listed by on the CN webpage, “Cognition is understood broadly to include the domains of perception, attention, planning, language, thinking, memory and action.” We further subdivided some of these domains (based only our intuitions about the domains investigated in the two journals). The topics are not at all meant to reflect an ontology of mental processes, and certainly they could have been categorized differently. We also recognize that the goals of the two methodologies are not dichotomous. Other authors have discussed how functional imaging (a CNS method) can be used to develop cognitive theory (e.g. see Humphreys & Price, 2001; Shallice, 2003).
We propose that a mismatch in the distribution across domains in the two journals indicates an opportunity for cognitive neuropsychologists to explore new domains – to identify dissociable cognitive processes underlying tasks like empathy and other “social cognition” tasks, decision-making, and various tasks of “executive function.” In most cases, these domains do not yet have adequately developed theories to be tested by the methods of CN. The theory could be developed by cognitive psychologists and evaluated in people with brain lesions (as in, for example, Caramazza & Hillis, 1989), or could be developed by cognitive neuropsychologists on the basis of patterns of impaired performance by brain injured patients (as in, for example, Samson et al., 2005, 2007). In either case, such an endeavor would provide the critical basis for neuroimaging and lesion studies to investigate neural regions or networks that support particular cognitive processes. By letting the horse pull the cart, we can develop a more coherent understanding of the how activity in, and interactions between, various parts of the brain support a wide range of cognitive tasks.
Methods:
Articles reporting experimental results from Issue 1-Volume 13, 1996 to Issue 5–6-Volume 33, 2016 (20 years) from Cognitive Neuropsychology (CN) were systematically categorized into one of 23 cognitive domains based on the primary hypothesis. Articles reporting experimental results from Issue 1-Volume 8, 1997 to Issue 12-Volume 28, 2016 from the Journal of Cognitive Neuroscience (JOCN) were also categorized into one of these cognitive domains. Articles where the major cognitive domain of study could not be clearly identified or was unique to that article were not included. Within each journal, articles were then subdivided into those studying computational models and/or patients with neurological impairment and those studying healthy participants (most frequently with functional neuroimaging). Methodology, opinion, theoretical studies, reviews, and rebuttals without original experimental work were not included in the analysis. Disparities between the CN and CNS approaches in the percentage of articles devoted to each domain of cognition were tested with chi squared tests, using STATA version 12. That is, we created two by two tables for each domain, with the rows being the number of articles investigating that domain and the number of articles not investigating that domain, and the columns were CN using CN (case study or case series) approach and JOCN using CNS approach.
For the statistical analysis, we excluded studies of lesion-symptom mapping, because these studies often involve methods of CN to evaluate specific cognitive processes that are impaired in individuals, but are designed to evaluate disruptions in neural circuitry and neural mechanisms that are responsible for those deficits (i.e. they represent an overlap between CN and CNS). Studies in CN that used healthy controls were not included in the statistical analyses, because we were most interested in evaluating the predominant approach (single case/case series methods of CN versus brain mapping methods of CNS) to studying each domain. Thus, we excluded even studies of healthy individuals that investigated the effects of temporary lesions (e.g. transcranial magnetic stimulation or direct cortical stimulation) on cognition because most of these used “temporary lesions” to address questions about the role of specific brain regions rather than cognitive mechanisms. We also excluded from the statistical analysis papers in JOCN that included neurologically impaired subjects, because virtually all of these studies used methods of CN with or without lesion-symptom mapping. However, the articles excluded from the statistical analyses are included in the table (columns 3 and 4).
Finally, articles in JOCN that studied domains of cognition rarely reported in CN were evaluated in more detail to elucidate the specific cognitive tasks that were investigated.
Results
Significant incongruities in the types of cognition investigated with the methods of CN versus the methods of CNS were identified. When comparing studies of patients with neurological impairment in CN versus healthy individuals in JOCN (columns 2 and 5 in Table 1), reading (χ2= 169.07), spelling (χ2 =96.60), grammatical processing (χ2= 16.18), naming (χ2=75.52), face identification (χ2 = 25.59) and semantics (χ2= 48.02) were significantly over-represented in studies of patients in CN. In JOCN, studies on executive functioning (χ2= 41.04), social cognition (χ2 = 20.18), reward/motivation (χ2 =15.48), auditory processing (χ2= 26.19), learning (χ2 = 41.29), and sensorimotor representation (χ2 =13.06) in healthy participants were significantly over-represented compared to studies with neurologically impaired patients in CN.
Table 1.
Distribution of Articles Across Domains of Research in each Journal
| Cognitive Neuropsychology (n=621) | Cognitive Neuroscience (n=2455) | |||
|---|---|---|---|---|
| Topic | Neurological Impairment | Healthy Individuals | Neurological Impairment | Healthy Individuals |
| Reading | 102 (16.4%) * | 1 (0.2%) | 8 (0.3%) | 72 (2.9%) |
| Spelling | 27 (4.4%) * | 0 (0%) | 0 (0%) | 2 (0.1%) |
| Word Comprehension | 25 (4.0%) | 1 (0.2%) | 2 (0.1%) | 57 (2.3%) |
| Grammatical Processing | 26 (4.2%) * | 0 (0%) | 9 (0.4%) | 39 (1.6%) |
| Sentence Processing | 18 (2.9%) | 0 (0%) | 8 (0.3%) | 77 (3.1%) |
| Naming | 35 (5.6%) * | 0 (0%) | 6 (0.2%) | 16 (0.7%) |
| General Language | 20 (3.2%) | 2 (0.3%) | 9 (0.4%) | 65 (2.7%) |
| Face Identification | 44 (7.1%) * | 14 (2.3%) | 9 (0.4%) | 69 (2.8%) |
| Vision | 33 (5.3%) | 3 (0.5%) | 46 (1.9%) | 235 (9.6%) |
| Semantics | 40 (6.4%) * | 1 (0.2%) | 9 (0.4%) | 38 (1.6%) |
| Object Representation | 20 (3.2%) | 2 (0.3%) | 6 (0.2%) | 76 (3.1%) |
| Body Representation | 13 (2.1%) | 2 (0.3%) | 3 (0.1%) | 20 (0.8%) |
| Mathematical Processing | 17 (2.7%) | 1 (0.2%) | 2 (0.1%) | 41 (1.7%) |
| Attention | 10 (1.6%) | 3 (0.5%) | 6 (0.2%) | 95 (3.9%) |
| Memory | 69 (11.1%) | 7 (1.1%) | 25 (1.0%) | 263 (10.7%) |
| Spatial Cognition | 33 (5.3%) | 3 (0.5%) | 20 (0.8%) | 80 (3.3%) |
| Auditory Processing | 7 (1.1%) | 0 (0%) | 11 (0.5%) | 153 (6.2%) * |
| Executive Function | 8 (1.3%) | 0 (0%) | 18 (0.7%) | 224 (9.1%) * |
| Learning | 0 (0%) | 2 (0.3%) | 19 (0.8%) | 155 (6.3%) * |
| Sensorimotor Representation | 19 (3.1%) | 4 (0.6%) | 16 (0.7%) | 173 (7.1%) * |
| Emotion | 4 (0.6%) | 0 (0%) | 9 (0.4%) | 71 (2.9%) |
| Reward/Motivation | 0 (0%) | 0 (0%) | 8 (0.3%) | 60 (2.4%) * |
| Social Cognition | 4 (0.6%) | 1 (0.2%) | 16 (0.7%) | 109 (4.4%) * |
In the domain of social cognition, there was a disproportionately low number of papers that used methods of CN. However, there were examples of how the cognitive neuropsychological approach can be applied to social cognition. In CN, patient studies of social cognition included participants with Williams syndrome, autism, and congenital prosopagnosia. For example, articles on social-perceptual abilities in individuals with Williams syndrome (Plesa Skwerer, Verbalis, Schofield, Faja, & Tager-Flusberg, 2006) and representation of others’ actions in those with autism (Sebanz, Knoblich, Stumpf, & Prinz, 2005) both investigated theory of mind in neurodevelopmental disorders. In JOCN, topics such as empathy, social emotion processing, social memory, sociability and interpersonal competence, self-referential processing, and perception of status cues were common topics of investigation. Some studies in JOCN did use methods of CN, studying patients with lesions to the amygdala (Adolphs, Baron-Cohen, & Tranel, 2002) or ventromedial prefrontal cortex (Karafin, Tranel, & Adolphs, 2004), or with frontotemporal dementia, autism, or Williams syndrome (Njomboro, Deb, & Humphreys, 2008). These studies of neurologically impaired individuals in JOCN (like studies of healthy individuals in CN) were not included in our statistical analyses.
Executive functioning was highly over-represented in JOCN compared to CN (Figure 1). Nevertheless, there were some studies of disruptions in executive function in neurologically impaired patients published in CN. These studies investigated topics such as selection equivocation and working memory (Morris, Miotto, Feigenbaum, Bullock, & Polkey, 1997), task switching (Kumada & Humphreys, 2006), and conflict adaptation (Funes, Lupiáñez, & Humphreys, 2010)2. There were also notable papers in JOCN that used the approach of CN to investigate disruptions in cognitive control (e.g. (Di Pellegrino, Ciaramelli, & Làdavas, 2007); working memory (e.g. (Müller, Machado, & Knight, 2002); and predictive judgments (Gomez-Beldarrain, Harries, Garcia-Monco, Ballus, & Grafman, 2004).
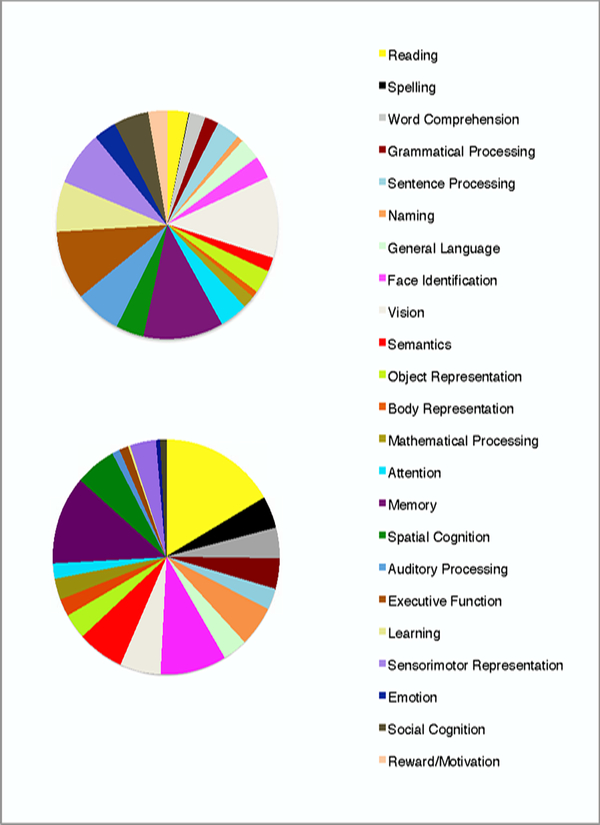
Figure 1.
Pie charts showing the distributions of papers across domains, for papers in CN (using CN methods; top) and papers in JOCN (using CNS methods; bottom)
One of the greatest disparities between CN and JOCN was noted in the domain of reward-related cognition. There were no studies in CN (using CN methods or otherwise) where the research focused on reward processing. In contrast, in JOCN reward-related topics included effects of reward expectation on cognition; reward valence; risk-taking; sensitivity to reward; and reward information coding. There were a few papers in JOCN using the methods of CN to study reward and decision-making, including papers on reward-related reversal learning after lesions in dorsolateral prefrontal or orbitofrontal cortex (Hornak et al., 2004) and evaluation of delayed rewards in individuals with ADHD compared to controls (Fassbender et al., 2014).
There were seven articles related to auditory domains of cognition in CN, and all of these articles focused on music processing. These studies included patients with congenital amusia (e.g. (Phillips-Silver, Toiviainen, Gosselin, & Peretz, 2013) and right hemisphere damage (e.g. (Schön, Lorber, Spacal, & Semenza, 2004). Although music was a common focus of auditory processing research in JOCN, articles on auditory attention, auditory priming, auditory working memory, and auditory discrimination were also sources of investigation in JOCN. Again there were a few papers using the approach of CN that were published in JOCN, including a study of enhanced pitch sensitivity in autism (Bonnel et al., 2003) and a study of neglect of auditory objects after right hemisphere lesions (Cusack, Carlyon, & Robertson, 2000).
Although many memory studies were published in CN, studies focused on the cognitive mechanisms underlying the process of learning per se were rarely published in CN. In JOCN, neuroplasticity, visuomotor learning, priming, reinforcement learning, language learning, and observational learning were all sources of investigation in healthy individuals. There were also a handful of studies applying methods of CN to investigate specific impairments in learning published in JOCN. Examples include studies of probabilistic learning and reversal associated with basal ganglia and hippocampal lesions (Shohamy, Myers, Hopkins, Sage, & Gluck, 2009) and startle habituation in Parkinson Disease (Chen et al., 2016).
Some cognitive tasks within the language domain (reading, grammatical processing, naming, and spelling) were overrepresented in CN compared JOCN. Studies of semantics were also published mostly in CN, including studies of category specific semantics (e.g. dissociations in recognition between living versus non-living things). Finally, investigations of identification of faces and facial expressions were more frequently published in CN than in JOCN. However, a few studies of the impaired cognitive mechanisms underlying congenital prosopagnosia appeared in JOCN (Bentin, DeGutis, D’Esposito, & Robertson, 2007).
Discussion
This brief review demonstrates that there is a mismatch in the domains of cognition that have been studied through the methods of CN (defined here as single case studies or case series of individuals with brain damage) and those that have been studied by the brain mapping methods of CNS. There has been an overwhelming emphasis on language, semantics, and face identification in CN single case/case series studies and a disproportionate emphasis on social cognition, decision-making (including reward/motivation), learning, and “executive function” in CNS brain mapping studies. This difference may be understandable because the latter domains seem to consist of tasks that are less clearly decomposable into distinct cognitive processes (based on the computational demands or selective deficits after brain damage). But it is not the case that domains of social cognition, decision-making, learning, and executive function are less affected by neurological disease. Finding patients with selective deficits in these domains may require evaluating patients with neurodegenerative disease (e.g. frontotemporal dementia), developmental disorders (autism, Williams syndrome), and traumatic brain injury, as well as patients with right hemisphere stroke. In fact, there have been many studies of theory of mind and some aspects of social cognition in individuals with developmental disorders like autism (cf Shu, Lung, Tien, & Chen, 2001; Travis & Sigman, 1998 for reviews) and Williams syndrome (cf Martens, Wilson, & Reutens, 2008 for review), published mostly in other journals. Likewise, there are many neuropsychological group studies of executive dysfunction in people with diffuse neurological diseases like dementia (Barba, Nedjam, & Dubois, 1999) and traumatic brain injury (Duncan, R., Johnson, M., Swales, C., Freer, J., 1997), as well as focal injury (Burgess, Veitch, de Lacy Costello, & Shallice, 2000), the majority of which are published in other journals (cf Duke & Kaszniak, 2000; Hill, 2004 for reviews). But single case studies could be carried out in individuals with focal brain damage and provide complementary evidence to refine the architectures in these domains.
The absence of focal lesions in some of these conditions might make it difficult to draw conclusions about the region of the brain responsible for the deficit, but would not preclude or even weaken the ability to draw inferences about what cognitive processes can be selectively impaired by brain damage. Studies of patients with impaired social cognition, for example, could provide evidence for the distinct cognitive processes underlying tasks such as recognition of affective prosody (Wright et al., 2015), recognition of emotional facial expression (Philippi, Mehta, Grabowski, Adolphs, & Rudrauf, 2009), affective empathy (Hillis, 2014; Samson, Apperly, Chiavarino, & Humphreys, 2004), and so on.
One explanation for the difference in domains investigated using CN single cases/case series methods versus CNS brain mapping methods is that the two approaches are simply more suitable for some processing domains than others. Single-case studies and case series are best conducted in domains where patterns of performance across tasks characterize and quantify each patient’s deficit, such that deficits can be contrasted (see Shallice, 2015 for detailed discussion). Nevertheless, some investigators have tackled the challenging task of examining selective deficits to component processes in brain damaged patients in the domain of executive function using the single case study approach (e.g. (Kumada & Humphreys, 2006).
In some domains, such as memory and object recognition, there has been a great deal of work using both methods of CN and CNS. In these domains, functional neuroimaging studies have identified neurocircuitry that supports discrete cognitive processes. Even in domains that have been studied more using CN methods, there have been excellent instances of combining both methods. Thus, for example, there are investigations of neural activation associated with discrete cognitive processes underlying reading (McCandliss, Cohen, & Dehaene, 2003), spelling (Purcell, Turkeltaub, Eden, & Rapp, 2011; Rapp & Dufor, 2011), naming (Kemeny et al., 2006), as well as studies of lesions associated with specific cognitive processes underlying reading (Rapcsak et al., 2009), spelling (Rapp, Purcell, Hillis, Capasso, & Miceli, 2016), and naming (DeLeon et al., 2007). We submit that these studies have been possible because of the decades of work developing a cognitive architecture of these tasks, in part using contrasting patterns of performance in brain damaged patients. We propose that to keep CN vital and impactful it is essential to expand the domains of investigation. It is difficult work, and may require studying populations of neurologically impaired individuals that have not been commonly studied by cognitive neuropsychologists, including patients with behavioral variant frontotemporal dementia, autism, and schizophrenia (to investigate the cognitive processes underlying specific social cognition tasks).
Limitations of our review include the fact that we only searched two journals for studies using each method. We recognize that studies using CN methods are published also in many other journals of cognition, language, psychology, aphasia, clinical neurology, and neuroscience. Likewise, studies using CNS methods are published in journals of neuroimaging, neurology, neuroscience, and so on. Nevertheless, we selected these two journals as representative of the respective approaches (as we have defined them). Another limitation is that it was not always possible to definitively classify articles into specific domains. Finally, we cannot rule out that the differences in types of studies published in each journal are influenced by preferences of the editorial board or the reviewers. It is also likely that authors tend to submit work to a journal that has published previous papers on the topic of their manuscript. These weaknesses were partially mitigated by excluding papers in CN that employed methods of CNS (as we defined it) and papers in JOCN that employed methods of CN from our statistical analyses.
Despite its limitations, this brief review underscores the need to develop cognitive architectures of a wide range of tasks. Brain mapping techniques such as functional imaging, if properly designed, can provide insights into the architecture of cognitive tasks (see Henson, 2005; Shallice & Cooper, 2011), although only a small percentage have been designed to inform cognitive theory (Tressoldi, Sella, Coltheart, and Umilta, 2012). Studies that examine activation or lesions associated with tasks, without considering or testing an architecture that specifies the underlying representations and processes of the task being investigated, are often premature. Thus, we believe there is an opportunity for cognitive neuropsychologists to use case studies or case series of brain damaged patients to develop and test cognitive theory in a broader range of processing domains.
Acknowledgements
The research reported in this paper was supported by the National Institutes of Health (National Institute of Deafness and Communication Disorders) through awards R01 DC05375 and P50 DC014664 and National Institute on Neurological Disorders and Stroke through award ROI NS047691. The content is solely the responsibility of the authors and does not necessarily represent the views the National Institutes of Health.
Footnotes
Here we refer to methods of CNS as those methods that are designed to identify neural circuitry or neural mechanisms that support cognitive processes or representations, such as functional neuroimaging. We have excluded (from statistical analyses, but have included in the Table) studies of lesion-symptom mapping, because these studies often involve methods of CN to evaluate specific cognitive processes that are impaired, but are designed to evaluate disruptions in neural circuitry and neural mechanisms that are responsible for those deficits.
It may be worth noting that a large proportion of studies using case study methodolgy of CN to investigate social cognition, decision-making (including reward/motivation), and “executive function” were published by the late Glyn Humphreys and his colleagues.
References
- Adolphs R, Baron-Cohen S, & Tranel D (2002). Impaired recognition of social emotions following amygdala damage. Journal of Cognitive Neuroscience, 14(8), 1264–1274. [DOI] [PubMed] [Google Scholar]
- Barba GD, Nedjam Z, & Dubois B (1999). Confabulation, executive functions, and source memory in alzheimer’s disease. Cognitive Neuropsychology, 16(3–5), 385–398. [Google Scholar]
- Bentin S, DeGutis JM, D’Esposito M, & Robertson LC (2007). Too many trees to see the forest: Performance, event-related potential, and functional magnetic resonance imaging manifestations of integrative congenital prosopagnosia. Journal of Cognitive Neuroscience, 19(1), 132–146. [DOI] [PubMed] [Google Scholar]
- Bonnel A, Mottron L, Peretz I, Trudel M, Gallun E, & Bonnel A (2003). Enhanced pitch sensitivity in individuals with autism: A signal detection analysis. Journal of Cognitive Neuroscience, 15(2), 226–235. [DOI] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, & Cohen JD (1999). Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature, 402(6758), 179–181. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Veitch E, de Lacy Costello A, & Shallice T (2000). The cognitive and neuroanatomical correlates of multitasking. Neuropsychologia, 38(6), 848–863. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, & Posner MI (2000). Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences, 4(6), 215–222. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, & Cohen JD (1998). Anterior cingulate cortex, error detection, and the online monitoring of performance. Science (New York, N.Y.), 280(5364), 747–749. [DOI] [PubMed] [Google Scholar]
- Chen K, Okerstrom KL, Kingyon JR, Anderson SW, Cavanagh JF, & Narayanan NS (2016). Startle habituation and midfrontal theta activity in parkinson disease. Journal of Cognitive Neuroscience , [DOI] [PMC free article] [PubMed]
- Cusack R, Carlyon RP, & Robertson IH (2000). Neglect between but not within auditory objects. Journal of Cognitive Neuroscience, 12(6), 1056–1065. [DOI] [PubMed] [Google Scholar]
- DeLeon J, Gottesman RF, Kleinman JT, Newhart M, Davis C, Heidler-Gary J, . . . Hillis AE (2007). Neural regions essential for distinct cognitive processes underlying picture naming. Brain: A Journal of Neurology, 130(Pt 5), 1408–1422. doi:awm011 [pii] [DOI] [PubMed] [Google Scholar]
- Di Pellegrino G, Ciaramelli E, & Làdavas E (2007). The regulation of cognitive control following rostral anterior cingulate cortex lesion in humans. Journal of Cognitive Neuroscience, 19(2), 275–286. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, & Davis KD (2001). The effect of task relevance on the cortical response to changes in visual and auditory stimuli: An event-related fMRI study. NeuroImage, 14(6), 1256–1267. [DOI] [PubMed] [Google Scholar]
- Duke LM, & Kaszniak AW (2000). Executive control functions in degenerative dementias: A comparative review. Neuropsychology Review, 10(2), 75–99. [DOI] [PubMed] [Google Scholar]
- Duncan R, Johnson M, Swales C, Freer J (1997). Frontal lobe deficits after head injury: Unity and diversity of function. Cognitive Neuropsychology, 14(5), 713–741. [Google Scholar]
- Fan Y, Duncan NW, de Greck M, & Northoff G (2011). Is there a core neural network in empathy? an fMRI based quantitative meta-analysis. Neuroscience & Biobehavioral Reviews, 35(3), 903–911. [DOI] [PubMed] [Google Scholar]
- Fassbender C, Houde S, Silver-Balbus S, Ballard K, Kim B, Rutledge KJ, . . . McClure SM (2014). The decimal effect: Behavioral and neural bases for a novel influence on intertemporal choice in healthy individuals and in ADHD. Journal of Cognitive Neuroscience, [DOI] [PMC free article] [PubMed]
- Funes M. a. J., Lupiáñez J, & Humphreys G (2010). Top-down and bottom-up deficits in conflict adaptation after frontal lobe damage. Cognitive Neuropsychology, 27(4), 360–375. [DOI] [PubMed] [Google Scholar]
- Gomez-Beldarrain M, Harries C, Garcia-Monco JC, Ballus E, & Grafman J (2004). Patients with right frontal lesions are unable to assess and use advice to make predictive judgments. Journal of Cognitive Neuroscience, 16(1), 74–89. [DOI] [PubMed] [Google Scholar]
- Gu X, Gao Z, Wang X, Liu X, Knight RT, Hof PR, & Fan J (2012). Anterior insular cortex is necessary for empathetic pain perception. Brain : A Journal of Neurology, 135(Pt 9), 2726–2735. doi: 10.1093/brain/aws199 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill EL (2004). Executive dysfunction in autism. Trends in Cognitive Sciences, 8(1), 26–32. [DOI] [PubMed] [Google Scholar]
- Hillis AE (2014). Inability to empathize: Brain lesions that disrupt sharing and understanding another’s emotions. Brain : A Journal of Neurology, 137(Pt 4), 981–997. doi: 10.1093/brain/awt317 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornak J, O’doherty J, Bramham J, Rolls E, Morris R, Bullock P, & Polkey C (2004). Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. Journal of Cognitive Neuroscience, 16(3), 463–478. [DOI] [PubMed] [Google Scholar]
- Karafin MS, Tranel D, & Adolphs R (2004). Dominance attributions following damage to the ventromedial prefrontal cortex. Journal of Cognitive Neuroscience, 16(10), 1796–1804. [DOI] [PubMed] [Google Scholar]
- Kemeny S, Xu J, Park GH, Hosey LA, Wettig CM, & Braun AR (2006). Temporal dissociation of early lexical access and articulation using a delayed naming task--an FMRI study. Cerebral Cortex (New York, N.Y.: 1991), 16(4), 587–595. doi:bhj006 [pii] [DOI] [PubMed] [Google Scholar]
- Kumada T, & Humphreys GW (2006). Dimensional weighting and task switching following frontal lobe damage: Fractionating the task switching deficit. Cognitive Neuropsychology, 23(3), 424–447. [DOI] [PubMed] [Google Scholar]
- Lamm C, Decety J, & Singer T (2011). Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage, 54(3), 2492–2502. [DOI] [PubMed] [Google Scholar]
- Martens MA, Wilson SJ, & Reutens DC (2008). Research review: Williams syndrome: A critical review of the cognitive, behavioral, and neuroanatomical phenotype. Journal of Child Psychology and Psychiatry, 49(6), 576–608. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, & Dehaene S (2003). The visual word form area: Expertise for reading in the fusiform gyrus. Trends in Cognitive Sciences, 7(7), 293–299. [DOI] [PubMed] [Google Scholar]
- Morris RG, Miotto EC, Feigenbaum JD, Bullock P, & Polkey CE (1997). Planning ability after frontal and temporal lobe lesions in humans: The effects of selection equivocation and working memory load. Cognitive Neuropsychology, 14(7), 1007–1027. [Google Scholar]
- Müller NG, Machado L, & Knight RT (2002). Contributions of subregions of the prefrontal cortex to working memory: Evidence from brain lesions in humans. Journal of Cognitive Neuroscience, 14(5), 673–686. [DOI] [PubMed] [Google Scholar]
- Njomboro P, Deb S, & Humphreys GW (2008). Dissociation between decoding and reasoning about mental states in patients with theory of mind reasoning impairments. Journal of Cognitive Neuroscience, 20(9), 1557–1564. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, & Raichle ME (1989). Positron emission tomographic studies of the processing of singe words. Journal of Cognitive Neuroscience, 1(2), 153–170. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Snyder AZ, & Raichle ME (1990). Activation of extrastriate and frontal cortical areas by visual words and word-like stimuli. Science (New York, N.Y.), 249(4972), 1041–1044. [DOI] [PubMed] [Google Scholar]
- Philippi CL, Mehta S, Grabowski T, Adolphs R, & Rudrauf D (2009). Damage to association fiber tracts impairs recognition of the facial expression of emotion. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 29(48), 15089–15099. doi: 10.1523/JNEUROSCI.0796-09.2009 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips-Silver J, Toiviainen P, Gosselin N, & Peretz I (2013). Amusic does not mean unmusical: Beat perception and synchronization ability despite pitch deafness. Cognitive Neuropsychology, 30(5), 311–331. [DOI] [PubMed] [Google Scholar]
- Plesa Skwerer D, Verbalis A, Schofield C, Faja S, & Tager-Flusberg H (2006). Social-perceptual abilities in adolescents and adults with williams syndrome. Cognitive Neuropsychology, 23(2), 338–349. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE, Fox PT, & Raichle ME (1988). Localization of cognitive operations in the human brain. Science (New York, N.Y.), 240(4859), 1627–1631. [DOI] [PubMed] [Google Scholar]
- Purcell J, Turkeltaub PE, Eden GF, & Rapp B (2011). Examining the central and peripheral processes of written word production through meta-analysis. Frontiers in Psychology, 2, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin KP, Gorno-Tempini ML, Allison SC, Stanley CM, Glenn S, Weiner MW, & Miller BL (2006). Structural anatomy of empathy in neurodegenerative disease. Brain : A Journal of Neurology, 129(Pt 11), 2945–2956. doi:awl254 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin KP, Kramer JH, & Miller BL (2005). Patterns of cognitive and emotional empathy in frontotemporal lobar degeneration. Cognitive and Behavioral Neurology : Official Journal of the Society for Behavioral and Cognitive Neurology, 18(1), 28–36. doi:00146965-200503000-00004 [pii] [DOI] [PubMed] [Google Scholar]
- Rapcsak SZ, Beeson PM, Henry ML, Leyden A, Kim E, Rising K, . . . Cho H (2009). Phonological dyslexia and dysgraphia: Cognitive mechanisms and neural substrates. Cortex, 45(5), 575–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp B, & Dufor O (2011). The neurotopography of written word production: An fMRI investigation of the distribution of sensitivity to length and frequency. Journal of Cognitive Neuroscience, 23(12), 4067–4081. [DOI] [PubMed] [Google Scholar]
- Rapp B, Purcell J, Hillis AE, Capasso R, & Miceli G (2016). Neural bases of orthographic long-term memory and working memory in dysgraphia. Brain : A Journal of Neurology, 139(Pt 2), 588–604. doi: 10.1093/brain/awv348 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson D, Apperly IA, Kathirgamanathan U, & Humphreys GW (2005). Seeing it my way: A case of a selective deficit in inhibiting self-perspective. Brain : A Journal of Neurology, 128(Pt 5), 1102–1111. doi:awh464 [pii] [DOI] [PubMed] [Google Scholar]
- Samson D, Apperly IA, Chiavarino C, & Humphreys GW (2004). Left temporoparietal junction is necessary for representing someone else’s belief. Nature Neuroscience, 7(5), 499–500. [DOI] [PubMed] [Google Scholar]
- Samson D, Apperly IA, & Humphreys GW (2007). Error analyses reveal contrasting deficits in “theory of mind”: Neuropsychological evidence from a 3-option false belief task. Neuropsychologia, 45(11), 2561–2569. [DOI] [PubMed] [Google Scholar]
- Schön D, Lorber B, Spacal M, & Semenza C (2004). A selective deficit in the production of exact musical intervals following right‐hemisphere damage. Cognitive Neuropsychology, 21(7), 773–784. [DOI] [PubMed] [Google Scholar]
- Sebanz N, Knoblich G, Stumpf L, & Prinz W (2005). Far from action-blind: Representation of others’ actions in individuals with autism. Cognitive Neuropsychology, 22(3–4), 433–454. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J, & Perry D (2009). Two systems for empathy: A double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain : A Journal of Neurology, 132(Pt 3), 617–627. doi: 10.1093/brain/awn279 [doi] [DOI] [PubMed] [Google Scholar]
- Shohamy D, Myers CE, Hopkins RO, Sage J, & Gluck MA (2009). Distinct hippocampal and basal ganglia contributions to probabilistic learning and reversal. Journal of Cognitive Neuroscience, 21(9), 1820–1832. [DOI] [PubMed] [Google Scholar]
- Shu B, Lung F, Tien AY, & Chen B (2001). Executive function deficits in non-retarded autistic children. Autism, 5(2), 165–174. [DOI] [PubMed] [Google Scholar]
- Travis LL, & Sigman M (1998). Social deficits and interpersonal relationships in autism. Mental Retardation and Developmental Disabilities Research Reviews, 4(2), 65–72. [Google Scholar]
- Wright A, Tippett DC, Davis C, Gomez Y, Posner J, Ross ED, . . . Hillis AE (2015). Affective-prosodic deficits during the hyperacute stage of right hemispheric ischemic infarction . Annual Meeting of the American Academy of Neurology, Washington, DC. [Google Scholar]