Abstract
Purpose
Systemic hypertension is a risk factor of neovascular age-related macular degeneration; consumption of dietary salt resulting in extracellular hyperosmolarity is a main cause of hypertension. Extracellular hyperosmolarity was shown to induce expression of angiogenic growth factors, such as vascular endothelial growth factor (VEGF) and placental growth factor (PlGF), in RPE cells. The aim of the present study was to determine whether the hyperosmotic expression of growth factor genes in RPE cells is mediated by activator protein-1 (AP-1), and whether c-Fos and c-Jun genes are regulated by extracellular osmolarity.
Methods
Hyperosmotic media were made up with the addition of NaCl or sucrose. Gene expression was quantified with real-time reverse transcription (RT)–PCR, and protein secretion was investigated with enzyme-linked immunosorbent assay (ELISA). Nuclear factor of activated T cell 5 (NFAT5) was depleted with siRNA. DNA binding of AP-1 protein was evaluated with electrophoretic mobility shift assay (EMSA).
Results
High NaCl and the addition of sucrose triggered expression of the c-Fos gene, but not of the c-Jun gene. High NaCl also increased the levels of c-Fos and phosphorylated c-Jun proteins and the level of DNA binding of AP-1. Hypoosmolarity decreased the expression of the c-Fos and c-Jun genes. NaCl-induced expression of the c-Fos gene was in part mediated by NFAT5. Autocrine/paracrine activation of fibroblast growth factor and adenosine A1 receptors is involved in mediating NaCl-induced expression of the c-Fos gene. Pharmacological inhibition of the AP-1 activity decreased the NaCl-induced expression of the HIF-1α, NFAT5, VEGF, PlGF, and TGF-β2 genes, and prevented the NaCl-induced secretion of PlGF but not of VEGF.
Conclusions
The data indicate that AP-1 is activated in RPE cells in response to extracellular hyperosmolarity and mediates in part via the NaCl-induced expression of VEGF and PlGF, and secretion of PlGF. It is suggested that high consumption of dietary salt may exacerbate the angiogenic response of RPE cells in part via activation of AP-1.
Introduction
Age-related macular degeneration (AMD) is the leading cause of visual impairment in developed countries [1]. The early stage of AMD is characterized by focal hyperpigmentation of the RPE, drusen deposition beneath the RPE, and slow degeneration of photoreceptors and RPE. The end stages of AMD are geographic atrophy (dry AMD) and choroidal neovascularization (wet AMD). Choroidal neovascularization is promoted by angiogenic growth factors, such as vascular endothelial growth factor (VEGF) and placental growth factor (PlGF) [2,3], which are produced, for example, in RPE cells [4,5].
AMD is a multifactorial disease; various different factors, including age, race, genetic variability, and lifestyle conditions (like sunlight exposure, cigarette smoking, and nutrition), are associated with the risk of AMD [6,7]. In addition, systemic hypertension is associated with the risk of AMD. This association has been documented in various population-based studies [8-11]. Some studies described an association between hypertension and neovascular AMD [12-16]. Cardiovascular disease and AMD share common risk factors, such as hypertension, atherosclerosis, systemic markers of inflammation, cigarette smoking, hyperlipidemia, and obesity [11,14,17,18].
The main cause of acute hypertension is the increase in the extracellular osmolarity following the intake of dietary salt [19,20]. Because the use of antihypertensive medication is not associated with the risk of AMD [12,21], it has been suggested that conditions that cause hypertension, such as high extracellular osmolarity and elevated extracellular salt (NaCl) levels, rather than hypertension per se may aggravate neovascular AMD [22]. It has been described that these conditions induce expression and secretion of angiogenic factors, like VEGF and PlGF, in RPE cells [5,22]. The NaCl-induced production of angiogenic factors was suggested to contribute to the development of neovascular AMD [23].
It has been shown that hyperosmotic stress induces expression of various transcription factors in RPE cells, including hypoxia-inducible transcription factor (HIF)-1α, nuclear factor (NF)-κB, and nuclear factor of activated T cell 5 (NFAT5) [22,24]. The hyperosmotic transcription of the VEGF (Gene ID 7422; OMIM 192240) and PlGF2 (Gene ID 5228; OMIM 601121) genes in RPE cells was shown to be partially induced by NFAT5; in addition, HIF activity participates to the hyperosmotic expression of the VEGF gene [5,22]. However, it is not known whether additional transcription factors, like activator protein-1 (AP-1), contribute to the osmotic induction of angiogenic growth factor expression in RPE cells, and whether the expression of c-Fos and c-Jun, which are components of AP-1 [25], depends upon extracellular osmolarity. The AP-1 family of transcription factors consists of homodimers and heterodimers of Fos (c-Fos, FosB, Fral, and Fra2), Jun (c-Jun, JunB, and JunD), activating transcription factor (ATF2, ATF3, and B-ATF), and JDP (JDP-1 and JDP-2) family members [26]. In the present study, we investigated the regulation of the expression of the c-Fos and c-Jun genes in response to osmotic stress and other pathogenic factors (like hypoxia and high extracellular glucose) which are known to be involved in mediating the pathogenesis of age-related retinal diseases [23,27]. In addition, we investigated the intra- and extracellular signaling pathways that mediate the hyperosmotic expression of the c-Fos gene (Gene ID, 2353; OMIM 164810), and the effects of AP-1 inhibition on the hyperosmotic expression of angiogenic growth factors and additional genes that are known to be regulated by extracellular osmolarity.
Methods
Human material
The study followed the tenets of Declaration of Helsinki for the use of human subjects and the ARVO statement on human subjects. The use of human material was approved by the Ethics Committee of the University of Leipzig (approval #745, 07/25/2011). Eyes were obtained from post-mortem cornea donors without reported eye disease within 48 h of death. Written informed consent for the use of retinal cells in basic research was obtained from the relatives of all donors.
Materials
All cell culture materials were purchased from Gibco BRL (Paisley, UK). Recombinant human basic fibroblast growth factor (bFGF), heparin-binding epidermal growth factor-like growth factor (HB-EGF), hepatocyte growth factor (HGF), interleukin-1β (IL-1β), IL-1 receptor antagonist, platelet-derived growth factor-BB (PDGF), transforming growth factor-β1 (TGF-β1), tumor necrosis factor-α (TNFα), and VEGF-A165 were obtained from R&D Systems (Abingdon, UK). Recombinant human PlGF-2 was obtained from Reliatech (Braunschweig, Germany). Cyclosporin A, 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), Gö6976, H-89, HIF inhibitor, LY294002, PD150606, PD98059, PP2, SP600125, SU1498, and U73122 were obtained from Calbiochem (Bad Soden, Germany). The following compounds were purchased from Tocris (Ellisville, MO): 666–15, A-438079, AR-C 118925XX, ARL-67156, caffeic acid phenethyl ester (CAPE), 8-(3-chlorostyryl) caffeine (CSC), GSK650394, MRS2179, SB203580, SR11302, the pannexin-blocking peptide 10panx, and the scrambled control peptide 10panxScr. Ac-YVAD-CMK, AG1478, and Stattic were from Enzo Life Science (Lausen, Switzerland). Dithiothreitol was obtained from Carl Roth (Karlsruhe, Germany), and PD173074 was kindly provided by Pfizer (Karlsruhe, Germany). Human-specific siRNA against NFAT5 and non-targeted control siRNA were obtained from Santa Cruz Biotechnology (Heidelberg, Germany). AG1296, adenosine-5′-O-(α,β-methylene)-diphosphate (AOPCP), apyrase, indomethacin, N-acetyl-L-cysteine, N-nitrobenzylthioinosine (NBTI), 1,10-phenanthroline, ruthenium red, SB431542, and all other agents used were from Sigma-Aldrich (Taufkirchen, Germany), unless stated otherwise.
The following antibodies were used: a rabbit anti-human β-actin (1:1,000; Cell Signaling, Frankfurt, Germany), a rabbit anti-c-Fos (1:1,000; Cell Signaling), a rabbit anti-c-Jun (1:1,000; Cell Signaling), a rabbit anti-phosphorylated c-Fos (1:1,000; ThermoFisher Scientific, Waltham, MA), a rabbit anti-phosphorylated c-Jun (1:1,000; Cell Signaling), a rabbit anti-cAMP response element-binding protein (CREB; 1:1,000; Cell Signaling), a rabbit anti-histone H3 (1:1,000; Cell Signaling), and anti-rabbit immunoglobulin G (IgG) conjugated with alkaline phosphatase (1:2,000; Cell Signaling).
Cell culture
The preparation and culture of RPE cells have been described previously [28]. After removing the vitreous and neural retina, RPE cells were mechanically harvested, separated by digestion with 0.05% trypsin and 0.02% ethylenediaminetetraacetic acid (EDTA), and washed two times with phosphate-buffered saline (PBS). The cells were suspended in complete Ham F-10 medium containing 10% fetal bovine serum, glutamax II, and gentamycin, and were cultured in laminin-coated T-75 tissue culture flasks (Greiner, Nürtingen, Germany) in 95% air/5% CO2 at 37 °C. Cell lines of passages 3–5 derived from a total of 34 Caucasian donors (mean ± standard deviation [SD] age, 76.4±11.3 years; 19 women, 15 men) were used; each line was used in three to ten different experiments. When a confluency of approximately 90% was achieved, the cells were cultured in serum-free medium for 16 h. Thereafter, test substances were added to the serum-free medium. Extracellular hyperosmolarity was induced with the addition of NaCl or sucrose to the culture medium. A decrease in the extracellular osmolarity to 60% of control was achieved with the addition of distilled water. Hypoxia was induced with the addition of the hypoxia mimetic CoCl2 (150 µM) or with cell culture in a 0.2% O2/5% CO2 atmosphere. Pharmacological inhibitors were preincubated for 30 min.
RNA extraction and cDNA synthesis
Total RNA was extracted with the InviTrap Spin Universal RNA Mini Kit (Stratec Molecular, Berlin, Germany). The A260/A280 ratio of the optical density of the RNA samples (measured with NanoDrop 1000; peQLab, Erlangen, Germany) was between 1.95 and 2.05, indicating adequate RNA quality. The RNA samples were treated with DNase I, and cDNA was synthesized from 0.5 µg RNA with a reverse transcription kit (ThermoFisher Scientific).
Real-time RT–PCR analysis
Real-time reverse transcription (RT)–PCR was performed with the MyiQ Single-Color Real-Time PCR Detection System (Bio-Rad, Munich, Germany). The primer sequences are given in Table 1. The amplification reaction mixture (15 μl) consisted of 7.5 μl of 2×iQ Sybr Green Supermix (Bio-Rad), a specific primer set (0.2 µM each), and 1 μl (1.25 ng) cDNA. The following protocol was used: one cycle of denaturation at 95 °C for 3 min, 45 cycles denaturation at 95 °C for 30 s, annealing at 58 °C for 20 s, extension at 72 °C for 45 s, and melting curve at 55 °C with the temperature gradually increased for 0.5 °C up to 95 °C. To prove the correct lengths of the PCR products, the samples were analyzed with agarose gel electrophoresis. RT–PCR for β-actin mRNA was used as an internal control. The results were analyzed with the 2-ΔΔCT method.
Table 1. Primer pairs used in PCR experiments.
| Gene and accession number | Primer sequences (5′→3′) | Product (bp) |
|---|---|---|
| ACTIN |
s ATGGCCACGGCTGCTTCCAGC |
237 |
|
NM_001101 |
as CATGGTGGTGCCGCCAGACAG |
|
| AQP5 |
s ACTGGGTTTTCTGGGTAGGG |
184 |
|
NM_001651.2 |
as GTGGTCAGCTCCATGGTCTT |
|
| AQP8 |
s TCCTGAGGAGAGGTTCTGGA |
156 |
|
NM_001169.2 |
as AGGGCCCTTTGTCTTCTCAT |
|
| AR (AKR1B1) |
s CCCATGTGTACCAGAATGAGAAT |
190 |
|
NM_001628.2 |
as AGGTAGAGGTCCAGGTAGTCCAG |
|
| COX2 |
s TGAGCATCTACGGTTTGCTG |
158 |
|
NM_000963 |
as TGCTTGTCTGGAACAACTGC |
|
| bFGF (FGF2) |
s AGAGCGACCCTCACATCAAG |
234 |
|
NM_002006 |
as ACTGCCCAGTTCGTTTCAGT |
|
| IL1B |
s TGGGCCTCAAGGAAAAGAATC |
358 |
|
NM_000576.2 |
as CTTTCTGTTCCCTTTCTGCCA |
|
| HBEGF |
s TGCCTGTAGCTTTCCTGGTCCC |
258 |
|
NM_001945 |
as CCCCACCTCCAACCTTCTCGG |
|
| HIF1A |
s CACAGAAATGGCCTTGTGAA |
214 |
|
NM_001530.3 |
as CCAAGCAGGTCATAGGTGGT |
|
| NFAT5 |
s TCACCATCATCTTCCCACCT |
174 |
|
NM_006599.3 |
as CTGCAATAGTGCATCGCTGT |
|
| PlGF2 |
s GGCGATGAGAATCTGCACTGT |
164 |
|
NM_001207012.1 |
as CACCTTTCCGGCTTCATCTTC |
|
| NLRP3 |
s AGACAGCATTGAAGAGGAGTGG |
169 |
|
NM_183395.2 |
as TTTGTTGAGGCTCACACTCTCA |
|
| TGFB2 |
s ACGTCTCAGCAATGGAGAAGA |
195 |
|
NM_001135599.2 |
as ATTCGCCTTCTGCTCTTGTTT |
|
| VEGFA188, 164, 120 |
s CCTGGTGGACATCTTCCAGGAGTA |
479; 407; |
|
NM_003376.5, NM_001287044.1, NM_001025370.2 |
as CTCACCGCCTCGGCTTGTCACA |
275 |
| CFOS |
s AGAATCCGAAGGGAAAGGAA |
150 |
|
NM_005252.3 |
as CTTCTCCTTCAGCAGGTTGG |
|
| CJUN |
s CCCCAAGATCCTGAAACAGA |
168 |
|
NM_002228.3 |
as CCGTTGCTGGACTGGATTAT |
|
| JUNB |
s TGGAACAGCCCTTCTACCAC |
241 |
|
NM_002229.2 |
as GAAGAGGCGAGCTTGAGAGA |
|
| JUND |
s TTCTACTCGGGGAACAAACG |
213 |
|
NM_005354.5 |
as GGCGAACCAAGGATTACAAA |
|
| FOSB |
s GAGAAGGAACGTCTGGAGTTTGT |
227 |
|
NM_001114171.1 |
as GTAAAGAGAGAAGCCGTCAGGTT |
|
| FOSL1 |
s AAACTGGAAGATGAGAAATCTGG |
202 |
|
NM_001300857.1 |
as GGGAAAGGGAGATACAAGGTACA |
|
| FOSL2 |
s ACAGTGATCACCTCCATGTCC |
162 |
|
NM_005253.3 |
as AGACAGCTGCTCATCTCTCCTC |
|
| BATF |
s AGCGAAGACCTGGAGAAACA |
189 |
|
NM_006399.3 |
as GGAGCTGACATGAGGTTGGT |
|
| ATF2 |
s CCCACATCAGCTATTGTTCGT |
153 |
|
NM_001256094.1 |
as CACAATTGGCCTGTTAGAGGA |
|
| ATF3 |
s TTGAGGATTTTGCTAACCTGACG |
233 |
|
NM_001674.3 |
as CTTCTTCTTGTTTCGGCACTTTG |
|
| JDP1 |
s AGTTGCTGCTCAGAGAAGTCG |
167 |
|
NM_018664.2 |
as ACATCTTCTCGTGCTCCTTCA |
|
| JDP2 |
s TGACTGTGGAGGAGCTGAAATAC |
171 |
|
NM_001135049.1 |
as CTGCTGCGACTTTGTTCTTCT |
|
| CREB1 |
s AACAGAAGCTGAAAACCAACAAAT |
160 |
| NM_004379.4 | as ATGACTCCATGGACTTGAACTGT |
s, sense. as, anti-sense.
Western blot analysis
The cells were seeded at 5 × 105 cells per well in 6-well plates and were cultured in fetal bovine serum (10%)-containing F-10 medium. When a confluency of 80–90% was achieved, the cells were growth arrested for 16 h in serum-free medium. Thereafter, NaCl (+ 100 mM) or CoCl2 (150 µM) was added for a further 2, 4, or 6 h. After the medium was removed, the cells were washed twice with prechilled PBS (pH 7.4; Invitrogen, Paisley, UK, product number 20012-019) and scraped into 180 µl of lysis buffer (50 mM Tris-HCl, pH 8.0, 5 mM EDTA, 150 mM NaCl, 0.5% NP-40, 1% protease inhibitor cocktail, and 1% phosphatase inhibitor cocktail). The cell lysates were centrifuged at 20,124 ×g for 10 min. Equal amounts of cytosolic or nuclear protein (30 or 35 µg) were separated with 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE). Immunoreactive bands were probed with primary and secondary antibodies, and visualized using 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium.
ELISA
Cells were seeded at 3 × 103 cells per well in 12-well plates. After reaching a confluency of approximately 90%, the medium was changed to a serum-free medium for 16 h. NaCl (+ 100 mM) or CoCl2 (150 µM) was added to the serum-free medium for 24 h. The levels of VEGF-A165 and PlGF in the cultured media (100 µl) were quantified with enzyme-linked immunosorbent assay (ELISA; R&D Systems).
siRNA transfection
Cells were seeded at 7 × 104 cells per well in 12-well plates. When a confluency of 60‒80% was achieved, NFAT5 siRNA or non-targeted siRNA (5 nM each) was transfected into the cells with HiPerFect reagent (Qiagen, Hilden, Germany) in serum (10%)-containing F-10 medium. After 48 h, the cells were cultured for 2 h in serum-free iso- or hyperosmotic medium (+ 100 mM NaCl). After RNA was extracted, the levels of the c-Fos and NFAT5 mRNAs were evaluated with real-time RT–PCR.
Electrophoretic mobility shift assay
Electrophoretic mobility shift assay (EMSA) was performed with the DIG Gel Shift Kit 2nd Generation (Roche, Mannheim, Germany) according to the manufacturer’s instructions. The following oligonucleotides that contained AP-1 binding sites (italics) were used: 5′-CGC TTG ATG ACT CAG CCG GAA-3′ and 5′-TTC CGG CTG AGT CAT CAA GCG-3′. The following oligonucleotides that contained CREB binding sites (italics) were used: 5′-AGA GAT TGC CTG ACG TCA GAG AGC TAG-3′ and 5′-CTA GCT CTC TGA CGT CAG GCA ATC TCT-3′. The gel shift reaction was performed with 4 µg of nuclear extract in a total volume of 20 µl for 15 min at room temperature. To check the specificity of the DNA–protein interaction, unlabeled double-stranded oligonucleotides were used as the competitor in 200-fold excess. Electrophoresis was performed with a 6% non-denaturing PAGE; the separated oligonucleotide-protein complexes were transferred to a positively charged nylon membrane with electroblotting. DIG-labeled DNA fragments were detected with an anti-DIG antibody, and immunoreactive bands were visualized using 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium.
Statistical analysis
Each test involved at least three experiments using cell lines of different donors. Data are shown as means ± standard error of the mean (SEM). Statistical analysis was performed with Prism (GraphPad Software, San Diego, CA). Comparisons between groups were performed with one-way ANOVA followed by Bonferroni’s multiple comparison test and the Mann–Whitney U test. A p value of less than 0.05 was considered statistically significant.
Results
Regulation of the expression of c-Fos and c-Jun genes
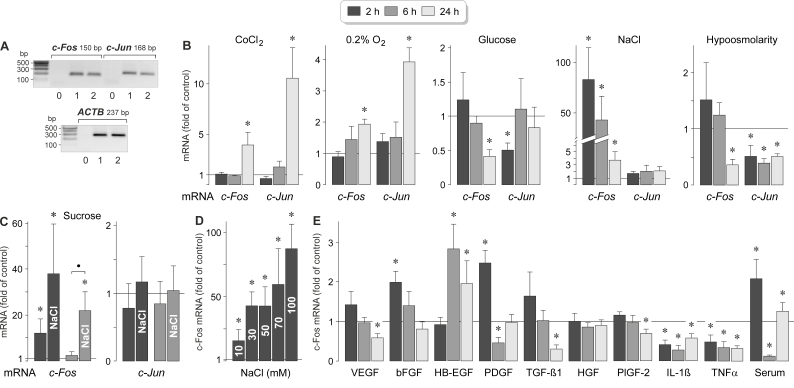
The transcription factor AP-1 is a heterodimer consisting of c-Fos and c-Jun [25]. Cultured human RPE cells expressed c-Fos and c-Jun genes (Figure 1A). To determine which pathogenic conditions induce expression of these genes, we stimulated RPE cells from different donors with the hypoxia mimetic CoCl2 [29], high (25 mM) glucose, and hyper- and hypoosmotic media. The gene expression levels were determined with real-time RT–PCR analysis. As shown in Figure 1B, CoCl2-induced chemical hypoxia induced statistically significant (p<0.05) increases in the expression of the c-Fos and c-Jun genes after 24 h of stimulation. Similar increases in the expression levels of both genes were found in cells that had been cultured in a low-O2 atmosphere (Figure 1B). In the presence of high glucose, the levels of c-Fos and c-Jun mRNAs were decreased after 24 and 2 h, respectively (Figure 1B). Extracellular hyperosmolarity was induced with the addition of 100 mM NaCl or 200 mM sucrose (which caused equal increases in the extracellular osmolarity). The addition of 100 mM NaCl to the culture medium induced a strong increase in the expression of the c-Fos gene after 2 and 6 h of stimulation while the expression of the c-Jun gene remained unaltered (Figure 1B). The addition of 200 mM sucrose induced a moderate increase in the expression of the c-Fos gene after 2 h of stimulation and had no effect on the expression of the c-Jun gene (Figure 1C). Coadministration of NaCl and sucrose induced higher expression of the c-Fos gene than the addition of sucrose alone; there was a statistically significant (p<0.05) difference in the c-Fos mRNA level between cells cultured under both conditions after 6 h of stimulation (Figure 1C). The data indicate that the NaCl-induced expression of the c-Fos gene is mainly mediated by the alteration of the transmembrane NaCl gradient and less by the elevation of the extracellular osmolarity. The effect of high NaCl on the c-Fos mRNA level was dose-dependent; a statistically significant (p<0.05) increase was already found when 10 mM NaCl was added to the culture medium (Figure 1D). A hypoosmotic medium decreased the c-Fos and c-Jun mRNA levels in RPE cells (Figure 1B).
Figure 1.
Regulation of the expression of the c-Fos and c-Jun genes in RPE cells. A: Presence of c-Fos and c-Jun gene transcripts in RPE cells. To confirm the correct lengths of the PCR products, agarose gel electrophoresis was performed using products obtained from two RPE cell lines (1, 2) derived from different post-mortem donors. Negative controls (0) were performed by adding double-distilled water instead of cDNA as template. The β-actin (ACTB) mRNA level was used to normalize the c-Fos and c-Jun mRNA levels. B–E: c-Fos and c-Jun mRNA levels, as determined with real-time reverse transcription (RT)–PCR analysis after stimulation of the cells for 2, 6, and 24 h (as indicated by the panels of the bars). The mRNA levels are expressed as folds of the unstimulated control. B: Effects of chemical hypoxia (induced with the addition of 150 µM CoCl2), culturing in a 0.2% O2 atmosphere, high (25 mM) glucose, extracellular hyperosmolarity induced with the addition of high (+ 100 mM) NaCl, and extracellular hypo-osmolarity (60% osmolarity) on the expression levels of the c-Fos and c-Jun genes. C: Effect of the addition of 200 mM sucrose (in the absence and presence of 100 mM NaCl) on the expression of the c-Fos and c-Jun genes. D: Dose-dependence of the effect of high extracellular NaCl on the c-Fos mRNA level. Ten to 100 mM NaCl were added to the culture medium, as indicated in the bars. E: Effects of inflammatory and growth factors on the expression of the c-Fos gene. The following factors were tested: vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), heparin-binding epidermal growth factor-like growth factor (HB-EGF), platelet-derived growth factor-BB (PDGF), transforming growth factor-β1 (TGF-β1), hepatocyte growth factor (HGF), placental growth factor-2 (PlGF-2), interleukin-1β (IL-1β), and tumor necrosis factor-α (TNFα). Each factor was applied at 10 ng/ml. In addition, fetal calf serum (10%) was tested. Each bar represents data obtained in three to ten independent experiments using cell lines from different donors. Significant difference versus unstimulated control: * p<0.05; ● p<0.05.
To determine whether the expression of the c-Fos gene in RPE cells is regulated by inflammatory and growth factors, we stimulated the cells with various cytokines and fetal calf serum, respectively. As shown in Figure 1E, exogenous bFGF and HB-EGF induced moderate increases in the c-Fos mRNA level after different time periods, PDGF and serum induced a biphasic regulation, and VEGF, TGF-β1, PlGF-2, IL-1β, and TNFα induced downregulation of the expression of the c-Fos gene after various time periods. The data indicate that the expression of the c-Fos gene in RPE cells is strongly induced by an elevation of the extracellular NaCl concentration while other factors, such as inflammatory and growth factors, induce moderate upregulation or downregulation of the expression of the c-Fos gene. In contrast, the expression of the c-Jun gene in RPE cells is not induced by high extracellular NaCl.
NaCl-induced expression of the genes of additional transcription factor proteins
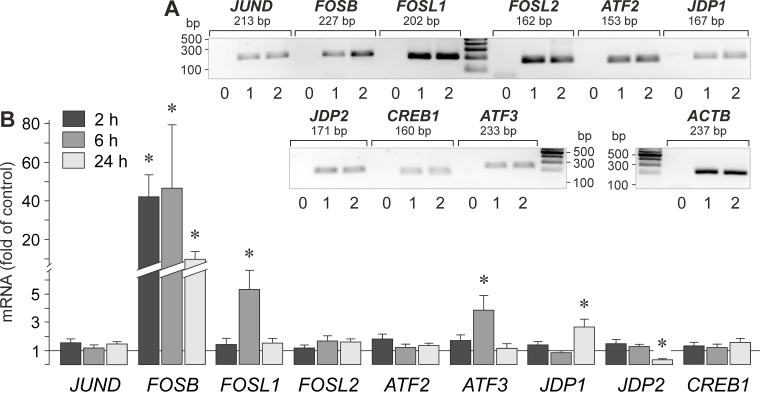
AP-1 is a heterodimer composed of proteins belonging to various protein families. We investigated whether the gene expression of additional proteins belonging to the Jun, Fos, ATF, JDP, and CREB protein families (Figure 2A) is altered by high extracellular osmolarity induced with the addition of 100 mM NaCl to the medium. As shown in Figure 2B, the addition of high NaCl induced a strong increase in the expression of FOSB (Gene ID 2354; OMIM 164772) while the expression of the following genes remained unaltered or was moderately altered: JUND (Gene ID 3727; OMIM 165162), FOSL1 (Gene ID 8061; OMIM 136515), FOSL2 (Gene ID 2355; OMIM 601575), ATF2 (Gene ID 1386; OMIM 123811), ATF3 (Gene ID 467; OMIM 603148), JDP1 (Gene ID 55509; OMIM 612470), JDP2 (Gene ID 122953; OMIM 608657), and CREB1 (Gene ID 1385; OMIM 123810). The levels of the JUNB and BATF transcripts were below the detection threshold of the RT–PCR analysis.
Figure 2.
High NaCl-induced gene expression of various members of the Jun, Fos, ATF, JDP, and CREB protein families. A: Presence of gene transcripts in RPE cells. To confirm the correct lengths of the PCR products, agarose gel electrophoresis was performed using products obtained from two RPE cell lines (1, 2) derived from different post-mortem donors. Negative controls (0) were performed by adding double-distilled water instead of cDNA as template. The β-actin (ACTB) mRNA level was used to normalize the c-Fos and c-Jun mRNA levels. B: Gene expression levels after the cells were stimulated for 2, 6, and 24 h (as indicated by the panels of the bars) with high (+ 100 mM) NaCl. mRNA levels were determined with real-time reverse transcription (RT)–PCR and are expressed as folds of the unstimulated control. Each bar represents data obtained in four independent experiments using cell lines from different donors. Significant difference versus unstimulated control: * p<0.05.
Regulation of the expression of c-Fos and c-Jun proteins
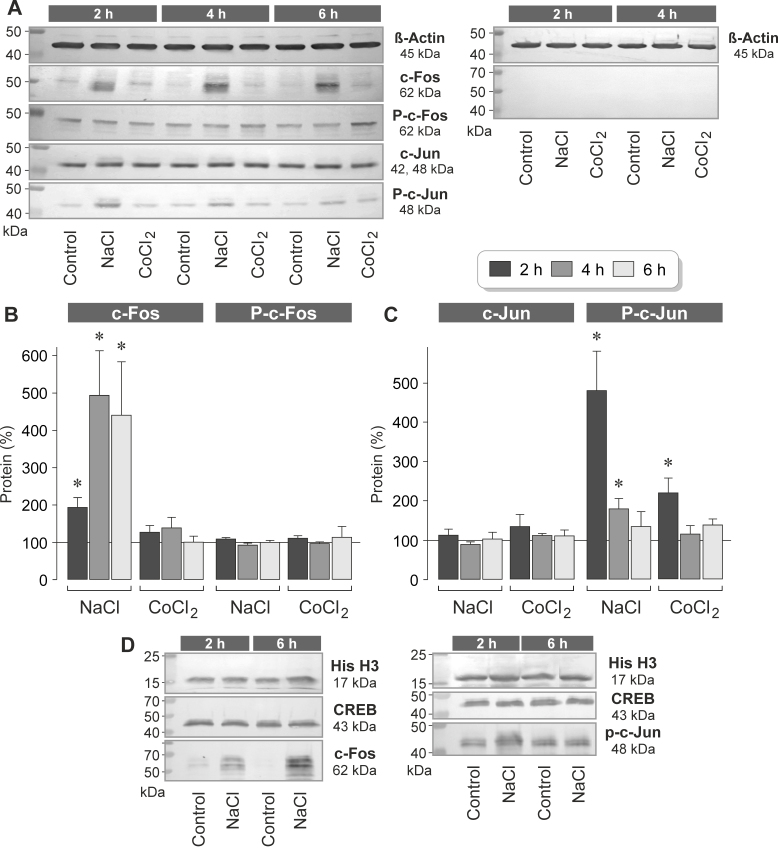
To determine whether NaCl-induced extracellular hyperosmolarity and CoCl2-induced chemical hypoxia induce alterations of the c-Fos and c-Jun protein levels in RPE cells, we determined the protein levels in cell lysates and nuclear extracts with western blot analysis. Under unstimulated control conditions, the cells contained a low level of c-Fos protein and a high level of c-Jun protein (Figure 3A), suggesting that c-Jun is constitutively produced by RPE cells. The addition of 100 mM NaCl to the culture medium induced a statistically significant (p<0.05) time-dependent increase in the cytosolic and nuclear levels of the c-Fos protein (Figure 3A,B,D). High NaCl had no effect on the cellular content of the c-Jun protein (Figure 3A,C). Chemical hypoxia did not alter the levels of the c-Fos and c-Jun proteins in RPE cells (Figure 3A–C).
Figure 3.
High extracellular NaCl induces elevation in the levels of c-Fos and phosphorylated c-Jun proteins in RPE cells. The cells were stimulated for 2, 4, and 6 h with high (+ 100 mM) NaCl or the hypoxia mimetic CoCl2 (150 µM). Protein levels were determined with western blot analysis of cell lysates (A–C) and nuclear extracts (D). A: Example of a western blot analysis in one cell line. Left: The levels of the following proteins were determined: β-actin, c-Fos, phosphorylated c-Fos (P-c-Fos), c-Jun, and phosphorylated c-Jun (P-c-Jun). Right: Negative control obtained with the omission of the first antibodies. B: Cytosolic levels of c-Fos (left) and phosphorylated c-Fos proteins (right), as determined with densitometric analysis of western blot data. C: Cytosolic levels of c-Jun (left) and phosphorylated c-Jun proteins (right). The data are normalized to the level of the β-actin protein and are expressed as a percentage of the unstimulated control (100%). Each bar represents data obtained in three to six independent experiments using cell lines from different donors. Significant difference versus unstimulated control: * p<0.05. D: Examples of western blot analysis of the nuclear extracts. The cells were stimulated for 2 and 6 h with high (+ 100 mM) NaCl. Note that the nuclear levels of histone H3 (His H3) and CREB proteins did not change in response to high NaCl.
Regulation of AP-1 activity
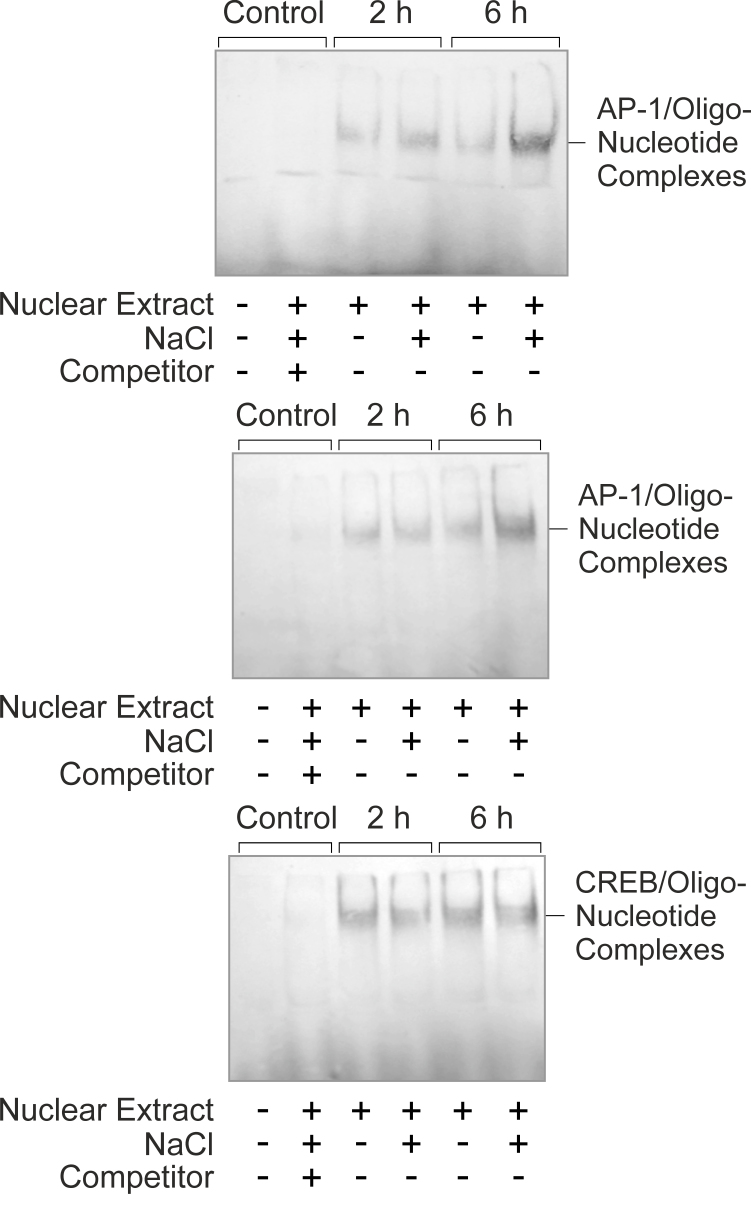
Phosphorylation of c-Jun by c-Jun NH2-terminal kinases (JNKs) or extracellular signal-regulated kinases 1 and 2 (ERK1/2) stimulates the AP-1-mediated transcription of the target genes [30,31]. Therefore, the level of phosphorylated c-Jun protein reflects the level of AP-1 activity. We found that stimulation of RPE cells with high NaCl induced statistically significant (p<0.05) increases in the cytosolic and nuclear levels of phosphorylated c-Jun protein after 2 and 6 h of stimulation (Figure 3A,C,D). The level of the phosphorylated c-Jun protein was also increased within 2 h of stimulation with the hypoxia mimetic CoCl2 (Figure 3C). The level of the phosphorylated c-Fos protein did not alter in response to high NaCl and CoCl2 (Figure 3A,B). EMSA showed that hyperosmolarity induced increased DNA binding of the AP-1 protein but not increased DNA binding of CREB (Figure 4). The data suggest that NaCl-induced extracellular hyperosmolarity induces activation and DNA binding of AP-1 in RPE cells.
Figure 4.
High extracellular NaCl induces DNA binding of AP-1 in RPE cells, as indicated by the increased level of the complexes of AP-1 protein and labeled oligonucleotides in electrophoretic mobility shift assay (EMSA; above and middle). Such an increase was not observed under isoosmotic conditions. High NaCl did not induce an increase in the level of the cAMP response element-binding protein (CREB)/oligonucleotide complexes (below). The cells were stimulated with the addition of 100 mM NaCl to the culture medium for 2 and 6 h. Control blots were obtained with nuclear extracts of cells cultured for 6 h. The addition of an excess of unlabeled oligonucleotides (Competitor) was used to show the specificity of the AP-1- and CREB/oligonucleotide complexes. Similar results were obtained in four independent experiments using cells from different donors.
Intracellular signaling involved in NaCl-induced expression of the c-Fos gene
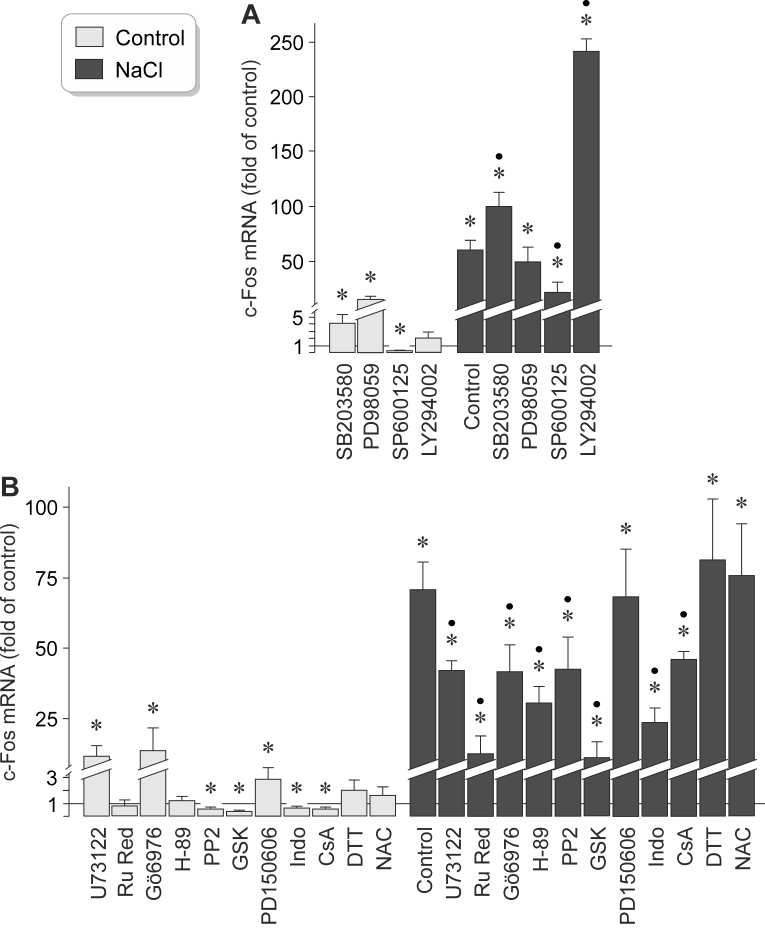
To investigate the intracellular signaling involved in mediating the NaCl-induced expression of the c-Fos gene in RPE cells, we tested pharmacological blockers of key intracellular signal transduction molecules in cell cultures that had been stimulated for 2 h with high (+ 100 mM) NaCl. As shown in Figure 5A, the expression of the c-Fos gene under control and high-NaCl conditions was statistically significantly (p<0.05) decreased by the JNK inhibitor SP600125. The expression of the c-Fos gene under high-NaCl conditions was statistically significantly (p<0.05) increased by the inhibitor of the p38 mitogen-activated protein kinase (p38 MAPK), SB203580, and the inhibitor of phosphatidylinositol-3 kinase (PI3K)-related kinases, LY294002, while the inhibitor of ERK1/2, PD98059, had no effect (Figure 5A). SB203580 and PD98059 increased the expression of the c-Fos gene under control conditions (Figure 5A). The data suggest that the constitutive and NaCl-induced expression of the c-Fos gene in RPE cells is (at least in part) mediated by the JNK signal transduction pathway, while the p38 MAPK and PI3K signal transduction pathways inhibit the NaCl-induced expression of the c-Fos gene.
Figure 5.
Intracellular signaling involved in mediating the NaCl-induced expression of the c-Fos gene in RPE cells. The level of c-Fos mRNA was determined with real-time reverse transcription (RT)–PCR analysis in cells cultured for 2 h in iso- (control) and hyperosmotic (+ 100 mM NaCl) media, and is expressed as a fold of the unstimulated control. A: The following compounds were tested: the inhibitor of p38 mitogen-activated protein kinase (MAPK) activation, SB203580 (10 µM), the inhibitor of extracellular signal-regulated kinases 1 and 2 (ERK1/2) activation, PD98059 (20 µM), the c-Jun NH2-terminal kinase (JNK) inhibitor SP600125 (10 µM), and the inhibitor of PI3K-related kinases, LY294002 (5 µM). B: The following compounds were tested: the inhibitor of PLCγ, U73122 (4 µM), the inhibitor of calcium-binding proteins, ruthenium red (Ru Red; 30 µM), the blocker of PKCα/β, Gö6976 (1 µM), the PKA blocker H-89 (1 µM), the inhibitor of Src tyrosine kinases, PP2 (100 nM), the serum and glucocorticoid-regulated kinase (SGK) blocker GSK650394 (GSK; 1 µM), the calpain inhibitor PD150606 (100 µM), the COX inhibitor indomethacin (Indo; 10 µM), the inhibitor of mitochondrial permeability transition, cyclosporin A (CsA; 1 µM), the reducing agent dithiothreitol (DTT; 300 µM), and the reactive oxygen species inhibitor N-acetyl-L-cysteine (NAC; 1 mM). Each bar represents data obtained in three to 14 independent experiments using cell lines from different donors. Significant difference versus unstimulated control: *p<0.05. Significant difference versus NaCl control: ●p<0.05.
The NaCl-induced expression of the c-Fos gene in RPE cells was also decreased by inhibitors of phospholipase Cγ (PLCγ; U73122), calcium-binding proteins (ruthenium red), protein kinases C (PKC) α/β (Gö6976), protein kinase A (PKA; H-89), and Src tyrosine kinases (PP2), and nearly completely suppressed by the inhibitor of the serum and glucocorticoid-regulated kinase (SGK), GSK650394 (Figure 5B). The calpain inhibitor PD150606 had no effect (Figure 5B). In addition, the cyclooxygenase (COX) inhibitor indomethacin and the inhibitor of mitochondrial permeability transition, cyclosporin A, decreased the NaCl-induced expression of the c-Fos gene (Figure 5B). The cell-permeable reducing agent dithiothreitol and the reactive oxygen species inhibitor N-acetyl-L-cysteine had no effects (Figure 5B). The data suggest that the expression of the c-Fos gene under high-NaCl conditions is (in part) mediated by the activities of PLCγ, PKCα/β, PKA, Src tyrosine kinases, SGK, and COX, as well as by a loss of the mitochondrial integrity.
Extracellular signaling involved in NaCl-induced expression of the c-Fos gene
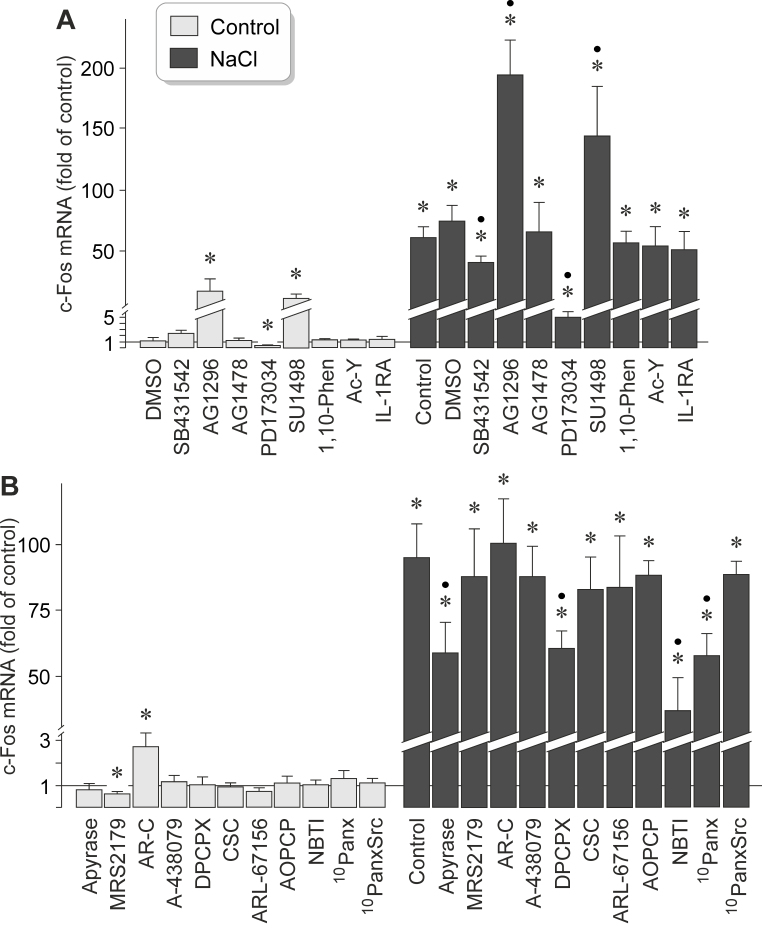
It has been shown that high extracellular NaCl induces the release of different growth factors, like VEGF, bFGF, and TGF-β1, from RPE cells [22,32]. Because various growth factors were found to induce expression of the c-Fos gene in RPE cells (Figure 1E), we determined whether autocrine/paracrine growth factor receptor signaling is required for the NaCl-induced expression of the c-Fos gene. We found that the NaCl-induced expression of the c-Fos gene in RPE cells was decreased by the inhibitor of TGF-β1 superfamily activin receptor-like kinase receptors, SB431542, and nearly completely suppressed by the inhibitor of the FGF receptor kinase, PD173074 (Figure 6A). Inhibition of the epidermal growth factor (EGF) receptor tyrosine kinase by AG1478 had no effect, while inhibition of the PDGF receptor tyrosine kinase by AG1296 and of the VEGF receptor-2 by SU1498 increased statistically significantly (p<0.05) the NaCl-induced expression of the c-Fos gene (Figure 6A). The broad-spectrum matrix metalloproteinase inhibitor 1,10-phenanthroline had no effect (Figure 6A), suggesting that shedding of growth factors from the extracellular matrix is not involved in mediating the NaCl-induced expression of the c-Fos gene. Furthermore, a caspase-1 inhibitor (Ac-YVAD-CMK) and a recombinant human IL-1 receptor antagonist had no effects (Figure 6A), suggesting that activation of the inflammasome [33] does not influence the NaCl-induced expression of the c-Fos gene. The data suggest that autocrine/paracrine activation of the FGF receptor kinase and (to a lesser extent) activation of TGF-β1 superfamily activin receptor-like kinase receptors are involved in mediating the NaCl-induced expression of the c-Fos gene in RPE cells. Autocrine/paracrine activation of the PDGF receptor tyrosine kinase and of the VEGF receptor-2 probably suppresses the NaCl-induced expression of the c-Fos gene.
Figure 6.
Receptor-mediated signaling involved in mediating NaCl-induced expression of the c-Fos gene in RPE cells. The level of c-Fos mRNA was determined with real-time reverse transcription (RT)–PCR analysis in cells cultured for 2 h in iso- (control) and hyperosmotic (+ 100 mM NaCl) media, and is expressed as a fold of the unstimulated control. A: The following inhibitors of receptor kinases were tested: the inhibitor of transforming growth factor-β1 (TGF-β1) superfamily activin receptor-like kinase receptors, SB431542 (10 µM), the inhibitor of the platelet-derived growth factor-BB (PDGF) receptor tyrosine kinase, AG1296 (10 µM), the blocker of the epidermal growth factor (EGF) receptor tyrosine kinase, AG1478 (600 nM), the inhibitor of the fibroblast growth factor (FGF) receptor kinase, PD173074 (500 nM), and the blocker of vascular endothelial growth factor (VEGF) receptor-2, SU1498 (10 µM). In addition, the broad-spectrum matrix metalloproteinase inhibitor 1,10-phenanthroline (1,10-Phen; 10 µM), the caspase-1 inhibitor Ac-YVAD-CMK (Ac-Y; 500 nM), and a recombinant human IL-1 receptor antagonist (IL-1RA; 1 µg/ml) were tested. Vehicle control was made with dimethyl sulfoxide (DMSO; 1:1,000). B: The following compounds were tested: the ATP/ADP phosphohydrolase apyrase (10 U/ml), the P2Y1 receptor antagonist MRS2179 (30 µM), the P2Y2 receptor antagonist AR-C 118925XX (AR-C; 10 µM), the P2X7 receptor antagonist A-438079 (50 nM), the adenosine A1 receptor antagonist DPCPX (50 nM), the adenosine A2A receptor antagonist CSC (200 nM), the ecto-ATPase inhibitor ARL-67156 (50 µM), the ectonucleotidase inhibitor AOPCP (250 µM), the antagonist of nucleoside transporters, NBTI (10 µM), the pannexin-blocking peptide 10panx (200 µM), and the scrambled control peptide 10panxScr (200 µM). Each bar represents data obtained in three to 14 independent experiments using cell lines from different donors. Significant difference versus unstimulated control: *p<0.05. Significant difference versus NaCl control: ●p<0.05.
It has been shown that high extracellular NaCl induces the release of adenosine 5′-triphosphate (ATP), a well-known cellular danger signal [34], from RPE cells [33]. To determine whether purinergic receptor signaling is involved in mediating the NaCl-induced expression of the c-Fos gene in RPE cells, we used pharmacological receptor antagonists. We found that the addition of the ATP-hydrolyzing enzyme apyrase to the culture medium decreased the NaCl-induced expression of the c-Fos gene in RPE cells (Figure 6B) to a similar extent as the inhibitor of PLCγ (U73122) and the blocker of PKCα/β (Gö6976), for example (Figure 5B). The NaCl-induced expression of the c-Fos gene was also statistically significantly (p<0.05) reduced by the adenosine A1 receptor antagonist DPCPX, the antagonist of nucleoside transporters, NBTI, and the pannexin-blocking peptide 10panx while a scrambled control peptide had no effect (Figure 6B). Antagonists of P2Y1 (MRS2179), P2Y2 (AR-C 118925XX), P2X7 (A-438079), and adenosine A2A receptors (CSC), as well as the ecto-ATPase inhibitor ARL-67156, had no effects on the NaCl-induced expression of the c-Fos gene (Figure 6B). The data suggest that a pannexin-mediated release of ATP from RPE cells, extracellular degradation of ATP, a transporter-mediated release of adenosine, and autocrine/paracrine activation of adenosine A1 receptors contribute to the full induction of the expression of the c-Fos gene under high-NaCl conditions.
Transcription factor activities involved in NaCl-induced expression of the c-Fos gene
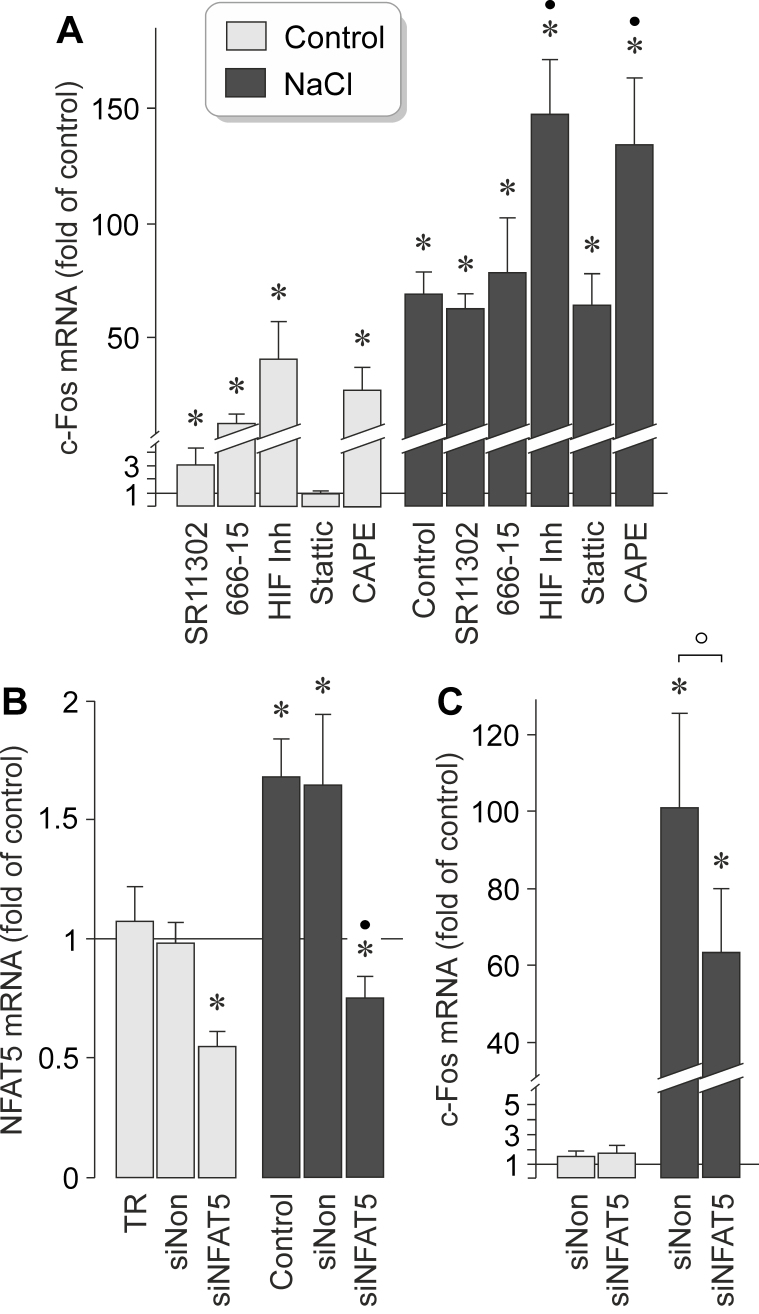
It has been shown that hyperosmotic stress induces expression of various transcription factors in RPE cells, such as HIF-1α, NF-κB, and NFAT5 [22,24]. To investigate which transcription factors mediate the NaCl-induced expression of the c-Fos gene in RPE cells, we used pharmacological blockers. As shown in Figure 7A, the NaCl-induced expression of the c-Fos gene was not altered in the presence of the AP-1 inhibitor SR11302, the CREB inhibitor 666–15, and the signal transducer and activator of transcription 3 (STAT3) inhibitor Stattic [35]. An HIF inhibitor [36] and the NF-κB inhibitor CAPE [37] increased statistically significantly (p<0.05) the expression of the c-Fos gene under the control and NaCl-stimulated conditions (Figure 7A), suggesting that HIF and NF-κB exert negative regulation of expression of the c-Fos gene in RPE cells. Negative regulation of the constitutive expression of the c-Fos gene exerted by various transcription factors, including AP-1, CREB, HIF, and NF-κB, is suggested by the statistically significant (p<0.05) increases in the c-Fos mRNA level under the unstimulated control conditions (Figure 7A).
Figure 7.
Transcription factor activities involved in mediating NaCl-induced expression of the c-Fos gene in RPE cells. mRNA levels were determined with real-time reverse transcription (RT)–PCR analysis in cells cultured for 2 h in iso- (control) and hyperosmotic (+ 100 mM NaCl) media, and are expressed as folds of the unstimulated control. A: The level of c-Fos mRNA was determined in cells cultured in the presence of the following inhibitory compounds: the AP-1 inhibitor SR11302 (5 µM), the cAMP response element-binding protein (CREB) inhibitor 666–15 (250 nM), a HIF inhibitor (HIF-Inh; 5 µM), the STAT3 inhibitor Stattic (1 µM), and the nuclear factor (NF)-κB inhibitor CAPE (5 µM). B, C: mRNA levels were determined in cells transfected with nuclear factor of activated T cell 5 (NFAT5) siRNA (siNFAT5; 5 nM) and non-targeted siRNA (siNon; 5 nM), respectively. B: Transfection of RPE cells with NFAT5 siRNA for 48 h resulted in a reduction of the NFAT5 mRNA level in cells cultured for 2 h in iso- and hyperosmotic media. Non-targeted siRNA had no effects. As negative control, transfection reagent (TR) without siRNA was tested. C: Knocking down NFAT5 with siRNA reduced the level of c-Fos mRNA under hyperosmotic conditions. Each bar represents data obtained in three to nine independent experiments using cell lines from different donors. Significant difference versus unstimulated control: *p<0.05. Significant difference versus NaCl control: ●p<0.05. ○ p<0.05.
In various cell systems, cellular survival in hyperosmotic stress depends on the transcriptional activity of NFAT5 [38]. It has been shown that high extracellular osmolarity increases the levels of the NFAT5 mRNA and protein, and induces DNA binding of the NFAT5 protein, in RPE cells [22]. To determine whether NFAT5 activity is involved in mediating the NaCl-induced expression of the c-Fos gene in RPE cells, we knocked down NFAT5 with transfection of the cells with NFAT5 siRNA. As negative control, non-targeted scrambled siRNA was used. As shown in Figure 7B, transfection with NFAT5 siRNA reduced the level of the NFAT5 transcripts by approximately 50% in the cells cultured under the control and high-NaCl conditions whereas transfection with non-targeted siRNA had no effect. Cells transfected with NFAT5 siRNA also displayed a statistically significant (p<0.05) lower level of c-Fos gene transcripts under high-NaCl conditions than cells transfected with non-targeted siRNA (Figure 7C). Knockdown of NFAT5 had no effect on the cellular level of the c-Fos gene transcripts under the control conditions (Figure 7C). The data suggest that the NaCl-induced expression of the c-Fos gene in RPE cells is (at least in part) mediated by the activity of NFAT5.
Role of AP-1 activity in NaCl-induced expression of genes
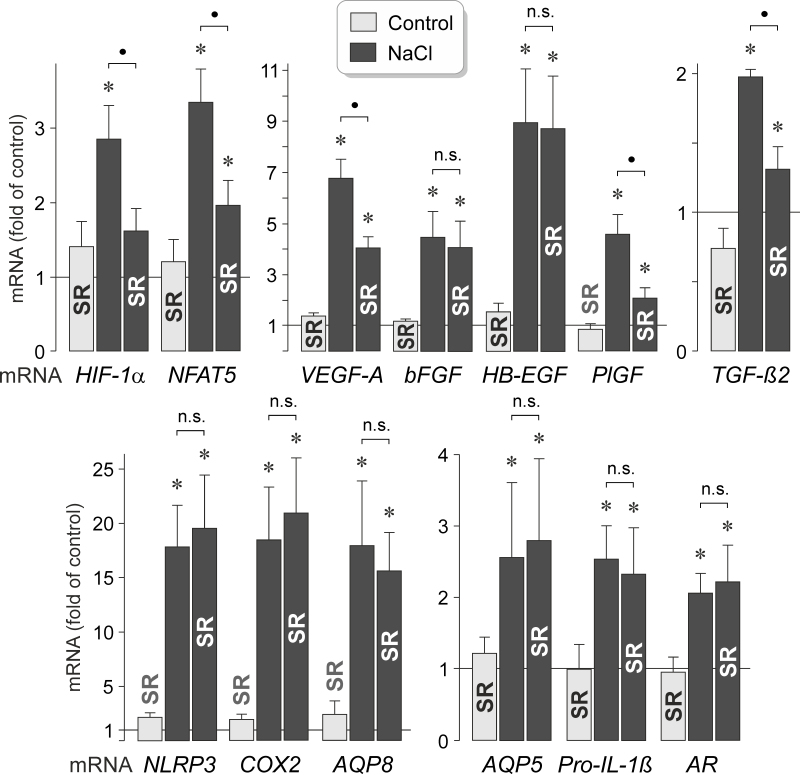
It has been shown that hyperosmotic stress induces expression of different genes in RPE cells, including genes that code for transcription factors, growth factors, NACHT, LRR and PYD domains-containing protein 3 (NLRP3), pro-IL-1β, aquaporin (AQP) water channels, COX, and aldose reductase [5,22,24,32,33,39]. To investigate which genes are transcriptionally activated by AP-1 activity in response to high extracellular NaCl, we stimulated RPE cells with high (+ 100 mM) NaCl in the absence and presence of the AP-1 inhibitor SR11302. As shown in Figure 8, the NaCl-induced expression of the the HIF-1α (Gene ID 3091; OMIM 603348) and NFAT5 (Gene ID 10725; OMIM 604708) genes was statistically significantly (p<0.05) reduced in the presence of SR11302. Inhibition of AP-1 activity also caused reductions in the NaCl-induced expression of the VEGF, PlGF, and TGF-β2 genes while the NaCl-induced expression of the bFGF (Gene ID 2247; OMIM 134920) and HB-EGF (Gene ID 1839; OMIM 126150) genes remained unaltered (Figure 8). Inhibition of AP-1 activity had no effects on the NaCl-induced expression of various other genes, including the NLRP3 (Gene ID 114548; OMIM 606416), pro-IL-1β (Gene ID 3553; OMIM 147720), AQP8 (Gene ID 343; OMIM 603750), AQP5 (Gene ID 362; OMIM 600442), COX-2 (Gene ID 5743; OMIM 600262), and aldose reductase genes (AKR1B1; Gene ID 231; OMIM 103880) genes (Figure 8). The data suggest that AP-1 activity is involved in mediating the NaCl-induced expression of various transcription factor (HIF-1α and NFAT5) and growth factor genes (VEGF, PlGF, and TGF-β2; Gene ID 7042; OMIM 190220).
Figure 8.
Inhibition of AP-1 activity alters NaCl-induced expression of various genes in RPE cells. The mRNA levels were determined with real-time reverse transcription (RT)–PCR analysis after stimulation of the cells for 6 h with high (+ 100 mM) NaCl, and are expressed as folds of the unstimulated control. AP-1 activity was inhibited with SR11302 (SR; 5 µM). The expression levels of the following genes were determined: HIF-1α, NFAT5, VEGF-A, bFGF, HB-EGF, PlGF, TGF-β2, NLRP3, COX-2, aquaporins 5 (AQP5) and 8 (AQP8), pro-IL-1β, and aldose reductase (AR). Each bar represents data obtained in five to eight independent experiments using cell lines from different donors. Significant difference versus unstimulated control: *p<0.05. ●p<0.05. n.s., not significant.
Involvement of AP-1 activity in NaCl-induced secretion of PlGF
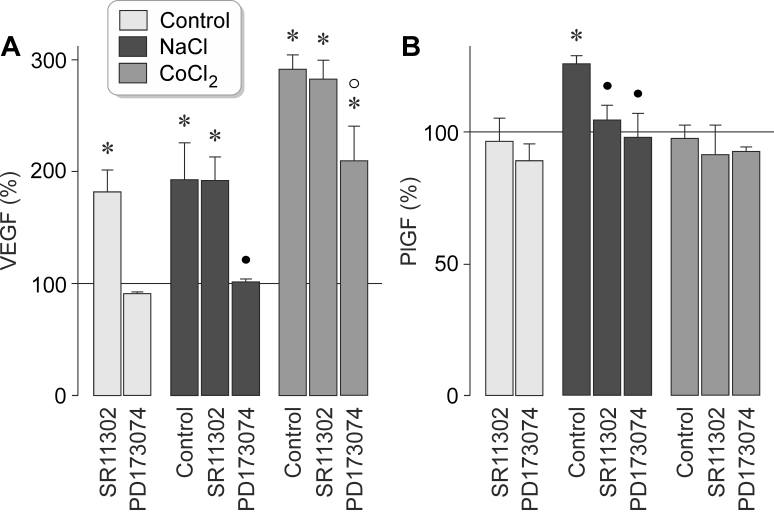
It has been shown that extracellular hyperosmolarity induces secretion of VEGF and PlGF from RPE cells [5,22]. To determine whether AP-1-mediated production of VEGF and PlGF contributes to the secretion of these factors, we inhibited AP-1 activity with SR11302. High extracellular NaCl induced the secretion of VEGF (Figure 9A) and PlGF (Figure 9B) from RPE cells, whereas chemical hypoxia induced the secretion of VEGF but not of PlGF (Figure 9A, B). SR11302 fully prevented the NaCl-induced secretion of PlGF (Figure 9B) and had no effects on the NaCl-induced and hypoxic secretion of VEGF (Figure 9A). However, the inhibitor of the FGF receptor kinase, PD173074, suppressed statistically significantly (p<0.05) the secretion of VEGF and PlGF under both conditions (Figure 9A, B). SR11302 increased the secretion of VEGF statistically significantly (p<0.05) under unstimulated control conditions (Figure 9A), suggesting that inhibition of the AP-1-mediated VEGF production may represent a stress condition for the cells.
Figure 9.
Inhibition of AP-1 activity suppresses the hyperosmotic secretion of PlGF from RPE cells. The levels of VEGF-A165 (A) and PlGF (B) proteins were determined with enzyme-linked immunosorbent assay (ELISA) in the media of cells cultured for 24 h under iso- (control) and hyperosmotic (+ 100 mM NaCl) conditions, and in the presence of the hypoxia mimetic CoCl2 (150 µM), respectively. The cells were cultured in the absence and presence of the inhibitor of AP-1 activity, SR11302 (SR; 5 µM), and the inhibitor of the FGF receptor kinase, PD173074 (500 nM), respectively. Each bar represents data obtained in four independent experiments using cell lines from different donors. Significant difference versus unstimulated control: *p<0.05. Significant difference versus NaCl control: ●p<0.05. Significant difference versus CoCl2 control: ○ p<0.05.
Discussion
Systemic hypertension is associated with a higher risk of neovascular AMD [12-16]. The main condition that causes acute hypertension is the increase in extracellular osmolarity following the intake of dietary salt [19,20]. Because the use of antihypertensive medications is not associated with a decreased risk of AMD [12,21], it was suggested that pathogenic conditions that cause hypertension, such as high extracellular NaCl and osmolarity, may induce cellular alterations that favor angiogenesis in the aged retina [22]. It has been shown that these conditions induce production of angiogenic factors, such as VEGF and PlGF, in RPE cells [5,22]. In the present study, we investigated whether the hyperosmotic expression of angiogenic factors in RPE cells depends upon the transcriptional activity of AP-1, and whether the expression of AP-1 components and the activity of AP-1 are regulated by extracellular osmolarity. We found that high extracellular osmolarity induces rapid expression of c-Fos and FOSB genes and has no effect on the gene expression of c-Jun and of other proteins that may be components of AP-1 (Figure 1B,C and Figure 2B). A decrease in the extracellular osmolarity induced downregulation of the expression of the c-Fos and c-Jun genes (Figure 1B). The data indicate that the expression of the c-Fos gene in RPE cells is dependent on the extracellular osmolarity. High extracellular NaCl also induced activation of AP-1, as indicated by the increase in the level of the phosphorylated c-Jun protein and of the level of DNA binding of the AP-1 protein (Figure 3A,C and Figure 4). Under high NaCl conditions, the c-Fos protein level was increased (Figure 3A,B) while the c-Jun protein level remained unaltered (Figure 3A,C). In contrast to the c-Fos protein, the c-Jun protein seems to be constitutively expressed at a high level in RPE cells (Figure 3A).
We found indications that activation of multiple signal transduction molecules is probably involved in mediating the NaCl-induced expression of the c-Fos gene in RPE cells. NaCl-induced expression of the c-Fos gene may be (in part) dependent on the activities of JNK, PLCγ, PKCα/β, PKA, Src tyrosine kinases, SGK, and COX, as well as on the loss of the mitochondrial integrity (Figure 3A,B). Inhibition of SGK resulted in nearly complete suppression of the NaCl-induced expression of the c-Fos gene (Figure 5B). SGK is a main mediator of cellular sodium homeostasis; it has been shown in various cell systems that SGK is induced by high extracellular NaCl and increases the protein abundance and activities of ion channels, carriers, and the sodium/potassium-ATPase [40,41].
We also found evidence that NaCl-induced expression of the c-Fos gene may depend on receptor-mediated signaling mechanisms. Exogenous bFGF induced expression of the c-Fos gene (Figure 1E), and inhibition of the FGF receptor kinase nearly completely suppressed the NaCl-induced expression of the c-Fos gene (Figure 6A). These data support the assumption that autocrine/paracrine FGF receptor signaling, likely induced by osmotically released bFGF [32], is crucially involved in mediating the effect of high NaCl on the expression of the c-Fos gene. The present data are consistent with previous studies which showed that bFGF induces increased expression of c-Fos in RPE cells via activation of FGF receptor-1 [42], and that the expression of c-Fos in RPE cells after experimental retinal detachment is likely induced by FGF receptor-1 signaling [43]. In addition to the FGF receptor signaling, autocrine/paracrine adenosine A1 receptor signaling is probably required for the full expression of the c-Fos gene in response to high NaCl (Figure 6B). Generally, adenosine is liberated from cells via nucleoside transporters and can be formed in the extracellular space by dephosphorylation of ATP [34]. We found that the inhibitor of nucleoside transporters, NBTI, suppressed the NaCl-induced expression of the c-Fos gene (Figure 6B). This finding suggest that adenosine is liberated from RPE cells by a transporter-mediated process. However, we also found that the ATP/ADP phosphohydrolase apyrase and a pannexin-blocking peptide decreased the NaCl-induced expression of the c-Fos gene (Figure 6B). These findings suggest that pannexin-mediated release of ATP, an extracellular dephosphorylation of ATP, and a subsequent activation of purinergic receptors contribute to the full induction of the expression of the c-Fos gene. The subtype of purinergic receptors involved in this signaling remains to be determined in future experiments. Activation of purinergic receptors may trigger a PLC-mediated calcium mobilization from internal stores and activation of PKC (Figure 5B). This signaling may result in the activation of nucleoside transporters. Because bFGF and adenosine are two major compounds implicated in the retinal defense against various stresses [34,44], the present data are consistent with the assumption that the expression of c-Fos is part of the RPE cell response to protect cells from the deleterious effects of hyperosmotic stress.
We found evidence that AP-1 activity is probably involved in mediating the NaCl-induced expression of various transcription factor genes (HIF-1α and NFAT5; Figure 8). Vice versa, the NaCl-induced expression of the c-Fos gene may depend (at least in part) on the transcriptional activity of NFAT5 (Figure 7C). The possible association between the expression of AP-1 and NFAT5 genes under high-NaCl conditions is in agreement with previous studies that showed functional cooperation between the two factors. It has been shown, for example, that AP-1 facilitates the NFAT5-mediated transcription of target genes upon stimulation with high NaCl, via the formation of complexes of both transcription factors [45,46]. In addition, the target genes of NFAT5 include genes of various protein kinases, such as SGK [47], which are involved in the regulation of the RPE cell response to osmotic stress and which may also regulate the expression of the c-Fos gene (Figure 5B).
AP-1 activity could be also involved in mediating NaCl-induced expression of various angiogenic growth factor genes (VEGF, PlGF, and TGF-β2; Figure 8). This functional role may, in part, be explained by the reducing effect of AP-1 inhibition on the expression of NFAT5 (Figure 8). It has been shown that NaCl-induced expression of the VEGF and PlGF genes (but not of the HB-EGF gene) in RPE cells is partially induced by the transcriptional activity of NFAT5 [5,22,32]. However, there are also genes, like bFGF, NLRP3, AQP5, and aldose reductase, which have been shown to be activated by NFAT5 in RPE cells [22,32,39], and which apparently are not regulated by AP-1 activity in the presence of high NaCl (Figure 8). The relationships between the activities of AP-1 and NFAT5 in RPE cells remain to be revealed in future investigations. The difference between the effects of the AP-1 inhibitor SR11302 on NaCl-induced secretion of VEGF and PlGF (Figure 9A,B) might be (in part) explained by the different amounts and half-life of both proteins; that is, it could be that the secreted PlGF protein was produced within the time period of NaCl stimulation whereas the secreted VEGF protein was produced before the time period of NaCl stimulation. However, the possibility cannot be ruled out that nonspecific effects of various pharmacological compounds used in the present study may contribute to the effects observed. Ruthenium red, for example, inhibits calcium-binding proteins and the mitochondrial calcium uniporter. We found that an inhibitor of mitochondrial permeability transition, cyclosporin A, also decreased the NaCl-induced expression of the c-Fos gene, albeit at a lower degree than ruthenium red (Figure 5B). Therefore, it seems to be likely that ruthenium red acted via inhibition of calcium-binding proteins and inhibition of the mitochondrial uniporter. SR11302 can—at higher concentrations—also block retinoic acid receptors. Further research is required to support the assumption that AP-1 activity contributes to the induction of angiogenic factors in RPE cells.
We found statistically significant effects of high extracellular NaCl on the expression of the c-Fos gene when 10 mM NaCl was added to the culture medium (Figure 1D). It is generally accepted that the highest pathological blood osmolarity in human subjects is around 360 mOsm/kg which can be achieved by the addition of 40 mM NaCl to the culture medium [41,48]. Therefore, the present results may be relevant for the interpretation of in vivo conditions.
In summary, we found that high extracellular NaCl induces expression of c-Fos and activation of AP-1 in RPE cells. NaCl-induced expression of the VEGF and PlGF genes, and NaCl-induced secretion of PlGF from RPE cells, may (partially) depend on the activity of AP-1. In addition to the activity of NFAT5, the AP-1-mediated transcriptional activation of target genes in RPE cells may link pathogenic conditions associated with hypertension, that is, high extracellular NaCl and osmolarity, to the development of AMD. The direct effects of both conditions on the gene expression of RPE cells [5,22], which proceed independently from hypertension, may explain the previous finding that the use of antihypertensive medications is not associated with a decreased risk of AMD [12,21]. In the developed world, the intake of dietary salt rapidly increased in the past along with higher consumption of processed foods that contain high amounts of salt [49]. Because hypertension is associated with a higher risk of neovascular AMD [12-16], and because high NaCl induces production of angiogenic factors in RPE cells [5,22,32], restriction of dietary salt intake, or increased intake of NaCl-lowering minerals, may be helpful to decelerate the progression of AMD [23]. In addition to the effects on the RPE, high salt stimulates proinflammatory responses of leukocytes [41,48,50,51]. Therefore, restriction of salt intake may be also helpful in limiting another factor that is believed to contribute to the initiation and progression of AMD: activation and chorioretinal infiltration of inflammatory cells (macrophages, lymphocytes, neutrophils, and mast cells) [52–55].
Acknowledgments
The authors thank Ute Weinbrecht for excellent technical assistance. This work was supported by a grant from the Geschwister Freter Stiftung (Hannover, Germany).
References
- 1.Klein R, Klein BE, Knudtson MD, Meuer SM, Swift M, Gangnon RE. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007;114:253–62. doi: 10.1016/j.ophtha.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 2.Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res. 2003;22:1–29. doi: 10.1016/s1350-9462(02)00043-5. [DOI] [PubMed] [Google Scholar]
- 3.Rakic JM, Lambert V, Devy L, Luttun A, Carmeliet P, Claes C, Nguyen L, Foidart JM, Noël A, Munaut C. Placental growth factor, a member of the VEGF family, contributes to the development of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3186–93. doi: 10.1167/iovs.02-1092. [DOI] [PubMed] [Google Scholar]
- 4.Hollborn M, Tenckhoff S, Seifert M, Köhler S, Wiedemann P, Bringmann A, Kohen L. Human retinal epithelium produces and responds to placenta growth factor. Graefes Arch Clin Exp Ophthalmol. 2006;244:732–41. doi: 10.1007/s00417-005-0154-9. [DOI] [PubMed] [Google Scholar]
- 5.Hollborn M, Reichmuth K, Prager P, Wiedemann P, Bringmann A, Kohen L. Osmotic induction of placental growth factor in retinal pigment epithelial cells in vitro: contribution of NFAT5 activity. Mol Biol Rep. 2016;43:803–14. doi: 10.1007/s11033-016-4016-9. [DOI] [PubMed] [Google Scholar]
- 6.Van Lookeren Campagne M, LeCouter J, Yaspan BL, Ye W. Mechanisms of age-related macular degeneration and therapeutic opportunities. J Pathol. 2014;232:151–64. doi: 10.1002/path.4266. [DOI] [PubMed] [Google Scholar]
- 7.Cheung CM, Wong TY. Is age-related macular degeneration a manifestation of systemic disease? New prospects for early intervention and treatment. J Intern Med. 2014;276:140–53. doi: 10.1111/joim.12227. [DOI] [PubMed] [Google Scholar]
- 8.Sperduto RD, Hiller R. Systemic hypertension and age-related maculopathy in the Framingham Study. Arch Ophthalmol. 1986;104:216–9. doi: 10.1001/archopht.1986.01050140070022. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg J, Flowerdew G, Smith E, Brody JA, Tso MO. Factors associated with age-related macular degeneration. An analysis of data from the first National Health and Nutrition Examination Survey. Am J Epidemiol. 1988;128:700–10. doi: 10.1093/oxfordjournals.aje.a115023. [DOI] [PubMed] [Google Scholar]
- 10.Klein R, Klein BE, Tomany SC, Cruickshanks KJ. The association of cardiovascular disease with the long-term incidence of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 2003;110:1273–80. doi: 10.1016/S0161-6420(03)00599-2. [DOI] [PubMed] [Google Scholar]
- 11.Van Leeuwen R, Ikram MK, Vingerling JR, Witteman JC, Hofman A, de Jong PT. Blood pressure, atherosclerosis, and the incidence of age-related maculopathy: the Rotterdam Study. Invest Ophthalmol Vis Sci. 2003;44:3771–7. doi: 10.1167/iovs.03-0121. [DOI] [PubMed] [Google Scholar]
- 12.Hyman L, Schachat AP, He Q, Leske MC. Hypertension, cardiovascular disease, and age-related macular degeneration. Age-Related Macular Degeneration Risk Factors Study Group. Arch Ophthalmol. 2000;118:351–8. doi: 10.1001/archopht.118.3.351. [DOI] [PubMed] [Google Scholar]
- 13.Age-Related Eye Disease Study Research Group Risk factors associated with age-related macular degeneration. A case-control study in the Age-Related Eye Disease Study: Age-Related Eye Disease Study report number 3. Ophthalmology. 2000;107:2224–32. doi: 10.1016/s0161-6420(00)00409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraser-Bell S, Wu J, Klein R, Azen SP, Hooper C, Foong AW, Varma R. Cardiovascular risk factors and age-related macular degeneration: the Los Angeles Latino Eye Study. Am J Ophthalmol. 2008;145:308–16. doi: 10.1016/j.ajo.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hogg RE, Woodside JV, Gilchrist SE, Graydon R, Fletcher AE, Chan W, Knox A, Cartmill B, Chakravarthy U. Cardiovascular disease and hypertension are strong risk factors for choroidal neovascularization. Ophthalmology. 2008;115:1046–52. doi: 10.1016/j.ophtha.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 16.Klein R, Cruickshanks KJ, Nash SD, Krantz EM, Nieto FJ, Huang GH, Pankow JS, Klein BE. The prevalence of age-related macular degeneration and associated risk factors. Arch Ophthalmol. 2010;128:750–8. doi: 10.1001/archophthalmol.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vingerling JR, Dielemans I, Bots ML, Hofman A, Grobbee DE, de Jong PTVM. Age-related macular degeneration is associated with atherosclerosis. The Rotterdam Study. Am J Epidemiol. 1995;142:404–9. doi: 10.1093/oxfordjournals.aje.a117648. [DOI] [PubMed] [Google Scholar]
- 18.Sato E, Feke GT, Appelbaum EY, Menke MN, Trempe CL, McMeel JW. Association between systemic arterial stiffness and age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2006;244:963–71. doi: 10.1007/s00417-005-0201-6. [DOI] [PubMed] [Google Scholar]
- 19.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–56. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 20.He FJ, Markandu ND, Sagnella GA, de Wardener HE, MacGregor GA. Plasma sodium: ignored and underestimated. Hypertension. 2005;45:98–102. doi: 10.1161/01.HYP.0000149431.79450.a2. [DOI] [PubMed] [Google Scholar]
- 21.Van Leeuwen R, Tomany SC, Wang JJ, Klein R, Mitchell P, Hofman A, Klein BE, Vingerling JR, Cumming RG, de Jong PT. Is medication use associated with the incidence of early age-related maculopathy? Pooled findings from 3 continents. Ophthalmology. 2004;111:1169–75. doi: 10.1016/j.ophtha.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 22.Hollborn M, Vogler S, Reichenbach A, Wiedemann P, Bringmann A, Kohen L. Regulation of the hyperosmotic induction of aquaporin 5 and VEGF in retinal pigment epithelial cells: Involvement of NFAT5. Mol Vis. 2015;21:360–77. [PMC free article] [PubMed] [Google Scholar]
- 23.Bringmann A, Hollborn M, Kohen L, Wiedemann P. Intake of dietary salt and drinking water: Implications for the development of age-related macular degeneration. Mol Vis. 2016;22:1437–54. [PMC free article] [PubMed] [Google Scholar]
- 24.Hollborn M, Fischer S, Wiedemann P, Bringmann A, Kohen L. Osmotic regulation of NFAT5 expression in RPE cells: the involvement of purinergic receptor signaling. Mol Vis. 2017;23:116–30. [PMC free article] [PubMed] [Google Scholar]
- 25.Halazonetis TD, Georgopoulos K, Greenberg ME, Leder P. c-Jun dimerizes with itself and with c-Fos, forming complexes of different DNA binding affinities. Cell. 1988;55:917–24. doi: 10.1016/0092-8674(88)90147-x. [DOI] [PubMed] [Google Scholar]
- 26.Karin M. Liu Zg, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–6. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 27.Schlingemann RO. Role of growth factors and the wound healing response in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2004;242:91–101. doi: 10.1007/s00417-003-0828-0. [DOI] [PubMed] [Google Scholar]
- 28.Chen R, Hollborn M, Grosche A, Reichenbach A, Wiedemann P, Bringmann A, Kohen L. Effects of the vegetable polyphenols epigallocatechin-3-gallate, luteolin, apigenin, myricetin, quercetin, and cyanidin in retinal pigment epithelial cells. Mol Vis. 2014;20:242–58. [PMC free article] [PubMed] [Google Scholar]
- 29.An WG, Kanekal M, Simon MC, Maltepe E, Blagosklonny MV, Neckers LM. Stabilization of wild-type p53 by hypoxia-inducible factor 1α. Nature. 1998;392:405–8. doi: 10.1038/32925. [DOI] [PubMed] [Google Scholar]
- 30.Pulverer BJ, Kyriakis JM, Avruch J, Nikolakaki E, Woodgett JR. Phosphorylation of c-jun mediated by MAP kinases. Nature. 1991;353:670–4. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- 31.Smeal T, Binetruy B, Mercola DA, Birrer M, Karin M. Oncogenic and transcriptional cooperation with Ha-Ras requires phosphorylation of c-Jun on serines 63 and 73. Nature. 1991;354:494–6. doi: 10.1038/354494a0. [DOI] [PubMed] [Google Scholar]
- 32.Veltmann M, Hollborn M, Reichenbach A, Wiedemann P, Kohen L, Bringmann A. Osmotic induction of angiogenic growth factor expression in human retinal pigment epithelial cells. PLoS One. 2016;11:e0147312. doi: 10.1371/journal.pone.0147312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prager P, Hollborn M, Steffen A, Wiedemann P, Kohen L, Bringmann A. High salt-induced priming of retinal pigment epithelial cells for NLRP3 inflammasome activation: contribution of P2Y1 receptor signaling. PLoS One. 2016;11:e0165653. doi: 10.1371/journal.pone.0165653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reichenbach A, Bringmann A. Purinergic signaling in retinal degeneration and regeneration. Neuropharmacology. 2016;104:194–211. doi: 10.1016/j.neuropharm.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 35.Schust J, Sperl B, Hollis A, Mayer TU, Berg T. Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem Biol. 2006;13:1235–42. doi: 10.1016/j.chembiol.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 36.Lee K, Lee JH, Boovanahalli SK, Jin Y, Lee M, Jin X, Kim JH, Hong YS, Lee JJ. (Aryloxyacetylamino)benzoic acid analogues: a new class of hypoxia-inducible factor-1 inhibitors. J Med Chem. 2007;50:1675–84. doi: 10.1021/jm0610292. [DOI] [PubMed] [Google Scholar]
- 37.Natarajan K, Singh S, Burke TR, Jr, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-κB. Proc Natl Acad Sci USA. 1996;93:9090–5. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheung CY, Ko BC. NFAT5 in cellular adaptation to hypertonic stress ‒ regulations and functional significance. J Mol Signal. 2013;8:5. doi: 10.1186/1750-2187-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winges A, Garcia TB, Prager P, Wiedemann P, Kohen L, Bringmann A, Hollborn M. Osmotic expression of aldose reductase in retinal pigment epithelial cells: involvement of NFAT5. Graefes Arch Clin Exp Ophthalmol. 2016;254:2387–400. doi: 10.1007/s00417-016-3492-x. [DOI] [PubMed] [Google Scholar]
- 40.Lang F, Böhmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev. 2006;86:1151–78. doi: 10.1152/physrev.00050.2005. [DOI] [PubMed] [Google Scholar]
- 41.Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, Regev A, Kuchroo VK. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496:513–7. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenthal R, Malek G, Salomon N, Peill-Meininghaus M, Coeppicus L, Wohlleben H, Wimmers S, Bowes Rickman C, Strauss O. The fibroblast growth factor receptors, FGFR-1 and FGFR-2, mediate two independent signalling pathways in human retinal pigment epithelial cells. Biochem Biophys Res Commun. 2005;337:241–7. doi: 10.1016/j.bbrc.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 43.Geller SF, Lewis GP, Fisher SK. FGFR1, signaling, and AP-1 expression after retinal detachment: reactive Müller and RPE cells. Invest Ophthalmol Vis Sci. 2001;42:1363–9. [PubMed] [Google Scholar]
- 44.Bringmann A, Iandiev I, Pannicke T, Wurm A, Hollborn M, Wiedemann P, Osborne NN, Reichenbach A. Cellular signaling and factors involved in Müller cell gliosis: neuroprotective and detrimental effects. Prog Retin Eye Res. 2009;28:423–51. doi: 10.1016/j.preteyeres.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Pan S, Tsuruta R, Masuda ES, Imamura R, Bazan F, Arai K, Arai N, Miyatake S. NFATz: a novel Rel similarity domain containing protein. Biochem Biophys Res Commun. 2000;272:765–76. doi: 10.1006/bbrc.2000.2831. [DOI] [PubMed] [Google Scholar]
- 46.Irarrazabal CE, Williams CK, Ely MA, Birrer MJ, Garcia-Perez A, Burg MB, Ferraris JD. Activator protein-1 contributes to high NaCl-induced increase in tonicity-responsive enhancer/osmotic response element-binding protein transactivating activity. J Biol Chem. 2008;283:2554–63. doi: 10.1074/jbc.M703490200. [DOI] [PubMed] [Google Scholar]
- 47.Chen S, Grigsby CL, Law CS, Ni X, Nekrep N, Olsen K, Humphreys MH, Gardner DG. Tonicity-dependent induction of Sgk1 expression has a potential role in dehydration-induced natriuresis in rodents. J Clin Invest. 2009;119:1647–58. doi: 10.1172/JCI35314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496:518–22. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Appel LJ, Frohlich ED, Hall JE, Pearson TA, Sacco RL, Seals DR, Sacks FM, Smith SC, Jr, Vafiadis DK, Van Horn LV. The importance of population-wide sodium reduction as a means to prevent cardiovascular disease and stroke: a call to action from the American Heart Association. Circulation. 2011;123:1138–43. doi: 10.1161/CIR.0b013e31820d0793. [DOI] [PubMed] [Google Scholar]
- 50.Junger WG, Liu FC, Loomis WH, Hoyt DB. Hypertonic saline enhances cellular immune function. Circ Shock. 1994;42:190–6. [PubMed] [Google Scholar]
- 51.Yi B, Titze J, Rykova M, Feuerecker M, Vassilieva G, Nichiporuk I, Schelling G, Morukov B, Choukèr A. Effects of dietary salt levels on monocytic cells and immune responses in healthy human subjects: a longitudinal study. Transl Res. 2015;166:103–10. doi: 10.1016/j.trsl.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Penfold PL, Killingsworth MC, Sarks SH. Senile macular degeneration: the involvement of immunocompetent cells. Graefes Arch Clin Exp Ophthalmol. 1985;223:69–76. doi: 10.1007/BF02150948. [DOI] [PubMed] [Google Scholar]
- 53.Sakurai E, Anand A, Ambati BK, van Rooijen N, Ambati J. Macrophage depletion inhibits experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3578–85. doi: 10.1167/iovs.03-0097. [DOI] [PubMed] [Google Scholar]
- 54.Espinosa-Heidmann DG, Suner IJ, Hernandez EP, Monroy D, Csaky KG, Cousins SW. Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3586–92. doi: 10.1167/iovs.03-0038. [DOI] [PubMed] [Google Scholar]
- 55.Jawad S, Liu B, Li Z, Katamay R, Campos M, Wei L, Sen HN, Ling D, Martinez Estrada F, Amaral J, Chan CC, Fariss R, Gordon S, Nussenblatt RB. The role of macrophage class A scavenger receptors in a laser-induced murine choroidal neovascularization model. Invest Ophthalmol Vis Sci. 2013;54:5959–70. doi: 10.1167/iovs.12-11380. [DOI] [PMC free article] [PubMed] [Google Scholar]