Abstract
Objective:
The efficacy of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in reducing cardiovascular outcomes in patients with diabetes and overt nephropathy is still a controversial issue.
Methods:
We systematically searched MEDLINE, Embase and Cochrane Library for randomised controlled trials.
Results:
Thirteen trials containing 4638 patients with diabetes and overt nephropathy were included. Compared with controls, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker treatment did not reduce the risk of cardiovascular events (odds ratio 0.94, 95% confidence interval 0.86 to 1.03, P=0.18; I2=0.0%, P=0.75). Angiotensin-converting enzyme inhibitor/angiotensin receptor blocker therapy reduced the odds of heart failure events by 29% (0.71, 0.61 to 0.83, P<0.001; I2=0%, P=0.78). The results indicated no significant differences between the two treatment regimens with regard to the frequency of MI (0.95, 0.76 to 1.19, P=0.64), stroke (1.20, 0.83 to 1.74, P=0.32), cardiovascular death (1.26, 0.96 to 1.65, P=0.09) and all-cause mortality (0.98, 0.86 to 1.12, P=0.73). Among all kinds of adverse effects, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker therapy increased the incidence of hyperkalemia (2.26, 1.42 to 3.61, P=0.001).
Conclusion:
This study demonstrated that angiotensin-converting enzyme inhibitors and angiotensin receptor blockers did not reduce cardiovascular events in patients with diabetes and overt nephropathy.
Keywords: Angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, cardiovascular outcomes, diabetes and overt nephropathy, meta-analysis
Introduction
Patients with diabetes and kidney disease (DKD) are at a high risk of cardiovascular disease (CVD), which is the leading cause of morbidity and mortality for individuals with diabetes mellitus (DM).1,2 Aggressive intervention against risk factors for CVD is very important in patients with DKD to reduce the incidence of cardiovascular outcomes. The role of angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) preserving kidney function in patients with diabetes and overt nephropathy is well documented.3,4 There is high-quality evidence from trials of patients with diabetes showing that ACEIs and ARBs improve cardiovascular outcomes.5,6 However, these studies did not focus on patients with clinically significant albuminuria. Some studies provided evidence that treatment with ARBs could reduce heart failure (HF) and marginally reduced the myocardial infarction (MI) rate,7 while others did not find any beneficial effect of ACEIs or ARBs.8 It is not clear whether these agents provide additional cardiovascular benefit over other antihypertensive medications.
This systematic review and meta-analysis was therefore undertaken to assess the effects of ACEIs and ARBs on cardiovascular outcomes in patients with diabetes and overt nephropathy.
Methods
Search strategy and search selection
We performed a systematic review of the published studies by searching MEDLINE by Ovid (from 1950 to May 2018), EMBASE (from 1966 to May 2018) and Cochrane Library databases using all spellings of MeSH headings and text words of known ACEIs, ARBs, DKD, diabetes and overt nephropathy, randomised controlled trials (RCTs), cardiovascular events, angina, MI, stroke, HF, cardiovascular mortality, death and adverse events. We included all studies that compared ACEIs and ARBs with placebo or other antihypertensive agents on the effects of cardiovascular outcomes, all-cause death, or drug-related adverse events in patients with diabetes and overt nephropathy, without language restriction. We also checked the reference lists of identified articles for the other potentially relevant trials. The literature was searched and identified by two investigators independently. Any divergence was resolved by consultation with a third author. Overt nephropathy was defined as urinary proteinuria excretion greater than 300 mg/day with or without defined as an estimated glomerular filtration rate less than 60 ml/min/1.73 m2 like before.8
Data extraction and quality of evidence
Two authors extracted data using standard data extraction forms, which include study name, interventions, type of DM, sample size, mean age, mean systolic blood pressure, albuminuria, mean serum creatinine and follow-up duration. We used a standard criterion to assess the quality of the trials.9 Cardiovascular outcomes were defined as a composite of angina, fatal or non-fatal MI, stroke, HF and cardiovascular mortality (cardiovascular death). Adverse events included hyperkalemia, cough, hypotension and oedema. Data abstracted by two authors were checked for coherence, and any differences were resolved by resorting to original context until a consensus was reached.
Statistical analysis
We calculated the odds ratio (OR) and 95% confidence interval (CI) for categorical variables by the random effects model. The I2 statistic was used to describe the percentage of variability that was due to heterogeneity beyond chance, and we analysed publication bias using Begg’s and Egger’s tests. A two-sided P<0.05 was considered statistically significant, and all statistical analyses were performed using STATA, version 12.0.
Results
The literature search yielded 2863 results for relevant articles, of which 25 were reviewed in full text. After a thorough and careful review, 13 trials which contained 4638 patients were included in the final analysis7,8,10–20 (Figure 1). The follow-up duration ranged from approximately 0.5 to 5.3 years. Four studied compared the efficacy of ACEIs and ARBS with placebo,7,8,11,16 eight compared ACEIs and ARBS with other active control,10,13–15,17–20 and one compared ACEIs and ARBS with placebo and other active control.12 Of the contained 13 trials, five studies enrolled patients with type 1 diabetes,13,14,16,18,20 seven studies enrolled patients with type 2 diabetes7,8,10,12,15,17,19 and the remaining one included patients with either type 1 or type 2 diabetes.11 Ten trials compared ACEIs with placebo or active control,10,11,13–20 three studies compared ARBs with placebo or active control.7,8,12 The characteristics of the included studies are presented in Table 1.
Figure 1.
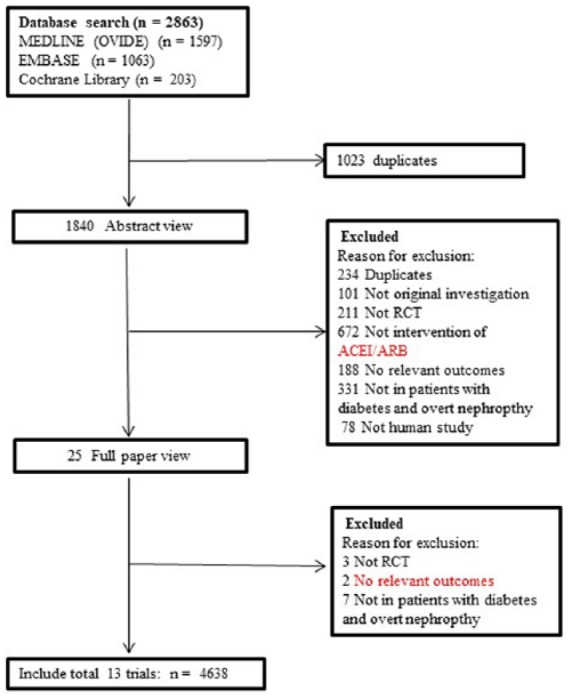
Process for identifying studies eligible for the meta-analysis.
Table 1.
Characteristics of patients with baseline of included studies.
| Trials | Treatment | Type of DM | No. of patients | Age (years) | Mean baseline SBP (mmHg) | Mean albuminuria | Serum creatinine (µmol/L) | Follow-up (years) |
|---|---|---|---|---|---|---|---|---|
| Parving, 1989 | ACEI/conventional antihypertensive agents | 1 | 32 | 32 | 128 | UAER (mg/day) 544.3 | NA | 1 |
| Bauer, 1992 | ACEI/placebo | 1 or 2 | 33 | 57 | 136 | Upro (mg/day) 2080 | NA | 1.5 |
| Bjorck, 1992 | ACEI/beta-blocker | 1 | 40 | 59.3 | 163 | UAER (mg/day) 2078 | NA | 2.2 |
| Lewis, 1993 | ACEI/placebo | 1 | 409 | 35 | 137 | UAER (mg/day) 2500 | 114.9 | 3 |
| Eiving, 1994 | ACEI/beta-blocker | 1 | 30 | 37 | 138 | UAER (mg/day) 2500 | 98 | 2 |
| BAKRIS, 1996 | ACEI/CCB/beta-blocker | 2 | 52 | 62 | 155 | Upro (mg/day) 3080 | 141 | 5.3 |
| Nielsen, 1997 | ACEI/beta-blocker | 2 | 43 | 61 | 172 | UAER (mg/day) 963 | NA | 3.5 |
| Fogari, 1999 | ACEI/CCB | 2 | 107 | 56.3 | 165 | UAER (mg/day) 792.2 | 176.8 | 2 |
| LISE, 2000 | ACEI/CCB | 1 | 52 | 35 | 155 | UAER (mg/day) 1556 | 128.1 | 4 |
| RENAAL, 2001 | ARB/placebo | 2 | 1513 | 60 | 152 | UACR (mg/g) 1237 | 168 | 3.4 |
| IDNT, 2003 | ARB/placebo/CCB | 2 | 1715 | 59.3 | 160 | Upro (mg/day) 3200 | 148 | 2.6 |
| Rachmani, 2004 | ACEI/diuretics | 2 | 46 | 58.8 | 128 | UACR (mg/g) 655.3 | 122 | 0.5 |
| ORIENT, 2011 | ARB/placebo | 2 | 566 | 57.9 | 140 | UACR (mg/mmol) 192 | 143 | 3.2 |
ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; CCB: calcium channel blocker; DM: diabetes mellitus; SBP: systolic blood pressure; UAER: urine albumin excretion rate; Upro: urine protein excretion rate; UACR: urinary albumin creatinine ratio; NA: not available.
Quantitative analysis
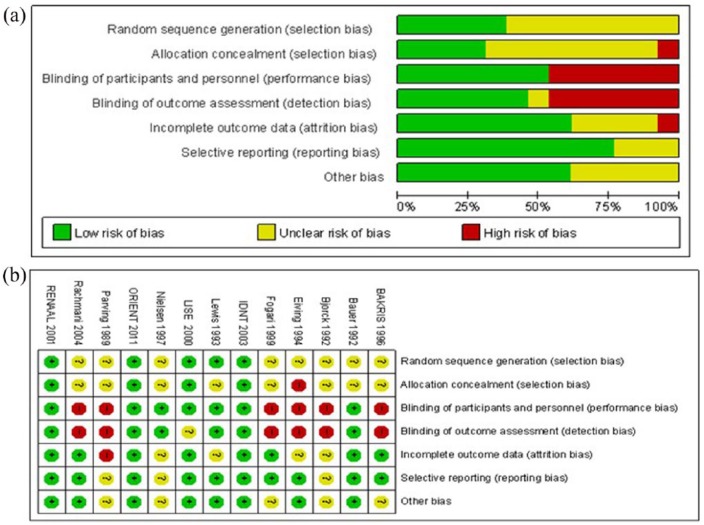
Sequence generation, allocation concealment, performance bias, detection bias, incomplete outcome data, selective reporting and other possible sources of bias were used to evaluate the quality of each study. The reported trial quality varied a little in different studies, and the summary of the risk of bias is presented in Figure 2.
Figure 2.
Risk of bias graph (a) and risk of bias summary (b).
Cardiovascular outcomes
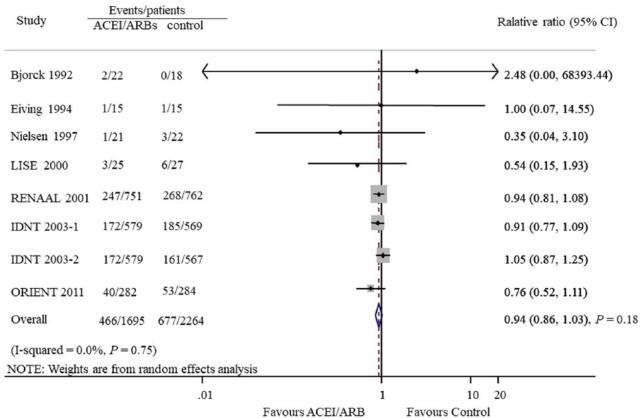
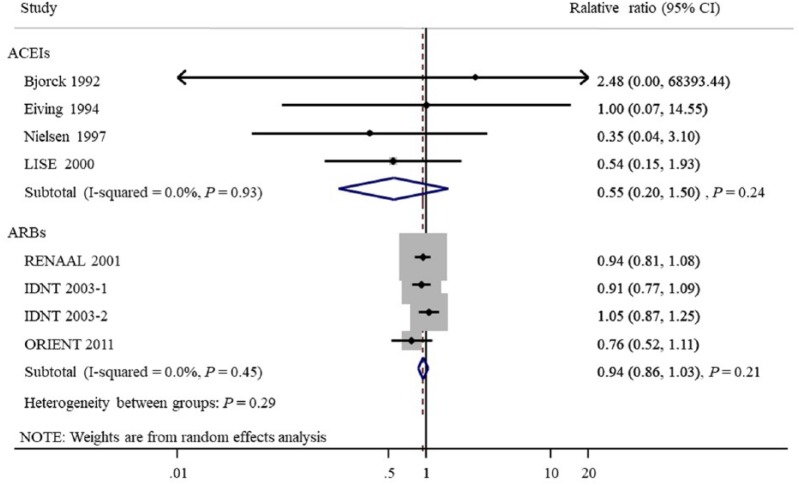
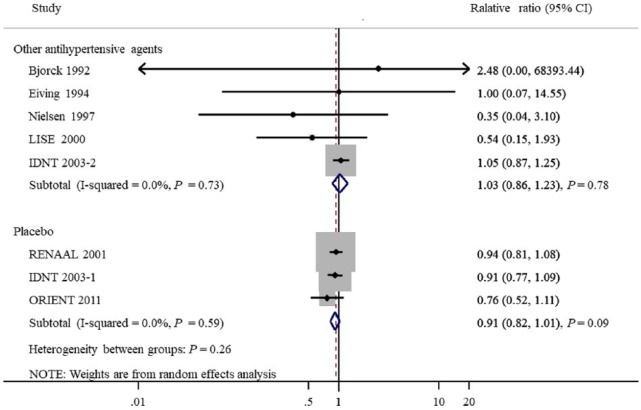
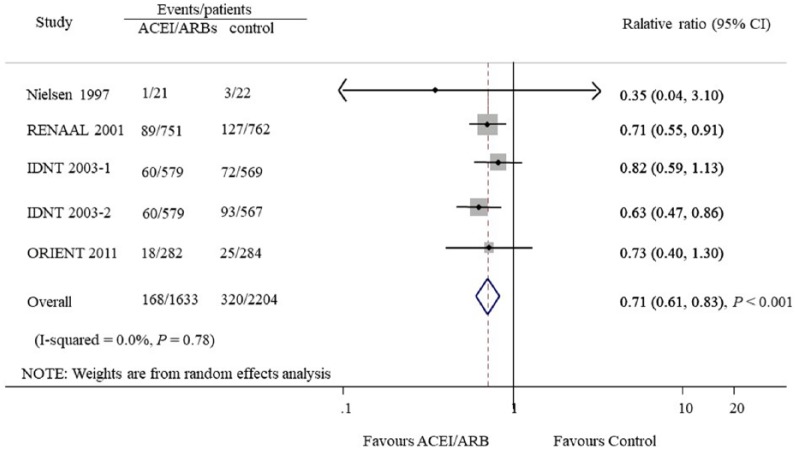
Seven studies provided 1143 CVD outcomes in 3959 patients included. Of the 1695 patients treated with ACEIs and ARBs there were 466 cardiovascular events (27.5%), and 677 events occurred in 2264 patients treated with placebo or active agents (29.9%). Overall, ACEI/ARB treatment did not reduce cardiovascular events compared with placebo or other antihypertensive agents (OR 0.94, 95% CI 0.86 to 1.03, P=0.18; I2=0.0%, Pfor heterogeneity=0.75, Figure 3). Subgroup analysis detected no significant difference between the two groups with regard to the different renin–angiotensin system inhibition category (ACEIs or ARBs, Figure 4) or control groups (placebo or active agents, Figure 5). Data regarding the effects of ACEIs and ARBs compared with placebo or active agents on HF were available from four trials with 168 events in 1633 patients with ACEI/ARB treatment (10.3%) and 320 events of the 2204 patients with placebo or active agent therapy (14.5%). Overall, ACEI/ARB therapy reduced the risk of HF events by 29% (0.71, 0.61 to 0.83, P<0.001) with no heterogeneity in the results of individual trials (I2=0%, P=0.78, Figure 6). This research included three trials on ARBs versus placebo and one trial on ACEIs versus beta-blockers. Subgroup analysis suggested that ARBs seemed to provide a higher probability of being beneficial for HF (0.71, 0.61 to 0.84).
Figure 3.
Effect of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers compared with placebo or other active agents on cardiovascular outcomes.
Figure 4.
Subgroup analysis of cardiovascular outcomes between angiotensin-converting enzyme inhibitors and angiotensin receptor blockers.
Figure 5.
Subgroup analysis of cardiovascular outcomes according to different control.
Figure 6.
Effect of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers compared with placebo or other active agents on heart failure.
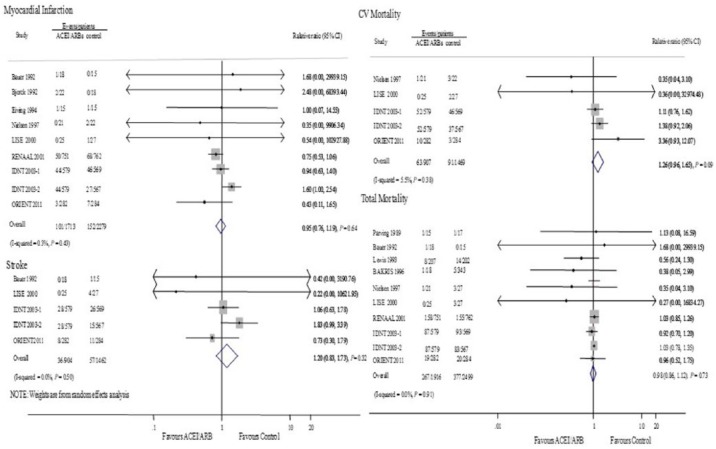
The events of MI, stroke and cardiovascular death were evaluated in eight, four and four studies, respectively. There were no significant differences between ACEIs and ARBs and the control group on the outcomes of MI (0.95, 0.76 to 1.19, P=0.64; I2=0%, P=0.43), stroke (1.20, 0.83 to 1.74, P=0.32; I2=0%, P=0.50) and cardiovascular death (1.26, 0.96 to 1.65, P=0.09; I2=0%, P=0.38, Figure 7).
Figure 7.
Effect of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers compared with placebo or other active agents on myocardial infarction, stroke, cardiovascular (CV) mortality and total mortality.
Total mortality
Nine studies involving 4415 patients evaluated the effect of ACEIs and ARBs on total mortality. A total of 276 deaths were available in 1916 patients in the ACEI/ARB group (14.4%), and 377 deaths in 2499 patients in the control group (15.1%). Total mortality did not differ significantly between the two groups (0.98, 0.86 to 1.12; P=0.73), with no evidence of heterogeneity (I2=0%, P=0.91, Figure 7).
Adverse effects
Eight trials reported at least one adverse event, and the data showed that 92 events occurred in 2030 patients with ACEI/ARB therapy treatment (4.5%) while 50 events occurred in 2608 patients with placebo (1.9%) (Table 2). Compared with control, ACEI/ARB therapy clearly increases the risk of adverse effects (OR 2.44, 95% CI 1.72 to 3.46, P<0.001). Among all kinds of adverse effects, ACEI/ARB therapy increased the incidence of hyperkalemia (2.26, 1.42 to 3.61, P=0.001), but did not increase the risk of cough (2.31, 0.80 to 6.67, P=0.12), hypotension (0.82, 0.00 to 1537.21, P=0.96) and oedema (0.46, 0.16 to 1.35, P=0.16).
Table 2.
Adverse events reported in the included randomised controlled trials.
| Adverse events | Studies reporting (n) | ACEI/ARB group (n/n) | Control group (n/n) | OR (95% CI) | P value |
|---|---|---|---|---|---|
| Total patients with adverse events | 8 | 92/2030 | 50/2608 | 2.44 (1.72–3.46) | <0.001* |
| Specific adverse events | |||||
| Hyperkalemia | 6 | 52/1855 | 25/2433 | 2.26 (1.42–3.61) | 0.001* |
| Hypotension | 5 | 23/141 | 9/159 | 2.31 (0.80–6.67) | 0.12 |
| Cough | 4 | 2/68 | 2/67 | 0.82 (0.00–1537.21) | 0.96 |
| Oedema | 4 | 4/97 | 14/114 | 0.46 (0.16–1.35) | 0.16 |
OR: odds ratio; CI: confidence interval.
P<0.05 was considered significant.
Risk of bias
Begg’s and Egger’s quantitative tests showed there was no evidence of publication bias for cardiovascular outcomes (P=0.90, Figure 8).
Figure 8.
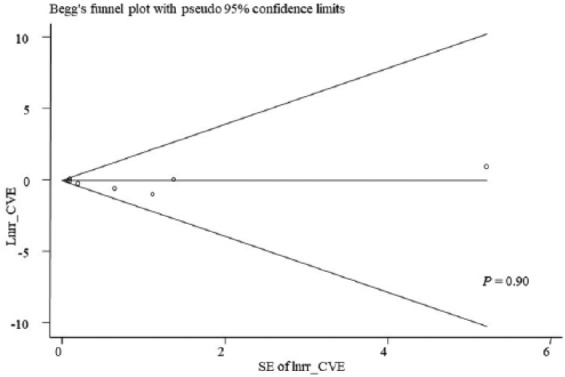
Forest plot for evaluation of publication bias for cardiovascular outcomes.
Discussion
The presence of kidney disease is associated with a markedly elevated risk of CVD and death in patients with DM. The beneficial effects of ACEIs or ARBs on cardiovascular outcomes in patients with diabetes and overt nephropathy remain controversial. This large quantitative review, including 13 trials, more than 4500 participants, 1143 cardiac vascular events, suggested that ACEI/ARB therapy did not confer cardiovascular protection and total mortality compared with control in patients with diabetes and overt nephropathy. It must be noted that patients in the ACEI/ARB group had a high risk of side effects such as hyperkalemia.
Diabetes patients with albuminuria are at increased risk of CVD as compared to diabetes patients with normal albumin excretion. Several studies have provided high-quality evidence that ACEIs and ARBs could reduce the risk of kidney outcomes in patients with diabetes and overt nephropathy; however, no clear effect on cardiovascular outcomes has been established.21–23 The question of whether ACEIs and ARBs exert a cardiovascular benefit if added after optimisation of supportive treatment is still unresolved. A systematic overview published in 2015 that included 119 RCTs and more than 60,000 patients with DKD by Xie et al., found that both ACEIs and ARBs produced odds reductions for cardiovascular outcomes versus control.24 However, that analysis included all types of DKD patients, we still did not definitively answer questions as to which patients might benefit more and which not. Palmer et al.25 have conducted a large-scale network meta-analysis with diabetes and kidney diseases and put forward the results that ARB monotherapy was superior to placebo for the prevention of MI, but stroke and cardiovascular mortality were not significant for either ACEIs or ARBs. Data for these outcomes come from patients who had micro or macro albuminuria. The Irbesartan Diabetic Nephropathy Trial (IDNT) in which 1715 patients reported 518 cardiovascular events showed that irbesartan did not confer cardiovascular protection compared with placebo or amlodipine.12 A similar neutrality trend was also noted in the study of Tarnow et al.20 Consistent with these negative effects, in this meta-analysis, we found there was no association between ACEI/ARB treatment and fewer cardiovascular events or lower total mortality. Further subgroup analysis did not show a significant modifying effect of cardiovascular outcomes according to different control groups or renin–angiotensin system inhibition type. One possible reason may be that some studies have excluded patients with clinically significant CVD, which lacked statistical power to make a definite answer. Another reason is that many diabetes patients with overt nephropathy have more than one risk factor, leading to an even higher risk of cardiovascular outcomes. These confounding factors, including disorders of dyslipidemia, thrombotic and embolic events, and fluid volume overload, could modify the beneficial effect of ACEIs and ARBs. These may explain the observations made regarding the negative effect of ACEIs and ARBs on CVD. Our results found that ACEI/ARB use reduced HF events in these individuals. HF, as for the only significant result, this research included three trials on ARBs compared with placebo and one trial on ACEIs compared with beta-blockers. A subgroup analysis was conducted and found that ARBs provided a higher probability of being beneficial for HF. So it may represent the positive effects for ARB monotherapy over placebo on the prevention of HF. The effectiveness of ARBs in reducing HF has only been assessed in three studies. Thus whether ARB therapy reduced cardiovascular events could not be conclusively determined. Therefore, studies with large samples are strongly recommended to confirm the effect of ACEIs or ARBs on HF events.
Safety is an important concern with the use of ACEIs and ARBs in patients with DM and overt nephropathy. It should be noted that in our meta-analysis ACEI/ARB therapy increased the risk of adverse events by 144%. We found hyperkalemia was the most common side effect, increased by 116% in the ACEI/ARB group. The incidence of cough, hypotension and oedema were not increased in the ACEI/ARB group. Hence, we still need to be cautious about using ACEIs and ARBs, because some side effects, especially hyperkalemia, can be triggered by this therapy.
The strengths of this meta-analysis were the large volume of cardiovascular outcomes included and the rigorous methodology used. However, our study also has the following limitations. First, this analysis was mainly dominated by three large studies (RENAAL 2001, IDNT 2003-2 and ORIENT 2011; accounting for 90% of the weight), although exclusion of these studies did not change the final results. Second, the existence of potential confounding factors could not be excluded. Different agents of ACEIs or ARBs might not have the same risk–benefit ratio in diabetes patients with overt nephropathy.
Conclusion
Overall, ACEIs and ARBs did not reduce cardiovascular morbidity and mortality in patients with diabetes and overt nephropathy. The clinical significance of the results requires confirmation with further high-quality RCTs.
Acknowledgments
The authors would like to thank all authors whose publications were included in our meta-analysis.
Footnotes
Author contribution: Dong Xue designed the study; Fan Shunan and Yuan Jiqing extracted and analysed data; Fan Shunan drafted the manuscript. All authors agree to be accountable for all aspects of the work.
Declaration of conflicting interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical privacy statement: No approval was required for ethical privacy because the study does not involve direct contact with patients or their personal information.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Dong Xue  https://orcid.org/0000-0002-4732-178X
https://orcid.org/0000-0002-4732-178X
References
- 1. Fox CS, Matsushita K, Woodward M, et al. ; Chronic Kidney Disease Prognosis Consortium. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet 2012; 380: 1662–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tonelli M, Muntner P, Lloyd A, et al. ; Alberta Kidney Disease Network. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet 2012; 380: 807–814. [DOI] [PubMed] [Google Scholar]
- 3. Perkovic V, Agarwal R, Fioretto P, et al. ; Conference Participants. Management of patients with diabetes and CKD: conclusions from a “kidney disease: improving global outcomes” (KDIGO) controversies conference. Kidney Int 2016; 90: 1175–1183. [DOI] [PubMed] [Google Scholar]
- 4. Roberts MA. Commentary on the KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Nephrology 2014; 19: 53–55. [DOI] [PubMed] [Google Scholar]
- 5. Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the Hope Study and Micro-Hope Substudy. Lancet 2000; 355: 253–259. [PubMed] [Google Scholar]
- 6. Lindholm LH, Ibsen H, Dahlof B, et al. ; Life Study Group. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention for Endpoint Reduction in Hypertension Study (LIFE): a randomised trial against atenolol. Lancet 2002; 359: 1004–1010. [DOI] [PubMed] [Google Scholar]
- 7. Brenner BM, Cooper ME, de Zeeuw D, et al. ; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345: 861–869. [DOI] [PubMed] [Google Scholar]
- 8. Imai E, Chan JC, Ito S, et al. ; ORIENT Study Investigators. Effects of olmesartan on renal and cardiovascular outcomes in type 2 diabetes with overt nephropathy: a multicentre, randomised, placebo-controlled study. Diabetologia 2011; 54: 2978–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Higgins JP, Altman DG, Gotzsche PC, et al. ; Cochrane Statistical Methods Group. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bakris GL, Copley JB, Vicknair N, et al. Calcium channel blockers versus other antihypertensive therapies on progression of niddm associated nephropathy. Kidney Int 1996; 50: 1641–1650. [DOI] [PubMed] [Google Scholar]
- 11. Bauer JH, Reams GP, Hewett J, et al. A randomized, double-blind, placebo-controlled trial to evaluate the effect of enalapril in patients with clinical diabetic nephropathy. Am J Kidney Dis: the official journal of the National Kidney Foundation 1992; 20: 443–457. [DOI] [PubMed] [Google Scholar]
- 12. Berl T, Hunsicker LG, Lewis JB, et al. ; Irbesartan Diabetic Nephropathy Trial, Collaborative Study Group. Cardiovascular outcomes in the irbesartan diabetic nephropathy trial of patients with type 2 diabetes and overt nephropathy. Ann Intern Med 2003; 138: 542–549. [DOI] [PubMed] [Google Scholar]
- 13. Bjorck S, Mulec H, Johnsen SA, et al. Renal protective effect of enalapril in diabetic nephropathy. BMJ 1992; 304: 339–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elving LD, Wetzels JF, van Lier HJ, et al. Captopril and atenolol are equally effective in retarding progression of diabetic nephropathy. Results of a 2-year prospective, randomized study. Diabetologia 1994; 37: 604–609. [DOI] [PubMed] [Google Scholar]
- 15. Fogari R, Zoppi A, Corradi L, et al. Long-term effects of ramipril and nitrendipine on albuminuria in hypertensive patients with type II diabetes and impaired renal function. J Hum Hypertens 1999; 13: 47–53. [DOI] [PubMed] [Google Scholar]
- 16. Lewis EJ, Hunsicker LG, Bain RP, et al. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The collaborative study group. N Engl J Med 1993; 329: 1456–1462. [DOI] [PubMed] [Google Scholar]
- 17. Nielsen FS, Rossing P, Gall MA, et al. Long-term effect of lisinopril and atenolol on kidney function in hypertensive niddm subjects with diabetic nephropathy. Diabetes 1997; 46: 1182–1188. [DOI] [PubMed] [Google Scholar]
- 18. Parving HH, Hommel E, Damkjaer Nielsen M, et al. Effect of captopril on blood pressure and kidney function in normotensive insulin dependent diabetics with nephropathy. BMJ 1989; 299: 533–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rachmani R, Slavachevsky I, Amit M, et al. The effect of spironolactone, cilazapril and their combination on albuminuria in patients with hypertension and diabetic nephropathy is independent of blood pressure reduction: a randomized controlled study. Diabetic Med: a journal of the British Diabetic Association 2004; 21: 471–475. [DOI] [PubMed] [Google Scholar]
- 20. Tarnow L, Rossing P, Jensen C, et al. Long-term renoprotective effect of nisoldipine and lisinopril in type 1 diabetic patients with diabetic nephropathy. Diabetes Care 2000; 23: 1725–1730. [DOI] [PubMed] [Google Scholar]
- 21. Maione A, Navaneethan SD, Graziano G, et al. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers and combined therapy in patients with micro- and macroalbuminuria and other cardiovascular risk factors: a systematic review of randomized controlled trials. Nephrol Dial Transplant 2011; 26: 2827–2847. [DOI] [PubMed] [Google Scholar]
- 22. Mann JF, Green D, Jamerson K, et al. Avosentan for overt diabetic nephropathy. J Am Soc Nephrol 2010; 21: 527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ravid M, Lang R, Rachmani R, et al. Long-term renoprotective effect of angiotensin-converting enzyme inhibition in non-insulin-dependent diabetes mellitus: a 7-year follow-up study. Arch Intern Med 1996; 156: 286–289. [PubMed] [Google Scholar]
- 24. Xie X, Liu Y, Perkovic V, et al. Renin–angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a Bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis: the official journal of the National Kidney Foundation 2016; 67: 728–741. [DOI] [PubMed] [Google Scholar]
- 25. Palmer SC, Mavridis D, Navarese E, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet 2015; 385: 2047–2056. [DOI] [PubMed] [Google Scholar]