Abstract
Objectives
Studies of older U.S. adults have consistently found that African Americans perform worse on cognitive measures than whites, but there are inconsistencies as to whether these findings hold over time. Moreover, studies have focused on adults 51 and older, without considering younger ages; thus it is unclear the age at which these disparities surface. The present study examines black-white disparities in mental status trajectories among adults as young as 25 years over a 25-year period.
Method
Data come from the Americans’ Changing Lives Study (ACL) (n = 3,617). Participants, ranging from ages 25–100 years old at baseline, were followed from 1986 to 2011 over 5 waves. Mental status was assessed at each wave using a 5-item Short Portable Mental Status Questionnaire. Growth models were used to estimate the associations between age, race, baseline status, and longitudinal changes in mental status, controlling for sociodemographic (e.g., education, income) and other health risk factors (diabetes, stroke, tobacco use, depression).
Results
Racial disparities were seen beginning in midlife and this relationship was curvilinear. Specifically, blacks had a steeper rate of mental status decline than whites and these disparities persisted after accounting for social and health risk factors (b = 0.0090, p < 0.0001).
Discussion
Study findings demonstrate disparities emerge at middles ages and worsen as age increases. This finding highlights the importance of addressing racial disparities in cognition across a larger part of the adult life course. By doing so, we may better be able to capture early-life exposures that influence later-life cognitive outcomes and ultimately lead to disparities.
Keywords: African Americans, Racial health disparities, Cognitive aging, USA
Introduction
Numerous studies of cognitive aging among older U.S. adults have found that, on average, blacks have lower cognitive test scores than whites (Weuve et al., 2018). We might expect that longitudinal studies would mimic cross-sectional findings, showing that blacks decline at a faster rate than whites over time. However, investigations of this question have produced mixed results and the literature is inconsistent as to whether blacks indeed decline at a faster rate as they age.
The inconsistencies in the literature may be due to the fact that many of these studies, if not all, have not considered the full adult life course. Most have primarily focused on adults who were 51 and older at baseline (Masel & Peek, 2009) or have followed participants for a limited length of time, e.g., 3–18 years (Weuve et al., 2018). Thus, it is unclear the age at which these disparities begin to emerge. One can postulate that if racial disparities in cognition emerge early in the life course they will persist into old ages. Yet, few studies have considered younger participants when examining cognitive aging trajectories in black and white adults.
The discrepancies in the current literature may also be due to the fact that several of these studies have used either small sample sizes (Early et al. 2013 – 116 black and 184 white; Wilson, Capuano, Sytsma, Bennett, & Barnes, 2015 – 647 black and 647 white) or been conducted in special populations, such as Medicare beneficiaries (Wolinsky et al., 2011), elders with mild cognitive impairment (Lee et al., 2012) or those clinically diagnosed with dementia as compared to those with normal cognition (Wilson et al., 2010). In addition, most of these studies have conducted secondary analyses of the same parent study, e.g., AHEAD data, but do not consistently report similar findings (Alley et al., 2007, Karlamangla et al., 2009, Sloan and Wang, 2005, Wolinsky et al., 2011). For example, some of these studies report that blacks have more rapid cognitive decline than whites, but these findings are limited to certain measures of cognition (e.g., memory score, TICS-7, immediate and delayed word recall tests) (Masel and Peek, 2009, Wolinsky et al., 2011).
Collectively, the evidence points to robust cross-sectional or baseline differences, such that older blacks have lower cognitive-test scores than whites (Alley et al., 2007, Karlamangla et al., 2009, Masel and Peek, 2009, Sloan and Wang, 2005, Weuve et al., 2018, Wilson et al., 2015, Wolinsky et al., 2011). Moreover, a recent systematic review of dementia incidence and prevalence in the U.S. indicates a higher risk of cognitive disease among blacks (Mehta & Yeo, 2017). Yet, current evidence on race specific change patterns in cognitive function remains less consistent. Whereas some studies show that blacks declined more over time (Sachs-Ericsson and Blazer, 2005, Sawyer et al., 2008), others have shown that whites declined at higher rates (Alley et al., 2007, Early et al., 2013, Karlamangla et al., 2009, Sloan and Wang, 2005, Weuve et al., 2018, Wilson et al., 2015). Hence, race differences in cognitive trajectories is not well understood (Early et al., 2013). More importantly, it remains unclear when these inequalities begin to appear in the life course.
Extant evidence not only indicates that blacks have a higher risk of cognitive disease (including lower baseline test scores, higher cognitive impairment, dementia incidence and prevalence); it further suggests that these disparities vary by age (Alzheimer’s Association, 2011, Lines and Wiener, 2014, Mehta and Yeo, 2017). For example, studies of racial disparities in cognitive performance and academic achievement during adolescence has shown that achievement gaps begin early and widen through the school years (Fryer and Levitt, 2004, Reardon, 2011, Reardon and Galindo, 2009). Specifically, national studies consistently show that non-Hispanic black students score well below the average on standardized tests of math and reading skills compared to non-Hispanic white students (Fryer and Levitt, 2004, Reardon, 2011). Minor and colleagues (2016), for instance, report that not only are black students less likely to be enrolled in advanced math courses, but once they do take advanced courses black math students have significantly lower test scores and are less likely to be proficient at all mathematics skill subdomains compared to their advanced white peers (Minor, 2016). Even when high-potential black students participate in intensive supplemental enrichment and accelerated programming to better prepare them for advanced math and science courses in high school, such as Project Excite, which is geared at increasing minority interest in STEM, racial differences in achievement gaps remain (Olszewski-Kubilius, Steenbergen-Hu, Thomson, & Rosen, 2017). One could posit that it is a challenge to overcome years of deficits in academic training and to expect a supplemental program is enough to narrow the achievement gap. Thus, the emergence of racial disparities in cognitive development and achievement during early childhood and adolescence suggests that these disparities will continue over the life course and into old age.
Yet, extant research has not typically included younger participants; thus, studies clarifying the effects of race on cognitive trajectories among younger aged adults are necessary to address current inconsistencies in terms of which groups are at the greatest disadvantage and at which age disparities emerge. Moreover, identifying potential risk factors contributing to race differences is also needed. Early detection of risks when differences are beginning to emerge, but individuals have yet to develop more severe symptoms, makes it possible to pinpoint the preclinical stages of the neurodegenerative processes that lead to later disease onset (DeKosky & Marek, 2003). This may also offer the most promise for reducing racial disparities.
The purpose of the present study was to evaluate how race relates to baseline and mental status trajectories among younger aged and older black and white adults. We expect that findings from this study would contribute to the ongoing debate on racial disparities in cognitive aging in two ways. First, we will better characterize how subtle cognitive deficits (e.g., cognitive errors) starting in early adulthood and middle-age unfold, as risk factors for cognitive dysfunction emerge and accumulate. Second, we will test whether, when, and to what extent trajectories of mental status differ between blacks and whites (aim 1), and assess how known social and health risk factors contribute to widening differences, if any (aim 2). Overall, we hypothesize that race would be associated with more acute cognitive errors, such that blacks would have higher baseline scores and a faster rate of longitudinal mental status decline than whites. Second, we posit that race differences would emerge at younger ages and persist in older adulthood, independent of other measured social (e.g., education, income) and health (e.g., diabetes, stroke, tobacco use, and depression) risk factors. This study was unique in that we used adults 25 and older at baseline to examine trajectories of mental status change over 25-years.
Methods
Participants
Participants were drawn from the Americans’ Changing Lives Survey (ACL), an ongoing longitudinal study designed to explore social disparities in health and aging with a particular emphasis on examining differences between black and white adults who are in middle and late life (House, Lantz, & Herd, 2005). The ACL cohort consists of a non-institutionalized sample of adults’ ages 25 years and older living in the U.S. in 1986. It oversamples, at twice the rate of others, blacks and adults 60 and over to increase the size of these groups and facilitate age and race comparisons. The individuals in the ACL cohort were interviewed face-to-face at baseline in 1986 (n = 3,617) and re-interviewed either by phone or face-to-face in 1989 (n = 2,867), 1994 (n = 2,559), 2001/2002 (n = 1,785), and 2011 (n = 1,427). As with all longitudinal samples, the sample declined over time, but the retention between waves was fairly good, ranging from a high of 83% between 1986 to 1994 to a low 76% between 1994 and 2001/2002.
Attrition
To address the losses to follow-up a maximum likelihood estimation (mle) was applied to the analysis. In addition, we conducted multiple sensitivity analyses on key demographics to see if attrition or mortality have an impact on black-white differences in mental status. These additional analyses are included as appendices. We found that blacks are no more likely to die in the ACL study compared to whites unadjusted OR = 1.14, p = 0.07, 95% CI [0.99, 1.32] (see Appendix 1). Those with higher levels of education at baseline are less likely to die compared to those with less education (Pearson χ2(3) = 406.27, p = 0.000). Individuals with diabetes at baseline (86.3%) are more likely to die during the study period than those without diabetes (48.3%) (Pearson χ2 (1) = 165.89, p = 0.000). 96.5% of participants who reported having a stroke died during the study period compared to 51.4% of those who did not have a stroke at baseline (Pearson χ2 (1) = 23.43, p =;0.000). Further, we conducted additional analyses examining the number of waves blacks and whites participated in the ACL study to determine whether race has an impact on attrition. We found that black-white differences were statically significant, such that on average, whites participated in 3.56 waves of the ACL compared to 3.10 for blacks; but more than half of this difference is accounted for by covariates that we control for in our models. Although blacks and whites do not differ in death status, all models control for both mortality status and the number of waves participated in to more robustly detect race gaps in mental status.
Measures
Mental status
Mental status was assessed at each wave using a shortened 5-item version of the Short Portable Mental Status Questionnaire (SPMSQ). The SPMSQ was designed to determine the presence and degree of intellectual impairment in the elderly (Pfeiffer, 1975). Specifically, it assesses the entire range of cognitive performance, from intact functioning to severe impairment (Pfeiffer, 1975). It has also been used in community samples to identify persons 65 years and older with cognitive impairment and clinically diagnosed Alzheimer's disease (Albert et al., 1991). Moreover, studies have used this scale as a measure of global cognition (Sachs-Ericsson and Blazer, 2005, Sawyer et al., 2008) and have validated the sensitivity of this instrument for interviewer error when assessing cognitive functioning among older adults (Malhotra et al., 2015). The measure tests a participant’s orientation (i.e., identifying today’s date and day of the week), knowledge of current and past affairs (i.e., naming the current and former Presidents of the United States), and working memory (i.e., using the serial 3s subtraction test). The serial 3s subtraction test asks participants to subtract 3 from 20, report the number they get, and to continue subtracting in increments of 3 from each new number they get. Successful cognitive performance on the serial 3s test consists of the number of times a respondent is able to correctly count backwards by three until arriving at the number two. Thus, the entire series beginning with the first subtraction (i.e., 20 − 3 = 17) and the remaining subtractions (e.g., 17 − 3=14, 14 − 3=11, etc.) must be performed accurately without missing any one of the subtractions in order to be scored as correct (0 = correct). Missing any one of the subtractions (or any error in the series) or refusing to attempt the series is scored as incorrect (1 = incorrect) (Pfeiffer, 1975, p. 441). The total mental status score was calculated by adding the number of errors or incorrect responses for each of the three domains (orientation, knowledge, and working memory) to form a single summary measure and continuous scale (0–5 errors), as done by other studies among blacks (Sawyer et al., 2008). Higher scores indicate more cognitive errors.
Race/Ethnicity
Race was self-reported as non-Hispanic white (referred to as whites; n = 2,205), non-Hispanic black (referred to as blacks; n = 1,156). Participants who were Native American (n = 44), Asian (n = 30) or Hispanic (n = 182) were excluded due to small sample size.
Control Variables
All analyses account for sociodemographic, chronic conditions, health behaviors and mental health variables that may influence cognitive functioning (Rexroth et al., 2013). These variables include sociodemographic characteristics (baseline age, age, age-squared, gender, education, income and marital status) and health risk factors (diabetes, stroke, newly diagnosed stroke, smoking status, physical activity, smoking status and depressive symptoms). Baseline age was measured in years and treated as a continuous measure. Age and age-squared were measured in years, centered at 75 years to facilitate interpretation of the intercept; this approach has been used by other studies that examine cognitive aging in black and white Americans (Weuve et al., 2018). Gender was dichotomized with male as the reference category. Level of education was self-reported and categorized as 0 = less than high school (reference category), 1 = high school graduate, 2 = some college, and 3 = college graduate and beyond. Income was measured using both the respondent’s and spouse’s income and treated as a continuous measure of family income; income was asked open-ended and respondents reported their income as actual dollar amounts. Some respondents refused to report or did not know the exact dollar amount of their income. In place of reporting a specific dollar amount, these respondents were asked to select from a range of incomes. To create a single measure of family income across all participants, respondents selecting from the range of incomes were then assigned a “mid-point” value. This mid-point value was based on the average income values for those respondents who reported an exact dollar amount for that income range. The final family income variable is based on these assigned averaged “mid-point” values across all participants, inflation-adjusted to 1986 dollars and is reported in thousands. Marital status was categorized as married (reference category), separated, divorced or widowed, and never married.
Chronic health conditions were based on two questions that asked participants whether they had experienced diabetes (including taking medication for high blood sugar) or stroke in the past 12 months. Given that these questions only asked respondents to report these health conditions during the last year, we created a variable for respondents who reported “yes” to having had a stroke in a previous wave to identify them in subsequent waves as a newly diagnosed stroke. In addition to accounting for stroke at baseline, a time-varying measure of self-reported incident stroke was also included. All of these variables were treated as dichotomous, with “no” as the reference category. Smoking status was categorized as currently (reference category), former, and never. Physical activity was based on three questions that asked participants how often they engage in the following activities: work in the garden or yard, engage in active sports or exercise, and take walks. Responses were (1) “often”, (2) “sometimes”, (3) “rarely” or (4) “never”. These categories were reverse coded so that higher scores indicated higher levels of physical activity. Sample range = 0 (never) to 9 (often). Body mass index (BMI) was calculated using self-reported weight (in kilograms) divided by self-reported height squared (in meters) and was dichotomized as overweight and other (reference category). Depressive symptoms were assessed using an 11-item standardized version of the Center for Epidemiologic Studies Depression Scale (CES-D) (Kohout, Berkman, Evans, & Cornoni-Huntley, 1993). This scale measures the extent to which a person feels depressed and has become a standard in community mental health surveys over the past three decades (Eaton and Kessler, 1981, Frerichs et al., 1981). Representative questions included: “I felt depressed”; “I felt that everything I did was an effort”; “My sleep was restless”; and “I could not get ‘going’.” Responses were (1) “never or hardly ever,” (2) “some of the time,” or (3) “most of the time.” Higher scores indicated more depressive symptoms. Sample range = 0 (hardly ever) to 32 (most of the time).
Analytic strategy
We used random intercept and slope linear mixed models to examine the associations between mental status (measured by the number of cognitive errors made) and our primary exposure variable (race) and covariates (social and health risk factors). Mixed models accommodate the non-independent nature of longitudinal data that has repeated measures over time within the same study subject (Masel & Peek, 2009). Another advantage of mixed-effects or multilevel growth models is their flexibility in accounting for varying numbers of assessments and the uneven spacing between measurements across individuals. Comprehensive theoretical and applied treatments of generalized linear mixed models are provided elsewhere (Singer & Willett 2003). We used standard modeling procedures as detailed in Singer and Willett (2003), and a maximum likelihood estimation (mle) feature, which provides an unbiased estimate that accommodates missing data on the dependent variable due to attrition over time. This estimation allows participants with missing data, or those lost in subsequent follow-up waves, to contribute to the model results (Masel & Peek, 2009). Linear mixed models allowed us to estimate fixed and random effects to 1) characterize the association between races and other model covariates and baseline level of mental status as well as rate of change (slope), and 2) quantify variations within (level 1) and between (level 2) individuals in mental status and the contributions of model covariates to explaining variance estimates. All analyses were conducted in Stata (version 13), using the “MIXED” functionality (StataCorp, 2017). Our modeling process included several incremental steps. First, the crude model was estimated and the effect of age on the growth of cognitive errors was examined. Age-squared was added as a predictor in the analyses to examine the non-linear relationship between age and mental status. Race was then added as a predictor to assess the impact of race on the increase in the number of cognitive errors. To determine whether the effect of age on cognitive errors varies by race, models included an interaction between race and age and race and age-squared. In addition, baseline age as well as the interaction between age and baseline age on the growth of cognitive errors were examined. Second, the effects of these variables in explaining the racial variability in the estimated baseline score and rate of change in the SMPSQ mental status measure, while controlling for sociodemographic, other health risk variables, mortality status and the number of waves participated in was tested.
The final model included the number of cognitive errors as the outcome predicted by age, age-squared, race, the interaction between race and age, the interaction between race and age-squared, baseline age, baseline age-squared, and the interaction between age and baseline age with a random intercept to test the variability in each person’s starting point and whether they tend to be above or below the sample mean at baseline. A random slope for age was also included to determine if there are individual changes in mental status performance over time. This analysis permitted the estimation of individual differences in mental status as a function of age and allowed us to assess whether variability in cognitive errors could be predicted by race, while controlling for sociodemographic, other health-related variables, and mortality and attrition status. To facilitate interpretation of our estimates, we generated empirical change plots to display the non-linear trends in the data and specified a quadratic (or non-linear) growth model that parameterized time as age, age-squared and baseline age, centered at 75 years.
Finally, to address the adequacy of statistical estimation procedures associated with random-effects growth modeling, additional diagnostics were done to evaluate the variance components associated with the random effects and within-subject error variance (Early et al., 2013, p. 8). Although, a random slope for age-squared was examined to test for individual differences in rates of change, it was not significant; thus, was not included in the final model.
Results
Descriptive Analyses
As shown in Table 1, there were significant racial differences on almost all of the study variables. Specifically, whites were older, less likely to be women, reported higher levels of education and income, and were more likely to be married. More than 60% of whites were married, and although nearly 40% of blacks were married, the majority were single either due to being separated, divorced, widowed or never married. Further, the two samples differed significantly in terms of their risk factors, with the exception of stroke. Nearly twice as many blacks (13.8%) reported having diabetes compared to whites (7.0%). Blacks (32.9%) were also more likely to report being a current smoker versus whites (27.5%). Additionally, blacks were significantly less likely to engage in physical activity and were more likely to report having an overweight BMI compared to whites. Blacks also reported experiencing more depressive symptoms than whites did.
Table 1.
Baseline characteristics for adult respondents, stratified by race/ethnicity and expressed as percentages unless otherwise specified (unweighted). Americans’ changing lives study, 1986.
| Characteristic | All participants (N = 3617) | Non-hispanic whites (N= 2205) | Non-hispanic blacks (N= 1156) | Race differencea |
|---|---|---|---|---|
| DEMOGRAPHICS | ||||
| Age, mean (SD) | 54.1 (17.63) | 55.4 (17.71) | 53.2 (17.34) | p < 0.001 |
| Female | 62.5 | 60.9 | 66.1 | p = 0.003 |
| Education | p < 0.001 | |||
| Less than High School | 37.3 | 28.5 | 52.0 | |
| High School Graduate | 29.1 | 32.9 | 23.7 | |
| Some College | 19.7 | 21.6 | 16.6 | |
| College Graduate and Beyond | 13.8 | 17.0 | 7.7 | |
| Family income (inflation-adjusted mid-points, per thousands) | p < 0.001 | |||
| $3125 | 14.8 | 7.5 | 27.6 | |
| $7250 | 17.7 | 15.1 | 22.2 | |
| $12,010 | 14.0 | 13.4 | 14.9 | |
| $17,210 | 10.3 | 11.7 | 7.9 | |
| $22,040 | 8.5 | 10.0 | 5.7 | |
| $26,910 | 8.0 | 9.3 | 5.7 | |
| $33,450 | 10.5 | 12.9 | 6.9 | |
| $46,940 | 10.2 | 12.3 | 6.7 | |
| $65,950 | 3.5 | 4.4 | 1.6 | |
| $85,230 | 2.5 | 3.5 | 0.9 | |
| Marital status | p < 0.001 | |||
| Married | 54.6 | 62.4 | 38.9 | |
| Separated, divorced or widowed | 34.2 | 29.6 | 44.5 | |
| Never Married | 11.2 | 8.1 | 16.6 | |
| RISK FACTORS | ||||
| Diabetes | p < 0.001 | |||
| Yes | 9.4 | 7.0 | 13.8 | |
| Stroke | p = 0.424 | |||
| Yes | 0.9 | 0.8 | 1.0 | |
| Smoking status | p < 0.001 | |||
| Former Smoker | 26.0 | 28.5 | 20.9 | |
| Never | 44.7 | 44.0 | 46.3 | |
| Physical activity, mean (SD) | 5.04 (2.48) | 5.35 (2.47) | 4.51 (2.44) | p < 0.001 |
| Body mass index | p < 0.001 | |||
| Overweight | 18.8 | 14.6 | 26.5 | |
| Depressive symptoms (CESD-11), mean (SD) | 15.66 (4.14) | 15.16 (3.89) | 16.51 (4.36) | p < 0.001 |
Note. SD = standard deviation. CESD-11 = Center for Epidemiologic Studies Depression Scale. Other races excluded were Non-Hispanic Native American (n = 44), Non-Hispanic Asian (n = 30) and Hispanic (n = 182).
Tests of difference between races was based on OLS regression for age and depressive symptoms; Pearson’s chi-square for gender, marital status, stroke, diabetes and BMI; multinomial logistic regression for smoking status with current smoker as the reference category; ordinal logit regression for education, income and level of physical activity.
Multilevel growth modeling analyses
The linear age term included in Model 1 in Table 2, the crude model, showed that the average linear change in cognitive errors for every year age increases was approximately 0.015 (SE = 0.003, p < 0.0001). However, in Model 1, we also found a significant positive quadratic age term (b = 0.0004, SE = 0.0001, p < 0.0001) indicating that cognitive errors increase non-linearly (curvilinear or convex relationship) with age such that as age increases cognitive errors are accelerating at a faster rate. This suggests that the linear age effect insufficiently captures the overall variability in each person’s trajectory of change (or slope) in cognitive errors over chronological age. And the quadratic age term better accounts for the individual variability in slopes.
Table 2.
Mixed models of mental status on sociodemographic, chronic conditions, Health behaviors, mental health characteristics, Mortality and attrition status for non-hispanic black and white respondents. Age centered at 75. Americans’ changing lives study, 1986–2011 (waves 1–5).
| Model 1: crude model | Model 2: adjusted model | Model 3: excludes mortality status and number of waves participated In | |
|---|---|---|---|
| Fixed effects | B [SE] | B [SE] | B [SE] |
| Age (centered at 75) | 0.01*** [0.003] | 0.01*** [0.004] | 0.01** [0.004] |
| Age-squared (centered at 75) | 0.00*** [0.000] | 0.00*** [0.000] | 0.00*** [0.000] |
| Black Race (ref=White) | 0.74*** [0.039] | 0.52*** [0.039] | 0.53*** [0.039] |
| Black Race#Age | 0.01*** [0.003] | 0.01*** [0.003] | 0.01*** [0.003] |
| Black Race#Age-squared | 0.00 [0.000] | 0.00 [0.000] | 0.00 [0.000] |
| Baseline Age (centered at 75) | 0.01** [0.004] | -0.00 [0.004] | 0.01 [0.004] |
| Baseline Age-squared | 0.00 [0.000] | 0.00 [0.000] | 0.00 [0.000] |
| Age#Baseline Age | −0.00 [0.000] | −0.00 [0.000] | −0.00 [0.000] |
| Female | 0.01 [0.023] | ||
| Education | |||
| High School Graduate | −0.36*** [0.030] | −0.37*** [0.030] | |
| Some College | −0.46*** [0.034] | −0.48*** [0.034] | |
| College Graduate and Beyond | −0.56*** [0.039] | −0.58*** [0.039] | |
| Family Income | −0.03*** [0.007] | −0.03*** [0.007] | |
| Marital status | |||
| Separated, Divorced or Widowed | 0.02 [0.022] | 0.02 [0.022] | |
| Never Married | 0.01 [0.035] | 0.02 [0.035] | |
| Diabetesa | 0.04 [0.031] | 0.04 [0.031] | |
| Strokea | 0.33*** [0.069] | 0.34*** [0.069] | |
| Newly Diagnosed Stroke | 0.20*** [0.059] | 0.18** [0.059] | |
| Smoking status | |||
| Former Smoker | −0.03 [0.030] | −0.04 [0.030] | |
| Never | 0.01 [0.026] | −0.01 [0.026] | |
| Physical Activitya | −0.01 [0.005] | −0.01* [0.005] | |
| Obese BMI | 0.00 [0.029] | −0.01 [0.029] | |
| Depressive Symptoms | 0.02* [0.006] | 0.02** [0.006] | |
| Died During ACL Study | −0.03 [0.037] | ||
| Number of waves Participated In | |||
| 2 | −0.12* [0.055] | ||
| 3 | −0.13* [0.052] | ||
| 4 | −0.27*** [0.053] | ||
| 5 | −0.30*** [0.056] | ||
| Intercept | 0.86*** [0.028] | 1.30*** [0.072] | 1.17*** [0.047] |
| Random effects (variance components) | |||
| Random Slope, τ11 | 0.01 [0.001] | 0.01 [0.001] | 0.01 [0.001] |
| Random Intercept, τ00 (between-person variance) | 0.71 [0.017] | 0.64 [0.016] | 0.65 [0.016] |
| Covariance, τ10 (correlation between intercept and slope) | 0.87 [0.037] | 0.88 [0.024] | 0.88 [0.024] |
| Residual, σε2 (within-person variance) | 0.71 [0.006] | 0.71 [0.006] | 0.71 [0.006] |
| X2 | 985.32*** | 1729.55*** | 1667.14*** |
| d.f. | 8 | 28 | 23 |
Note. SE = standardized error. BMI = body mass index.
1=condition or level of physical activity reported.
p < .05;
p < .01;
p < .001
Moreover, in Model 1 we accounted for a linear age and race interaction term to test whether there is differential change in the slopes of cognitive errors between blacks and whites. The significant interaction between linear age and race demonstrates that the effect of age on cognitive errors varies by race; such that cognitive errors increased at a greater rate over time for blacks compared to whites (b = 0.014, SE = 0.003, p < 0.0001). The black-white gap in mean cognitive errors widened by 0.014 per year of age, indicating that blacks on average made more errors and demonstrated a more rapid rate of mental status decline than whites. Controlling for sociodemographic, chronic conditions, health behaviors, mental health characteristics, and mortality and attrition status in Model 2 attenuated but did not explain the race differences evidenced in Model 1. The main effect of race was reduced by 30% [0.744-0.518/0.744].
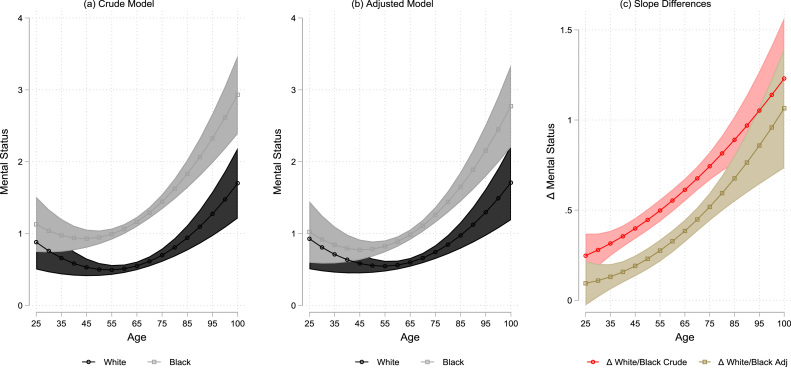
Fig. 1 plots the adjusted trajectories by race based on Models 1 and 2 derived estimates of the post-estimation contrasts of group differences in marginal means and their 95% confidence intervals over the age spectrum. This figure helps to quantify the white advantages in mental status performance over the age continuum; whereas black-white differences are initially insignificant in early adulthood, black disadvantage becomes apparent in midlife and increasingly so into older age. More specifically, the incremental changes in cognitive errors (or slope of change) between blacks and whites becomes more pronounced and increasingly accelerated starting in middle age (around age 45). Although whites are declining in mental status ability as they age, blacks are declining at a faster rate due to the fact that they make more cognitive errors, after adjusting for model covariates. Overall this figure indicates that beginning in middle age, blacks and whites show a diverging and distinct pattern in their predicted values or slopes of cognitive errors that becomes wider at older ages.
Fig. 1.
The relationship between Centered Age at 75 and mental status as moderated by race, Americans’ changing lives study (Ages 25–100). Note. Graph of Models 1 and 2 from Table 2 adjusted for sociodemographic, chronic conditions, health behaviors, mental health characteristics, mortality status and number of waves participated in.
The explained between-person intercept variance that resulted from incremental adjustment of each model was 0.64/0.71 = 0.90 between Model 1 and Model 2. Overall, 90.1% of the between-person variance is explained by sociodemographic, chronic conditions, health behaviors, mental health characteristics, and mortality and attrition status.
Discussion
The purpose of this study was to determine whether black-white differences in mental status exist and evaluate whether these disparities persist over time. Two primary findings are notable. First, this study found evidence of baseline differences in mental status at older ages, given that black participants performed worse, on average, on the Short Portable Mental Status Questionnaire (SPMSQ) than white participants at baseline (age 75, the reference value of age). This finding is consistent with other studies that have examined the effects of race/ethnicity on cognition among older (and middle age) blacks and whites and likewise found baseline differences at age 75 and above (Diaz-Venegas et al., 2016, Rexroth et al., 2013, Tang et al., 2001, Weuve et al., 2018, Weuve et al., 2015). The second major finding is that race is associated with change in mental status over time: blacks experienced a faster rate of decline in their cognitive abilities than whites; and sociodemographic and other health risk factors only partially accounted for these race differences.
This finding is consistent with some studies; yet inconsistent with others. For instance, some studies have found that blacks experience a more rapid rate of cognitive decline (Sachs-Ericsson and Blazer, 2005, Sawyer et al., 2008), while others have found that whites experience a more rapid rate of cognitive decline (Alley et al., 2007, Early et al., 2013, Karlamangla et al., 2009, Sloan and Wang, 2005, Weuve et al., 2018, Wilson et al., 2015), some have also found no difference (Atkinson et al., 2005, Castora-Binkley et al., 2013, Marsiske et al., 2013) and others present mixed results within the same study (Masel and Peek, 2009, Wolinsky et al., 2011). The inconsistencies in reporting racial differences may be due to how attrition was addressed across studies. For example, some studies handle attrition statistically using various approaches, including inverse probability-of-continuation weights (Weuve et al., 2018) or controlling for mortality to determine whether the results remain unchanged (Early et al., 2013), while others make no mention of it (Sawyer et al., 2008). The present study handles attrition using a maximum likelihood estimation as well as conducting multiple sensitivity analyses on key demographics to see if attrition or mortality have an impact on black-white differences in mental status, which offers some advantages over prior approaches.
The discrepancies may also be due to the target population included in these studies, specifically with respect to what age groups are represented, which are primarily older aged participants. Thus, we may not be capturing enough of the life course to fully understand when race disparities in cognitive outcomes begin to emerge. Understanding cognitive disease, like many other diseases, requires widening the investigative lens to cover as much of the lifespan as possible.
Given the discrepancies in the literature, the present study is valuable because it helps to address some of these inconsistencies by clarifying the effects of race on a continuous measure of mental status change. While previous studies have focused on only middle ages and/or older adults at baseline such as ages 51 and above (Masel & Peek, 2009), this longitudinal study is innovative as it examines racial differences in mental status trajectories across a greater portion of the adult life course; by studying a nationally representative sample of black and white adults who were ages 25 and older at baseline. Thus, to our knowledge, this study is the first to consider changes in mental status in younger ages and over a longer time frame.
The study findings further demonstrate that blacks made more cognitive errors than whites at middle ages and these disparities worsen with age, even after adjusting for sociodemographic and other health-related factors. With differences emerging at middle ages this further highlights that racial disparities begin earlier than previously identified, but are nonetheless consistent with other studies that have examined longitudinal changes in cognitive functioning in older black and white adults using the SPMSQ (Sachs-Ericsson and Blazer, 2005, Sawyer et al., 2008), the same measure used in the current study. The findings further show that disparities also widen, especially beginning in middle age and this pattern persist over time. Yet, social and health risk factors only partially accounted for these differences. This may then explain the higher reported prevalence rates and cognitive disease incidence among blacks (Mehta & Yeo, 2017). Indeed, studies have found a link between greater vascular burden among black elders and cognitive changes (Carmasin, Mast, Allaire, & Whitfield, 2014). Research further shows that the health of blacks, particularly black women, deteriorates at earlier ages than whites, as measured by allostatic load scores. Allostatic load (AL) is defined as “the cumulative impact of physiological stress responses that chronically exceed optimal operating ranges and causes wear and tear on the body’s regulatory systems” (Chyu & Upchurch, 2011, p. 575). Chyu and Upchurch (2011), for example, report that black women have the highest predicted AL scores compared to any other racial/ethnic group and there is a marked black-white gap in AL scores that persists across all age groups. Specifically, black women 40–49 years old have AL scores that are 1.14 times higher than white women 50–59 years old, suggesting earlier health deterioration and the poorer health status of black women in their middle ages.
Moreover, studies have examined the link between health behaviors (i.e., smoking, drinking, physical inactivity, obesity, and a healthy eating index score) and mortality among middle to older age black men (Griffith and Thorpe, 2016, Thorpe et al., 2013). For example, Thorpe et al. (2013) found that being a current smoker was associated with an increased risk of mortality in black men aged 25- to 44-year old, whereas being physically inactive was associated with an increased risk of mortality in the 45- to 64-year age-group (Thorpe et al., 2013). Similarly, in the present study, blacks had much higher rates of cardiovascular risk factors and poor health behaviors at baseline, including diabetes, being a current smoker, less physically active and an overweight BMI. Based on our attrition/mortality analyses, we found that those with diabetes and a higher BMI at baseline were also more likely to die during the study period. As such, this may explain why black-white differences in mental status emerge in middle age, yet persists and widen into older age. It may be that the overall poor health status and behaviors of blacks compared to whites is a major contributing factor to the racial differences in mental status trajectories.
The present study further contributes to this literature by following participants for a longer period time given that the ACL tracked individuals for 25 years from 1986–2011. Most studies have followed participants on average for 3–12 years, less than half the time of the current study. The ability to identify risks at early onset, when differences first emerge and begin to widen, makes it possible to pinpoint the neurodegenerative processes that lead to later disease development but are occurring in the preclinical stages (DeKosky & Marek, 2003).
Several limitations should be recognized. First, although ACL is the oldest ongoing nationally representative longitudinal study, the lag time between waves varied; and different periods between interviews may influence the strength, statistical significance, and associations of variables over time. For example, the time span between adjacent waves keeps increasing (e.g., the time gap between waves 1 and 2 = 2.5 years, waves 2 and 3 = 5 years, waves 3 and 4 = 7.5 years and waves 4 and 5 = 10 years). So it is more likely that attrition occurred at later waves of the ACL survey. Future work that follows individuals longitudinally should consider the time between waves of data collection. It is possible that using uniform or evenly spaced periods of time could yield a different pattern. Hence, future studies should validate these findings with additional waves of follow-up to more accurately detect race differences in trajectories of mental status and bring more consistency, given the mixed and seemingly conflicting findings that race differences exist in cognitive changes over time.
Second, the SPMSQ, the primary outcome measure, is not commonly used today to assess cognitive functioning, but when the ACL study began in 1986 it was one of the most prominent tools employed. Moreover, given that the ACL spans 25 years and has consistently used this tool as a short test of mental status, its continued use for the data analysis in this study is appropriate. However, future work should be done to validate these findings using different scales and more comprehensive tests, given that a brief global measure tends to represent fewer cognitive abilities versus specific domains of cognition. Further, the central insights may change depending on the measure used since measurement precision varies across domains. For example, studies show that on certain domains (e.g., executive functioning) blacks have faster cognitive decline than whites (Masel and Peek, 2009, Sawyer et al., 2008), while others show that whites decline at a faster rate (Early et al., 2013, Weuve et al., 2018).
Third, the measures of health status and sociodemographic factors used in this study were self-reported and suffer the same challenges as all self-report data. Specifically, these measures make it difficult to determine whether the associations found are valid or the extent to which they may reflect some other underlying factor such as early-stage dementia (Schulz et al., 2006). Dohrenwend (2006) also notes that recall bias, unreliability of recall, and criterion and construct validity are all potential problems of self-reported measures (Dohrenwend, 2006).
Fourth, although we did not directly assess the moderating effects of education on racial differences in this manuscript, it is an important issue that deserves future examination. In addition, the quality of education should be evaluated given its link to Alzheimer’s and cognitive decline (Mehta et al., 2009) as well as early education considering the link between cognitive performance and academic achievement during adolescence (Minor, 2016, Olszewski-Kubilius et al., 2017, Reardon, 2011). Likewise, this study did not evaluate whether similar declines occur for black men versus black women (race-sex differences), given the focus of this research on understanding whether blacks and whites experience similar rates of decline in mental status from young adulthood to later life; however, race by sex by age differences present an intriguing argument and the authors plan to look at these differences in a future study.
Finally, age effects (and race by age effects) as reported in this study aggregate and extrapolate from varying individual periods of observation. Longitudinal age changes may also diverge from our reported common trajectory, given that these trajectories can be altered due to cohort specific systemic changes. To address this, we control for age at baseline in our models and include data generated from long follow-up periods. Still, the trajectories reported are based on a wide baseline age range, which deserves serious consideration when assessing our reported findings and other replication studies with longer time series and across multiple cohorts.
In conclusion, these findings from one of the longest on-going studies to date of black and white Americans, sharpen the pattern from other cohorts and suggest that disparities emerge earlier in the life course than previously identified, widens and accelerates over time. Future studies examining trajectories of mental status changes in different racial/ethnic groups composed of individuals who represent a comprehensive view of the life course from young to old age are particularly relevant to understanding the adult life span of cognitive aging.
Ethics approval statement
Ethics approval was obtained by the University of Michigan. For additional information regarding the ethical review board or any other pertinent information regarding the Americans’ Changing Lives Survey contact the Principal Investigator:
Acknowledgements
The authors are grateful to Dr. Byrd’s other doctoral committee members, including Steven Wallace, Megan Sweeney and Chandra Ford. We would also like to thank Dr. Byrd’s post-doctoral mentor, Keith E. Whitfield, for his generous support of this manuscript. The authors benefited from facilities and resources provided by the California Center for Population Research at UCLA (CCPR), which receives core support (P2C-HD041022) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD).
Appendix A
See Table A1
Table A1.
Logistic regression models for non-hispanic black and white respondents who died during the ACL study period on sociodemographic, chronic conditions, Health behaviors and mental health characteristics.
| Model 1: Race Only | Model 2: Race + Covariates | |
|---|---|---|
| B [95% CI] | B [95% CI] | |
| DEMOGRAPHICS | ||
| Black Race | 1.14 [0.99;1.32] | 1.34* [1.07;1.69] |
| Age | 1.13*** [1.12;1.14] | |
| Female | 0.62*** [0.50;0.78] | |
| Education | ||
| High School Graduate | 0.63*** [0.49;0.80] | |
| Some College | 0.57*** [0.42;0.75] | |
| College Graduate and Beyond | 0.52*** [0.37;0.73] | |
| Marital Status | ||
| Separated, Divorced or Widowed | 1.38** [1.10;1.73] | |
| Never Married | 1.38 [0.95;2.00] | |
| RISK FACTORS | ||
| Diabetes | 3.51*** [2.32;5.32] | |
| Stroke | 8.72 [0.93;81.66] | |
| Newly Diagnosed Stroke | 0.95 [0.60;1.51] | |
| Smoking Status | ||
| Former Smoker | 0.50*** [0.38;0.65] | |
| Never Smoked | 0.42*** [0.32;0.54] | |
| Body Mass Index | ||
| Overweight | 1.22 [0.95;1.57] | |
| Intercept | 1.03 [0.95;1.12] | 0.003*** [0.002;0.005] |
| R-squared | 0.00 | 0.45 |
Note. CI = confidence interval.
p < .05.
p < .01.
p < .001.
Appendix B
See Table B1
Table B1.
Linear regression models of the number of waves participated in the ACL study for non-hispanic black and white respondents on sociodemographic, chronic conditions, health behaviors and mental health characteristics.
| Model 1: Race only | Model 2: Race + Covariates | |
|---|---|---|
| B [SE] | B [SE] | |
| DEMOGRAPHICS | ||
| Black Race | −0.46*** [0.05] | −0.38*** [0.05] |
| Age | −0.03*** [0.00] | |
| Female | 0.26*** [0.04] | |
| Education | ||
| High School Graduate | 0.23*** [0.05] | |
| Some College | 0.34*** [0.06] | |
| College Graduate and Beyond | 0.50*** [0.07] | |
| Marital Status | ||
| Separated, Divorced or Widowed | −0.24*** [0.05] | |
| Never Married | −0.46*** [0.07] | |
| RISK FACTORS | ||
| Diabetes | −0.34*** [0.07] | |
| Stroke | −0.60** [0.22] | |
| Newly Diagnosed Stroke | 0.34*** [0.10] | |
| Smoking Status | ||
| Former Smoker | 0.26*** [0.06] | |
| Never Smoked | 0.33*** [0.05] | |
| Body Mass Index | ||
| Overweight | 0.20*** [0.05] | |
| Intercept | 3.56*** [0.03] | 4.90*** [0.10] |
| R-squared | 0.026 | 0.289 |
Note. SE = standard error.
p < .01.
p < .001.
References
- Albert M., Smith L.A., Scherr P.A., Taylor J.O., Evans D.A., Funkenstein H.H. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer’s disease. International Journal of Neuroscience. 1991;57(3-4):167–178. doi: 10.3109/00207459109150691. [DOI] [PubMed] [Google Scholar]
- Alley D., Suthers K., Crimmins E. Education and cognitive decline in older Americans: Results from the AHEAD sample. Res Aging. 2007;29(1):73–94. doi: 10.1177/0164027506294245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association (2011). . 2010 Alzheimer’s disease facts and figures. Retrieved from 〈https://www.alz.org/documents_custom/report_alzfactsfigures2010.pdf〉. [DOI] [PubMed]
- Atkinson H.H., Cesari M., Kritchevsky S.B., Penninx B.W., Fried L.P., Guralnik J.M., Williamson J.D. Predictors of combined cognitive and physical decline. Journal of the American Geriatrics Society. 2005;53(7):1197–1202. doi: 10.1111/j.1532-5415.2005.53362.x. [DOI] [PubMed] [Google Scholar]
- Carmasin J.S., Mast B.T., Allaire J.C., Whitfield K.E. Vascular risk factors, depression, and cognitive change among African American older adults. International Journal of Geriatric Psychiatry. 2014;29(3):291–298. doi: 10.1002/gps.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castora-Binkley M., Peronto C.L., Edwards J.D., Small B.J. A longitudinal analysis of the influence of race on cognitive performance. The Journals of Gerontology Series B Psychological Sciences and Social Sciences. 2013 doi: 10.1093/geronb/gbt112. [DOI] [PubMed] [Google Scholar]
- Chyu L., Upchurch D.M. Racial and ethnic patterns of allostatic load among adult women in the United States: Findings from the national health and nutrition examination survey 1999–2004. Journal of Womens Health. 2011;20(4):575–583. doi: 10.1089/jwh.2010.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky S.T., Marek K. Looking backward to move forward: Early detection of neurodegenerative disorders. Science. 2003;302(5646):830–834. doi: 10.1126/science.1090349. [DOI] [PubMed] [Google Scholar]
- Diaz-Venegas C., Downer B., Langa K.M., Wong R. Racial and ethnic differences in cognitive function among older adults in the USA. International Journal of Geriatric Psychiatry. 2016;31(9):1004–1012. doi: 10.1002/gps.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrenwend B.P. Inventorying stressful life events as risk factors for psychopathology: Toward resolution of the problem of intracategory variability. Psychological Bulletin. 2006;132(3):477–495. doi: 10.1037/0033-2909.132.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early D.R., Widaman K.F., Harvey D., Beckett L., Park L.Q., Farias S.T., Mungas D. Demographic predictors of cognitive change in ethnically diverse older persons. Psychol Aging. 2013;28(3):633–645. doi: 10.1037/a0031645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton W., Kessler L.G. Rates of symptoms of depression in a national sample. American Journal of Epidemiology. 1981;114(4):528–538. doi: 10.1093/oxfordjournals.aje.a113218. [DOI] [PubMed] [Google Scholar]
- Frerichs R.R., Aneshensel C.S., Clark V.A. Prevalence of depression in Los Angeles County. American Journal of Epidemiology. 1981;113(6):691–699. doi: 10.1093/oxfordjournals.aje.a113149. [DOI] [PubMed] [Google Scholar]
- Fryer R., Levitt S. Understanding the black-white test score gap in the first two years of school. The Review of Economics and Statistics. 2004;86(2):447–464. [Google Scholar]
- Griffith D.M., Thorpe R.J., Jr. Men’s physical health and health behaviors. In: Wong Y.J., Wester S.R., editors. APA handbooks in psychology series. APA handbook of men and masculinities. American Psychological Association; Washington, DC: 2016. [Google Scholar]
- House J.S., Lantz P.M., Herd P. Continuity and change in the social stratification of aging and health over the life course: Evidence from a nationally representative longitudinal study from 1986 to 2001/2002 (Americans' Changing Lives Study) The Journals of Gerontology: Series B. 2005;60(Special_Issue_2):S15–S26. doi: 10.1093/geronb/60.special_issue_2.s15. [DOI] [PubMed] [Google Scholar]
- Karlamangla A.S., Miller-Martinez D., Aneshensel C.S., Seeman T.E., Wight R.G., Chodosh J. Trajectories of cognitive function in late life in the United States: Demographic and socioeconomic predictors. American Journal of Epidemiology. 2009;170(3):331–342. doi: 10.1093/aje/kwp154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohout F.J., Berkman L.F., Evans D.A., Cornoni-Huntley J. Two shorter forms of the CES-D (Center for Epidemiological Studies Depression) depression symptoms index. Journal of Aging Health. 1993;5(2):179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- Lines L.M., Wiener J.M. Research Triangle Institute (RTI) International; Washington, DC: 2014. Racial and ethnic disparities in Alzheimer's disease: A literature review. (Retrieved from) [Google Scholar]
- Malhotra R., Haaland B.A., Chei C.L., Chan A., Malhotra C., Matchar D.B. Presence of and correction for interviewer error on an instrument assessing cognitive function of older adults. Geriatrics & Gerontology International. 2015;15(3):372–380. doi: 10.1111/ggi.12331. [DOI] [PubMed] [Google Scholar]
- Marsiske M., Dzierzewski J.M., Thomas K.R., Kasten L., Jones R.N., Johnson K.E., Rebok G.W. Race-related disparities in 5-year cognitive level and change in untrained ACTIVE participants. Journal of Aging Health. 2013;25(8 Suppl):103S–127S. doi: 10.1177/0898264313497794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masel M.C., Peek M.K. Ethnic differences in cognitive function over time. Annals of Epidemiology. 2009;19(11):778–783. doi: 10.1016/j.annepidem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta K.M., Stewart A.L., Langa K.M., Yaffe K., Moody-Ayers S., Williams B.A., Covinsky K.E. “Below average” self-assessed school performance and Alzheimer’s disease in the aging, demographics, and memory study. Alzheimers & Dementia. 2009;5(5):380–387. doi: 10.1016/j.jalz.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta K.M., Yeo G.W. Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimers & Dementia. 2017;13(1):72–83. doi: 10.1016/j.jalz.2016.06.2360. [DOI] [PubMed] [Google Scholar]
- Minor E.C. Racial differences in mathematics test scores for advanced mathematics students. The High School Journal. 2016;99(3):193–210. [Google Scholar]
- Olszewski-Kubilius P., Steenbergen-Hu S., Thomson D., Rosen R. Minority achievement gaps in STEM:Findings of a longitudinal study of project excite. Gifted Child Quarterly. 2017;61(1):20–39. [Google Scholar]
- Pfeiffer E. A short portable mental status questionnaire for the assessment of arganic brain deficit in elderly patients. Journal of the American Geriatrics Society. 1975;23(10):433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- Reardon S.F. The widening academic achievement gap between the rich and the poor: New evidence and possible explanations. In: Murnane R., Duncan G., editors. Whither opportunity? Rising inequality and the uncertain life chances of low-income children. Russell Sage Foundation Press; New York: 2011. [Google Scholar]
- Reardon S.F., Galindo C. The hispanic-white achievement gap in math and reading in the elementary grades. American Educational Research Journal. 2009;46(3):853–891. [Google Scholar]
- Rexroth D.F., Tennstedt S.L., Jones R.N., Guey L.T., Rebok G.W., Marsiske M.M., Unverzagt F.W. Relationship of demographic and health factors to cognition in older adults in the ACTIVE study. Journal of Aging Health. 2013;25(8 Suppl):128S–146S. doi: 10.1177/0898264313498415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs-Ericsson N., Blazer D.G. Racial differences in cognitive decline in a sample of community-dwelling older adults: The mediating role of education and literacy. The American Journal of Geriatric Psychiatry. 2005;13(11):968–975. doi: 10.1176/appi.ajgp.13.11.968. [DOI] [PubMed] [Google Scholar]
- Sawyer K., Sachs-Ericsson N., Preacher K.J., Blazer D.G. Racial differences in the influence of the APOE epsilon 4 allele on cognitive decline in a sample of community-dwelling older adults. Gerontology. 2008;55(1):32–40. doi: 10.1159/000137666. [DOI] [PubMed] [Google Scholar]
- Schulz A.J., Gravlee C.C., Williams D.R., Israel B.A., Mentz G., Rowe Z. Discrimination, symptoms of depression, and self-rated health among African American women in Detroit: Results from a longitudinal analysis. American Journal of Public Health. 2006;96(7):1265–1270. doi: 10.2105/AJPH.2005.064543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer J.D., Willett J.B. Applied Longitudinal Data Analysis: Modeling Change and Event. Oxford University Press; New York, NY: 2003. Doing data analysis with the multilevel model for change; pp. 75–137. [Google Scholar]
- Sloan F.A., Wang J. Disparities among older adults in measures of cognitive function by race or ethnicity. The Journals of Gerontology Series B Psychological Sciences and Social Sciences. 2005;60(5):P242–P250. doi: 10.1093/geronb/60.5.p242. [DOI] [PubMed] [Google Scholar]
- StataCorp . StataCorp LLC; College Station, TX: 2017. Stata statistical software: Release 15. [Google Scholar]
- Tang M.X., Cross P., Andrews H., Jacobs D.M., Small S., Bell K., Mayeux R. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56(1):49–56. doi: 10.1212/wnl.56.1.49. [DOI] [PubMed] [Google Scholar]
- Thorpe R.J., Wilson-Frederick S.M., Bowie J.V., Coa K., Clay O.J., LaVeist T.A., Whitfield K.E. Health behaviors and all-cause mortality in African American Men. American Journal of Men’States Health. 2013;7(4_suppl):8S–18S. doi: 10.1177/1557988313487552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weuve J., Barnes L.L., Mendes de Leon C.F., Rajan K.B., Beck T., Aggarwal N.T., Evans D.A. Cognitive aging in black and white Americans: Cognition, cognitive decline, and incidence of Alzheimer disease dementia. Epidemiology. 2018;29(1):151–159. doi: 10.1097/EDE.0000000000000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weuve J., Hebert L.E., Scherr P.A., Evans D.A. Prevalence of Alzheimer disease in US states. Epidemiology. 2015;26(1):e4–e6. doi: 10.1097/EDE.0000000000000199. [DOI] [PubMed] [Google Scholar]
- Wilson R.S., Capuano A.W., Sytsma J., Bennett D.A., Barnes L.L. Cognitive aging in older black and white persons. Psychology and Aging. 2015;30(2):279–285. doi: 10.1037/pag0000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky F.D., Bentler S.E., Hockenberry J., Jones M.P., Weigel P.A., Kaskie B., Wallace R.B. A prospective cohort study of long-term cognitive changes in older Medicare beneficiaries. BMC Public Health. 2011;11:710. doi: 10.1186/1471-2458-11-710. [DOI] [PMC free article] [PubMed] [Google Scholar]