Abstract
Prostate cancer is one of the most common cancers in men worldwide, and primary prostate cancer is typically treated with surgery, radiation, androgen deprivation, or a combination of these therapeutic modalities. Despite technical advances, approximately 30% of men will experience biochemical recurrent within 10 years of definitive treatment. Upon detection of a rise in serum prostate specific antigen (PSA), there is great need to accurately stage these patients to help guide further therapy. As a result, there are considerable efforts underway to establish the role of positron emission tomography (PET) in the diagnostic algorithm of biochemically recurrent prostate cancer. This manuscript provides an overview of PET tracers used for the detection and localization of prostate cancer in the setting of biochemical recurrence with a focus on PET tracers that are currently being used in clinical practice in the United States.
Keywords: Biochemical recurrence, positron emission tomography (PET)/computed tomography (CT), PET/magnetic resonance imaging (MRI), prostate cancer
Introduction
Prostate cancer is one of the most common cancers in men worldwide (1). Radical prostatectomy can cure appropriately selected patients with localized disease, as can radiation therapy. Imaging for staging at the time of diagnosis varies slightly depending on the patient’s overall risk of extraprostatic disease, but patients classified as intermediate risk or higher will undergo skeletal scintigraphy and either an abdominopelvic CT or pelvic MRI for comprehensive staging per the National Comprehensive Center Network (NCCN) clinical practice guidelines (2). Locally advanced prostate cancer is defined by the NCCN as T3b-T4 tumors, tumors with primary Gleason pattern of 5, or tumors with greater than four cores of Gleason grade group 4 or 5 (2). Patients are deemed metastatic at initial staging if any distant or nodal metastasis is detected on conventional anatomic imaging (2).
Following definitive treatment, patients will undergo serial serum PSA sampling to evaluate for response to treatment and to detect recurrence. Approximately 20–40% of patients undergoing radical prostatectomy and 30–50% of patients undergoing radiation therapy will experience biochemical recurrence within 10 years (3-5). The American Urological Association defines biochemical recurrence in patients who have undergone prostatectomy as an initial PSA value of at least 0.2 ng/mL followed by a subsequent confirmatory PSA value of 0.2 ng/mL or more (6). In patients who have undergone definitive primary radiation therapy, biochemical recurrence is defined as a rise of 2 ng/mL or more above the nadir PSA level (7). Common locations of local recurrence are within the prostate gland, prostatectomy surgical bed, regional nodal metastases, and osseous metastases. Computed tomography (CT) and magnetic resonance imaging (MRI) are non-invasive modalities that have been utilized for detection of metastatic nodal disease, largely relying on morphologic characteristics such as size and shape. A meta-analysis found a pooled sensitivity of 42% and specificity of 82% for CT imaging and similar 39% sensitivity and 82% specificity for detection of metastatic lymph nodes (8). Metastatic nodal disease is determined largely by size when using CT and MR, with a threshold of 1.0 cm in short axis of oval nodes and 0.8 cm for round nodes used as indicators of likelihood of metastatic disease (9). However, more than half of lymph nodes involved with metastatic prostate cancer may be less than 1 cm (10). Furthermore, non-metastatic nodes may be enlarged due to reactive hyperplasia, leading to false positives. Imaging remains a crucial aspect of evaluation of the patient with biochemical recurrence, as a recurrence in the prostate bed or pelvic lymph nodes may be treated with surgery or salvage radiotherapy, while a more distant pattern of metastasis may require systemic treatment with either androgen deprivation therapy or chemotherapy (2). However, anatomic imaging with CT and MRI alone has limited sensitivity for detection of nodal metastases from prostate cancer.
Positron emission tomography (PET) is an imaging technique that utilizes radiolabeled drugs to image molecular targets, metabolic pathways, and functional processes in cancer. A number of PET tracers have been developed that can increase the accuracy of prostate cancer detection compared to morphological imaging alone, particularly in the setting of biochemical recurrence. Significant research is currently being conducted to develop effective PET tracers that can be used for both initial staging and localization of biochemical recurrence in patients with prostate cancer. Additionally, several of the tracers that will be discussed including PSMA ligands are being evaluated not only for their diagnostic capabilities, but also for molecularly targeted radionuclide therapy. These agents are often called theranostics, and in this scenario imaging is used to select appropriate patients for therapy by non-invasively confirming the presence of the target prior to treatment. For example, the same agent can be labeled with a positron-emitting radionuclide such as gallium-68 for PET or with a therapeutic radionuclide such as lutetium-177 for treatment. In this article we will discuss and compare the PET tracers available for detection of prostate cancer in patients with biochemical recurrence.
Methods
A search was conducted through PubMed to assess the existing literature regarding the use of PET imaging in the setting of biochemically recurrent prostate cancer. Terms searched included “prostate cancer”, “PET prostate cancer”, and “PET biochemical recurrence”. These searches were conducted on 12/4/2017. Articles were reviewed and included in the paper if they were scientific articles regarding PET imaging of biochemically recurrent prostate cancer, provided needed statistics on prostate cancer recurrence rates and/or mortality, or provided a useful summary or meta-analysis of the prostate cancer PET literature. Articles were excluded if they did not discuss PET imaging of biochemically recurrent prostate cancer or focused on non-PET imaging modalities of prostate cancer.
Basics of prostate cancer PET imaging and available tracers
PET is currently performed in combination with CT or MR for attenuation correction and anatomic localization of PET findings. The vast majority of clinical PET scanners currently are PET/CT systems, although PET/MRI is in clinical use with an increasing installation base. In PET imaging for prostate cancer, a positron-emitting radiopharmaceutical is administered intravenously, and images are acquired through the detection of coincident gamma rays of 511 keV that result from the annihilation of a positron with an electron in a tissue (11). PET images can provide quantitative localization of radioactivity throughout the body at a single or multiple time points after tracer injection.
The timing of imaging acquisition after PET tracer administration varies depending on the pharmacokinetics of the tracer. In some cases, image acquisition within 5 minutes after PET tracer administration is desirable to minimize the concentration of radioactivity in the urinary bladder and ureters. PET provides higher spatial and temporal resolution than conventional single photon computed tomography (SPECT). However, one of the limitations of PET is resolution, with decreased sensitivity of the detection and characterization of PET tracer uptake in lesions less than 8 mm. Additionally, PET tracers are expensive compared to CT and MRI contrast agents, and reimbursement for PET studies can be challenging even when the PET tracers are approved by the Food and Drug Administration (FDA).
A wide range of radionuclides have been used to label PET tracers for prostate cancer imaging. The radionuclides that have been used most commonly for PET tracers targeting prostate cancer are carbon-11, fluorine-18, and gallium-68, which vary in terms of their physical half-life and their chemical properties (Table 1). Fluorine-18 is widely available, and its 110 min half-life is sufficient for production in large quantities and distribution to other sites that do not have onsite production capabilities. Carbon-11 is readily produced on medical cyclotrons, but its 20 min half-life markedly limits distribution to other sites and requires significant coordination between the production and imaging team to obtain multiple patients dosages from a single carbon-11 production. Gallium-68 has a 68 min half-life and is typically produced using germanium-68 generator systems which facilitates on-site and on-demand production of 68Ga-labeled tracers. There are efforts to develop cyclotron-based production methods for gallium-68, but this technology is not currently widely available.
Table 1. Radionuclides commonly used for PET imaging of prostate cancer.
| Radionuclide | Half-life (min) | Production method |
|---|---|---|
| Carbon-11 [11C] | 20 | Cyclotron |
| Gallium-68 [68Ga] | 68 | Generator |
| Fluorine-18 [18F] | 110 | Cyclotron |
PET, positron emission tomography.
In general, PET tracers used for evaluation of biochemical recurrence all demonstrate increasing rates of positivity as the serum PSA levels rise (12-16). However, more recent studies demonstrate increased sensitivity of PSMA ligands and [18F]fluciclovine when compared to choline ligands (17,18). There is little current head-to-head comparison between PSMA ligands and [18F]fluciclovine in the literature, but an early case series suggests possible superiority of [68Ga]PSMA-11 over [18F]fluciclovine for lesion detection (19).
PET tracers that have been developed for the evaluation of prostate cancer recurrence include 2-deoxy-2-[18F]fluoro-D-glucose (FDG), [11C]acetate, [18F]DCFPyl and [68Ga]PSMA-11 (also known as HBED-CC), [68Ga]DOTA-bombesin, [18F]fluoro-5α-dihydrotestosterone (FDHT), [11C]choline, and [18F]fluciclovine (Table 2). Currently in the United States, [18F]FDG, sodium [18F]fluoride, [18F]fluciclovine, and [11C]choline are FDA-approved for use in the setting of biochemical recurrence, although [18F]FDG does not have an established role at the time of initial biochemical recurrence. The use of sodium [18F]fluoride, [18F]fluciclovine, and [11C]choline in prostate cancer is included in the National Comprehensive Cancer Network Guidelines version 1.2018 (2).
Table 2. Selected PET radiotracers used for prostate cancer imaging.
| PET radiotracers | Mechanism of action |
|---|---|
| [18F]FDG | Glucose metabolism |
| Na[18F]F | Bone chemisorption |
| [18F]choline, [11C]choline | Cell membrane metabolism |
| [18F]DCFPyl, [68Ga]PSMA-11 | PSMA binding |
| [11C]acetate | Fatty acid metabolism |
| [18F]fluciclovine | Amino acid transport |
| [68Ga]DOTA-bombesin | GRPR receptor binding |
| [18F]FDHT | Androgen receptor binding |
Reproduced with permission from the World Journal of Radiology article Zarzour JG et al. Lymph node imaging in initial staging of prostate cancer: an overview and update. PET, positron emission tomography; [18F]FDG, [18F]fluoro-D-glucose; [18F]NaF, sodium [18F]fluoride; [18F] DCFPyl, [18F] 2-(3-(1-carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl)-amino]-pentyl)-ureido)-pentanedioic acid; [18F]FDHT, [18F]fluorodehydrotestosterone; GRPR, gastrin-releasing peptide receptor.
[18F]FDG and [18F]Sodium fluoride
[18F]FDG is the most commonly used PET agent for clinical oncologic imaging but has limited clinical utility for the staging of prostate cancer at the time of diagnosis and in the setting of initial biochemical recurrence. In particular, there is an inverse relationship between levels of [18F]FDG uptake and tumoral differentiation with well-differentiated tumors demonstrating low-level [18F]FDG uptake and poorly-differentiated tumors demonstrating increasing [18F]FDG uptake (20,21). Another significant limitation of [18F]FDG-PET is the urinary excretion of the radiotracer, which can limit evaluation of the pelvic lymph nodes and prostate surgical bed (22). [18F]FDG may have a limited role in staging and monitoring response to castrate-resistant metastatic prostate cancer or biopsy-proven poorly differentiated recurrent prostate cancer (23).
Sodium [18F]fluoride was the first PET radiotracer approved for the evaluation of osseous metastases and was initially approved in the United States in the 1970s (Figure 1). Sodium [18F]fluoride is approved for both the initial evaluation of osseous metastatic disease and evaluation of response to treatment. When compared to 99mTc-labelled diphosphonates, sodium [18F]fluoride offers shorter scanning times, higher spatial resolution, and better image quality (24). A study has reported a sensitivity and specificity of 100% for sodium [18F]fluoride-PET/CT compared to 70% sensitivity and 57% specificity for planar bone scintigraphy (25), although it is not clear that [18F]fluoride-PET/CT reaches this high degree of specificity in clinical practice. Sodium [18F]fluoride-PET/CT also allows for earlier detection of osseous metastases compared to conventional bone scintigraphy and detection of occult osseous metastases when compared to conventional imaging (26,27). Additionally, sodium [18F]fluoride-PET/CT positivity tends to associate with increasing PSA levels in men who have undergone prostatectomy and may occur at lower levels of PSA than expected (27). Although the NCCN guidelines state that sodium [18F]fluoride can be used when planar bone scans are negative but there is high suspicion for osseous metastases or when there is disease progression, there is concern about its use in the treatment response setting. This concern is largely due to established guidelines being based on planar bone scintigraphy. The impact of sodium [18F]fluoride-PET/CT has been demonstrated through the initial results of the National Oncologic PET Registry, where 40% of treatment plans were revised following sodium [18F]fluoride-PET/CT (28). Despite this, there are limitations of [18F]sodium fluoride-PET/CT, including cost, availability of PET/CT scanners, and lack of soft tissue evaluation which can be performed with other PET tracers.
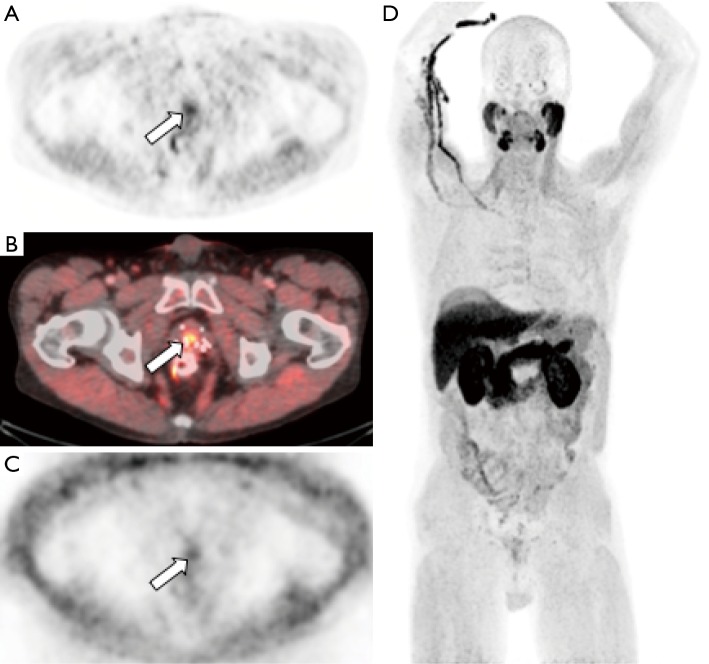
Figure 1.
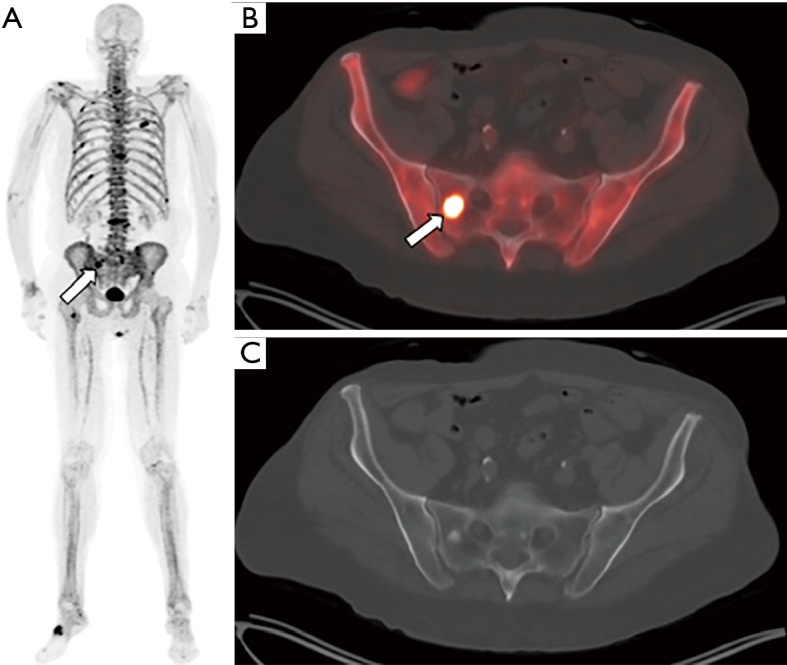
Sodium [18F]fluoride-PET/CT images from a patient with prostate cancer status post external beam radiation therapy, androgen-deprivation therapy, and brachytherapy presenting with elevated PSA and biochemical recurrence. The maximum intensity projection image (MIP, panel A) demonstrates numerous foci of increased tracer activity in several ribs and the bony pelvis. The fused PET/CT (B) and CT only (C) images demonstrate a right sacral metastasis (arrow) with a sclerotic correlate.
[11C]Choline
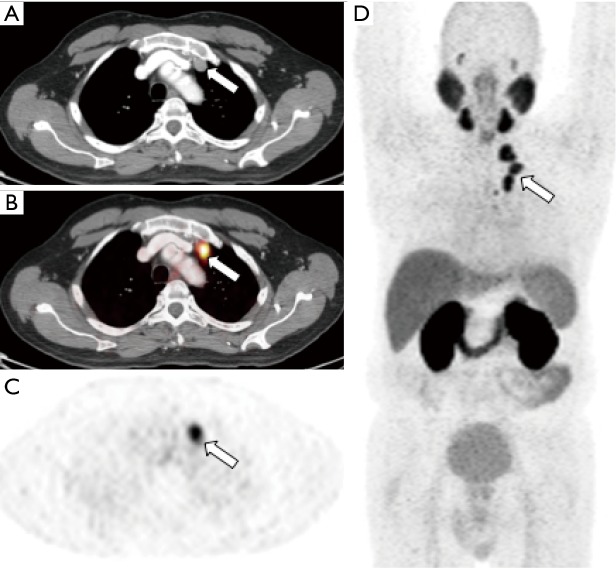
Choline is a substrate for the synthesis of phosphatidylcholine, which is the major phospholipid in the cell membrane (29). Choline is incorporated into tumor cells after transport into prostate cancer cells and phosphorylation by choline kinase, which is upregulated in prostate cancer (30). [11C]choline has been approved by the FDA for use in the detection and localization of suspected biochemically recurrent prostate cancer. [11C]choline offers an advantage over 18F-labeled choline derivatives in that the metabolite of [11C]choline demonstrates less urinary excretion than fluorinated choline compounds, which is advantageous in the evaluation of the prostatectomy bed and pelvic lymph nodes (Figure 2). However, a comparative analysis between [11C]choline and [18F]choline demonstrated comparable results in the detection of disease (31). Fanti et al. performed a meta-analysis and critical review of literature consisting of 12 studies including 1,270 patients, specifically looking at the ability of [11C]choline detect prostate cancer in the setting of biochemical recurrence after definitive treatment, with a derived pooled sensitivity and specificity of 89% (32). This derived pool data is similar to other published meta-analyses of [11C]choline-PET/CT (33-35). The performance of [11C]choline-PET/CT is better for larger lymph nodes, but studies have shown decreased sensitivity in lymph nodes measuring less than 7 mm (36). A meta-analysis of [11C]choline-PET/CT demonstrated a pooled rate of detection of 36% for nodal disease and 25% for osseous metastases (32). The same meta-analysis demonstrated a pooled detection rate for locally recurrent disease of 27%, with a pooled sensitivity of 61% and a pooled specificity of 97% (32). This result is consistent with comparative studies that have shown that multiparametric MRI with endorectal coil is superior to [11C]choline for detection of local recurrence, while [11C]choline-PET/CT was shown to be superior to MRI for pelvic lymph node metastases and equal with respect to bone metastases (37). The NCCN guidelines 1.2018 state that [11C]choline-PET/CT or PET/MRI can be considered for recurrence or disease progression after definitive therapy or for disease progression during systemic therapy (2).
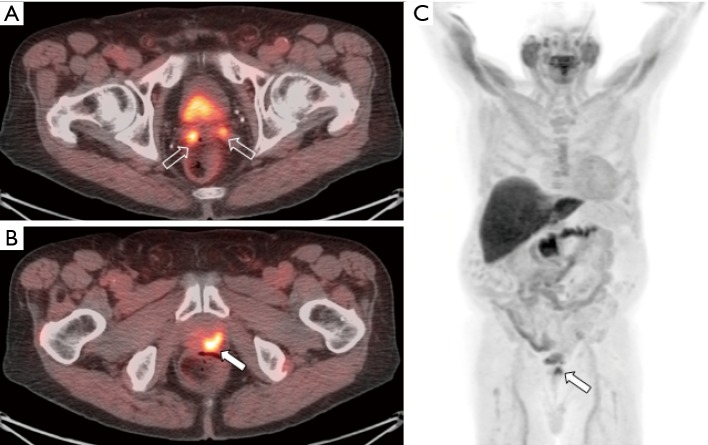
Figure 2.
[11C]choline-PET/CT images from a patient with prostate cancer status post brachytherapy, now with elevated PSA. Attenuation corrected PET (A) and fused PET/CT (B) images demonstrate focal tracer activity within the prostate gland (arrow) suspicious for recurrent tumor within the gland. Non-attenuation corrected PET images (C) demonstrate that the activity is not due to attenuation correction artifact from adjacent brachytherapy seeds. Whole body MIP images (D) demonstrates no additional distant metastases. Patient subsequently underwent prostatectomy which confirmed recurrent prostate cancer.
[18F]Fluciclovine
Amino acid transport is another important target for the imaging of prostate cancer due to upregulation of transmembrane amino acid transport (38) (Figures 3,4). The most work with radiolabeled amino acids for prostate cancer imaging has been with [18F]fluciclovine, a non-natural alicyclic amino acid. [18F]fluciclovine targets the LAT1 and ACST2 transmembrane transporters, both of with are overexpressed in prostate cancer cells (39,40). [18F]fluciclovine was approved by the FDA in 2016 for use in biochemically recurrent prostate cancer. An advantage of [18F]fluciclovine is relatively little urinary excretion of radiotracer, which leads to improved evaluation of potential recurrent disease in the pelvis (41,42). However, a limitation of [18F]fluciclovine is that it is unable to reliably differentiate malignant intraprostatic lesions from nodules due to benign prostatic hypertrophy, although new research suggests that delayed PET of the pelvis on PET/MRI shows promise for the differentiation between malignant and benign tissue (43,44). Additionally, the transport of [18F]fluciclovine is bidirectional which can lead to washout from prostate cancer at later time points after injection (45).
Figure 3.
Fused [18F]fluciclovine-PET/CT images (A and B) and whole body MIP image (C) from a patient with prostate cancer initially treated with radiation therapy and androgen-deprivation therapy reaching a PSA nadir of 0.7 ng/mL. The patient’s PSA continued to rise and measured 5.0 ng/mL at time of imaging which demonstrated local recurrence within the prostate gland (filled arrow) and invasion of the bilateral seminal vesicles (open arrows).
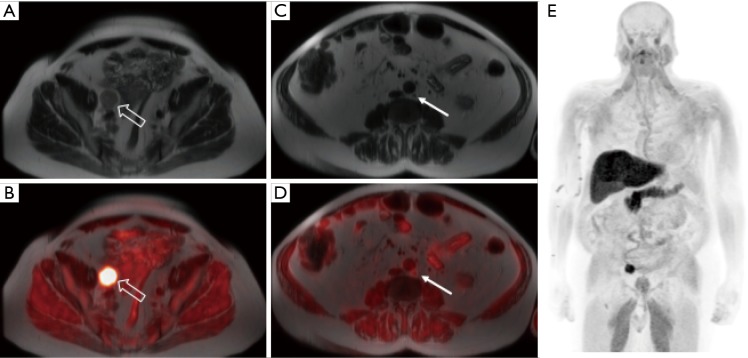
Figure 4.
[18F]fluciclovine-PET/MRI images from a patient with prostate cancer status post radical prostatectomy with subsequent biochemical recurrence and a PSA of 8.8 ng/mL. The T2 single shot fast spin echo (SSFSE) MR images demonstrate a large right internal iliac lymph node (A, open arrow) with markedly increased tracer activity on the fused PET/MR image (B) and a normal-sized left paraaortic lymph node (C, filled arrow) with increased activity on the fused PET/MR image (D), suspicious for an additional nodal metastasis. Whole body MIP image (E) demonstrates no additional distant metastases.
[18F]fluciclovine has been shown to have an overall detection rate of approximately 68% in the setting of biochemical recurrence, including in patients with low PSA levels (detection rate 41% for PSA <0.79 ng/mL) (46). When compared to conventional imaging (CT and bone scan), [18F]fluciclovine has demonstrated the ability to detect subcentimeter lymph node metastases and osseous metastases that were not detected on conventional imaging (47). Schuster et al. reported specificities of 40% and 97% for prostate bed and extraprostatic lesions, respectively (48). Odewole et al. demonstrated similar specificities of 56% and 100% for prostate bed and extraprostatic lesions, respectively (49). A meta-analysis evaluated [18F]fluciclovine PET, including 6 studies and 251 patients with biochemical recurrence (50). The pooled sensitivity and specificity on a per-patient analysis was 87% and 66%, respectively (50). One study examining the effect of [18F]fluciclovine-PET/CT on consideration of salvage radiation therapy in the setting of biochemical recurrence demonstrated a change in management in 41% of patients (51).
A study comparing the diagnostic performance of [18F]fluciclovine-PET/CT to [111In] capromab pendetide-SPECT/CT demonstrated superior performance for [18F]fluciclovine-PET/CT in the detection of both intraprostatic and extraprostatic disease (48). Additionally, a study comparing the diagnostic performance of [18F]fluciclovine-PET/CT to [11C]choline-PET/CT in biochemical recurrence following definitive therapy revealed superior performance of [18F]fluciclovine-PET/CT (17). In this study, [18F]fluciclovine demonstrated a sensitivity of 37% compared to 32% for [11C]choline and a specificity of 67% compared to 40% for [11C]choline. A small study in 10 patients with recurrent prostate cancer comparing [68Ga]PSMA-11 to [18F]fluciclovine suggested that [68Ga]PSMA-11 provides higher sensitivity for prostate cancer detection (19). However, larger studies are needed to determine the diagnostic accuracy of PSMA-PET versus [18F]fluciclovine for initial staging and the detection of recurrent prostate cancer. The NCCN guidelines 1.2018 for [18F]fluciclovine are very similar to those for [11C]choline and state that [18F]fluciclovine-PET/CT or PET/MRI can be considered for recurrence or disease progression after definitive therapy or for disease progression during systemic therapy (2).
[11C]Acetate
Acetate is a naturally occurring substance that can enter the fatty acid metabolic pathway, which is overexpressed in prostate cancer cells (52) (Figure 5). Currently, [11C]acetate-PET/CT is used at fewer sites than choline radiotracers (53). [11C]acetate-PET/CT has demonstrated good performance in the evaluation of recurrence in the prostatectomy bed, with 15 of the 18 patients with biopsy-proven recurrent disease demonstrating positivity on [11C]acetate-PET/CT and none of the patients with negative biopsies demonstrating [11C]acetate-PET/CT positivity (54). The same study also demonstrated comparable results between [11C]choline-PET/CT and [11C]acetate-PET/CT (54). At this point in time, no conclusions have been reached about the superiority of [11C]acetate-PET/CT versus [11C]choline-PET/CT with additional studies demonstrating similar results between the two radiotracers (55,56). A similar limitation between the two radiotracers is that the sensitivity for detection of disease is correlated with PSA levels, with decreased performance for PSA levels of <2 ng/mL (53,57,58).
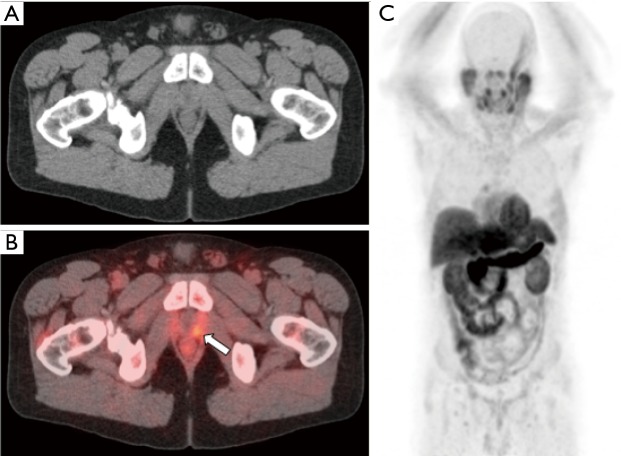
Figure 5.
[11C]acetate-PET/CT in a patient with elevated PSA of 0.38 ng/mL status post prostatectomy. CT images (A) and fused PET/CT images (B) demonstrate focal soft tissue with increased tracer activity in the prostatectomy bed (arrow). Whole body MIP image (C) demonstrates no distant metastases. Patient was subsequently referred to radiation oncology for salvage pelvic radiotherapy.
Prostate specific membrane antigen (PSMA) ligands
PSMA is a transmembrane protein expressed by the prostate and overexpressed in prostate cancer (59) (Figure 6). PSMA has long been a target for imaging patients with metastatic prostate cancer and was originally the target of [111In]indium capromab pendetide (tradename Prostascint), a radiolabeled monoclonal antibody targeting the intracellular portion of the transmembrane PSMA protein. Although this was an improvement at the time over existing imaging techniques, the sensitivity and specificity of the examination were significantly limited due to the targeting of the intracellular portion of the PSMA protein which permitted radiotracer binding only in the setting of cellular apoptosis or necrosis (36,59-62). An additional practical limitation was that the kinetics of [111In]indium capromab pendetide mandated imaging at 5–7 days following radiotracer injection.
Figure 6.
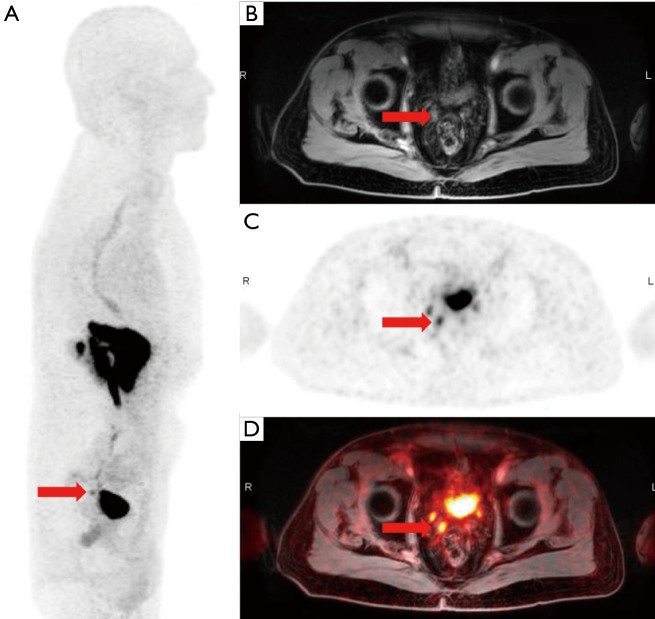
[68Ga]PSMA-11-PET/CT in a patient with prostate cancer initially treated with radiation therapy in 2013 with subsequent biochemical recurrence treated with salvage pelvic radiation therapy. Patient PSA continued to be elevated to 7.7 ng/mL. CT image (A), fused [68Ga]PSMA-11-PET/CT (B), [68Ga]PSMA-11-PET (C), and whole body MIP (D) images demonstrate increased tracer activity in several enlarged left internal mammary lymph nodes (arrow). (Images courtesy of Tom Hope, MD, University of California San Francisco).
New small molecular imaging PSMA ligands have been developed such as [18F]DCFBC, [18F]DCFPyl, and [68Ga]PSMA-11, which bind irreversibly to the extracellular component of PSMA and have been shown to improve detection of metastatic prostate cancer (63,64). The most commonly used PSMA ligands in Europe is [68Ga]PSMA-11. It is important to note that less than 10% of prostate cancers have no uptake on PSMA PET (65). In addition, the agent is rapidly cleared from non-target tissue. In the setting of biochemical recurrence, [68Ga]PSMA-11-PET/CT demonstrated higher tumor-to-background and higher detections rates than [18F]choline-PET/CT, particularly at lower PSA levels (66). A large retrospective study demonstrated that [68Ga]PSMA-11-PET/CT demonstrated uptake in 82% of patients with evidence of biochemical recurrence (67). Among the lesions that underwent surgical intervention or biopsy, 30 false-negative results were observed in a total of 4 patients with the rest of the lesion (n=416) classified as either true-positive or true-negative (67). Of note, this study also demonstrated a 50% detection rate in patients with serum PSA of less than 0.5 ng/mL, a significant improvement compared to the existing literature for choline and acetate and a finding that has been replicated in additional studies (67,68). A recent meta-analysis was performed by Perera et al., in which 16 articles including 1,309 patients were evaluated (69). When evaluating on a per-patient basis, the summary sensitivity and specificity were identical at 86% (69). When analyzed on a per-lesion basis, summary sensitivity was 80% and specificity was 97% (69).
A current limitation of widespread use of [68Ga]PSMA-11-PET/CT in the United States is that it is not currently approved by the FDA. Additionally, production of [68Ga]PSMA ligands currently requires an onsite generator for most sites as the radius of distribution of 68Ga-labeled compounds is limited by the relatively short half-life. However, research is currently ongoing regarding the development of PSMA analogues labeled with [18F]fluorine, which has potential to significantly reduce the cost and increase the availability of PSMA ligand through batch production and remote distribution (70). A potential future in targeted molecular imaging of metastatic prostate carcinoma is the use of PSMA for targeted therapy with alpha or beta-emitters {including [90Y] and [177Lu]}, currently being utilized in clinical trials for patients with biochemical recurrence, but not currently approved for use in the United States (71).
Emerging and experimental PET radiotracers for prostate cancer
Gastrin-releasing peptide receptor (GRPR) is a current investigational target for prostate cancer imaging (Figure 7). GRPR is overexpressed in prostate cancer cells, but demonstrates lower levels of expression in benign prostate tissue (72). Increases in GRPR expression have been shown to be present in 63–100% of intraprostatic prostate cancers and 50–85% of nodal and osseous metastases (73). To target and image GRPR overexpression, several peptide-based bombesin and gastrin-related peptide (GRP) analogs have been developed and labeled with a number of radioisotopes (74-76). A recent study examined the use of [68Ga]RM2-PET/CT (GRPR antagonist) in patients with known biochemical recurrence and negative or equivocal [18F]fluoroethylcholine-PET/CT and demonstrated that [68Ga]RM2-PET/CT was helpful in localizing the recurrence in a majority of the cases (77). An additional study examining the use of a 68Ga-labeled bombesin analog for PET/CT in patients with both initial diagnosis of prostate cancer and biochemical recurrence demonstrated a sensitivity of 88%, specificity of 81%, and accuracy of 83% for primary prostate cancer and 70% sensitivity for detection of metastatic lymph nodes (78). The same bombesin analog also correctly identified two of three cases of local recurrence both in the prostatectomy surgical bed and regional lymph nodes when compared to [11C]acetate-PET/CT (78). Similar to PSMA ligands, bombesin agents are being examined for their potential theranostic capabilities in delivering targeted radiation via alpha-emitters or beta-emitters (79).
Figure 7.
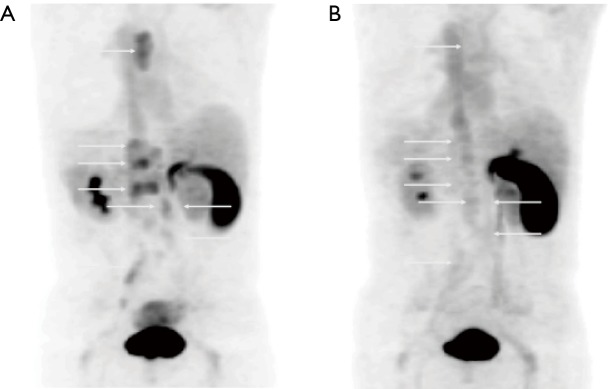
Whole body MIP (A), MR (B), [68Ga]RM2-PET (C), and fused [68Ga]RM2-PET/MRI images (D) in a 73-year-old patient with biochemical recurrence status post radiation therapy (PSA =1.32 ng/mL) demonstrate activity in the right seminal vesicle, suspicious for local recurrence (arrow). (Images courtesy of Andrei Iagaru, MD, Stanford University).
Therapies targeting androgen receptor signalling are a cornerstone of the treatment algorithm of prostate carcinoma, both for treatment-naïve patients and patients diagnosed with biochemical recurrence. Although not currently utilized in the clinical setting, PET imaging targeting the androgen receptor would therefore make sense in the setting of prostate cancer. The majority of preclinical and initial in-human work for PET imaging of androgen receptors has been with 16β-[18F]DFHT (Figure 8). An initial study examining the [18F]FDHT in patients with progressive metastatic prostate cancer demonstrated uptake is 46/59 lesions compared with [18F]FDG uptake in 57/59 (80). An additional pilot study demonstrated [18F]FDHT PET positivity in 63% of patients with advanced prostate cancer and demonstrated an additional 17 unsuspected lesions in a total of 10 patients (81). Interestingly, when the patients underwent a repeat [18F]FDHT-PET/CT one day following the initiation of flutamide, there was an immediate decrease in the radiotracer activity of greater than 50% that suggests [18F]FDHT-PET may be useful in tracking response to androgen deprivation therapy (81). This finding was later replicated in a phase 1/2 study evaluating the use of [18F]FDHT-PET to assess androgen blockade in patients with castration-resistant prostate cancer (82). The optimal use of androgen receptor PET imaging in prostate cancer remains to be established, but the evidence is promising that certain niche applications of [18F]FDHT-PET may emerge in the future.
Figure 8.
[18F]FDHT-PET images of a patient with biochemically recurrent metastatic prostate cancer (PSA =62 ng/mL) before (A) and after (B) initiation of flutamide demonstrate osseous and lymph node metastases (arrows) and subsequent treatment response. (Images courtesy of Farrokh Dehdashti, MD, Washington University in St. Louis).
PET/CT vs. PET/MRI for prostate cancer
PET/MRI scanners are being implemented throughout the world for routine clinical applications with increasing frequency. These scanners are capable of acquiring PET and MRI data simultaneously with the potential for more accurate image co-registration. In the setting of biochemical recurrence following both prostatectomy or definitive radiation therapy, MRI of the pelvis remains a cornerstone of evaluation for potential sites of local recurrence owing to its superior soft tissue contrast. Given the development of multiple PET radiotracers that show excellent sensitivity and specificity for recurrent prostate cancer {e.g., [8F]fluciclovine, [68Ga]PSMA-11}, it is logical that PET/MRI may become the optimal imaging modality for patients with biochemically recurrent prostate cancer. Several studies have shown a high detection rate of PET/MRI for pelvic recurrence in the setting of biochemical recurrence (83,84). As PET/MRI scanners become increasingly common, further research is needed to demonstrate the added value of PET/MRI both in the pretreatment and posttreatment settings.
Conclusions
Molecular imaging of prostate cancer continues to evolve as new radiotracers are studied and put into clinical practice. The use of both PET/CT and PET/MRI are an important diagnostic consideration for patients with biochemically recurrent prostate cancer, particularly if locoregional therapy is being considered. As newer PET radiotracers become approved, it will be important for larger prospective head-to-head studies to be performed if an optimal molecular imaging algorithm is to be developed. At this point in time, it appears that both [18F]fluciclovine and PSMA tracers are superior to the older PET tracers, but little existing data exists directly comparing the two. Given the existing research in theranostics with both PSMA and GRPR tracers, these imaging agents may be the optimal agent if consideration is being given to targeted molecular therapy with alpha-emitters or beta-emitters.
Acknowledgements
None.
Footnotes
Conflicts of Interest: Drs. Galgano and McConathy receive research support from Blue Earth Diagnostics. Dr. Valentin has no conflicts of interest to declare.
References
- 1.Global Burden of Disease Cancer Collaboration , Fitzmaurice C, Allen C, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2017;3:524-48. 10.1001/jamaoncol.2016.5688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer Version 2.2018. June 3, 2018; Available online: https://www.nccn.org/professionals/physician_gls/PDF/prostate.pdf
- 3.Roehl KA, Han M, Ramos CG, et al. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol 2004;172:910-4. 10.1097/01.ju.0000134888.22332.bb [DOI] [PubMed] [Google Scholar]
- 4.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA 2005;294:433-9. 10.1001/jama.294.4.433 [DOI] [PubMed] [Google Scholar]
- 5.Kupelian PA, Mahadevan A, Reddy CA, et al. Use of different definitions of biochemical failure after external beam radiotherapy changes conclusions about relative treatment efficacy for localized prostate cancer. Urology 2006;68:593-8. 10.1016/j.urology.2006.03.075 [DOI] [PubMed] [Google Scholar]
- 6.Cookson MS, Aus G, Burnett AL, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol 2007;177:540-5. 10.1016/j.juro.2006.10.097 [DOI] [PubMed] [Google Scholar]
- 7.Roach M, 3rd, Hanks G, Thames H, Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 2006;65:965-74. 10.1016/j.ijrobp.2006.04.029 [DOI] [PubMed] [Google Scholar]
- 8.Hövels AM, Heesakkers RA, Adang EM, et al. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol 2008;63:387-95. 10.1016/j.crad.2007.05.022 [DOI] [PubMed] [Google Scholar]
- 9.Jager GJ, Barentsz JO, Oosterhof GO, et al. Pelvic adenopathy in prostatic and urinary bladder carcinoma: MR imaging with a three-dimensional TI-weighted magnetization-prepared-rapid gradient-echo sequence. AJR Am J Roentgenol 1996;167:1503-7. 10.2214/ajr.167.6.8956585 [DOI] [PubMed] [Google Scholar]
- 10.Davis GL. Sensitivity of frozen section examination of pelvic lymph nodes for metastatic prostate carcinoma. Cancer 1995;76:661-8. [DOI] [PubMed] [Google Scholar]
- 11.Fortuin A, Rooij Md, Zamecnik P, et al. Molecular and functional imaging for detection of lymph node metastases in prostate cancer. Int J Mol Sci 2013;14:13842-75. 10.3390/ijms140713842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grubmüller B, Baltzer P, D'Andrea D, et al. (68)Ga-PSMA 11 ligand PET imaging in patients with biochemical recurrence after radical prostatectomy - diagnostic performance and impact on therapeutic decision-making. Eur J Nucl Med Mol Imaging 2018;45:235-42. 10.1007/s00259-017-3858-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanli Y, Kuyumcu S, Sanli O, et al. Relationships between serum PSA levels, Gleason scores and results of 68Ga-PSMAPET/CT in patients with recurrent prostate cancer. Ann Nucl Med 2017;31:709-17. 10.1007/s12149-017-1207-y [DOI] [PubMed] [Google Scholar]
- 14.Almeida FD, Yen CK, Scholz MC, et al. Performance characteristics and relationship of PSA value/kinetics on carbon-11 acetate PET/CT imaging in biochemical relapse of prostate cancer. Am J Nucl Med Mol Imaging 2017;7:1-11. [PMC free article] [PubMed] [Google Scholar]
- 15.Evangelista L, Cimitan M, Hodolič M, et al. The ability of 18F-choline PET/CT to identify local recurrence of prostate cancer. Abdom Imaging 2015;40:3230-7. 10.1007/s00261-015-0547-0 [DOI] [PubMed] [Google Scholar]
- 16.Kairemo K, Rasulova N, Partanen K, et al. Preliminary clinical experience of trans-1-Amino-3-(18)F-fluorocyclobutanecarboxylic Acid (anti-(18)F-FACBC) PET/CT imaging in prostate cancer patients. Biomed Res Int 2014;2014:305182. 10.1155/2014/305182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nanni C, Zanoni L, Pultrone C, et al. (18)F-FACBC (anti1-amino-3-(18)F-fluorocyclobutane-1-carboxylic acid) versus (11)C-choline PET/CT in prostate cancer relapse: results of a prospective trial. Eur J Nucl Med Mol Imaging 2016;43:1601-10. 10.1007/s00259-016-3329-1 [DOI] [PubMed] [Google Scholar]
- 18.Schwenck J, Rempp H, Reischl G, et al. Comparison of (68)Ga-labelled PSMA-11 and (11)C-choline in the detection of prostate cancer metastases by PET/CT. Eur J Nucl Med Mol Imaging 2017;44:92-101. 10.1007/s00259-016-3490-6 [DOI] [PubMed] [Google Scholar]
- 19.Calais J, Fendler WP, Herrmann K, et al. Comparison of (68)Ga-PSMA-11 and (18)F-Fluciclovine PET/CT in a Case Series of 10 Patients with Prostate Cancer Recurrence. J Nucl Med 2018;59:789-94. 10.2967/jnumed.117.203257 [DOI] [PubMed] [Google Scholar]
- 20.Effert P, Beniers AJ, Tamimi Y, et al. Expression of glucose transporter 1 (Glut-1) in cell lines and clinical specimens from human prostate adenocarcinoma. Anticancer Res 2004;24:3057-63. [PubMed] [Google Scholar]
- 21.Jadvar H. Is There Use for FDG-PET in Prostate Cancer? Semin Nucl Med 2016;46:502-6. 10.1053/j.semnuclmed.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shreve PD, Grossman HB, Gross MD, et al. Metastatic prostate cancer: initial findings of PET with 2-deoxy-2-[F-18]fluoro-D-glucose. Radiology 1996;199:751-6. 10.1148/radiology.199.3.8638000 [DOI] [PubMed] [Google Scholar]
- 23.Jadvar H. Imaging evaluation of prostate cancer with 18F-fluorodeoxyglucose PET/CT: utility and limitations. Eur J Nucl Med Mol Imaging 2013;40 Suppl 1:S5-10. 10.1007/s00259-013-2361-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Segall G, Delbeke D, Stabin MG, et al. SNM practice guideline for sodium 18F-fluoride PET/CT bone scans 1.0. J Nucl Med 2010;51:1813-20. Erratum in: J Nucl Med 2011;52:495. 10.2967/jnumed.110.082263 [DOI] [PubMed] [Google Scholar]
- 25.Even-Sapir E, Metser U, Mishani E, et al. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP Planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J Nucl Med 2006;47:287-97. [PubMed] [Google Scholar]
- 26.Apolo AB, Lindenberg L, Shih JH, et al. Prospective Study Evaluating Na18F PET/CT in Predicting Clinical Outcomes and Survival in Advanced Prostate Cancer. J Nucl Med 2016;57:886-92. 10.2967/jnumed.115.166512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jadvar H, Desai B, Ji L, et al. Prospective evaluation of 18F-NaF and 18F-FDG PET/CT in detection of occult metastatic disease in biochemical recurrence of prostate cancer. Clin Nucl Med 2012;37:637-43. 10.1097/RLU.0b013e318252d829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hillner BE, Siegel BA, Hanna L, et al. Impact of 18F-fluoride PET in patients with known prostate cancer: initial results from the National Oncologic PET Registry. J Nucl Med 2014;55:574-81. 10.2967/jnumed.113.130005 [DOI] [PubMed] [Google Scholar]
- 29.Zeisel SH. Dietary choline: biochemistry, physiology, and pharmacology. Annu Rev Nutr 1981;1:95-121. 10.1146/annurev.nu.01.070181.000523 [DOI] [PubMed] [Google Scholar]
- 30.Ackerstaff E, Glunde K, Bhujwalla ZM. Choline phospholipid metabolism: a target in cancer cells? J Cell Biochem 2003;90:525-33. 10.1002/jcb.10659 [DOI] [PubMed] [Google Scholar]
- 31.von Eyben FE, Kairemo K. Meta-analysis of (11)C-choline and (18)F-choline PET/CT for management of patients with prostate cancer. Nucl Med Commun 2014;35:221-30. 10.1097/MNM.0000000000000040 [DOI] [PubMed] [Google Scholar]
- 32.Fanti S, Minozzi S, Castellucci P, et al. PET/CT with (11)C-choline for evaluation of prostate cancer patients with biochemical recurrence: meta-analysis and critical review of available data. Eur J Nucl Med Mol Imaging 2016;43:55-69. 10.1007/s00259-015-3202-7 [DOI] [PubMed] [Google Scholar]
- 33.Evangelista L, Zattoni F, Guttilla A, et al. Choline PET or PET/CT and biochemical relapse of prostate cancer: a systematic review and meta-analysis. Clin Nucl Med 2013;38:305-14. 10.1097/RLU.0b013e3182867f3c [DOI] [PubMed] [Google Scholar]
- 34.Umbehr MH, Müntener M, Hany T, et al. The role of 11C-choline and 18F-fluorocholine positron emission tomography (PET) and PET/CT in prostate cancer: a systematic review and meta-analysis. Eur Urol 2013;64:106-17. 10.1016/j.eururo.2013.04.019 [DOI] [PubMed] [Google Scholar]
- 35.Shen G, Deng H, Hu S, et al. Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a meta-analysis. Skeletal Radiol 2014;43:1503-13. 10.1007/s00256-014-1903-9 [DOI] [PubMed] [Google Scholar]
- 36.Fortuin AS, Deserno WM, Meijer HJ, et al. Value of PET/CT and MR lymphography in treatment of prostate cancer patients with lymph node metastases. Int J Radiat Oncol Biol Phys 2012;84:712-8. 10.1016/j.ijrobp.2011.12.093 [DOI] [PubMed] [Google Scholar]
- 37.Kitajima K, Murphy RC, Nathan MA, et al. Detection of recurrent prostate cancer after radical prostatectomy: comparison of 11C-choline PET/CT with pelvic multiparametric MR imaging with endorectal coil. J Nucl Med 2014;55:223-32. 10.2967/jnumed.113.123018 [DOI] [PubMed] [Google Scholar]
- 38.Schuster DM, Nanni C, Fanti S. Evaluation of Prostate Cancer with Radiolabeled Amino Acid Analogs. J Nucl Med 2016;57:61S-6S. 10.2967/jnumed.115.170209 [DOI] [PubMed] [Google Scholar]
- 39.Segawa A, Nagamori S, Kanai Y, et al. L-type amino acid transporter 1 expression is highly correlated with Gleason score in prostate cancer. Mol Clin Oncol 2013;1:274-80. 10.3892/mco.2012.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oka S, Okudaira H, Ono M, et al. Differences in transport mechanisms of trans-1-amino-3-[18F]fluorocyclobutanecarboxylic acid in inflammation, prostate cancer, and glioma cells: comparison with L-[methyl-11C]methionine and 2-deoxy-2-[18F]fluoro-D-glucose. Mol Imaging Biol 2014;16:322-9. 10.1007/s11307-013-0693-0 [DOI] [PubMed] [Google Scholar]
- 41.McParland BJ, Wall A, Johansson S, et al. The clinical safety, biodistribution and internal radiation dosimetry of [18F]fluciclovine in healthy adult volunteers. Eur J Nucl Med Mol Imaging 2013;40:1256-64. 10.1007/s00259-013-2403-1 [DOI] [PubMed] [Google Scholar]
- 42.Nye JA, Schuster DM, Yu W, et al. Biodistribution and radiation dosimetry of the synthetic nonmetabolized amino acid analogue anti-18F-FACBC in humans. J Nucl Med 2007;48:1017-20. Erratum in: J Nucl Med 2016;57:804. 10.2967/jnumed.107.040097 [DOI] [PubMed] [Google Scholar]
- 43.Turkbey B, Mena E, Shih J, et al. Localized prostate cancer detection with 18F FACBC PET/CT: comparison with MR imaging and histopathologic analysis. Radiology 2014;270:849-56. 10.1148/radiol.13130240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elschot M, Selnæs KM, Sandsmark E, et al. A PET/MRI study towards finding the optimal [(18)F]Fluciclovine PET protocol for detection and characterisation of primary prostate cancer. Eur J Nucl Med Mol Imaging 2017;44:695-703. 10.1007/s00259-016-3562-7 [DOI] [PubMed] [Google Scholar]
- 45.Schuster DM, Votaw JR, Nieh PT, et al. Initial experience with the radiotracer anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid with PET/CT in prostate carcinoma. J Nucl Med 2007;48:56-63. [PubMed] [Google Scholar]
- 46.Bach-Gansmo T, Nanni C, Nieh PT, et al. Multisite Experience of the Safety, Detection Rate and Diagnostic Performance of Fluciclovine ((18)F) Positron Emission Tomography/Computerized Tomography Imaging in the Staging of Biochemically Recurrent Prostate Cancer. J Urol 2017;197:676-83. 10.1016/j.juro.2016.09.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suzuki H, Inoue Y, Fujimoto H, et al. Diagnostic performance and safety of NMK36 (trans-1-amino-3-[18F] fluorocyclobutanecarboxylic acid)-PET/CT in primary prostate pancer: multicenter Phase IIb clinical trial. Jpn J Clin Oncol 2017;47:283. 10.1093/jjco/hyw177 [DOI] [PubMed] [Google Scholar]
- 48.Schuster DM, Nieh PT, Jani AB, et al. Anti-3-[(18)F]FACBC positron emission tomography-computerized tomography and (111)In-capromab pendetide single photon emission computerized tomography-computerized tomography for recurrent prostate carcinoma: results of a prospective clinical trial. J Urol 2014;191:1446-53. 10.1016/j.juro.2013.10.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Odewole OA, Tade FI, Nieh PT, et al. Recurrent prostate cancer detection with anti-3-[(18)F]FACBC PET/CT: comparison with CT. Eur J Nucl Med Mol Imaging 2016;43:1773-83. 10.1007/s00259-016-3383-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ren J, Yuan L, Wen G, et al. The value of anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid PET/CT in the diagnosis of recurrent prostate carcinoma: a meta-analysis. Acta Radiol 2016;57:487-93. 10.1177/0284185115581541 [DOI] [PubMed] [Google Scholar]
- 51.Akin-Akintayo OO, Jani AB, Odewole O, et al. Change in Salvage Radiotherapy Management Based on Guidance With FACBC (Fluciclovine) PET/CT in Postprostatectomy Recurrent Prostate Cancer. Clin Nucl Med 2017;42:e22-8. 10.1097/RLU.0000000000001379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vāvere AL, Kridel SJ, Wheeler FB, et al. 1-11C-acetate as a PET radiopharmaceutical for imaging fatty acid synthase expression in prostate cancer. J Nucl Med 2008;49:327-34. 10.2967/jnumed.107.046672 [DOI] [PubMed] [Google Scholar]
- 53.Brogsitter C, Zöphel K, Kotzerke J. 18F-Choline, 11C-choline and 11C-acetate PET/CT: comparative analysis for imaging prostate cancer patients. Eur J Nucl Med Mol Imaging 2013;40 Suppl 1:S18-27. 10.1007/s00259-013-2358-2 [DOI] [PubMed] [Google Scholar]
- 54.Kotzerke J, Volkmer BG, Neumaier B, et al. Carbon-11 acetate positron emission tomography can detect local recurrence of prostate cancer. Eur J Nucl Med Mol Imaging 2002;29:1380-4. 10.1007/s00259-002-0882-6 [DOI] [PubMed] [Google Scholar]
- 55.Vees H, Buchegger F, Albrecht S, et al. 18F-choline and/or 11C-acetate positron emission tomography: detection of residual or progressive subclinical disease at very low prostate-specific antigen values (<1 ng/mL) after radical prostatectomy. BJU Int 2007;99:1415-20. 10.1111/j.1464-410X.2007.06772.x [DOI] [PubMed] [Google Scholar]
- 56.Lamanna G, Tabouret-Viaud C, Rager O, et al. Long-term Results of a Comparative PET/CT and PET/MRI Study of 11C-Acetate and 18F-Fluorocholine for Restaging of Early Recurrent Prostate Cancer. Clin Nucl Med 2017;42:e242-6. 10.1097/RLU.0000000000001609 [DOI] [PubMed] [Google Scholar]
- 57.Giovacchini G, Picchio M, Coradeschi E, et al. Predictive factors of [(11)C]choline PET/CT in patients with biochemical failure after radical prostatectomy. Eur J Nucl Med Mol Imaging 2010;37:301-9. 10.1007/s00259-009-1253-3 [DOI] [PubMed] [Google Scholar]
- 58.Rinnab L, Simon J, Hautmann RE, et al. [(11)C]choline PET/CT in prostate cancer patients with biochemical recurrence after radical prostatectomy. World J Urol 2009;27:619-25. 10.1007/s00345-009-0371-7 [DOI] [PubMed] [Google Scholar]
- 59.Ghosh A, Heston WD. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem 2004;91:528-39. 10.1002/jcb.10661 [DOI] [PubMed] [Google Scholar]
- 60.Rosenthal SA, Haseman MK, Polascik TJ. Utility of capromab pendetide (ProstaScint) imaging in the management of prostate cancer. Tech Urol 2001;7:27-37. [PubMed] [Google Scholar]
- 61.Ponsky LE, Cherullo EE, Starkey R, et al. Evaluation of preoperative ProstaScint scans in the prediction of nodal disease. Prostate Cancer Prostatic Dis 2002;5:132-5. 10.1038/sj.pcan.4500570 [DOI] [PubMed] [Google Scholar]
- 62.Troyer JK, Beckett ML, Wright GL., Jr Location of prostate-specific membrane antigen in the LNCaP prostate carcinoma cell line. Prostate 1997;30:232-42. [DOI] [PubMed] [Google Scholar]
- 63.Rowe SP, Macura KJ, Ciarallo A, et al. Comparison of Prostate-Specific Membrane Antigen-Based 18F-DCFBC PET/CT to Conventional Imaging Modalities for Detection of Hormone-Naïve and Castration-Resistant Metastatic Prostate Cancer. J Nucl Med 2016;57:46-53. 10.2967/jnumed.115.163782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eder M, Eisenhut M, Babich J, et al. PSMA as a target for radiolabelled small molecules. Eur J Nucl Med Mol Imaging 2013;40:819-23. 10.1007/s00259-013-2374-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Budäus L, Leyh-Bannurah SR, Salomon G, et al. Initial Experience of (68)Ga-PSMA PET/CT Imaging in High-risk Prostate Cancer Patients Prior to Radical Prostatectomy. Eur Urol 2016;69:393-6. 10.1016/j.eururo.2015.06.010 [DOI] [PubMed] [Google Scholar]
- 66.Afshar-Oromieh A, Holland-Letz T, Giesel FL, et al. Diagnostic performance of (68)Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging. 2017. Aug;44(8):1258-1268. Epub 2017 May 12. Erratum in: Eur J Nucl Med Mol Imaging 2017;44:1781. 10.1007/s00259-017-3711-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Afshar-Oromieh A, Avtzi E, Giesel FL, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging 2015;42:197-209. 10.1007/s00259-014-2949-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eiber M, Maurer T, Souvatzoglou M, et al. Evaluation of Hybrid 68Ga-PSMA Ligand PET/CT in 248 Patients with Biochemical Recurrence After Radical Prostatectomy. J Nucl Med 2015;56:668-74. Erratum in: J Nucl Med 2016;57:1325. 10.2967/jnumed.115.154153 [DOI] [PubMed] [Google Scholar]
- 69.Perera M, Papa N, Christidis D, et al. Sensitivity, Specificity, and Predictors of Positive (68)Ga-Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol 2016;70:926-37. 10.1016/j.eururo.2016.06.021 [DOI] [PubMed] [Google Scholar]
- 70.Giesel FL, Hadaschik B, Cardinale J, et al. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur J Nucl Med Mol Imaging 2017;44:678-88. 10.1007/s00259-016-3573-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rahbar K, Ahmadzadehfar H, Kratochwil C, et al. German Multicenter Study Investigating 177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J Nucl Med 2017;58:85-90. 10.2967/jnumed.116.183194 [DOI] [PubMed] [Google Scholar]
- 72.Wibmer AG, Burger IA, Sala E, et al. Molecular Imaging of Prostate Cancer. Radiographics 2016;36:142-59. 10.1148/rg.2016150059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mansi R, Fleischmann A, Mäcke HR, et al. Targeting GRPR in urological cancers--from basic research to clinical application. Nat Rev Urol 2013;10:235-44. 10.1038/nrurol.2013.42 [DOI] [PubMed] [Google Scholar]
- 74.Dijkgraaf I, Franssen GM, McBride WJ, et al. PET of tumors expressing gastrin-releasing peptide receptor with an 18F-labeled bombesin analog. J Nucl Med 2012;53:947-52. 10.2967/jnumed.111.100891 [DOI] [PubMed] [Google Scholar]
- 75.Mansour N, Paquette M, Ait-Mohand S, et al. Evaluation of a novel GRPR antagonist for prostate cancer PET imaging: [(64)Cu]-DOTHA(2)-PEG-RM26. Nucl Med Biol 2018;56:31-8. 10.1016/j.nucmedbio.2017.10.006 [DOI] [PubMed] [Google Scholar]
- 76.Mitran B, Thisgaard H, Rosenström U, et al. High Contrast PET Imaging of GRPR Expression in Prostate Cancer Using Cobalt-Labeled Bombesin Antagonist RM26. Contrast Media Mol Imaging 2017;2017:6873684. 10.1155/2017/6873684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wieser G, Popp I, Christian Rischke H, et al. Diagnosis of recurrent prostate cancer with PET/CT imaging using the gastrin-releasing peptide receptor antagonist (68)Ga-RM2: Preliminary results in patients with negative or inconclusive [(18)F]Fluoroethylcholine-PET/CT. Eur J Nucl Med Mol Imaging 2017;44:1463-72. 10.1007/s00259-017-3702-8 [DOI] [PubMed] [Google Scholar]
- 78.Kähkönen E, Jambor I, Kemppainen J, et al. In vivo imaging of prostate cancer using [68Ga]-labeled bombesin analog BAY86-7548. Clin Cancer Res 2013;19:5434-43. 10.1158/1078-0432.CCR-12-3490 [DOI] [PubMed] [Google Scholar]
- 79.Chatalic KL, Konijnenberg M, Nonnekens J, et al. In Vivo Stabilization of a Gastrin-Releasing Peptide Receptor Antagonist Enhances PET Imaging and Radionuclide Therapy of Prostate Cancer in Preclinical Studies. Theranostics 2016;6:104-17. 10.7150/thno.13580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Larson SM, Morris M, Gunther I, et al. Tumor localization of 16beta-18F-fluoro-5alpha-dihydrotestosterone versus 18F-FDG in patients with progressive, metastatic prostate cancer. J Nucl Med 2004;45:366-73. [PubMed] [Google Scholar]
- 81.Dehdashti F, Picus J, Michalski JM, et al. Positron tomographic assessment of androgen receptors in prostatic carcinoma. Eur J Nucl Med Mol Imaging 2005;32:344-50. 10.1007/s00259-005-1764-5 [DOI] [PubMed] [Google Scholar]
- 82.Scher HI, Beer TM, Higano CS, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet 2010;375:1437-46. 10.1016/S0140-6736(10)60172-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kranzbühler B, Nagel H, Becker AS, et al. Clinical performance of (68)Ga-PSMA-11 PET/MRI for the detection of recurrent prostate cancer following radical prostatectomy. Eur J Nucl Med Mol Imaging 2018;45:20-30. 10.1007/s00259-017-3850-x [DOI] [PubMed] [Google Scholar]
- 84.Freitag MT, Radtke JP, Afshar-Oromieh A, et al. Local recurrence of prostate cancer after radical prostatectomy is at risk to be missed in (68)Ga-PSMA-11-PET of PET/CT and PET/MRI: comparison with mpMRI integrated in simultaneous PET/MRI. Eur J Nucl Med Mol Imaging 2017;44:776-87. 10.1007/s00259-016-3594-z [DOI] [PubMed] [Google Scholar]