SUMMARY
The incidence of glioblastoma (GBM) is increasing among the elderly, who now account for up to half of all the adult cases of GBM. This trend has resulted in the recent development of clinical research specifically dedicated to this fragile population. Some studies have investigated surgical resection, radiotherapy and chemotherapy with temozolomide, and ongoing research is currently addressing the use of combined radiochemotherapy in this population. Although older patients with GBM have a significantly worse life expectancy compared with their younger counterparts, etiologic treatments should not be withheld from these patients solely because of their age. On the contrary, results from prospective studies suggest that active care of these patients has a significant positive impact on survival without affecting quality of life or cognition. To optimize both symptomatic and etiologic treatment, neuro-oncology multidisciplinary teams must take into account performance and cognitive status, the resectability of the tumor, and associated comorbidities.
Practice Points.
The incidence of glioblastoma is increasing in the aging population of industrialized countries.
Elderly patients may benefit from specific treatment such as surgery, radiotherapy or chemotherapy.
Performance and cognitive status, quality of life, and associated comorbidities are important factors to be considered before treatment onset.
When feasible, extensive resection can be performed safely and has a positive impact on survival and quality of life compared with biopsy.
Radiotherapy alone improves survival without deterioration of quality of life or cognitive functions in elderly patients with glioblastoma and good performance status (Karnofsky performance score ≥70%). Short schedules of irradiation seem equally effective but more convenient than standard courses of radiation in this population.
Temozolomide alone can be an alternative, particularly in patients with a poor Karnofsky performance score (<70%) and in patients with a methylated status of the MGMT promoter.
The benefit of a combined radiochemotherapy regimen remains to be demonstrated by ongoing studies.
Glioblastoma (GBM) is the most frequent and malignant primary brain tumor in adults [1]. Its incidence has significantly increased over recent decades, especially among the older segments of the population [2–6]. According to recent epidemiologic studies, elderly patients, aged over 65–70 years, may account for up to 50% of GBM cases in adults [7]. This frequency is expected to keep rising in coming years [8] because of the progressive aging of the populations in developed countries and as a result of the more widespread use of vigorous diagnostic work-ups in older patients [9].
This latter trend reflects a cultural change in the concept of ‘elderly’ over recent decades. Indeed, because physiological age does not necessarily match with chronological age, ages that were once considered ‘old’ are now considered ‘young’ [9,10].
In this setting, the management of GBM in elderly patients is becoming an increasingly important and challenging topic in neuro-oncology [8]. Advanced age is a well-recognized pejorative prognostic factor in GBM [11]. Recent analyses of population-based registries and institutional databases have revealed that elderly patients with GBM are less likely to undergo resection and receive adjuvant therapies, such as radiation or chemotherapy, than their younger counterparts, and advanced age has been given as the primary reason for this treatment decision [12–16]. In fact, until recently these patients have been systematically excluded from clinical trials and effective therapies because of the preconceived ideas that they would be unable to tolerate the treatment and that the therapies would be less effective [17–20]. As a consequence, many of these patients have been offered only palliative care [20]. The neuro-oncology community has realized that it is no longer acceptable to continue treating so many patients without relying on data from evidence-based medicine. Thus, important efforts have recently been made to develop clinical trials for elderly patients with GBM, not only focused on survival benefits, but also on quality of life and neurocognitive issues (Table 1).
Table 1. . Results from selected recent prospective studies in elderly patients with glioblastoma.
| Procedure | Age (years) | KPS (%) | Conclusions | Ref. |
|---|---|---|---|---|
| Elderly GBM patients with good performance status | ||||
| Surgery | ||||
| Stereotactic biopsy vs open craniotomy and tumor resection | ≥65 | ≥60 | Longer OS is achieved after open craniotomy and tumor resection | [40] |
| Radiation therapy | ||||
| Standard RT (50 Gy/28 fr) vs best supportive care | ≥70 | ≥70 | RT increases PFS and OS without reducing the quality of life or cognition | [43] |
| Standard (60 Gy/30 fr) vs short-course RT (40 Gy/15 fr) | ≥60 | ≥50 | Short-course RT has similar efficacy as standard RT | [46] |
| Chemotherapy | ||||
| TMZ (200 mg/m2/day × 5 days) vs standard RT (60 Gy/30 fr) vs hypofractionated RT (34 Gy/10 fr) | ≥60 | ≥60 | Standard RT, hypofractioned RT and TMZ have similar efficacy | [50] |
| Dose-dense TMZ (100 mg/m2/day, days 1–7/15–21) vs RT (60 Gy/30 fr) | ≥65 | ≥60 | TMZ is not inferior to RT in terms of survival | [51] |
| Combined radiochemotherapy | ||||
| Short-course RT (40 Gy/15 fr) plus concomitant (75 mg/m2/day) and adjuvant (200 mg/m2/day × 5 days) TMZ (uncontrolled trial) | ≥70 | ≥60 | Combined therapy is well tolerated and may prolong survival | [62] |
| Elderly GBM patients with impaired performance status | ||||
| Chemotherapy | ||||
| TMZ (200 mg/m2/day × 5 days) in elderly patients with impaired functional status (uncontrolled trial) | ≥70 | ≤60 | TMZ has an acceptable tolerance, is associated with improvement of functional status and may increase survival | [53] |
fr: Fraction; GBM: Glioblastoma; KPS: Karnofsky performance score; OS: Overall survival; PFS: Progression-free survival; RT: Radiotherapy; TMZ: Temozolomide.
The aim of this article is to review the current management of elderly patients with GBM, which has evolved over the past decade from a purely supportive treatment to specific antitumor therapies that are based on clinical trials.
Symptomatic treatments
Peritumoral brain edema, seizures, thromboembolic complications, fatigue and depression are common among elderly patients with GBM and account for significant morbidity, altered quality of life and mortality [21]. Thus, supportive care, which aims to reduce the symptoms and signs of the disease, must be offered to all elderly patients with GBM [22]. Symptomatic treatment primarily consists of corticosteroids and anticonvulsants.
The former remain the mainstay of treatment for symptomatic peritumoral edema. They alleviate the symptoms and signs related to intracranial hypertension, such as headaches, vomiting and blurred vision, as well as edema-related neurological deficits. Because corticosteroids are associated with a long list of deleterious side effects and drug interactions, which could be more pronounced in the elderly [23,24], only symptomatic patients should be treated. These patients should always receive the lowest effective dose for the shortest time period possible [25]. Additionally, preventive measures that diminish the risk of harmful effects, such as lymphopenia and osteoporosis, must be seriously considered. In particular, prophylactic therapy against Pneumocystis carinii pneumonia with trimethoprim sulfamethoxazole or aerosolized pentamidine should be carefully considered owing to the decline in immunity that is related to age [26] and combined steroids.
The general principles for the management of epilepsy apply to elderly patients with GBM. Antiepileptic drugs must be limited to patients with a previous history of seizures [27]. When indicated, patients should be treated with a single agent at the lowest dose that effectively controls the seizures. If the initial drug does not work at the highest tolerated dose, then patients should be switched to monotherapy with a second drug. The use of multiple anticonvulsants should be reserved for refractory cases, as the side effects increase with the number of drugs used [25]. When choosing the appropriate anticonvulsant, multiple factors other than efficacy must be considered, such as drug interactions, changes in pharmacokinetics, altered CNS pharmacodynamics and the side effects, particularly those that affect alertness and cognitive function [28]. Nonenzyme-inducing antiepileptic drugs are preferred in order to reduce the risk of interactions with corticosteroids, chemotherapeutic agents and the frequent polypharmacy in the elderly. Among these drugs, lamotrigine and levetiracetam might be especially helpful in the elderly [29,30]. Additionally, older patients may require lower doses of anticonvulsants than their younger counterparts to achieve therapeutic concentrations [31].
The risk of deep venous thrombosis is increased in elderly GBM patients who often have reduced mobility. Adequate prophylaxis with active and passive mobilization, compression stockings or low-molecular-weight heparin is frequently necessary.
Apart from these symptomatic treatments, depression must be promptly recognized because patients might benefit from antidepressants and anxiolytics, drugs that should be introduced at low doses and escalated slowly. Psychosocial support is also crucial to help these severely impaired patients face the illness. If fatigue, a common secondary effect of specific treatments such as radiotherapy and chemotherapy, becomes a limiting symptom, psychostimulants such as methylphenidate can be used in selected cases to provide relief. Treatment with psychostimulants must also be initiated at a low dose with subsequent slow titration. Whenever possible, physical and cognitive rehabilitation can also have a major impact on the patient's quality of life.
Further studies specifically assessing the impact of these symptomatic measures on cognition and quality of life are needed in such a severely impaired population.
Specific treatments
▪ Surgery
With rare exceptions, histological examination of the tumor tissue remains mandatory for a definitive diagnosis. First, it is the only procedure that can rule out a nontumoral cause, a feature that is particularly important for curable lesions such as infection. Second, it allows for a precise histological examination and the correct classification of the glial tumor as a high-grade lesion. In fact, up to 40% of low-grade gliomas in elderly patients may show contrast enhancement on MRI, thus simulating a high-grade glioma on neuroimaging [32].
In rare cases, when the patient is in very poor general condition or suffers from severe polypathologies, the risks of a direct approach may appear too high. In these exceptional circumstances, a working diagnosis of probable high-grade glioma is sometimes made when the clinical context is suggestive, when whole-body CT or PET scans are negative, and when spectroscopy/perfusion features of the lesion on brain MRI are also suggestive of high-grade glioma. However, the risk of committing a diagnostic error still exists, and the patient and their family must be informed of this risk [10].
It has traditionally been believed that elderly patients recover more slowly from surgery and are at a higher risk of developing postoperative neurologic complications [33,34]. However, several retrospective studies have shown that older patients tolerate aggressive surgery without increased surgery-related morbidity [35,36]. Moreover, according to these studies, extensive resection of the tumor appears to have a positive impact on survival when compared with needle biopsy alone [14,35,37,38].
The indication of the extent of the surgical procedure is based on the characteristics of the tumor and the frequently associated comorbidities of the aged patient [39]. The effectiveness of extensive resection in elderly patients with GBM was confirmed by a small prospective Finnish trial [40]. Patients older than 65 years with a malignant glioma, mostly GBM, were assigned to undergo either stereotactic biopsy or open craniotomy and resection of the tumor, followed by radiotherapy in both cases. With the constraint of the small number of included patients, this study showed improved survival for patients who underwent resection (5.6 months) when compared with those who only received a needle biopsy (2.8 months). Furthermore, resection was also associated with an improved quality of life. In addition to this trial, the extent of surgery has also been found to be independently associated with survival in several studies [14,36,41,42], some of which were prospective trials that primarily evaluated other specific therapies [43].
▪ Radiotherapy
The role of radiation therapy in the management of elderly patients with GBM has recently been established by a multicenter Phase III trial [43]. This study included 81 patients aged 70 years or older with newly diagnosed GBM and a Karnofsky performance score (KPS) of 70% or higher. After biopsy or surgery, the patients were randomly assigned to receive either supportive care alone or supportive care and radiotherapy. Radiotherapy consisted of fractioned focal irradiation at a dose of 1.8 Gy per fraction given once daily for 5 days per week for a total dose of 50 Gy. The addition of radiotherapy to the supportive care significantly increased survival by a median time of 21 weeks (29 weeks for the group of patients receiving combined therapy versus 17 weeks for the group of patients treated with the best palliative care alone; p = 0.004). Importantly, radiation therapy was well tolerated and did not have a negative impact on performance status, health-related quality of life or cognitive functions.
Although the benefit of radiotherapy is unequivocal [14,34,43,44], the optimal schedule of irradiation is still uncertain. Apart from the associated morbidity, conventional courses of radiotherapy delivering 60 Gy over a 6-week period [34] appear too long and inconvenient for these fragile patients, for whom survival times tend to be short. Several retrospective studies have suggested that short-term radiotherapy might be an effective and safe alternative for this select population [38,44,45]. This issue has been addressed in a prospective randomized trial of GBM patients aged 60 years or older [46]. Patients were assigned to receive either a standard course of radiotherapy (60 Gy in 30 fractions over 6 weeks) or a shorter course (40 Gy in 15 fractions over 4 weeks). No difference in survival was observed between the patients receiving the standard and those receiving the shorter radiotherapy course (5.1 vs 5.6 months, respectively; p = 0.57). Moreover, apart from a reduced time of treatment, patients receiving the abbreviated course of radiotherapy had a lower rate of premature discontinuation of radiotherapy (10 vs 26%) and required a lower increase in their post-treatment corticosteroid dosage, thus indicating an improved tolerance.
▪ Chemotherapy
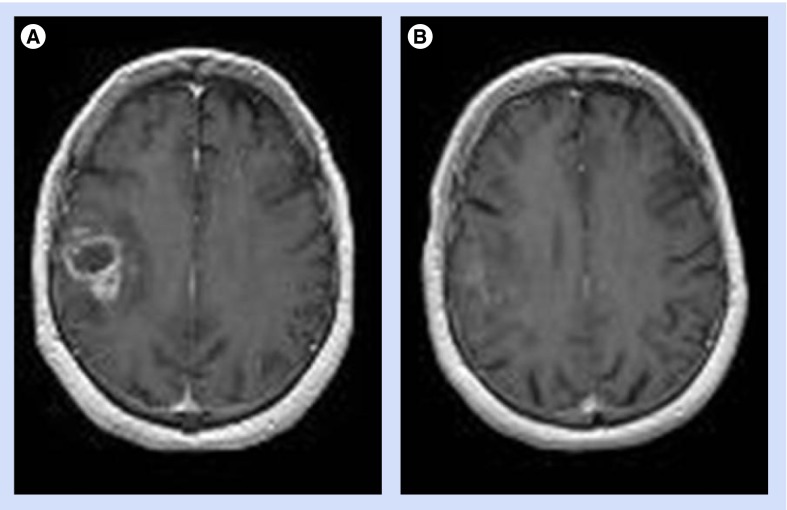
Because of its oral administration and favorable toxicity profile, temozolomide alone is sometimes used as an upfront treatment in elderly GBM patients, and cases of tumor response have been reported (Figure 1) [47]. Several retrospective studies also suggest that temozolomide alone might be as effective as radiation in this population [47–49], thus constituting a reasonable therapeutic alternative because of its facile administration and good tolerance.
Figure 1. Near-complete response in an 80-year-old patient with glioblastoma and a Karnofsky performance score of 70%, who refused radiation therapy and received chemotherapy with temozolomide alone.
MRI (A) at baseline and (B) after five cycles of temozolomide.
Recently, the results of two large prospective studies addressing this issue have been reported. A northern European randomized study compared two different radiotherapy schedules (i.e., standard and hypofractionated radiotherapy) with single-agent temozolomide chemotherapy [50]. Similarly to the aforementioned retrospective studies, no significant differences were found in survival between the three treatment arms: 6 months for the standard radiotherapy group, 7.5 months for the hypofractionated radiation group and 8 months for the temozolomide group (p = 0.14). Also, a large randomized German study showed the noninferiority of dose-intensified temozolomide alone (median overall survival of 8.6 months) compared with standard radiotherapy alone (median overall survival of 9.6 months) in elderly patients with high-grade glioma. Median event-free survival did not significantly differ between the treatment groups, and was longer in patients with MGMT promoter methylation who received temozolomide than in those who underwent radiotherapy. In terms of toxicity, grade 2–4 adverse events were in general more frequent in the dose-dense temozolomide group than in the radiation therapy group [51].
For practical purposes, chemotherapy alone appears to be the only conceivable option for dependent and bedridden elderly patients with GBM [52]. Radiotherapy, even delivered in short courses, is considered inconvenient for these severely impaired patients with such a short survival period because it requires daily trips to the hospital and results in increased fatigue. In this context, a Phase II prospective multicenter study showed that chemotherapy with temozolomide alone in elderly (≥70 years of age) GBM patients with poor KPS (<70%) allowed 25% of these patients to achieve KPS >70% – that is, becoming capable of self-care [53]. The observed median survival of 25 weeks was an encouraging finding that far exceeded the 12-week median survival time assumed for a similar patient population treated only with supportive care. This study also confirmed that the toxicity profile of temozolomide was acceptable in elderly patients, even in those with a poor performance status. Grade 3 or 4 neutropenia occurred in 13% of patients, and thrombocytopenia occurred in 14% of them, which are comparable to the toxicities observed in younger patients [54]. Thus, temozolomide alone may be a useful alternative in severely disabled patients to whom supportive care is commonly offered as the sole therapeutic option.
Recently, a subgroup analysis of a clinical trial evaluating the role of bevacizumab in the first-line treatment of GBM suggested that patients with a clinically determined poor prognosis, such as elderly patients, might benefit the most from upfront therapy with this antiangiogenic agent that targets VEGF [55]. An ongoing Phase II trial is currently evaluating the combination of temozolomide and bevacizumab in this neurologically impaired population.
▪ Chemoradiation
The question that arises in the management of elderly GBM patients is whether adding concomitant and adjuvant chemotherapy with temozolomide to radiation therapy increases survival when compared with radiation therapy alone. The European Organization for Research and Treatment of Cancer, and National Cancer Institute of Canada randomized Phase III trial, which included only patients up to 70 years of age, showed that the administration of chemotherapy with temozolomide during and after radiotherapy significantly increased the median overall survival when compared with radiotherapy alone (12.6 vs 14.6 months, respectively) [54,56]. Since then, this regimen has become the standard of care for younger patients with GBM.
The main concern about transferring this multimodality treatment scheme to the elderly is the development of severe hematologic and neurological toxicity, which could affect the quality of life of these patients. Whether the addition of temozolomide to radiotherapy may increase the risk of neurocognitive deficits in elderly patients is an unresolved question [57,58]. A few retrospective studies have shown promising survival advantages, with overall survival times ranging from 10 to 14 months in elderly patients treated with radiotherapy plus concomitant and adjuvant temozolomide [58–63]. Patients with low comorbidity might benefit the most [63,64]. However, high rates of neurologic treatment-related toxicity have been reported with this combined therapeutic regimen [59–62].
Only one recent prospective multicenter nonrandomized Phase II trial has addressed this issue [65]. This trial specifically evaluated the efficacy and safety of an abbreviated course of radiotherapy plus concomitant and adjuvant temozolomide in elderly patients with GBM. The observed median survival time was 12 months. Importantly, all patients completed the planned program of radiation therapy, and grade 3 and 4 neutropenia and thrombocytopenia occurred in 15% of patients. Grade 3 neurologic treatment-related toxicity was reported in only one patient during the adjuvant phase.
However, definitive results concerning the efficacy and toxicity of this combined regimen, and its impact on cognition and quality of life, must be validated by a randomized Phase III trial. Answers to these questions will be provided by a currently ongoing randomized clinical trial conducted by the National Cancer Institute of Canada and the European Organization for Research and Treatment of Cancer, which is comparing short-course radiotherapy alone with combined radiochemotherapy in GBM patients older than 65 years.
Possible specificities of molecular alterations in elderly GBM patients
The poorer outcome attributed to elderly patients with GBM might not be explained solely by an age-related suboptimal pattern of care [66]. Indeed, specific genetic alterations in the GBM tumors of elderly patients are also suspected to play a role. A recent retrospective study showed that the prognostic effects of TP53 mutations, EGFR amplification, CDKN2A/p16 alterations and the loss of chromosome 1p were dependent on the age of the patients [67]. TP53 mutations and CDKN2A alterations had negative prognostic effects in older patients, whereas EGFR amplification and the loss of chromosome 1p had a positive impact on the survival of these patients. Particularly, the prognostic effect of EGFR and TP53 alterations among elderly patients with GBM was the opposite of the effect observed in their younger counterparts. The different prognostic impact of EGFR amplification or overexpression according to age, with a better outcome in older patients and a shorter survival in younger patients, has also been found in other recent analyses [68,69]. Further studies are still needed to clarify if GBM in elderly patients is biologically different from that of younger patients.
On the other hand, the predictive impact of MGMT promoter methylation on response to alkylating agents appears to be independent of age. Epigenetic silencing of the MGMT gene by promoter methylation results in a diminished resistance to alkylating agents and constitutes the most important prognostic biomarker in younger GBM patients treated with radiation therapy and temozolomide [70–72]. Also, several retrospective studies [73,74] and prospective trials addressing this issue have confirmed the favorable impact of MGMT promoter methylation on survival in elderly GBM patients treated with temozolomide alone [51,53], or in combination with radiation therapy [61,65]. Some authors suggest that treatment of elderly patients should be tailored according to the MGMT status of the tumor, favoring temozolomide in ‘MGMT methylated’ patients and radiotherapy in ‘unmethylated’ patients [51]. However, this remains questionable because some nonmethylated patients may also respond to chemotherapy and because techniques to detect the methylation status of the MGMT promoter are not perfectly optimized. Nowadays, as noted above, a key issue is in fact to know if combined radiochemotherapy improves survival as compared with each modality alone without affecting quality of life. If combined treatment is proved superior, it is possible that knowledge of the MGMT methylation status, as in younger patients, will not influence the standard regimen, but may prompt specific clinical trials.
Conclusion & future perspective
Many questions remain concerning the role of surgery and its extension, the place of either chemotherapy with temozolomide or radiation therapy at the onset of care, and the efficacy and toxicity profiles of a combined therapeutic regimen. Currently ongoing prospective clinical trials will further provide helpful answers in the coming years. Nevertheless, currently available data from already published prospective studies suggest that the active care of these patients can be helpful. Given that the already large number of elderly patients suffering from GBM will continue to rise in the future, efforts to develop adapted and validated regimens in this population should be pursued with a particular attention to comorbidities, quality of life and neurocognitive issues.
Footnotes
Financial & competing interests disclosure
The authors have received no funding support for this article. J-Y Delattre receives research support from the Institut National du Cancer. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: ▪ of interest ▪▪ of considerable interest
- 1.Hess KR, Broglio KR, Bondy ML. Adult glioma incidence trends in the United States, 1977–2000. Cancer. 2004;101(10):2293–2299. doi: 10.1002/cncr.20621. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman S, Propp JM, McCarthy BJ. Temporal trends in incidence of primary brain tumors in the United States, 1985–1999. Neuro Oncol. 2006;8(1):27–37. doi: 10.1215/S1522851705000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wrensch M, Minn Y, Chew T, Bondy M, Berger MS. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro. Oncol. 2002;4(4):278–299. doi: 10.1093/neuonc/4.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleury A, Menegoz F, Grosclaude P, et al. Descriptive epidemiology of cerebral gliomas in France. Cancer. 1997;79(6):1195–1202. doi: 10.1002/(sici)1097-0142(19970315)79:6<1195::aid-cncr19>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 5.Chakrabarti I, Cockburn M, Cozen W, Wang YP, Preston-Martin S. A population-based description of glioblastoma multiforme in Los Angeles County, 1974–1999. Cancer. 2005;104(12):2798–2806. doi: 10.1002/cncr.21539. [DOI] [PubMed] [Google Scholar]
- 6.Elia-Pasquet S, Provost D, Jaffre A, et al. Incidence of central nervous system tumors in Gironde, France. Neuroepidemiology. 2004;23(3):110–117. doi: 10.1159/000075953. [DOI] [PubMed] [Google Scholar]
- 7.Werner MH, Phuphanich S, Lyman GH. The increasing incidence of malignant gliomas and primary central nervous system lymphoma in the elderly. Cancer. 1995;76(9):1634–1642. doi: 10.1002/1097-0142(19951101)76:9<1634::aid-cncr2820760921>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 8.Yovino S, Grossman SA. Treatment of glioblastoma in ‘elderly’ patients. Curr. Treat. Options Oncol. 2011;12(3):253–262. doi: 10.1007/s11864-011-0158-0. [DOI] [PubMed] [Google Scholar]
- 9.Laigle-Donadey F, Navarro S, Delattre JY. Gliomas in the elderly. Rev. Neurol. (Paris) 2008;164(6–7):542–546. doi: 10.1016/j.neurol.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Delattre JY. Glioblastoma in the elderly. Rev. Neurol. (Paris) 2011;167(10):680–682. doi: 10.1016/j.neurol.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Li J, Wang M, Won M, et al. Validation and simplification of the Radiation Therapy Oncology Group recursive partitioning analysis classification for glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2011;81(3):623–630. doi: 10.1016/j.ijrobp.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barnholtz-Sloan JS, Williams VL, Maldonado JL, et al. Patterns of care and outcomes among elderly individuals with primary malignant astrocytoma. J. Neurosurg. 2008;108(4):642–648. doi: 10.3171/JNS/2008/108/4/0642. [DOI] [PubMed] [Google Scholar]
- 13.Iwamoto FM, Reiner AS, Panageas KS, Elkin EB, Abrey LE. Patterns of care in elderly glioblastoma patients. Ann. Neurol. 2008;64(6):628–634. doi: 10.1002/ana.21521. [DOI] [PubMed] [Google Scholar]
- 14.Scott J, Tsai YY, Chinnaiyan P, Yu HH. Effectiveness of radiotherapy for elderly patients with glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2011;81(1):206–210. doi: 10.1016/j.ijrobp.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 15.Kita D, Ciernik IF, Vaccarella S, et al. Age as a predictive factor in glioblastomas: population-based study. Neuroepidemiology. 2009;33(1):17–22. doi: 10.1159/000210017. [DOI] [PubMed] [Google Scholar]
- 16.Gulati S, Jakola AS, Johannesen TB, Solheim O. Survival and treatment patterns of glioblastoma in the elderly: a population-based study. World Neurosurg. 2011 doi: 10.1016/j.wneu.2011.12.008. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 17.Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N. Engl. J. Med. 1999;341(27):2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 18.Gross CP, Herrin J, Wong N, Krumholz HM. Enrolling older persons in cancer trials: the effect of sociodemographic, protocol, and recruitment center characteristics. J. Clin. Oncol. 2005;23(21):4755–4763. doi: 10.1200/JCO.2005.14.365. [DOI] [PubMed] [Google Scholar]
- 19.Yee KW, Pater JL, Pho L, Zee B, Siu LL. Enrollment of older patients in cancer treatment trials in Canada: why is age a barrier? J. Clin. Oncol. 2003;21(8):1618–1623. doi: 10.1200/JCO.2003.12.044. [DOI] [PubMed] [Google Scholar]
- 20.Aapro MS, Kohne CH, Cohen HJ, Extermann M. Never too old? Age should not be a barrier to enrollment in cancer clinical trials. Oncologist. 2005;10(3):198–204. doi: 10.1634/theoncologist.10-3-198. [DOI] [PubMed] [Google Scholar]
- 21.Pruitt AA. Medical management of patients with brain tumors. Curr. Treat. Options Neurol. 2011;13(4):413–426. doi: 10.1007/s11940-011-0132-y. [DOI] [PubMed] [Google Scholar]
- 22.Ricard D, Idbaih A, Ducray F, Lahutte M, Hoang-Xuan K, Delattre JY. Primary brain tumours in adults. Lancet. 2012;379(9830):1984–1996. doi: 10.1016/S0140-6736(11)61346-9. [DOI] [PubMed] [Google Scholar]
- 23.Tatsuno I, Sugiyama T, Suzuki S, et al. Age dependence of early symptomatic vertebral fracture with high-dose glucocorticoid treatment for collagen vascular diseases. J. Clin. Endocrinol. Metab. 2009;94(5):1671–1677. doi: 10.1210/jc.2008-1578. [DOI] [PubMed] [Google Scholar]
- 24.Chevalet P, Masseau-Imbert A, Durand-Fix MH, et al. Giant cell arteritis after the age of 75. Ann. Med. Interne (Paris) 2002;153(6):373–377. [PubMed] [Google Scholar]
- 25.Drappatz J, Schiff D, Kesari S, Norden AD, Wen PY. Medical management of brain tumor patients. Neurol. Clin. 2007;25(4):1035–1071, ix. doi: 10.1016/j.ncl.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Desai A, Grolleau-Julius A, Yung R. Leukocyte function in the aging immune system. J. Leukoc. Biol. 2010;87(6):1001–1009. doi: 10.1189/jlb.0809542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glantz MJ, Cole BF, Forsyth PA, et al. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;54(10):1886–1893. doi: 10.1212/wnl.54.10.1886. [DOI] [PubMed] [Google Scholar]
- 28.Sommer BR, Fenn HH. Review of topiramate for the treatment of epilepsy in elderly patients. Clin. Interv. Aging. 2010;5:89–99. doi: 10.2147/cia.s3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arif H, Buchsbaum R, Pierro J, et al. Comparative effectiveness of 10 antiepileptic drugs in older adults with epilepsy. Arch. Neurol. 2010;67(4):408–415. doi: 10.1001/archneurol.2010.49. [DOI] [PubMed] [Google Scholar]
- 30.Zeber JE, Copeland LA, Pugh MJ. Variation in antiepileptic drug adherence among older patients with new-onset epilepsy. Ann. Pharmacother. 2010;44(12):1896–1904. doi: 10.1345/aph.1P385. [DOI] [PubMed] [Google Scholar]
- 31.Contin M, Mohamed S, Albani F, Riva R, Baruzzi A. Levetiracetam clinical pharmacokinetics in elderly and very elderly patients with epilepsy. Epilepsy Res. 2012;98(2–3):130–134. doi: 10.1016/j.eplepsyres.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 32.Kaloshi G, Psimaras D, Mokhtari K, et al. Supratentorial low-grade gliomas in older patients. Neurology. 2009;73(24):2093–2098. doi: 10.1212/WNL.0b013e3181c6781e. [DOI] [PubMed] [Google Scholar]
- 33.Laigle-Donadey F, Sanson M. Pattern of care of high-grade gliomas. Rev. Prat. 2006;56(16):1779–1786. [PubMed] [Google Scholar]
- 34.Marijnen CA, van den Berg SM, van Duinen SG, Voormolen JH, Noordijk EM. Radiotherapy is effective in patients with glioblastoma multiforme with a limited prognosis and in patients above 70 years of age: a retrospective single institution analysis. Radiother. Oncol. 2005;75(2):210–216. doi: 10.1016/j.radonc.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Chaichana KL, Garzon-Muvdi T, Parker S, et al. Supratentorial glioblastoma multiforme: the role of surgical resection versus biopsy among older patients. Ann. Surg. Oncol. 2011;18(1):239–245. doi: 10.1245/s10434-010-1242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oszvald A, Guresir E, Setzer M, et al. Glioblastoma therapy in the elderly and the importance of the extent of resection regardless of age. J. Neurosurg. 2012;116(2):357–364. doi: 10.3171/2011.8.JNS102114. [DOI] [PubMed] [Google Scholar]
- 37.Kelly PJ, Hunt C. The limited value of cytoreductive surgery in elderly patients with malignant gliomas. Neurosurgery. 1994;34(1):62–66. [PubMed] [Google Scholar]
- 38.Pierga JY, Hoang-Xuan K, Feuvret L, et al. Treatment of malignant gliomas in the elderly. J. Neurooncol. 1999;43(2):187–193. doi: 10.1023/a:1006262918694. [DOI] [PubMed] [Google Scholar]
- 39.Chaichana KL, Chaichana KK, Olivi A, et al. Surgical outcomes for older patients with glioblastoma multiforme: preoperative factors associated with decreased survival. Clinical article. J. Neurosurg. 2011;114(3):587–594. doi: 10.3171/2010.8.JNS1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vuorinen V, Hinkka S, Farkkila M, Jaaskelainen J. Debulking or biopsy of malignant glioma in elderly people – a randomised study. Acta Neurochir. (Wien) 2003;145(1):5–10. doi: 10.1007/s00701-002-1030-6. [DOI] [PubMed] [Google Scholar]; ▪ Small randomized trial demonstrating longer survival and improved quality of life with resection compared with biopsy.
- 41.Ewelt C, Goeppert M, Rapp M, Steiger HJ, Stummer W, Sabel M. Glioblastoma multiforme of the elderly: the prognostic effect of resection on survival. J. Neurooncol. 2011;103(3):611–618. doi: 10.1007/s11060-010-0429-9. [DOI] [PubMed] [Google Scholar]
- 42.Iwamoto FM, Cooper AR, Reiner AS, Nayak L, Abrey LE. Glioblastoma in the elderly: the Memorial Sloan-Kettering Cancer Center Experience (1997–2007) Cancer. 2009;115(16):3758–3766. doi: 10.1002/cncr.24413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keime-Guibert F, Chinot O, Taillandier L, et al. Radiotherapy for glioblastoma in the elderly. N. Engl. J. Med. 2007;356(15):1527–1535. doi: 10.1056/NEJMoa065901. [DOI] [PubMed] [Google Scholar]; ▪▪ Initial randomized trial that established the benefit of adding radiotherapy to supportive care for elderly glioblastoma (GBM) patients with good performance status.
- 44.Idbaih A, Taillibert S, Simon JM, et al. Short course of radiation therapy in elderly patients with glioblastoma multiforme. Cancer Radiother. 2008;12(8):788–792. doi: 10.1016/j.canrad.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Hoegler DB, Davey P. A prospective study of short course radiotherapy in elderly patients with malignant glioma. J. Neurooncol. 1997;33(3):201–204. doi: 10.1023/a:1005750111883. [DOI] [PubMed] [Google Scholar]
- 46.Roa W, Brasher PM, Bauman G, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J. Clin. Oncol. 2004;22(9):1583–1588. doi: 10.1200/JCO.2004.06.082. [DOI] [PubMed] [Google Scholar]; ▪ Randomized trial comparing short-course radiotherapy with standard-course irradiation.
- 47.Laigle-Donadey F, Figarella-Branger D, Chinot O, et al. Upfront temozolomide in elderly patients with glioblastoma. J. Neurooncol. 2010;99(1):89–94. doi: 10.1007/s11060-009-0110-3. [DOI] [PubMed] [Google Scholar]
- 48.Chinot OL, Barrie M, Frauger E, et al. Phase II study of temozolomide without radiotherapy in newly diagnosed glioblastoma multiforme in an elderly populations. Cancer. 2004;100(10):2208–2214. doi: 10.1002/cncr.20224. [DOI] [PubMed] [Google Scholar]
- 49.Glantz M, Chamberlain M, Liu Q, Litofsky NS, Recht LD. Temozolomide as an alternative to irradiation for elderly patients with newly diagnosed malignant gliomas. Cancer. 2003;97(9):2262–2266. doi: 10.1002/cncr.11323. [DOI] [PubMed] [Google Scholar]
- 50.Malmstrom A, Gronberg BH SR, et al. Glioblastoma (GBM) in elderly patients: a randomized Phase III trial comparing survival in patients treated with 6-week radiotherapy (RT) versus hypofractionated RT over 2 weeks versus temozolomide single-agent chemotherapy (TMZ) J. Clin. Oncol. 2010;28(Suppl.) Abstract. [Google Scholar]
- 51.Wick W, Platten M, Meisner C, et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, Phase 3 trial. Lancet Oncol. 2012;13(7):707–715. doi: 10.1016/S1470-2045(12)70164-X. [DOI] [PubMed] [Google Scholar]; ▪ Large Phase III randomized trial demonstrating the noninferiority of temozolomide compared with radiotherapy in elderly patients with malignant astrocytomas.
- 52.Ducray F, Benouaich-Amiel A, Idbaih A, et al. Complete response after one cycle of temozolomide in an elderly patient with glioblastoma and poor performance status. J. Neurooncol. 2008;88(2):185–188. doi: 10.1007/s11060-008-9546-0. [DOI] [PubMed] [Google Scholar]
- 53.Gallego Perez-Larraya J, Ducray F, Chinot O, et al. Temozolomide in elderly patients with newly diagnosed glioblastoma and poor performance status: an ANOCEF Phase II trial. J. Clin. Oncol. 2011;29(22):3050–3055. doi: 10.1200/JCO.2011.34.8086. [DOI] [PubMed] [Google Scholar]; ▪ Nonrandomized trial showing acceptable tolerance and improvement of functional status in elderly GBM patients with Karnofsky performance score <70% treated with temozolomide alone.
- 54.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 55.Lai A, Tran A, Nghiemphu PL, et al. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J. Clin. Oncol. 2011;29(2):142–148. doi: 10.1200/JCO.2010.30.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised Phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 57.Combs SE, Wagner J, Bischof M, et al. Postoperative treatment of primary glioblastoma multiforme with radiation and concomitant temozolomide in elderly patients. Int. J. Radiat. Oncol. Biol. Phys. 2008;70(4):987–992. doi: 10.1016/j.ijrobp.2007.07.2368. [DOI] [PubMed] [Google Scholar]
- 58.Sijben AE, McIntyre JB, Roldan GB, et al. Toxicity from chemoradiotherapy in older patients with glioblastoma multiforme. J. Neurooncol. 2008;89(1):97–103. doi: 10.1007/s11060-008-9593-6. [DOI] [PubMed] [Google Scholar]
- 59.Minniti G, De SV, Muni R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma in elderly patients. J. Neurooncol. 2008;88(1):97–103. doi: 10.1007/s11060-008-9538-0. [DOI] [PubMed] [Google Scholar]
- 60.Minniti G, Salvati M, Arcella A, et al. Correlation between O6-methylguanine-DNA methyltransferase and survival in elderly patients with glioblastoma treated with radiotherapy plus concomitant and adjuvant temozolomide. J. Neurooncol. 2011;102(2):311–316. doi: 10.1007/s11060-010-0324-4. [DOI] [PubMed] [Google Scholar]
- 61.Brandes AA, Franceschi E, Tosoni A, et al. Temozolomide concomitant and adjuvant to radiotherapy in elderly patients with glioblastoma: correlation with MGMT promoter methylation status. Cancer. 2009;115(15):3512–3518. doi: 10.1002/cncr.24406. [DOI] [PubMed] [Google Scholar]
- 62.Gerstein J, Franz K, Steinbach JP, et al. Postoperative radiotherapy and concomitant temozolomide for elderly patients with glioblastoma. Radiother. Oncol. 2010;97:382–386. doi: 10.1016/j.radonc.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 63.Balducci M, Fiorentino A, De Bonis P, et al. Impact of age and co-morbidities in patients with newly diagnosed glioblastoma: a pooled data analysis of three prospective mono-institutional Phase II studies. Med. Oncol. 2012 doi: 10.1007/s12032-012-0263-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 64.Fiorentino A, Caivano R, Chiumento C, et al. Comorbidity assessment and adjuvant radiochemotherapy in elderly affected by glioblastoma. Med. Oncol. 2012 doi: 10.1007/s12032-012-0246-4. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 65.Minniti G, Lanzetta G, Scaringi C, et al. Phase II study of short-course radiotherapy plus concomitant and adjuvant temozolomide in elderly patients with glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2012;83(1):93–99. doi: 10.1016/j.ijrobp.2011.06.1992. [DOI] [PubMed] [Google Scholar]; ▪ Phase II trial suggesting an increased survival and acceptable tolerance to combined chemoradiation therapy in elderly GBM patients.
- 66.Lutterbach J, Bartelt S, Momm F, Becker G, Frommhold H, Ostertag C. Is older age associated with a worse prognosis due to different patterns of care? A long-term study of 1346 patients with glioblastomas or brain metastases. Cancer. 2005;103(6):1234–1244. doi: 10.1002/cncr.20895. [DOI] [PubMed] [Google Scholar]
- 67.Batchelor TT, Betensky RA, Esposito JM, et al. Age-dependent prognostic effects of genetic alterations in glioblastoma. Clin. Cancer Res. 2004;10(1 Pt 1):228–233. doi: 10.1158/1078-0432.ccr-0841-3. [DOI] [PubMed] [Google Scholar]
- 68.Simmons ML, Lamborn KR, Takahashi M, et al. Analysis of complex relationships between age, p53, epidermal growth factor receptor, and survival in glioblastoma patients. Cancer Res. 2001;61(3):1122–1128. [PubMed] [Google Scholar]
- 69.Kleinschmidt-DeMasters BK, Lillehei KO, Varella-Garcia M. Glioblastomas in the older old. Arch. Pathol. Lab. Med. 2005;129(5):624–631. doi: 10.5858/2005-129-0624-GITOO. [DOI] [PubMed] [Google Scholar]
- 70.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 71.Gorlia T, van den Bent MJ, Hegi ME, et al. Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981-22981/CE.3. Lancet Oncol. 2008;9(1):29–38. doi: 10.1016/S1470-2045(07)70384-4. [DOI] [PubMed] [Google Scholar]
- 72.Weller M, Felsberg J, Hartmann C, et al. Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: a prospective translational study of the German Glioma Network. J. Clin. Oncol. 2009;27(34):5743–5750. doi: 10.1200/JCO.2009.23.0805. [DOI] [PubMed] [Google Scholar]
- 73.Gerstner ER, Yip S, Wang DL, Louis DN, Iafrate AJ, Batchelor TT. MGMT methylation is a prognostic biomarker in elderly patients with newly diagnosed glioblastoma. Neurology. 2009;73(18):1509–1510. doi: 10.1212/WNL.0b013e3181bf9907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reifenberger G, Hentschel B, Felsberg J, et al. Predictive impact of MGMT promoter methylation in glioblastoma of the elderly. Int. J. Cancer. 2011;131(6):1342–1350. doi: 10.1002/ijc.27385. [DOI] [PubMed] [Google Scholar]