Abstract
Background
In the current study we used contrast-enhanced T1 subtraction maps to test whether early changes in enhancing tumor volume are prognostic for overall survival (OS) in newly diagnosed glioblastoma (GBM) patients treated with chemoradiation with or without bevacizumab (BV).
Methods
Seven hundred ninety-eight patients (404 BV and 394 placebo) with newly diagnosed GBM in the AVAglio trial (NCT00943826) had baseline MRI scans available, while 337 BV-treated and 269 placebo-treated patients had >4 MRI scans for response evaluation. The volume of contrast-enhancing tumor was quantified and used for subsequent analyses.
Results
A decrease in tumor volume during chemoradiation was associated with a longer OS in the placebo group (hazard ratio [HR] = 1.578, P < 0.0001) but not BV-treated group (HR = 1.135, P = 0.4889). Results showed a higher OS in patients on the placebo arm with a sustained decrease in tumor volume using a post-chemoradiation baseline (HR = 1.692, P = 0.0005), and a trend toward longer OS was seen in BV-treated patients (HR = 1.264, P = 0.0724). Multivariable Cox regression confirmed that sustained response or stable disease was prognostic for OS (HR = 0.7509, P = 0.0127) when accounting for age (P = 0.0002), KPS (P = 0.1516), postsurgical tumor volume (P < 0.0001), O6-methylguanine-DNA methyltransferase status (P < 0.0001), and treatment type (P = 0.7637) using the post-chemoradiation baseline.
Conclusions
The post-chemoradiation timepoint is a better baseline for evaluating efficacy in newly diagnosed GBM. Early progression during the maintenance phase is consequential in predicting OS, supporting the use of progression-free survival rates as a meaningful surrogate for GBM.
Keywords: bevacizumab, clinical trials, GBM, glioblastoma, MRI, response assessment, T1 subtraction
Importance of the study
Significant questions remain regarding the usefulness of contrast enhancement in determining tumor response and treatment failure in GBM. To address practical clinical questions relating to the best use of contrast-enhancing tumor burden to predict outcome, we utilized contrast-enhanced T1 subtraction maps to increase lesion conspicuity, then tested whether early changes in contrast enhancement during chemoradiation or maintenance therapy are prognostic for OS in patients with newly diagnosed GBM treated with concomitant radiation and temozolomide with or without BV. Results suggest the first post-chemoradiation timepoint may be a better baseline for evaluating therapeutic efficacy in newly diagnosed GBM. Data suggest that early progression during the maintenance phase is more consequential in predicting OS, supporting the use of progression-free survival rates as a meaningful surrogate.
Significant questions surround the usefulness of contrast enhancement in determining tumor response and failure within the context of newly diagnosed glioblastoma (GBM). Following initial surgical resection, for example, there may not be sufficient measurable enhancing disease to monitor treatment response. Additionally, the presence of pseudoprogression, or treatment-related inflammatory changes mimicking tumor growth,1–3 often confounds interpretation of tumor response in the upfront setting, particularly during concomitant radiation and temozolomide.4,5 Moreover, ongoing investigations into the use of anti-angiogenic agents for treatment of GBM raise additional doubts about the reliability of contrast enhancement as a meaningful surrogate of treatment efficacy.6–8
Clinicians have developed novel strategies to mitigate these challenges during routine clinical care; however, these approaches have not been thoroughly evaluated and have yet to be translated into routine use in multicenter clinical trials. For example, the postoperative MRI examination is often used as a baseline for upfront GBM clinical trials, but this scan often occurs without a standardized acquisition protocol because it is attained prior to enrollment and the scans are often confounded with postsurgical changes including blood products. To isolate contrast enhancement from blood products, or to identify true disease-related enhancement from occult enhancement during anti-angiogenic therapy, clinicians can use digital subtraction techniques,9–11 or “T1 subtraction maps,” or wait for subsequent scans to better understand the current state of the disease. Because clinicians often observe transient imaging changes during chemoradiation due to pseudoprogression, pseudoresponse (in case of anti-angiogenic treatments), or true tumor progression, many choose to use the first post-chemoradiation MRI examination as a “new baseline” for evaluating subsequent changes during routine clinical care. Thus, there appears at least some level of divergence between how physicians evaluate treatment efficacy in routine clinical practice and how efficacy is measured during a clinical trial setting, likely owing to inadequate scientific evidence relating these differing strategies to clinical value or predicting long-term overall survival (OS).
To address these practical questions we utilized contrast-enhanced T1 subtraction maps to increase lesion conspicuity, then tested whether early changes in contrast enhancement during chemoradiation or maintenance therapy are prognostic for OS in patients with newly diagnosed GBM treated with concomitant radiation (RT) and temozolomide (TMZ) with or without bevacizumab (BV), a potent anti-angiogenic agent, using data from AVAglio, a large multicenter phase III clinical trial. We aimed to determine whether early volume changes occurring during the initial chemoradiation phase were meaningful for predicting OS, or whether using a post-chemoradiation baseline and evaluating subsequent changes during the maintenance phase would improve the ability to predict OS independently of therapy by removing uncertainty that often occurs as a result of pseudoprogression or pseudoresponse. We hypothesized that use of the post-chemoradiation timepoint as the baseline for evaluating subsequent changes could predict OS in newly diagnosed GBM.
Methods
Study Design, Treatment, and Patients
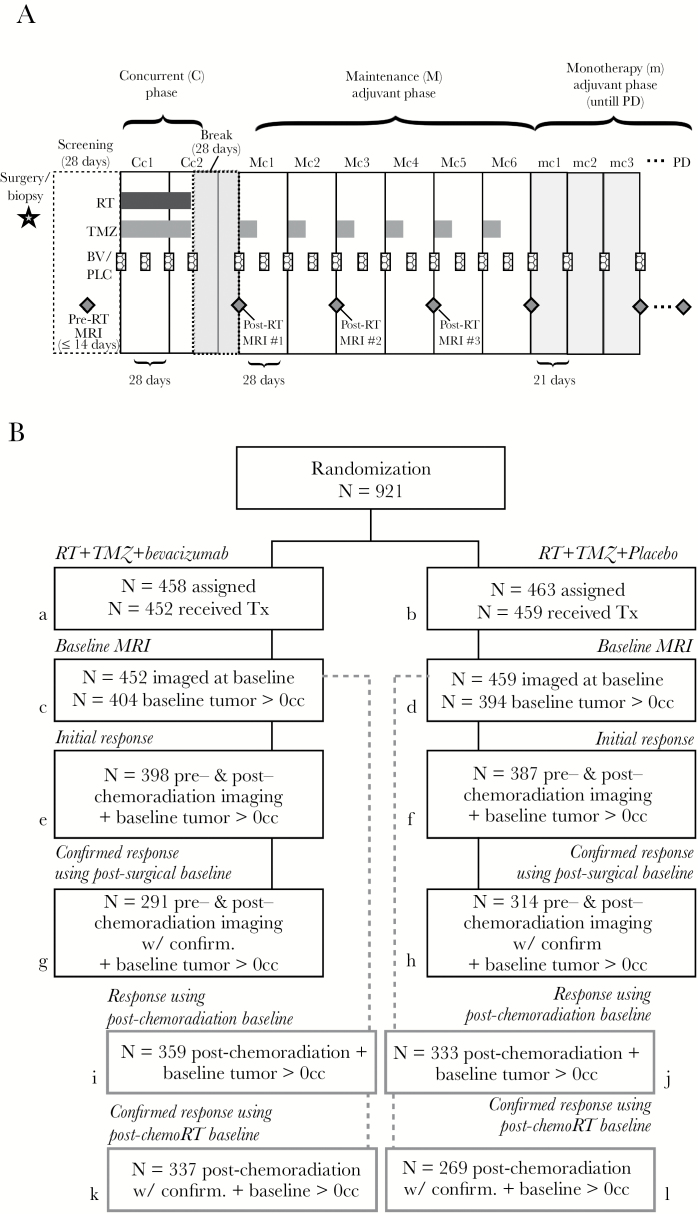
A total of 921 patients were enrolled in AVAglio, a randomized, double-blind, placebo-controlled phase III trial sponsored by F. Hoffmann-La Roche (ClinicalTrials.gov #NCT00943826; clinicaltrials.gov/ct2/show/NCT00943826) and conducted at 120 sites in 23 countries. The protocol was approved by the applicable independent ethics committee and institutional review board at each institution. All patients provided written informed consent to participate. The study adhered to the principles of the Declaration of Helsinki and the Guidelines for Good Clinical Practice.Patient demographics are provided in Table 1 and are available in Chinot et al.12 Detailed methodology for patient selection is outlined in the Supplementary material.
Table 1.
Patient demographics (from Chinot et al12)
| Patient Characteristic | Chemoradiation + Bevacizumab (N = 458) | Chemoradiation + Placebo (N = 463) |
|---|---|---|
| Age, y (range) | 57 (20–84) | 56 (18–79) |
| Sex, M/F | 282 (62%) / 176 (38%) | 298 (64%) / 165 (36%) |
| KPS at baseline (% of patients) | 50–80 (33%) 90–100 (67%) |
50–80 (30%) 90–100 (70%) |
| MGMT status (% of patients) | Methylated (26%) Unmethylated (49%) Missing (25%) |
Methylated (26%) Unmethylated (51%) Missing (23%) |
| Primary vs secondary GBM | Primary (452/458) Secondary (6/458) |
Primary (461/463) Secondary (2/458) |
| Median progression-free survival | 10.6 mo | 6.2 mo |
| Median OS | 16.8 mo | 16.7 mo |
Of the 921 patients initially enrolled, 458 were randomized to the BV group and 463 to the placebo group (Fig. 1), while 452 patients in the BV group and 459 patients in the placebo group received treatment. All patients were 18 years of age or older and had newly diagnosed supratentorial GBM on histological confirmation. Patients were excluded if they had no detectable disease at baseline (volume = 0 cc) or if they had recent symptomatic intracranial hemorrhage or prior radiotherapy, chemotherapy, or immunotherapy. After undergoing surgical resection or biopsy, patients underwent concurrent RT (60 Gy administered as 2-Gy fractions 5 days/wk) and oral TMZ (75 mg/m2 of body surface area per day for a maximum of 49 days) along with either intravenous BV (10 mg/kg of body weight) or placebo every 2 weeks (Fig. 1A). Patients then underwent a 28-day break in treatment followed by maintenance with oral TMZ (150 mg/m2/d on days 1 to 5 during the first cycle and 200 mg/m2/d during subsequent cycles if unacceptable toxic effects did not develop) plus either intravenous BV (10 mg/kg body weight) or placebo, with no crossover between arms, every 2 weeks for six 4-week cycles (24 wk). In the final monotherapy phase, either BV (15 mg/kg body weight) or placebo alone was administered every 3 weeks until disease progression or development of adverse effects. OS for these patients was measured from the date of randomization in the trial. Additional details regarding the clinical trial protocol and general efficacy outcomes are available from Chinot et al.12
Fig. 1.
Treatment schema and patient groups used for imaging analysis. (A) Treatment schema showing timing of radiation therapy (RT), temozolomide (TMZ), bevacizumab (BV), and placebo (PLC) treatment along with timing of MRI examinations during the concurrent phase (C), maintenance (M) adjuvant phase, and monotherapy (m) adjuvant phase, which is continued until progressive disease (PD) is noted at the local site. (B) Randomization, treatment, and follow-up patient groups and numbers used for the subsequent imaging analysis. Detailed methodology for patient selection is outlined in the Supplementary material.
Magnetic Resonance Imaging
After surgical resection or biopsy, all patients received imaging prior to the start of radiochemotherapy (postoperative), as well as 28 days after completion of concurrent radiochemotherapy (post-chemoradiation). Patients also received imaging approximately every 8 weeks during the maintenance phase, every 9 weeks during the monotherapy phase, and at the time of disease progression. At each MRI examination, patients received T1-weighted MR images both before (T1) and after (T1+C) injection of a gadolinium-based contrast agent. Any MRI examination without a precontrast T1-weighted image was excluded from the study. Additional anatomic MR images included T2-weighted fluid-attenuated inversion recovery and T2-weighted turbo spin echo images, but these were not used in the current study.
Tumor Segmentation
Contrast-enhanced T1-weighted subtraction maps were calculated by standard techniques outlined by Ellingson et al.11 Briefly, linear registration was performed between T1 and T1+C images, including trilinear interpolation if image resolution was not equivalent. Then, Gaussian normalization of both T1 and T1+C images was executed by dividing the signal intensity values in each image voxel by the standard deviation across the entire brain. Lastly, voxelwise subtraction of the normalized T1 image from the normalized T1+C image was performed. Lesion segmentation was performed using a semiautomated thresholding method described previously.11,13,14 First, a volume of interest was placed on the lesion of interest and voxels with positive signal intensities on T1 subtraction maps were retained to delineate the tumor. Lastly, manual editing of the tumor contour was performed to eliminate extraneous voxels or nontumor tissue. Contours were edited to include regions of necrosis, which were rare, but not areas thought to be part of the resection cavity. Final measures of tumor volume, in cubic centimeters (cc), were calculated by multiplying the number of voxels within the tumor contour by the voxel resolution.
Hypothesis Testing and Statistical Analysis
Initial change in tumor volume during chemoradiation with or without concomitant BV
To test whether the initial change in tumor volume during chemoradiation with or without concomitant BV was predictive of OS in newly diagnosed GBM, we first examined whether the absolute change in tumor volume before and after chemoradiation with or without concomitant BV was linearly correlated with OS. We hypothesized that patients with large decreases in tumor volume would have a longer OS. We also explored whether a sustained decrease in tumor volume was a better predictor of OS compared with patients exhibiting either an unsustained decrease (initial decrease followed by rebound consisting of an increase in volume greater than baseline) or increase in tumor volume using similar univariate and multivariate analyses.
Volumetric response during maintenance (adjuvant) phase using post-chemoradiation baseline
Next, we tested whether evaluation of volumetric changes evolving during the maintenance phase, after completion of chemoradiation, was a significant predictor of OS. We used both univariate and multivariate analyses to examine whether patients with a sustained decrease in tumor volume during the maintenance phase had a significant survival advantage compared with patients exhibiting an increase in tumor volume or an unsustained decrement in volume from the post-chemoradiation baseline.
Volumetric equivalent Response Assessment in Neuro-Oncology (RANO) response during chemoradiation or maintenance phase with or without concomitant BV
To test whether early response or failure using the RANO criteria could be used to predict OS we implemented “volumetric equivalent” RANO response categories based on thresholds described previously.15–18 A series of univariate and multivariate analyses were conducted to examine the association between radiographic responders (partial response [PR] and complete response [CR] corresponding to decreased enhancing tumor volume >65%) and those with stable (SD) or progressing disease (PD, defined as >40% increase in tumor volume) using both postsurgical and post-chemoradiation baselines. Additionally, a separate analysis was conducted to compare survival between sustained responders and nonresponders or unsustained responders. Lastly, we performed univariate and multivariate analyses comparing patients with early PD, including unsustained PR or CR along with unsustained SD, with patients exhibiting continually shrinking or stable tumor.
All multivariable analyses controlled for age, KPS, O6-methylguanine-DNA methyltransferase (MGMT) methylation status, and treatment arm. log-rank analysis on Kaplan–Meier data and Cox proportional hazards regression models were used to understand the relationship between radiographic variables and OS under a variety of conditions. No adjustments for multiple comparisons were performed. All statistical tests were performed using GraphPad Prism v6.0h, Stata v12, or Matlab (R2017a; Mathworks).
Results
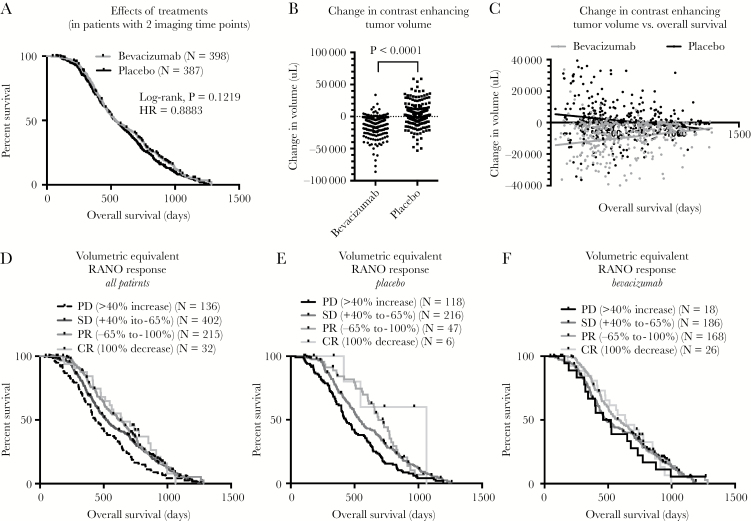
No difference in OS was observed between treatment groups in patients with imaging available at both the pre- and post-chemoradiation timepoints (Fig. 2A; log-rank, P = 0.1219, HR = 0.8883); however, a significant difference in the change in tumor volume between these 2 timepoints was observed (Fig. 2B; t-test, P < 0.0001), suggesting BV patients had a significantly larger decrease in enhancing tumor volume after initial therapy compared with placebo (−9.7 cc vs +1.2 cc change, respectively). Intriguingly, investigation into the relationship between initial change in tumor volume during concurrent chemoradiation and resulting OS resulted in dramatically different results between the 2 treatment arms (Fig. 2C). As expected, a strong association was observed between significant decreases in tumor volume and increased OS in patients within the placebo group (Fig. 2C; linear regression, P = 0.0019; slope = −7.93 cc change in volume for every day of increased OS); however, an opposite association between change in tumor volume and OS was observed within the BV group, suggesting patients with the largest decrease in tumor volume actually had worse prognosis (Fig. 2C; linear regression, P = 0.0007; slope = +8.64 cc change in volume for every day of increased OS).
Fig. 2.
Effects of change in tumor volume and volumetric equivalent RANO responses during concurrent phase on OS in newly diagnosed GBM treated with or without concomitant bevacizumab. (A) Kaplan–Meier plots showing effect of treatment arms in patients with available imaging prior to and after chemoradiation. (B) Continuous change in enhancing tumor volume during chemoradiation between treatment arms (t-test, P < 0.0001) demonstrating a significant decrease in tumor volume after administration of bevacizumab. (C) Linear correlation between continuous change in enhancing tumor volume (μL) during chemoradiation and OS from randomization. (D) Kaplan–Meier plots showing impact of volumetric equivalent RANO responses on OS for all patients, pooled across treatment groups. (E) Kaplan–Meier plots showing impact of volumetric equivalent RANO responses on OS for patients in the placebo arm. (F) Kaplan–Meier plots showing impact of volumetric equivalent RANO responses on OS for patients in the bevacizumab arm.
Volumetric Equivalent RANO Response During Chemoradiation with or without Concomitant Bevacizumab
Classification of initial change in tumor size into “volumetric equivalent” RANO response categories indicated a higher proportion of patients with PR (42.2%) and CR (6.5%) within the BV arm compared with the placebo arm (12.1% and 1.6%, respectively). Log-rank test for trends indicated a significant increase in median OS with better response when examining all subjects pooled together (Fig. 2D; log-rank test for trends, P < 0.0001; median OS = 436 days, 509 days, 580 days, and 642 days for PD, SD, PR, and CR, respectively). Stratification of patients by treatment indicated this trend was present in the placebo arm (Fig. 2E; log-rank test for trends, P < 0.0001; median OS = 434 days, 540 days, 687 days, and 1064 days for PD, SD, PR, and CR, respectively) but not the BV arm (Fig. 3F; log-rank test for trends, P = 0.2869; median OS = 491 days, 485 days, 589 days, and 638 days for PD, SD, PR, and CR, respectively).
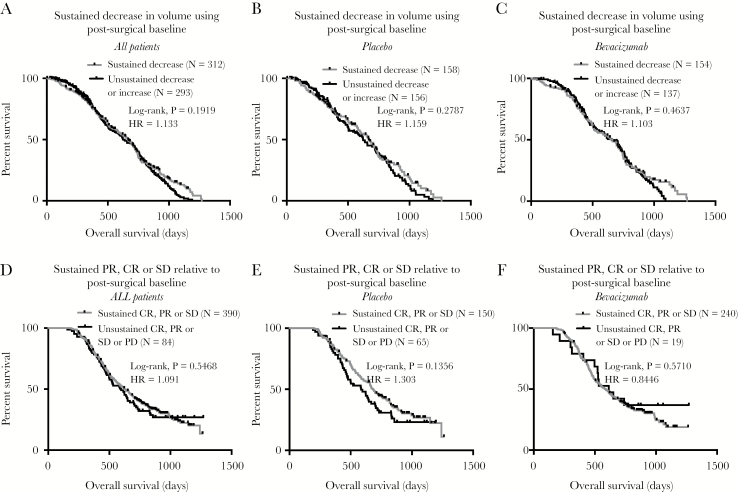
Fig. 3.
Initial volumetric response during the concurrent phase and the impact on OS in newly diagnosed GBM treated with or without concomitant bevacizumab. (A) Kaplan–Meier curves for OS in all patients stratified by whether they exhibited a sustained decrease in tumor volume (log-rank, P = 0.1919). (B) Kaplan–Meier curves for OS for patients in the placebo arm stratified by whether they exhibited a sustained decrease in tumor volume (log-rank, P = 0.2787). (C) Kaplan–Meier curves for OS for patients in the bevacizumab arm stratified by whether they exhibited a sustained decrease in tumor volume (log-rank, P = 0.4637). (D) Kaplan–Meier plots for OS in all patients, pooled across treatment arms, stratified by early progression during the concurrent phase (log-rank, P = 0.5468). (E) Kaplan–Meier plots for OS in patients on the placebo arm stratified by early progression during the concurrent phase (log-rank, P = 0.1356). (F) Kaplan–Meier plots for OS in patients on the bevacizumab arm stratified by early progression during the concurrent phase (log-rank, P = 0.5710). PD = progressive disease; SD = stable disease; PR = partial response; CR = complete response.
Initial Change in Tumor Volume During Chemoradiation with or without Concomitant Bevacizumab
No survival differences were observed between pooled patients exhibiting a sustained decrease in tumor volume compared with patients exhibiting an unsustained or increased tumor size while using the postsurgical scan as the baseline for evaluation (Fig. 3A; log-rank, P = 0.1919, HR = 1.133). The same trend was observed in both the placebo (Fig. 3B; log-rank, P = 0.2787, HR = 1.103) and BV arm (Fig. 3C; log-rank, P = 0.4637, HR = 1.103).
Multivariable Cox regression demonstrated a significant association between continuous measures of initial change in tumor burden and OS (Supplementary Table S1; Cox, P < 0.0001, HR = 1.0142) after accounting for treatment arm (Cox, P = 0.8024), age (Cox, P < 0.0001, HR = 1.0209), KPS (Cox, P = 0.1647), MGMT methylation status (Cox, P < 0.0001, HR = 0.4227), and continuous measures of postsurgical, residual contrast-enhancing tumor volume (Cox, P < 0.0001, HR = 1.0229). Additional models investigating continuous measures in percentage change in tumor volume (Cox, P = 0.8549) did not show a significant association after accounting for other covariates.
Patients were then stratified into initial “radiographic responders” (PR or CR) or “radiographic nonresponders” (SD or PD) based on volumetric equivalent calculations to the RANO criteria using the postoperative baseline. Univariate log-rank analysis of data pooled across treatment arms suggested that patients with radiographic response had significantly longer OS compared with nonresponders (Supplementary Fig. S1A; log-rank, P = 0.0058, HR = 1.260). This effect was stronger when examining the placebo arm alone (Supplementary Fig. S1B; log-rank, P = 0.0155, HR = 1.508); however, it is important to note that only 13.7% of patients demonstrated a response in this treatment arm. Consistent with trends observed when analyzing absolute changes in tumor size, patients on the BV arm who experienced radiographic response (PR or CR) did not demonstrate a significant survival advantage compared with patients exhibiting SD or PD (Supplementary Fig. S1C; log-rank, P = 0.2615).
Next, patients were stratified based on whether they maintained a sustained or confirmed response (CR or PR) based on the volume changes on the subsequent scan after the initial response. In general, patients with a sustained response (CR or PR) did not demonstrate a significant survival advantage when pooled across treatment arms (Supplementary Fig. S1D; log-rank, P = 0.885, HR = 1.022) using the postsurgical baseline timepoint. This was true for both the placebo (Supplementary Fig. S1E; log-rank, P = 0.6198, HR = 1.133) and BV arm (Supplementary Fig. S1F; log-rank, P = 0.0928, HR = 1.046) when evaluated separately.
Early progression, defined as an unsustained CR, PR, or SD, from the postsurgical baseline timepoint also was not prognostic compared with patients exhibiting a sustained CR, PR, or SD state when evaluated for all patients (Fig. 3D; log-rank, P = 0.5468, HR = 1.091) or patients stratified into the placebo (Fig. 3E; log-rank, P = 0.1356, HR = 1.303) or BV treatment arms (Fig. 3F; log-rank, P = 0.5710, HR = 0.8446).
Cox multivariable regression comparing sustained radiographic responders to nonresponders while controlling for treatment, age, postoperative residual tumor volume, MGMT methylation status, and KPS confirmed the univariate observations, suggesting that MGMT status (Supplementary Table S2; Cox, P < 0.0001, HR = 0.3554), age (Cox, P = 0.0002, HR = 1.0197), and postoperative tumor volume (Cox, P < 0.0001, HR = 1.0170) were significant prognostic factors for OS, while radiographic response (Cox, P = 0.5398, HR = 0.9217), treatment arm (P = 0.9003, HR = 1.0153), and KPS (P = 0.2066, HR = 0.8548) were not. A Cox multivariable regression model suggested that patients with early progression, defined as an unsustained CR, PR, or SD, as well as early PD, from the postsurgical baseline timepoint, trended toward shorter OS (Supplementary Table S3; Cox, P = 0.0761) when accounting for other covariates.
Volumetric Response During Maintenance (Adjuvant) Temozolomide with or without Concomitant Bevacizumab Using a Post-Chemoradiation Baseline
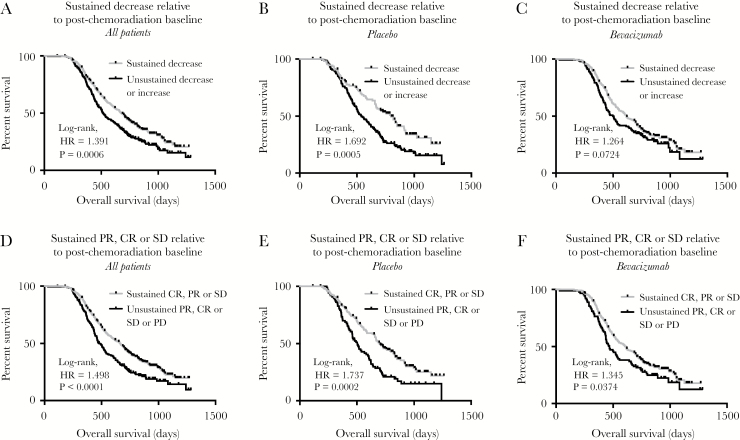
In general, a sustained decrease in tumor volume during the maintenance (adjuvant) treatment phase with TMZ with or without BV resulted in a favorable prognosis (Fig. 4). Stratification of patients based on a sustained decreasing tumor volume (Fig. 4A; log-rank, P = 0.0006, HR = 1.391) or whether they had progressive disease during this period appeared to be the strongest predictor (Fig. 4D; log-rank, P < 0.0001, HR = 1.498), whereas sustained response did not reflect a long-term survival advantage (Supplementary Fig. S2A; log-rank, P = 0.7296, HR = 1.050). Patients in the placebo arm treated with TMZ during the maintenance phase who displayed a sustained decrease in tumor volume had significantly longer survival (Fig. 4B; log-rank, P = 0.0005, HR = 1.692), whereas patients treated with TMZ and BV during the same period who exhibited a decrease in tumor volume trended to have longer survival (Fig. 4C; log-rank, P = 0.0724, HR = 1.264). Patients demonstrating a sustained response (CR or PR) in the placebo (Supplementary Fig. S2B; log-rank, P = 0.0849, HR = 1.690) or BV arm (Supplementary Fig. S2C; log-rank, P = 0.4972, HR = 0.8963) did not exhibit significantly longer survival; however, patients exhibiting early progression or an unsustained response treated with either TMZ alone (Fig. 4E; log-rank, P = 0.0002, HR = 1.737) or with concomitant BV (Fig. 4F; log-rank, P = 0.0374, HR = 1.345) had a significantly worse outcome.
Fig. 4.
Volumetric responses during the maintenance phase using a post-chemoradiation baseline and the impact on OS in newly diagnosed GBM treated with or without concomitant bevacizumab. (A) Kaplan–Meier plots for OS in all patients, pooled across treatment arms, stratified by sustained decrease in tumor volume during the maintenance phase (log-rank, P = 0.0006). (B) Kaplan–Meier plots for OS in patients on the placebo arm stratified by sustained decrease in tumor volume during the maintenance phase (log-rank, P = 0.0005). (C) Kaplan–Meier plots for OS in patients on the bevacizumab arm stratified by sustained decrease in tumor volume during the maintenance phase (log-rank, P = 0.0724). (D) Kaplan–Meier plots for OS in all patients, pooled across treatment arms, stratified by early progression during the maintenance phase (log-rank, P < 0.0001). (E) Kaplan–Meier plots for OS in patients on the placebo arm stratified by early progression during the maintenance phase (log-rank, P = 0.0002). (F) Kaplan–Meier plots for OS in patients on the bevacizumab arm stratified by early progression during the maintenance phase (log-rank, P = 0.0374).
Consistent with univariate observations that a sustained response, defined as a confirmed PR or CR, does not translate into a significant survival advantage using univariate analyses, multivariable Cox regression analysis did not establish a relationship between sustained response and OS (Supplementary Table S4; Cox, P = 0.2020) when also accounting for covariates including age (P = 0.0002, HR = 1.0196), MGMT status (P < 0.0001, HR = 0.3531), KPS (P = 0.2390), treatment (P = 0.8063), and postoperative tumor volume (P < 0.0001, HR = 1.0169).
Multivariable Cox regression demonstrated a significant survival advantage in examining patients with a sustained decrease in tumor volume during the maintenance phase with respect to the post-chemoradiation baseline (Supplementary Table S5; Cox, P = 0.0437, HR = 0.7758 for patients without a sustained decrease) when accounting for other covariates including age (P = 0.0003; HR = 1.0201), MGMT status (P < 0.0001, HR = 0.3538), KPS (P = 0.2104, HR = 0.8540), treatment arm (P = 0.9561, HR = 1.0065), and postsurgical tumor volume (P < 0.0001, HR = 1.0175). In addition, multivariate analyses suggested that patients with early tumor progression, defined as having early PD or unsustained PR, CR, or SD within the maintenance phase with respect to the post-chemoradiation baseline, had a significantly shorter OS compared with patients exhibiting a sustained response or stable disease (Table 2; Cox, P = 0.0127, HR = 0.7509 for patients with sustained PR, CR, or SD) after accounting for age (P = 0.0002, HR = 1.0195), MGMT status (P < 0.0001, HR = 0.3652), KPS (P = 0.1516, HR = 0.8380), treatment arm (P = 0.8796, HR = 1.0173), and postsurgical residual tumor volume (P < 0.0001, HR = 1.0175). Together, these results suggest that early evidence of tumor growth during the maintenance phase, relative to the post-chemoradiation examination, is a strong predictor of OS in newly diagnosed GBM.
Table 2.
Multivariable Cox regression model results for patients with early failure during maintenance phase (post-chemoradiation baseline), treatment arm, age, KPS, and MGMT status
| Variable | Coefficient | Hazard Ratio | 95% CI | P-value |
|---|---|---|---|---|
| Post-chemoradiation failure vs disease control (PD vs SD/PR/CR) | −0.2864 ± 0.1149 | 0.7509 | (0.5995–0.9406) | 0.0127 |
| Postsurgical tumor volume (continuous; cc) | 0.0173 ± 0.0026 | 1.0175 | (1.0122–1.0228) | <0.0001 |
| Treatment (placebo vs bevacizumab) | 0.0171 ± 0.1131 | 1.0173 | (0.8151–1.2697) | 0.8796 |
| Age, y | 0.0193 ± 0.0053 | 1.0195 | (1.0090–1.0301) | 0.0002 |
| KPS (50–80 vs 90–100) | −0.1768 ± 0.1233 | 0.8380 | (0.6581–1.0670) | 0.1516 |
| MGMT status (methylated vs unmethylated) | −1.0072 ± 0.1286 | 0.3652 | (0.2838–0.4700) | <0.0001 |
Note: N = 478 of 606 patients had all variables available for multivariate Cox analysis.
Discussion
Contrast enhancement on computed tomography (CT) or MRI has been the gold standard for brain tumor detection, diagnosis, clinical monitoring, and response assessment to new therapies for more than 40 years. The first article to use contrast-enhanced CT to visualize intracranial tumors was published in 1974,19 but it wasn’t until the early 1990s that clinical use of contrast-enhanced T1-weighted MRI became relatively routine, after studies showed comparable lesion measurements with CT.20,21 Although contrast enhancement on CT or MRI has been used routinely in the clinic, questions have remained regarding the optimal timing of measurements and overall utility of contrast-enhancing tumor volume measurements in predicting long-term outcomes and determining drug efficacy in the context of newly diagnosed GBM.
The current study is consistent with multiple previous investigations demonstrating that postsurgical contrast-enhancing tumor volume is a significant, independent prognostic factor for OS in newly diagnosed GBM. Although both postsurgical contrast-enhancing volume22–26 and extent of resection26–38 appear to be prognostic, studies suggest that postsurgical residual tumor volume may be a stronger predictor of OS.26 Additionally, postsurgical residual volume may be a more practical measurement to obtain, since presurgical MRI scans are often not available or collected as part of clinical trials, partly because patients are not enrolled until after surgery and subsequent diagnosis. Despite the abundant evidence suggesting that residual tumor size may predict OS, the current study may be the largest to date (N = 798) using a homogeneous approach to quantify enhancing tumor volume without contamination from postsurgical blood products.
Results from the current study suggest the post-chemoradiation MRI examination may be a better baseline for evaluating therapeutic efficacy compared with the postsurgical, pre-chemoradiation examination. Although results are biased toward patients who can tolerate chemoradiation and are able to obtain at least 3 MRI examinations during the maintenance phase, the use of the post-chemoradiation timepoint as the baseline has a number of practical and scientific advantages. First, use of the post-chemoradiation timepoint as the baseline reduces ambiguity associated with postsurgical changes, off-protocol (pre-study) scan parameter variation, and potential variation of timing after surgical intervention, which may be hours to days, or variation in timing from surgery to start of therapy, as some tumor growth may occur between surgery and the start of cytotoxic therapy. Additionally, there would inherently be less emphasis on the transient changes known to occur during chemoradiation (ie, pseudoprogression) or transient changes that may occur with new experimental therapeutics including anti-angiogenic agents (ie, pseudoresponse) or immunotherapies (ie, inflammatory changes). Moreover, the use of the first post-chemoradiation timepoint as the baseline more accurately reflects current clinical management of patients with newly diagnosed GBM, as questions regarding the interpretation of transient changes during chemoradiation often occur. Perhaps most importantly, scientific results from the current study support use of the post-chemoradiation timepoint as the baseline for evaluating changes in tumor size as a meaningful, treatment agnostic predictor of long-term survival both in standard therapy and in combination with anti-angiogenic agents.
Another principal observation in the current study is the limited value of early radiographic response in newly diagnosed GBM. Data using either the postsurgical or post-chemoradiation timepoint as a baseline for evaluation showed no survival benefit for patients who had a confirmed or sustained partial or complete radiographic response (>65% decrease in tumor volume) compared with patients exhibiting stable or progressing disease. Importantly, results did suggest a significant survival disadvantage in patients with early radiographic progression, independent of treatment arm, age, KPS, and MGMT status, particularly when using the post-chemoradiation timepoint as a baseline and when including patients with measurable disease after surgery (Fig. 4). Together, these data corroborate previous views that objective response rates are not clinically meaningful in newly diagnosed GBM, and may suggest that a measure of early progression-free survival or treatment failure rates during the maintenance phase (eg, progression-free survival rates) may be extremely useful for predicting long-term outcome, independently of other clinical variables.
An interesting finding in the current study was that simple measures of absolute increasing versus decreasing tumor volume appeared to be informative in terms of predicting OS, particularly when probing univariate results. The ability to quickly evaluate whether a tumor is growing or shrinking as a meaningful and early measure of treatment efficacy was the basis of the early Levin criteria39 and is more consistent with clinical management of GBM patients, where progression is typically noted after evidence of continual tumor growth and not after this growth has reached an empirical threshold. Despite this observation, survival differences in patients with growing versus shrinking tumors were not maintained after evaluating using a multivariate Cox regression model including common clinical variables, suggesting that this may not be the most sensitive method for evaluating efficacy and predicting OS in newly diagnosed GBM.
Study Limitations
There are a few limitations that should be addressed. Since postsurgical scans are often acquired out of the trial and with nonstandardized acquisition parameters, the results from the endpoint comparisons may have been influenced by these technical differences. Additionally, if pre- and postcontrast T1-weighted images were not acquired identically, there is a chance that the resulting enhancing tumor measurements on T1 subtraction maps could be inaccurate.
Conclusion
In summary, results from the current study suggest that the post-chemoradiation timepoint may be a better baseline for evaluating therapeutic efficacy in newly diagnosed GBM compared with the postsurgical timepoint often used as the baseline in upfront trials, particularly in standard-of-care chemoradiation and chemoradiation plus therapies that may transiently modulate vascular permeability like BV. Data suggest that early progression during the maintenance phase is more consequential than early radiographic response in predicting OS, supporting the use of progression-free survival rates as a meaningful surrogate for outcome in newly diagnosed GBM. Further evaluation in future clinical trials is essential to confirm these observations.
Funding
This work was supported by the National Brain Tumor Society (NBTS) Research Grant (Ellingson, Cloughesy); American Cancer Society (ACS) Research Scholar Grant (RSG-15-003-01-CCE) (Ellingson); Roche/Genentech Research Grant (Ellingson, Cloughesy); Art of the Brain (Cloughesy); UCLA SPORE in Brain Cancer (NIH/NCI 1P50CA211015-01A1) (Ellingson, Pope, Cloughesy).
Supplementary Material
Conflict of interest statement. None declared.
Disclosures:
Ellingson—Advisory Board—Hoffman La-Roche; Siemens; Nativis; Medicenna; MedQIA; Bristol Meyers Squibb; Imaging Endpoints; Agios. Paid Consultant—Nativis; MedQIA; Siemens; Hoffman La-Roche; Imaging Endpoints; Medicenna; Agios. Grant Funding—Hoffman La-Roche; Siemens; Agios; Janssen
Mason—Consultant—Roche, Merck, Abbvie, Celgene, Triphase
Cloughesy—Advisory Board—Roche/Genentech, Amgen, Tocagen, NewGen, LPath, Proximagen, Celgene, Vascular Biogenics Ltd, Insys, Agios, Cortice Bioscience, Pfizer, Human Longevity, BMS, Merck, Notable Lab, MedQIA
References
- 1. Van Mieghem E, Wozniak A, Geussens Y, et al. Defining pseudoprogression in glioblastoma multiforme. Eur J Neurol. 2013;20(10):1335–1341. [DOI] [PubMed] [Google Scholar]
- 2. de Wit MC, de Bruin HG, Eijkenboom W, Sillevis Smitt PA, van den Bent MJ. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology. 2004;63(3):535–537. [DOI] [PubMed] [Google Scholar]
- 3. Brandes AA, Tosoni A, Spagnolli F, et al. Disease progression or pseudoprogression after concomitant radiochemotherapy treatment: pitfalls in neurooncology. Neuro Oncol. 2008;10(3):361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brandes AA, Franceschi E, Tosoni A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26(13):2192–2197. [DOI] [PubMed] [Google Scholar]
- 5. Gerstner ER, McNamara MB, Norden AD, Lafrankie D, Wen PY. Effect of adding temozolomide to radiation therapy on the incidence of pseudo-progression. J Neurooncol. 2009;94(1):97–101. [DOI] [PubMed] [Google Scholar]
- 6. Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70(10):779–787. [DOI] [PubMed] [Google Scholar]
- 7. Ellingson BM, Wen PY, van den Bent MJ, Cloughesy TF. Pros and cons of current brain tumor imaging. Neuro Oncol. 2014;16(Suppl 7):vii2–vii11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: Response Assessment in Neuro-Oncology Working Group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 9. Suto Y, Caner BE, Tamagawa Y, et al. Subtracted synthetic images in Gd-DTPA enhanced MR. J Comput Assist Tomogr. 1989;13(5):925–928. [DOI] [PubMed] [Google Scholar]
- 10. Lee VS, Flyer MA, Weinreb JC, Krinsky GA, Rofsky NM. Image subtraction in gadolinium-enhanced MR imaging. AJR Am J Roentgenol. 1996;167(6):1427–1432. [DOI] [PubMed] [Google Scholar]
- 11. Ellingson BM, Kim HJ, Woodworth DC, et al. Recurrent glioblastoma treated with bevacizumab: contrast-enhanced T1-weighted subtraction maps improve tumor delineation and aid prediction of survival in a multicenter clinical trial. Radiology. 2014;271(1):200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 13. Ellingson BM, Cloughesy TF, Lai A, Nghiemphu PL, Mischel PS, Pope WB. Quantitative volumetric analysis of conventional MRI response in recurrent glioblastoma treated with bevacizumab. Neuro Oncol. 2011;13(4):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ellingson BM, Nguyen HN, Lai A, et al. Contrast-enhancing tumor growth dynamics of preoperative, treatment-naive human glioblastoma. Cancer. 2016;122(11):1718–1727. [DOI] [PubMed] [Google Scholar]
- 15. Chappell R, Miranpuri SS, Mehta MP. Dimension in defining tumor response. J Clin Oncol. 1998;16(3):1234. [DOI] [PubMed] [Google Scholar]
- 16. Ellingson BM, Wen PY, Cloughesy TF. Modified criteria for radiographic response assessment in glioblastoma clinical trials. Neurotherapeutics. 2017;14(2):307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. James K, Eisenhauer E, Christian M, et al. Measuring response in solid tumors: unidimensional versus bidimensional measurement. J Natl Cancer Inst. 1999;91(6):523–528. [DOI] [PubMed] [Google Scholar]
- 18. Shah GD, Kesari S, Xu R, et al. Comparison of linear and volumetric criteria in assessing tumor response in adult high-grade gliomas. Neuro Oncol. 2006;8(1):38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. New PF, Scott WR, Schnur JA, Davis KR, Taveras JM. Computerized axial tomography with the EMI scanner. Radiology. 1974;110(1):109–123. [DOI] [PubMed] [Google Scholar]
- 20. Earnest F 4th, Kelly PJ, Scheithauer BW, et al. Cerebral astrocytomas: histopathologic correlation of MR and CT contrast enhancement with stereotactic biopsy. Radiology. 1988;166(3):823–827. [DOI] [PubMed] [Google Scholar]
- 21. Claussen C, Laniado M, Kazner E, Schörner W, Felix R. Application of contrast agents in CT and MRI (NMR): their potential in imaging of brain tumors. Neuroradiology. 1985;27(2):164–171. [DOI] [PubMed] [Google Scholar]
- 22. Andreou J, George AE, Wise A, et al. CT prognostic criteria of survival after malignant glioma surgery. AJNR Am J Neuroradiol. 1983;4(3):488–490. [PMC free article] [PubMed] [Google Scholar]
- 23. Wood JR, Green SB, Shapiro WR. The prognostic importance of tumor size in malignant gliomas: a computed tomographic scan study by the Brain Tumor Cooperative Group. J Clin Oncol. 1988;6(2):338–343. [DOI] [PubMed] [Google Scholar]
- 24. Vecht CJ, Avezaat CJ, van Putten WL, Eijkenboom WM, Stefanko SZ. The influence of the extent of surgery on the neurological function and survival in malignant glioma. A retrospective analysis in 243 patients. J Neurol Neurosurg Psychiatry. 1990;53(6):466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Albert FK, Forsting M, Sartor K, Adams HP, Kunze S. Early postoperative magnetic resonance imaging after resection of malignant glioma: objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurgery. 1994;34(1):45–60; discussion 60–61. [DOI] [PubMed] [Google Scholar]
- 26. Grabowski MM, Recinos PF, Nowacki AS, et al. Residual tumor volume versus extent of resection: predictors of survival after surgery for glioblastoma. J Neurosurg. 2014;121(5):1115–1123. [DOI] [PubMed] [Google Scholar]
- 27. Ammirati M, Vick N, Liao YL, Ciric I, Mikhael M. Effect of the extent of surgical resection on survival and quality of life in patients with supratentorial glioblastomas and anaplastic astrocytomas. Neurosurgery. 1987;21(2):201–206. [DOI] [PubMed] [Google Scholar]
- 28. Curran WJ Jr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85(9):704–710. [DOI] [PubMed] [Google Scholar]
- 29. Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190–198. [DOI] [PubMed] [Google Scholar]
- 30. Laws ER, Parney IF, Huang W, et al. ; Glioma Outcomes Investigators Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg. 2003;99(3):467–473. [DOI] [PubMed] [Google Scholar]
- 31. Bauchet L, Mathieu-Daude H, Fabbro-Peray P, et al. Oncological patterns of care and outcome for 952 patients with newly diagnosed glioblastoma in 2004. Neuro Oncol. 2010;12(7):725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li J, Wang M, Won M, et al. Validation and simplification of the Radiation Therapy Oncology Group recursive partitioning analysis classification for glioblastoma. Int J Radiat Oncol Biol Phys. 2011;81(3):623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115(1):3–8. [DOI] [PubMed] [Google Scholar]
- 34. Chaichana KL, Jusue-Torres I, Navarro-Ramirez R, et al. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol. 2014;16(1):113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oppenlander ME, Wolf AB, Snyder LA, et al. An extent of resection threshold for recurrent glioblastoma and its risk for neurological morbidity. J Neurosurg. 2014;120(4):846–853. [DOI] [PubMed] [Google Scholar]
- 36. Pan IW, Ferguson SD, Lam S. Patient and treatment factors associated with survival among adult glioblastoma patients: a USA population-based study from 2000–2010. J Clin Neurosci. 2015;22(10):1575–1581. [DOI] [PubMed] [Google Scholar]
- 37. Chaichana KL, Cabrera-Aldana EE, Jusue-Torres I, et al. When gross total resection of a glioblastoma is possible, how much resection should be achieved?World Neurosurg. 2014;82(1-2):e257–e265. [DOI] [PubMed] [Google Scholar]
- 38. Jeremic B, Shibamoto Y, Grujicic D, et al. Pre-irradiation carboplatin and etoposide and accelerated hyperfractionated radiation therapy in patients with high-grade astrocytomas: a phase II study. Radiother Oncol. 1999;51(1):27–33. [DOI] [PubMed] [Google Scholar]
- 39. Levin VA, Crafts DC, Norman DM, Hoffer PB, Spire JP, Wilson CB. Criteria for evaluating patients undergoing chemotherapy for malignant brain tumors. J Neurosurg. 1977;47(3):329–335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.