Abstract
Background
Current clinical nomograms such as American Urological Association/National Comprehensive Cancer Network (AUA/NCCN) risk categories or CAPRA may not always reflect prostate cancer (PCa) risk among African American men. We evaluated the usefulness of adding a commercially available cell cycle progression (CCP) score to improve risk stratification in a community-based African American population.
Methods
Biopsy tissues from 150 African American and 60 Caucasian men were obtained from a single community urologic oncology practice in Memphis, TN. The biopsy samples were evaluated with a commercially available CCP panel (Prolaris). Clinical variables such as Gleason score, prostate-specific antigen (PSA), age, clinical stage, and extent of disease were combined to determine a single category of low-, intermediate-, or high-risk. AUA risk stratification for cancer aggressiveness was then compared between the CCP score vs. the clinical parameters to determine potential risk improvement by the CCP score.
Results
Based on the clinical parameters, of the 150 African American men evaluated, 20% were classified as low-risk, 40% were classified as intermediate-risk, and 40% were classified as high-risk. Of the 60 Caucasian men evaluated, 42% were low-risk, 42% were intermediate-risk, and 17% were high-risk. However, when re-evaluating the African American patients using the CCP score, 30% of the patients were determined to be more aggressive than the clinical low-risk category. Similarly, 21.67% of the patients were found to be more aggressive than the clinical intermediate-risk category, and 23.33% of the patients were more aggressive than the high-risk category. When compared to our Caucasian cohort, 12% of the low-risk patients, 8% of the intermediate-risk patients, and 10% of the high-risk patients were found to be more aggressive by the CCP score. Overall, 24% of African American men vs. 10% of Caucasian men were reclassified to a higher risk by CCP score. When we compared the mean CCP score in the African American population vs. the Caucasian population, the mean CCP score in the AUA low-risk was 3.2 vs. 2.9; 3.4 vs. 3.2 in the AUA intermediate-risk; and 3.8 vs. 3.5 in the AUA high-risk category, respectively. Despite the higher mean CCP score in the African American population, the difference between the African American men and the Caucasian men was not significant (P=0.064 for low-risk, P=0.204 for intermediate-risk, and P=0.209 for high-risk).
Conclusions
Our data extends the evidence that CCP score derived from a biopsy specimen can be clinically useful. Our findings showed that the CCP score could stratify 10-year mortality risk in African American men beyond the current clinicopathologic features, which may better prepare patients for follow-up visits and discussions with their health care provider(s) and enhance their ability to select the most appropriate treatment option.
Keywords: African American, prostate cancer (PCa), risk stratification, cell cycle progression score (CCP score), combined clinical risk (CCR)
Introduction
Prostate cancer (PCa) kills more men annually in the United States than any other malignancy except lung cancer (1). African American men have a higher incidence of PCa than men from other ethnic groups and their mortality rates are 2.5 times that of Whites and Native Americans and five times that of Asian/Pacific Islanders (1). PCa in African American men is often detected at a much later stage, usually more aggressive, and harder to treat. Hence, the decline in overall incidence and mortality in African American men has significantly lagged behind that of other ethnic groups (2,3). Even African American men with very low risk PCa, as defined by the National Comprehensive Cancer Network (NCCN), have been noted to have higher rates of non-organ confined disease, positive surgical margins, adverse pathological and poorer oncologic outcomes, and larger tumor volumes (2-4). Some of the factors purported to account for the disproportionate burden of PCa among African American men include unequal access to health care services, diagnosis at later stages of disease, faster cancer growth rates, health education and literacy challenges or deficits, adverse knowledge-attitude and behavior patterns, and inadequate clinical decision support (2-4).
Current nomograms such as Partin tables used in PCa provide a risk of extraprostatic extension, seminal vesicle involvement, and lymph node metastasis (5). Other nomograms such as the D’Amico risk stratification provide prognostic information on biochemical recurrence for patients treated with radiation therapy and radical prostatectomy (6), and the Kattan nomogram exist for preoperative and postoperative predictions as well as for identification of metastatic disease (7,8). The Cancer of the Prostate Strategic Urologic Research Endeavor/Center for Prostate Disease Research (CaPSURE/CPDR) equation offers an assessment of recurrence risk based on pretreatment prostate-specific antigen (PSA), Gleason score, organ confinement and ethnicity (9). Unfortunately, all of the above nomograms suffer from a lack of ethnic diversity among the patient population and have a very small percentage of African American participants. The ideal nomogram, algorithm or model should be accurate, culturally unbiased, pathologist or surgeon independent, and applicable to patients in the community as well as population in tertiary care institutions. The addition of biomarkers that improve these current nomograms could decrease prognostic uncertainty and enable more appropriate treatment decision.
The cell cycle progression (CCP) score is based on measuring CCP gene expression and is strongly associated with PCa outcomes (10-16). In all of the previously published data, CCP score derived from a biopsy specimen can predict subsequent tumor aggressiveness (10-16). We now report on the usefulness of adding CCP score to improve risk stratification in a community-based African American population.
Methods
Patient
Biopsy tissues from 150 African American and 60 Caucasian men were obtained from a single community urologic oncology practice in Memphis, TN, USA.
Sample preparation and CCP score
Formalin fixed, paraffin-embedded tumor blocks containing the diagnostic biopsy were analyzed at Myriad Genetics (Salt Lake City, Utah, USA). The test begins with H&E stained slides from each case reviewed by a board-certified anatomical pathologist to determine whether there was sufficient cancer in the biopsy for RNA extraction, i.e., ≥0.5 mm linear tumor and/or ≥75% tumor. In addition, samples were reviewed to determine that the specimen contains enough appropriate tissue type, i.e., prostatic acinar adenocarcinoma. The pathologist removed areas containing the cancer from unstained sections and total RNA was extracted from the tissue. We cut ten 10-µm sections for RNA extraction.
Select carcinoma regions were macrodissected according to pathologist instructions. Carcinoma was deparaffinized and RNA was extracted using miRNeasy (Qiagen, Germany) as described by the manufacturer. Gene expression was measured using TaqMan Low Density Arrays as previously described (10,11). All samples were run in triplicate.
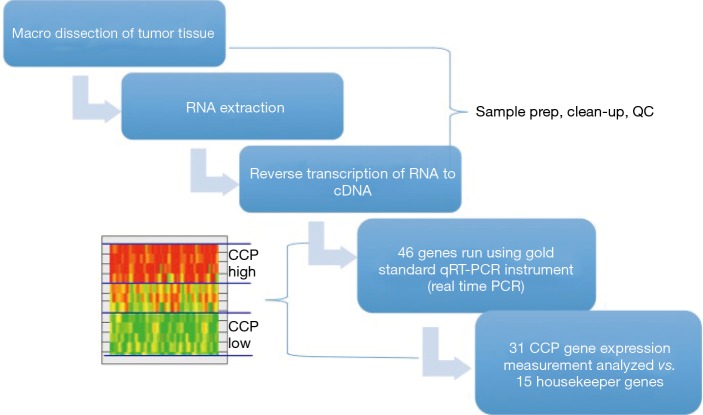
The CCP score was calculated from the expression data of 31 CCP genes normalized by the expression of 15 housekeeper genes as previously described (10,11). CCP scores were rejected if more than nine CCP genes were missing or the SD of CCP scores in the triplicate value was greater than 0.5. Figure 1 illustrates the process of the CCP testing.
Figure 1.
The process of CCP sample testing. The CCP score was calculated from the expression data of 31 CCP genes normalized by the expression of 15 housekeeper genes. CCP, cell cycle progression; PCR, polymerase chain reaction.
Risk stratification
Clinical variables such as Gleason score, PSA, age, clinical stage, and extent of disease were combined to determine a single category of American Urological Association (AUA) low-, intermediate-, or high-risk. Risk parameters were used to determine potential risk improvement by the CCP score. The combined score, CCR, is calculated using a linear combination of the Cancer of the Prostate Risk Assessment (CAPRA) and CCP scores. The predicted 10-year rate of PCa mortality rises regularly from 1% to over 50%.
Statistical analysis
The 2-sample t-test was used to test for a difference in means for CCP scores. The Wilcoxon rank-sum test was used to test for continuous clinicopathological variables while Fisher’s exact test was used for categorical variables. Statistical significance was set at the 5% level, thus P<0.05 was considered statistically significant.
Results
Cohort description by race
Table 1 lists the clinical and pathological characteristics distribution of patients enrolled. We had 150 African American and 60 Caucasian males in this cohort. Median age of the African American men was 66 years and the median age of the Caucasian men was 65 years. PSA ranged from 4.0 to 8.8 ng/mL in the African American men (median 5.6 ng/mL) and 3.6 to 6.9 ng/mL in the Caucasian men (median 4.8 ng/mL). African American men had lower percentages of Gleason score 6, but higher 3+4 and >7 graded disease (P=0.020).
Table 1. Cohort clinical description by race.
| Variables | African-American (n=150) | Caucasian (n=60) | P* |
|---|---|---|---|
| Age (years), median [IQR] | 66 [61–71] | 65 [60–71] | 0.61 |
| PSA (ng/mL), median [IQR] | 5.6 [4.0–8.8] | 4.8 [3.6–6.9] | 0.093 |
| Gleason score [n (%)] | 0.020 | ||
| <7 | 36 [24] | 27 [45] | |
| 3+4 | 78 [52] | 25 [42] | |
| 4+3 | 3 [2] | 1 [2] | |
| >7 | 33 [22] | 7 [12] | |
| Clinical stage [n (%)] | 0.0022 | ||
| T1c | 118 [79] | 56 [93] | |
| T2 | 30 [20] | 2 [3] | |
| % positive cores, median [IQR] | 25.0 [16.7–41.7] | 25.0 [16.7–33.3] | 0.048 |
| AUA risk [n (%)] | 0.00058 | ||
| Low | 30 [20] | 25 [42] | |
| Intermediate | 60 [40] | 25 [42] | |
| High | 60 [40] | 10 [17] |
*, Wilcoxon rank sum test for continuous variables; Fisher’s exact test for categorical variables. IQR, inter quartile range.
CCP scores distribution by AUA risks and race
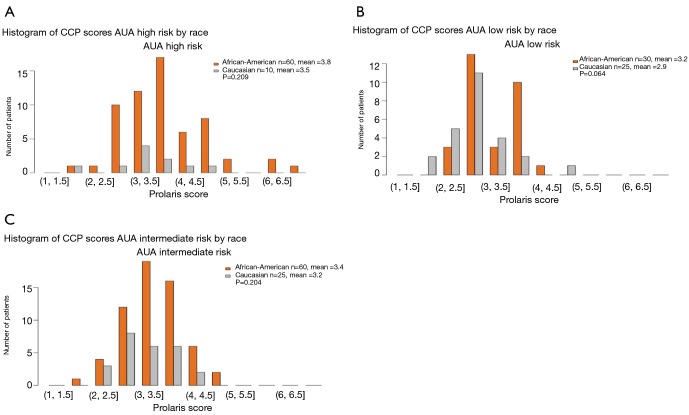
Figure 2 illustrates the CCP score distribution by AUA risk and race. When analyzed by the total cohort, there were some African American men with a higher CCP score than the Caucasian men (CCP score >5.0; Figure 2). However, when analyzed by AUA risk category, many of the African American men with a CCP score >5.0 were high-risk patients (Figure 3A). In fact, in the AUA low-risk category, some of the Caucasian men had a CCP score that was higher than the African American men (Figure 3B). When we compared the mean of the CCP score in the African American population versus the Caucasian population, the mean CCP score in the AUA low-risk was 3.2 vs. 2.9; 3.4 vs. 3.2 in the AUA intermediate-risk; and 3.8 vs. 3.5 in the AUA high-risk category (Figure 3). Despite the higher CCP mean in the African American population, the difference between the African American men and the Caucasian men was not significant (P=0.064 for the low-risk, P=0.204 for the intermediate-risk, and P=0.209 for the high-risk, respectively).
Figure 2.
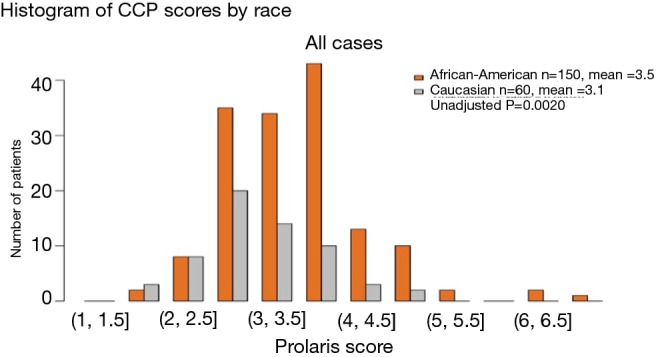
CCP distribution by race from the entire cohort of both African American men and Caucasian men. Prolaris score on the x-axis is the CCP score. CCP, cell cycle progression.
Figure 3.
CCP scores distribution by AUA risk category and race. (A) Histogram of CCP scores AUA high risk by rice; (B) histogram of CCP scores AUA low risk by rice; (C) histogram of CCP scores AUA intermediate risk by rice. CCP, cell cycle progression; AUA, American Urological Association.
Reclassification of patient risk by CCP score
When based solely on the clinical parameters of the 150 African American men, Table 1 shows that 20% were classified as AUA low-risk (PSA ≤10 ng/mL, Gleason score ≤6, and clinical T-stage of T1c–T2a), 40% were classified as AUA intermediate-risk (PSA between 10–20 ng/mL, Gleason score of 7, or clinical stage T2b), and 40% were classified as AUA high-risk (PSA >20 ng/mL, Gleason score of 8 to 10, or clinical stage T2c). Of the 60 Caucasian men evaluated, 42% were AUA low-risk, 42% were AUA intermediate-risk, and 17% were high-risk. However, when re-evaluating the African American patients using the CCP score, 30% of the patients were determined to be more aggressive than the clinical low-risk category. Similarly, 22% of the patients were found to be more aggressive than the clinical intermediate-risk category, and 24% of the patients were more aggressive than the high-risk category [hazard ratio (HR) is 2.08 in a multivariate model, P=0.04] (Table 2).
Table 2. Reclassification of patient risk by CCP score—African American population.
| AUA risk category | Considerably less aggressive [n (%)] | Less aggressive [n (%)] | Consistent [n (%)] | More aggressive [n (%)] | Considerably more aggressive [n (%)] | Total |
|---|---|---|---|---|---|---|
| Low | 0 [0] | 9 [30] | 12 [40] | 9 [30] | 0 [0] | 30 |
| Intermediate | 1 [2] | 16 [27] | 30 [50] | 12 [20] | 1 [2] | 60 |
| High | 2 [3] | 21 [35] | 23 [38] | 10 [17] | 4 [7] | 60 |
| Total | 3 [2] | 46 [31] | 65 [43] | 31 [21] | 5 [3] | 150 |
CCP, cell cycle progression.
When compared to our Caucasian cohort in Table 3, 12% of the low-risk Caucasian men, 8% of the intermediate-risk men, and 10% of the high-risk men were found to be more aggressive by the CCP score.
Table 3. Reclassification of patient risk by CCP score—Caucasian population.
| AUA risk category | Considerably less aggressive [n (%)] | Less aggressive [n (%)] | Consistent [n (%)] | More aggressive [n (%)] | Considerably more aggressive [n (%)] | Total |
|---|---|---|---|---|---|---|
| Low | 1 [4] | 12 [48] | 9 [36] | 2 [8] | 1 [4] | 25 |
| Intermediate | 0 [0] | 11 [44] | 12 [48] | 2 [8] | 0 [0] | 25 |
| High | 1 [10] | 5 [50] | 3 [30] | 1 [10] | 0 [0] | 10 |
| Total | 2 [3] | 28 [47] | 24 [40] | 5 [8] | 1 [2] | 60 |
CCP, cell cycle progression.
CCP stratifies beyond clinical features
For African American men, we clearly showed that CCP could help stratify beyond various clinical features. Figure 4 shows the effects of incorporating the CCP score together with the CAPRA score to define a CCR (combined clinical risk) prediction. It demonstrates that CCR can stratify 10-year mortality risk beyond Gleason score alone. The 10-year median risk for PCa for Gleason 6 was 2%, but the CCR score ranged from 0.8% to 4.5%. Median risk for Gleason 7 was 4.8% whereas for CCR it ranged from 1.5% to 20%. For Gleason >7, median risk estimated at 12% while CCR predictions ranged from 4% to 50%. Similarly Figure 5 demonstrates that CCP can stratify 10-year mortality risk beyond PSA levels alone. For each PSA range (0–6, 6–10, 10–20, 20–30, and 30–100) CCR was able to provide risk stratification beyond median risk for each PSA range. Finally, Figure 6 demonstrates that CCR can stratify 10-year mortality risk beyond low, intermediate and high CAPRA scores.
Figure 4.
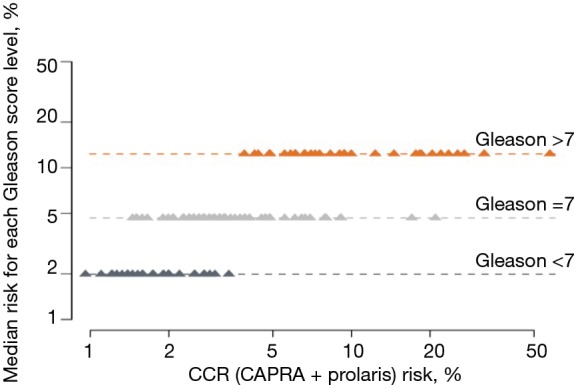
CCP score stratifies 10-year mortality risk beyond Gleason score alone in the African American men. CCR, combined clinical risk; CAPRA, Cancer of the Prostate Risk Assessment.
Figure 5.
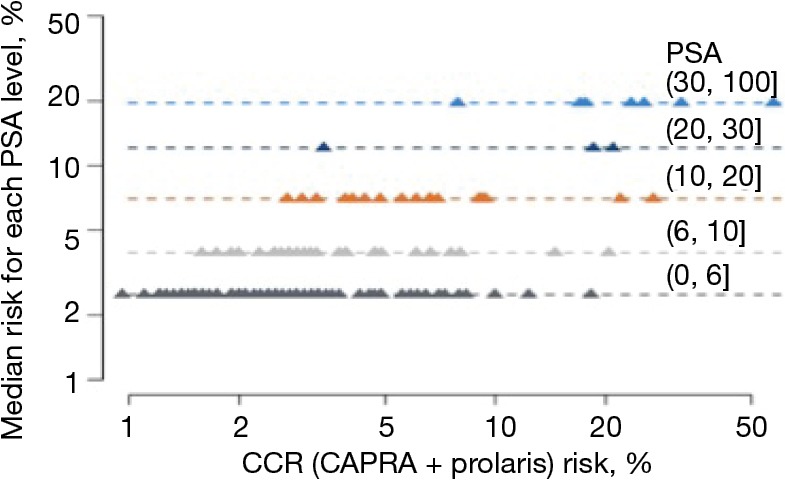
CCP score stratifies 10-year mortality risk beyond PSA alone in African American men. CCR, combined clinical risk; PSA, prostate-specific antigen.
Figure 6.
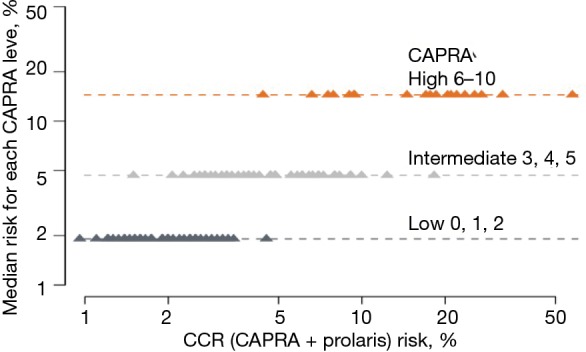
CCP score stratifies 10-year mortality risk beyond the CAPRA nomogram in African American men. CCR, combined clinical risk; CAPRA, Cancer of the Prostate Risk Assessment.
Discussion
The main goals of a cancer diagnosis and treatment are to cure or considerably prolong the life of patients and ensure the best possible quality of life for the cancer survivor. Localized PCa is a clinically heterogeneous disease and result in variability in patient outcomes even within the same clinical risk category such as the NCCN or AUA risk category, or within the same Gleason scores. It is vital to increase our ability to better stratify patient outcomes over-and-beyond routine clinicopathologic variables (T-stage, PSA, Gleason scores, etc.) from biopsies and/or pathology specimens. In addition, while multivariable nomograms such as CAPRA or the prostate nomogram from Memorial Sloan Kettering Cancer Center (MSKCC) can be used to predict the risk of many clinically relevant outcomes, results between nomograms are not directly comparable and these current nomograms such as the Partin tables, D’Amico tables, and CaPSURE Database do not have sufficient representation of African American men in their cohort. Thus, there is a clear need to identify and validate prediction models and/or prognostic biomarkers for African American men newly diagnosed with PCa.
A 31-gene panel consisting of CCP genes, along with 15 housekeeping genes, was developed for PCa by Myriad Genetics, Inc. The CCP score is validated on biopsies and helps to better determine if immediate or deferred (active surveillance or conservatively managed) treatment is the better course. The oncologic endpoints for the CCP score has been validated for disease-specific mortality, biochemical recurrence following radical prostatectomy or external beam radiation, and metastasis following initial treatment such as radical prostatectomy or external beam radiation (12-14). Cuzick et al., in two separate studies (10,11), examined the CCP prognostic value for PCa death in two independent conservatively managed needle biopsy cohorts (10,11). Their results showed improved prognostic ability over clinicopathologic variables. In fact, the CCP score generated from needle biopsies predicted PCa death more accurately than any other known factors.
Although studies involving CCP included African American men, the majority of the cohorts in the studies were non-African American men. In this report, we evaluated whether adding CCP score to the clinical parameters improves risk stratifications in a community-based predominantly African American population.
Figure 1 illustrates the process, as described in the methods section, for obtaining the CCP score. When the overall CCP distribution of the entire cohort was compared, there were some African American patients who had a higher CCP score (CCP score >5.0) than the Caucasian men (Figure 2). These patients were mainly found in the AUA high-risk category and upon further analysis, the CCP distribution by AUA risk between the races was not significant (Figure 3, P=0.064 for low-risk, P=0.204 for intermediate-risk, and P=0.209 for high-risk, respectively). These results were consistent with the more recent findings from Ochsner Health System where they compared the prognostic utility of CCP score for predicting metastatic disease in African American men and non-African American men with PCa (15). Their results also concluded that there was no evidence of an interaction between race (HR =0.5; 95% CI: 0.22–1.04) (15).
From a clinical utility perspective, reclassification of both African American and Caucasian men was seen in our study (Tables 2,3). When we compared the reclassification rate between African American and Caucasian men, there was a slight trend that the African American men were more likely to be reclassified by the CCP score to a more aggressive disease (compare Table 2 to Table 3). However, the reclassification rate in the African American men did not appear to be significantly different from other published reclassification rates in the general population (15). Nonetheless, the CCP score can stratify the 10-year mortality risk in African American men beyond their Gleason score (Figure 4), PSA (Figure 5), and CAPRA (Figure 6). An apparent clinical utility of the CCP score is to help identify low-risk patients who can be safely managed by active surveillance. This is particularly important for African American men newly diagnosed with PCa since active surveillance recommendations were mostly derived from a majority Caucasian population and there is disagreement as to whether African American men should undergo active surveillance (17). The CCP score may also help stratify intermediate-risk and high-risk localized PCa into an unfavorable group that is at high risk of developing metastases and require multi-modal therapies vs. a favorable group that could be treated with local therapy alone. This information is relevant and may prove useful in helping guide treatment decisions: neoadjuvant, adjuvant, localized treatment alone, or salvage therapy. African American men are likely to benefit from this stratification using the CCP score, which may improve decision making at the time of diagnosis. Moreover, the selection of more appropriate and effective definitive treatment may help to eliminate disparate outcomes in this disease. Although we did not show the data for the Caucasian men, multiple studies have been published demonstrating the utility of the CCP score to stratify the 10-year mortality risk in the general population (16,18).
Conclusions
Our data extends the evidence that CCP score derived from a biopsy specimen can be clinically useful. A limitation of our current report is that our data is derived from a single practice site, and a larger community-based predominantly African American cohort would be required to validate our findings. Regardless, our findings showed that the CCP score could stratify 10-year mortality risk in African American men beyond the current clinicopathologic features, which may better prepare patients for follow-up visits and discussions with their health care provider(s) and enhance their ability to select the most appropriate treatment option.
Acknowledgements
None.
Ethical Statement: The study is a retrospective assessment of our experience using the cell cycle progression score, and all specific patient identifying information was removed from analysis, so ethical approval and patient informed consent are not required.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Powell IJ. Epidemiology and pathophysiology of prostate cancer in African-American men. J Urol 2007;177:444-9. 10.1016/j.juro.2006.09.024 [DOI] [PubMed] [Google Scholar]
- 2.Underwood W, De Monner S, Ubel P, et al. Racial/ethnic disparities in the treatment of localized/regional prostate cancer. J Urol 2004;171:1504-7. 10.1097/01.ju.0000118907.64125.e0 [DOI] [PubMed] [Google Scholar]
- 3.Schwartz K, Powell IJ, Underwood W, 3rd, et al. Interplay of race, socioeconomic status, and treatment on survival of patients with prostate cancer. Urology 2009;74:1296-302. 10.1016/j.urology.2009.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moul JW, Douglas TH, McCarthy WF, et al. Black race is an adverse prognostic factor for prostate cancer recurrence following radical prostatectomy in an equal access health care setting. J Urol 1996;155:1667-73. 10.1016/S0022-5347(01)66160-3 [DOI] [PubMed] [Google Scholar]
- 5.Eifler JB, Feng Z, Lin BM, et al. An updated prostate cancer staging nomogram (Partin tables) based on cases from 2006 to 2011. BJU Int 2013;111:22-9. 10.1111/j.1464-410X.2012.11324.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998;280:969-74. 10.1001/jama.280.11.969 [DOI] [PubMed] [Google Scholar]
- 7.Kattan MW, Eastham JA, Stapleton AM, et al. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst 1998;90:766-71. 10.1093/jnci/90.10.766 [DOI] [PubMed] [Google Scholar]
- 8.Kattan MW, Wheeler TM, Scardino PT. Postoperative nomogram for disease recurrence after radical prostatectomy for prostate cancer. J Clin Oncol 1999;17:1499-507. 10.1200/JCO.1999.17.5.1499 [DOI] [PubMed] [Google Scholar]
- 9.Moul JW, Connelly RR, Lubeck DP, et al. Predicting risk of prostate specific antigen recurrence after radical prostatectomy with the Center for Prostate Disease Research and Cancer of the Prostate Strategic Urologic Research Endeavor databases. J Urol 2001;166:1322-7. 10.1016/S0022-5347(05)65761-8 [DOI] [PubMed] [Google Scholar]
- 10.Cuzick J, Berney DM, Fisher G, et al. Prognostic value of a cell cycle progression signature for prostate cancer death in a conservatively managed needle biopsy cohort. Br J Cancer 2012;106:1095-9. 10.1038/bjc.2012.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuzick J, Stone S, Fisher G, et al. Validation of an RNA cell cycle progression score for predicting death from prostate cancer in a conservatively managed needle biopsy cohort. Br J Cancer 2015;113:382-9. 10.1038/bjc.2015.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freedland SJ, Gerber L, Reid J, et al. Prognostic utility of cell cycle progression score in men with prostate cancer after primary external beam radiation therapy. Int J Radiat Oncol Biol Phys 2013;86:848-53. 10.1016/j.ijrobp.2013.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooperberg MR, Simko JP, Cowan JE, et al. Validation of a cell-cycle progression gene panel to improve risk stratification in a contemporary prostatectomy cohort. J Clin Oncol 2013;31:1428-34. 10.1200/JCO.2012.46.4396 [DOI] [PubMed] [Google Scholar]
- 14.Bishoff JT, Freedland SJ, Gerber L, et al. Prognostic utility of the cell cycle progression score generated from biopsy in men treated with prostatectomy. J Urol 2014;192:409-14. 10.1016/j.juro.2014.02.003 [DOI] [PubMed] [Google Scholar]
- 15.Bardot S, Reid J, Latsis M, et al. Evaluating the prognostic utility of the CCP score for predicting prostate cancer aggressiveness in African American men. J Urol 2017;197:e346 10.1016/j.juro.2017.02.832 [DOI] [Google Scholar]
- 16.Tosoian JJ, Chappidi MR, Bishoff JT, et al. Prognostic utility of biopsy-derived cell cycle progression score in patients with National Comprehensive Cancer Network low-risk prostate cancer undergoing radical prostatectomy: implications for treatment guidance. BJU Int 2017;120:808-14. 10.1111/bju.13911 [DOI] [PubMed] [Google Scholar]
- 17.Tosoian JJ, Carter HB, Lepor A, et al. Active surveillance for prostate cancer: current evidence and contemporary state of practice. Nat Rev Urol 2016;13:205-15. 10.1038/nrurol.2016.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehdaie B, Stone S, Bernhisel R, et al. The impact of clinical CCP testing in men with localized prostate cancer for expanding the population of men eligible for active surveillance. J Urol 2017;197:e517 10.1016/j.juro.2017.02.1236 [DOI] [Google Scholar]