Abstract
Background:
The aim of the study was to compare and correlate glycated high-density lipoprotein (GHDL-C) and glycated low-density lipoprotein (GLDL-C) plasma levels with adiposity indices [weight/hip ratio (WHR) and body adiposity index (BAI)], lipid ratios and hematological indices [platelet/lymphocyte ratio (PLR), monocyte/lymphocyte ratio (MLR)].
Methods:
This was a cross-sectional study of 30 nondiabetic metabolic syndrome (MetS) patients, 30 prediabetic or type 2 diabetes mellitus (T2DM) patients and 30 normoglycemic controls.
Results:
Remarkably both GHDL-C and GLDL-C levels lacked any intergroup statistically significant discrepancy in either MetS or MetS-pre/T2DM versus control (p > 0.05). Unlike GLDL-C/LDL-C ratios for either MetS groups; there were highly significant intergroup differences in the means of GHDL-C/HDL-C ratios when comparing both nondiabetic MetS and MetS-pre/T2DM groups versus controls (p = 0.001). In MetS patients; GHDL-C and GLDL-C proportionally correlated with WHR (p < 0.05). Also, MetS GHDL-C correlated inversely with MLR and monocytes (p < 0.05). In MetS-pre/T2DM; GLDL-C directly correlated with BAI, platelet count and PLR (p < 0.05).
Conclusion:
GLDL-C and GHDL-C are dysfunctional glucolipotoxicity lipoproteins and may present putatively surrogate biomarkers for prediction/prevention of metabolic disturbances.
Keywords: Glycated low-density lipoprotein cholesterol (GLDL-C), glycated high-density lipoprotein cholesterol (HDL-C), type 2 diabetes mellitus, metabolic syndrome, prediabetes
Introduction
Diabetes mellitus (DM) is a metabolic disorder identified by hyperglycemia and consists of several types. Of which, type 2 DM (T2DM) could occur due to insulin resistance (IR) and obesity.1 Whereas, prediabetes (pre-DM) is a state where glucose levels are elevated but individuals are neither DM patients nor normal healthy individuals.2 Closely related to DM is metabolic syndrome (MetS) which is a cluster of risk factors caused mainly by IR and abdominal obesity, leading to precipitating risk of cardiovascular diseases (CVD), stroke and diabetes.2,3 Thus IR and hyperinsulinemia are hallmarks of the metabolic syndrome.4 The hyperinsulinemic/euglycemic clamp has recently emerged as the gold standard method to detect IR, though clinically impractical to do so. Subsequently a surrogate marker for IR is needed. Recently, several studies have reported that the lipid ratios triglyceride (TG)/high-density lipoprotein (HDL)-C, total cholesterol (TC)/HDL-C, and low-density lipoprotein (LDL)-C/HDL-C are surrogate marker of IR.5 Coupled with hyperglycemia is glycation. Glycation is a normal nonenzymatic reaction between reducing sugars and proteins, lipids or nucleic acids, however unfavorable.6 Resulting from glycation are products called circulating advanced glycation end-products which bind to lipoproteins and delay their clearance.7 Hyperglycemia increases the formation of oxidized and glycated LDL-C (GLDL-C), which are important modulators of atherosclerosis and CVD.8 T2DM features important modification of both LDL-C and HDL-C.9 Plasma levels of HDL-C are decreased during T2DM due to increased catabolism of HDL-C particles. Furthermore, qualitative abnormalities of HDL-C are described; such as significant reduction in their antioxidative and endothelium-dependent vasorelaxant properties.10 TGs are also affected in T2DM. Hypertriglyceridemia produces atherogenic, small, dense forms of LDL-C and decreases cholesterol transport to the liver by HDL-C. Therefore, high value of the TG/HDL-C ratio in the present work can enable identification of diabetic patients at a higher risk of CVD.11 Other emerging indices are hematological indices, such as mean platelet volume (MPV), platelet (PLT) count and white blood cell subtypes. They are among the inflammatory markers that are considered as reliable prognostic indicators for diabetes complications.12,13 In addition, atherogenic index of plasma (AIP; log TG/HDL-C) has lately been considered as a surrogate estimate of the atherogenicity dyslipidemia among T2DM patients.14,15 Also, adiposity markers such as conicity index (CI), body adiposity index (BAI) and waist-height ratio (WHtR) are investigated for the estimation of the risk of coronary heart diseases.16,17
Participants, materials and methods
Study participants
The study was conducted at The University of Jordan Hospital (UoJH) at the diabetes and endocrinology outpatient clinics. The candidates were divided into: healthy lean normoglycemic control group with 30 participants, 30 patients in a non-DM MetS group, and 30 patients in a pre-DM/newly diagnosed T2DM MetS group. The study was approved by the scientific research committee at the School of Pharmacy at The University of Jordan and by the UoJH institutional review board. All potential candidates were approached and informed thoroughly about the study, with a written informed consent.
Anthropometric measurements
Weight and height were measured using a balance mounted stadiometer. Waist circumference (WC) was measured using a nonstretchable tape at the midpoint between the last rib and the upper iliac crest, and hip circumference (HC) was measured around the widest section of the buttocks. Body mass index (BMI) was calculated as body weight (kg) divided by the square of height (m²). WHR and WHtR were calculated by dividing the WC (cm) by HC (cm) and height respectively. CI and Body adiposity index (BAI) were calculated using the following formulae. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured using a digital blood pressure meter.
CI formula by Nadeem:17
Body adiposity index (BAI) formula by Geliebter:18
Hematological and biochemical analysis
Blood samples were drawn using lithium heparin and ethylenediaminetetraacetic acid (EDTA)-coated test tubes, centrifuged and stored at −20°C for analysis. Analysis of Complete Blood Count (CBC), HbA1c, fasting blood glucose (FBG) and lipid profile were conducted, and AIP was calculated. Metabolic biomarkers GLDL-C and glycated HDL-C (GHDL-C) serum levels were estimated using sandwich enzyme-linked immunosorbent assay (ELISA) based on the instructions of the manufacturer (human ELISA kits; MyBioscience, USA). See Table 1 for the ELISA kit precision.
Table 1.
ELISA kit precision.
| %CV | GLDL-C | Inter-assay <10% |
| Intra-assay <10% | ||
| GHDL-C | Inter-assay <15% | |
| Intra-assay <15% |
CV, Coefficient Of Variation; GHDL, glycated high-density lipoprotein; GLDL, glycated low-density lipoprotein.
Statistical analysis
Data were expressed as mean ± SE (standard error). Comparisons were performed by analysis of covariance (ANCOVA) with age as a covariate. A Spearman correlation was implemented for correlations and p < 0.05 was determined as statistically significant. Data were analyzed using SPSS version 22 (SPSS, Inc.,Chicago Illinois, USA).
Results
General and clinical characteristics
Table 2 shows the clinical characteristics of study participants. The mean age is significantly higher in the MetS pre-DM/T2DM group compared with the nondiabetic MetS and control group. The BMI, WC, HC and lipid profile were also significantly greater in both MetS groups. Mean FBG and HbA1c were significantly higher in the pre-DM/T2DM MetS group compared with the nondiabetic and control group.
Table 2.
Comparison of metabolic biomarkers, clinical characteristics, adiposity, atherogenicity and hematological indices between study groups.
| Parameter | MetS pre-DM/T2DM | Nondiabetic MetS | Control | p-value* | p-value** | p-value*** |
|---|---|---|---|---|---|---|
| Molecular metabolic risk biomarkers | ||||||
| GHDL-C (mg/dl) | 25.6 ± 3.9 | 23.9 ± 4.8 | 21.9 ± 3.4 | 0.842 | 0.420 | 0.769 |
| GHDL-C (mmol/l) | 1.42 ± 0.27 | 1.33 ± 0.19 | 1.22 ± 0.21 | 0.769 | 0.420 | 0.842 |
| GLDL-C (mg/dl) | 35.9 ± 0.25 | 33.6 ± 0.22 | 31.3 ± 0.33 | 0.812 | 0.797 | 0.920 |
| GLDL-C (mmol/l) | 0.81 ± 0.33 | 0.86 ± 0.22 | 0.93 ± 0.25 | 0.920 | 0.797 | 0.812 |
| GLDL-C (mg/dl)/LDL-C (mg/dl) | 0.27 ± 0.09 | 0.28 ± 0.05 | 0.32 ± 0.020 | 0.606 | 0.056 | 0.23 |
| GHDL-C (mg/dl)/HDL-C (mg/dl) | 0.64 ± 0.31 | 0.58 ± 0.16 | 0.45 ± 0.06 | 0.131 | 0.001 | 0.001 |
| Clinical parameters | ||||||
| Agea (years) | 51.80 | 44.79 | 30.48 | |||
| BMI (kg\m²) | 34.44 ±.91 | 33.64 ± 0.82 | 21.48 ± 0.99 | 0.001a | 0.001b | 0.390c |
| WC (cm) | 105.64 ± 1.77 | 103.22 ± 1.60 | 78.67 ± 1.92 | 0.001 | 0.001 | 0.324 |
| HC (cm) | 119.28 ± 1.97 | 116.40 ± 1.78 | 93.96 ± 2.14 | 0.001 | 0.001 | 0.150 |
| SBP (mmHg) | 131. 21 ± 2.93 | 131. 69 ± 2.65 | 112.16 ± 3.17 | 0.001 | 0.001 | 0.888 |
| DBP (mmHg) | 81.22 ± 2 | 79.67 ± 1.81 | 69.90 ± 2.17 | 0.001 | 0.001 | 0.643 |
| FBG (mg/dl) | 122.48 ± 4.72 | 91.81 ± 4.26 | 85.80 ± 5.11 | 0.404 | 0.001 | 0.001 |
| HbA1c% | 6.37 ± 0.14 | 5.21 ± 0.13 | 5.13 ± 0.15 | 0.724 | 0.001 | 0.001 |
| TG (mg/dl) | 211.34 ± 18.34 | 187.67 ± 16.57 | 64.93 ± 19.86 | 0.001 | 0.001 | 0.296 |
| LDL-C (mg/dl) | 134.36 ± 6.92 | 122.10 ± 6.25 | 98.24 ± 7.49 | 0.016 | 0.002 | 0.161 |
| LDL-C (mmol/l) | 7.46 ± 0.38 | 6.77 ± 0.34 | 5.45 ± 0.41 | 0.016 | 0.002 | 0.161 |
| HDL-C (mg/dl) | 39.98 ± 2.08 | 40.90 ± 1.88 | 47.64 ± 2.26 | 0.203 | 0.071 | 0.368 |
| HDL-C (mmol/l) | 2.21 ± 0.115 | 2.26 ± 0.104 | 2.64 ± 0.125 | 0.203 | 0.071 | 0.368 |
| TC (mg/dl) | 216.38 ± 7.79 | 200.48 ± 7.04 | 159.77 ± 8.44 | 0.001 | 0.001 | 0.153 |
| Adiposity indices | ||||||
| WHR | 0.89 ± .01 | 0.89 ± .01 | 0.84 ± 0.01 | 0.993 | 0.033 | 0.016 |
| WHtR | 0.63 ± .01 | 0.62 ± .01 | 0.48 ± 0.01 | 0.433 | <0.001 | <0.001 |
| CI | 1.28 ± .01 | 1.26 ± .01 | 1.21 ± .01 | 0.460 | 0.002 | 0.005 |
| BAI | 36.68 ± 1.23 | 35.94 ± 1.12 | 26.47 ± 1.34 | 0.647 | <0.001 | <0.001 |
| Hematological indices | ||||||
| RDW-CV % | 13.82 ± 0.21 | 13.52 ± 0.19 | 13.47 ± 0.22 | 0.217 | 0.318 | 0.88 |
| MPV (fl) | 8.66 ± 0.39 | 9.03 ± 0.35 | 6.92 ± 0.42 | 0.467 | 0.009 | <0.001 |
| PLT count (×10^3/l) | 262.35 ± 12.15 | 275.98 ± 10.98 | 234.76 ± 13.16 | 0.393 | 0.175 | 0.023 |
| Monocytes (×10^3/l) | 0.43 ± 0.04 | 0.55 ± 0.04 | 0.24 ± 0.05 | 0.040 | 0.003 | <0.001 |
| Neutrophils (×10^3/l) | 4.66 ± 0.30 | 4.42 ± 0.273 | 4.91 ± 0.327 | 0.541 | 0.627 | 0.273 |
| Lymphocytes (×10^3/l) | 2.88 ± 0.16 | 2.35 ± 0.14 | 2.54 ± 0.17 | 0.012 | 0.206 | 0.396 |
| MLR | 0.16 ± 0.09 | 0.24 ± 0.02 | 0.10 ± .020 | 0.001 | 0.052 | <0.001 |
| NLR | 1.70 ± 0.14 | 1.94 ± 0.13 | 2.10 ± 0.16 | 0.202 | 0.096 | 0.449 |
| PLR | 96.40 ± 8.39 | 124.91 ± 7.58 | 105.92 ± 9.08 | 0.011 | 0.495 | 0.125 |
| Atherogenicity indices | ||||||
| LDL-C/HDL-C | 3.53 ± 0.20 | 3.08 ± 0.18 | 2.11 ± 0.22 | 0.098 | <0.001 | 0.002 |
| TC/HDL-C | 5.69 ± 0.28 | 5.14 ± 0.26 | 3.37 ± 0.31 | 0.100 | <0.001 | <0.001 |
| AIP | 0.69 ± 0.05 | 0.64 ± 0.05 | 0.19 ± 0.05 | 0.436 | <0.001 | <0.001 |
MetS pre-DM/T2DM versus nondiabetic MetS.
MetS pre-DM/T2DM versus control.
Nondiabetic MetS versus control.
Data obtained by analysis of covariance.
Covariates appearing in the model are evaluated at the following values: age = 42.47.
AIP, atherogenic index of plasma; BAI, body adiposity index; BMI, body mass index (kg\m²); CI, conicity index; DBP, diastolic blood pressure (mmHg); DM, diabetes mellitus; FBG, fasting blood glucose (mg/dl); GHDL-C, glycated high-density lipoprotein; GLDL-C, glycated low-density lipoprotein (mg/dl); HbA1c, hemoglobin A1c (%); HC, hip circumference (cm); HDL-C, high-density lipoprotein (mg/dl); LDL-C, low-density lipoprotein (mg/dl); MetS, metabolic syndrome; MLR, monocyte-to-lymphocyte ratio (×10^3/l); MPV, mean platelet volume (fl); NLR, neutrophil-to-lymphocyte ratio (×10^3/l); PLT, platelet count (×10^3/l); PLR, platelet-to-lymphocyte ratio; RDW, red blood cell distribution width (CV%); SBP, systolic blood pressure (mmHg); T2DM, type 2 diabetes mellitus; TC, total cholesterol (mg/dl); TG, triglycerides (mg/dl); WC, waist circumference (cm); WHR, waist-to-hip ratio; WHtR, waist to height ratio.
Comparisons of indices
Table 2 shows the WHtR (p < 0.05), CI (p < 0.01) and BAI (p < 0.001) were higher in the MetS and MetS-pre/T2DM group when compared with controls. As for hematological indices, the PLR showed intergroup difference in nondiabetic MetS patients versus MetS-pre/T2DM with significance (p < 0.05) being higher in the MetS-nondiabetic group. Whereas MPV in both MetS groups was higher than in controls.
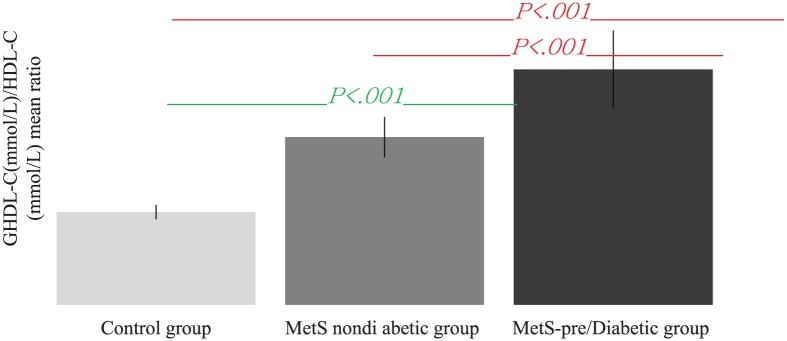
TG/HDL-C and AIP had significant intergroup difference (p < 0.001) when compared between healthy participants and both MetS and MetS-pre/T2DM. GHDL (mmol/l) and GLDL (mmol/l) lacked any statistically significant variations in nondiabetic MetS and MetS-pre/T2DM groups (p > 0.05; Table 3). Interestingly, when introducing the ratio of the means of GHDL-C/HDL-C, there were highly significant intergroup differences (p < 0.001) when comparing both nondiabetic MetS and MetS-pre/T2DM groups versus the healthy control group (Table 3 and Figure 1). However, GLDL-C/LDL-C did not show the same behavior for both MetS groups versus controls (Figure 2).
Table 3.
The intra-group correlations of the metabolic biomarkers with adiposity, hematologic and atherogenic indices.
| Parameter | Correlation | MetS-pre-DM/T2DM | Nondiabetic MetS | Control | |||
|---|---|---|---|---|---|---|---|
| GHDL-C (mg/dl) | GLDL-C (mg/dl) | GHDL-C (mg/dl) |
GLDL-C (mg/dl) |
GHDL-C (mg/dl) |
GLDL-C (mg/dl) |
||
| Adiposity indices | |||||||
| CI | * R | −0.208 | −0.192 | −0.134 | 0.062 | −0.196 | 0.581 ** |
| Sig (2-tailed) | 0.271 | 0.309 | 0.490 | 0.746 | 0.299 | 0.001 | |
| N | 30 | 30 | 29 | 30 | 30 | 29 | |
| BAI | R | 0.134 | 0.465** | 0.162 | −0.106 | −0.088 | −0.061 |
| Sig (2-tailed) | 0.479 | 0.010 | 0.402 | 0.577 | 0.645 | 0.752 | |
| N | 30 | 30 | 29 | 30 | 30 | 29 | |
| WHR | R | −0.254 | −0.188 | 0.377* | 0.601** | 0.099 | 0.415* |
| Sig (2-tailed) | 0.175 | 0.321 | 0.044 | 0.000 | 0.602 | 0.025 | |
| N | 30 | 30 | 29 | 30 | 30 | 29 | |
| WHtR | R | −0.064 | 0.313 | 0.187 | 0.175 | 0.108 | 0.518** |
| Sig (2-tailed) | 0.737 | 0.092 | 0.331 | 0.356 | 0.570 | 0.004 | |
| N | 30 | 30 | 29 | 30 | 30 | 29 | |
| Hematological indices | |||||||
| RDW-CV% | R | 0.017 | −0.076 | −0.141 | 0.038 | −0.157 | 0.220 |
| Sig (2-tailed) | 0.931 | 0.689 | 0.465 | 0.842 | 0.406 | 0.252 | |
| N | 30 | 30 | 29 | 30 | 30 | 29 | |
| MPV (fl) | R | 0.258 | 0.000 | −0.265 | 0.066 | −0.464** | −0.439* |
| Sig (2-tailed) | 0.169 | 1.000 | 0.165 | 0.729 | 0.010 | 0.017 | |
| N | 30 | 30 | 29 | 30 | 30 | 29 | |
| PLT count (×10^3/l) | R | 0.067 | 0.424 * | 0.161 | 0.133 | −0.272– | −0.018 |
| Sig (2-tailed) | 0.723 | 0.020 | 0.403 | 0.482 | 0.146 | 0.925 | |
| N | 30 | 30 | 29 | 30 | 30 | 29 | |
| Monocytes (×10^3/l) | R | 0.063 | 0.328 | −0.388 * | 0.167 | 0.116 | 0.045 |
| Sig (2-tailed) | 0.741 | 0.077 | 0.037 | 0.377 | 0.542 | 0.816 | |
| N | 30 | 30 | 29 | 30 | 30 | 29 | |
| Neutrophils (×10^3/l) | R | 0.159 | 0.262 | −0.118 | −0.111 | 0.382 * | 0.010 |
| Sig (2-tailed) | 0.401 | 0.161 | .540 | 0.559 | 0.037 | .958 | |
| N | 30 | 30 | 29 | 30 | 30 | 29 | |
| Lymphocytes (×10^3/l) |
R | −0.175 | −0.110 | 0.088 | 0.075 | 0.062 | −0.073 |
| Sig (2-tailed) | 0.356 | 0.564 | 0.651 | 0.695 | 0.746 | 0.706 | |
| N | 30 | 30 | 29 | 30 | 30 | 29 | |
| MLR | R | −0.016 | 0.351 | −0.465* | 0.118 | 0.098 | 0.061 |
| Sig (2-tailed) | 0.934 | 0.057 | 0.011 | 0.534 | 0.605 | 0.753 | |
| N | 30 | 30 | 29 | 30 | 30 | 29 | |
| NLR | R | 0.223 | 0.344 | −0.236 | −0.143 | 0.304 | 0.153 |
| Sig (2-tailed) | 0.236 | 0.063 | 0.218 | 0.449 | 0.103 | 0.429 | |
| N | 30 | 30 | 29 | 30 | 30 | 29 | |
| PLR | R | 0.109 | 0.363 * | 0.039 | 0.057 | −0.128 | 0.047 |
| Sig (2-tailed) | 0.566 | 0.048 | 0.840 | 0.766 | 0.500 | 0.809 | |
| N | 30 | 30 | 29 | 30 | 30 | 29 | |
| Atherogenicity indices | |||||||
| AIP | R | 0.320 | −0.020 | 0.038 | −0.139 | −0.018 | 0.267 |
| Sig (2-tailed) | 0.085 | 0.915 | 0.846 | 0.464 | 0.926 | 0.161 | |
| N | 30 | 30 | 29 | 30 | 30 | 29 | |
| TC/HDL-C ratio | R | 0.172 | 0.079 | 0.132 | −0.133 | 0.061 | 0.289 |
| Sig (2-tailed) | 0.362 | 0.677 | 0.495 | 0.483 | 0.748 | 0.129 | |
| N | 30 | 30 | 29 | 30 | 30 | 29 | |
| LDL-C/HDL-C ratio | R | 0.015 | 0.055 | 0.129 | −0.101 | 0.053 | 0.245 |
| Sig (2-tailed) | 0.938 | 0.771 | 0.504 | 0.595 | 0.783 | 0.199 | |
| N | 30 | 30 | 29 | 30 | 30 | 29 | |
Spearman correlation coefficient (r) was used, r = 0.1–.0.29 low relationship, r = 0.3–0.49 moderate relationship, r > 0.5 good relationship.
AIP, atherogenic index of plasma; BAI, body adiposity index; CI, conicity index; DM, diabetes mellitus; GHDL-C, glycated high-density lipoprotein; GLDL-C, glycated low-density lipoprotein; MetS, metabolic syndrome; MLR, monocyte-to-lymphocyte ratio; MPV, mean platelet volume; NLR, neutrophil-to-lymphocyte ratio; PLT, platelet; PLR, platelet-to-lymphocyte ratio; RDW, red blood cell distribution width; T2DM, type 2 diabetes mellitus; WHR, waist-to-hip ratio; WHtR, waist to height ratio.
Figure 1.
Intergroup comparisons of means of GHDL-C/HDL-C ratios.
GHDL-C, glycated high-density lipoprotein-cholesterol; GLDL-C, glycated low-density lipoprotein-cholesterol; HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol; MetS, metabolic syndrome.
Figure 2.
Intergroup comparisons of means of GLDL-C/LDL-C ratios.
GHDL-C, glycated high-density lipoprotein-cholesterol; GLDL-C, glycated low-density lipoprotein-cholesterol; HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol; MetS, metabolic syndrome.
Metabolism-related biomarkers GHDL-C and GLDL-C
The glucolipotoxicity-related metabolism biomarkers GHDL (mmol/l) and GLDL (mmol/l) were observed as higher in MetS pre/T2DM when compared with normoglycemic lean controls. Interestingly, GHDL-C and GLDL-C plasma levels lacked any statistically significant variations.
Correlation between indices and metabolic biomarkers
Table 3 displays the potential associations and p values between indices and biomarkers. Starting with adiposity indices, both GHDL-C and GLDL-C showed positive proportionality with WHR p < 0.05 only in nondiabetic MetS patients. Notably GLDL-C in pre/diabetic MetS participants associated positively with BAI. Direct relationships were identified for whole population GLDL-CI pairs. In all study arms, CI had an inverse correlation with GHDL-C, but showed a positive correlation with GLDL-C only in controls. For hematological indices, only GLDL-C correlated directly with PLT count (p < 0.01). Both biomarkers correlated inversely (p < 0.05) with MPV in the control group. In the nondiabetic MetS group, GHDL-C correlated inversely with both monocyte count and MLR.
Finally, there are potential correlations between atherogenicity indices and metabolic biomarkers in each individual study arm. Collectively, the total population atherogenic indices showed potential significant correlations in comparison with each study arm, which lacked those correlations. As such, GHDL-C correlated positively and significantly with all indices; however, only GLDL-C significantly correlated with the TC/HDL-C ratio (p < 0.01). Interestingly, in the MetS-nondiabetic group GLDL-C inversely correlated with each index. Exceptionally, AIP of the pre/diabetic MetS group showed a similar inverse correlation.
Discussion
This study aimed to evaluate the intergroup discrepancies in plasma levels of those proteins in nondiabetic MetS patients as well as MetS-pre/T2DM patients, and to examine the intra-group associations of those biomarkers with each other, with adiposity indices (WHR, WHtR, CI, and BAI), with hematological indices [red blood cell distribution width (RDW), MPV, neutrophil-to-lymphocyte ratio (NLR), PLR, MLR] and with atherogenic indices (AIP, TC/HDL-C and LDL-C/HDL-C ratios). Our goal was to provide a therapeutic evidence for using GLDL-C and GHDL-C as drug targets in treating metabolic disturbances like obesity, MetS and T2DM among the Middle Eastern populations. Their utility as surrogate molecular biomarkers for prediction/prevention of metabolic derangements is strongly suggested.
In line with the effect of glycation of lipoproteins such as GLDL-C and GHDL-C and their significant role in the pathophysiology of diabetes complications,18 several studies have reported that dyslipidemia in T2DM individuals is very common, and this phenomenon is associated with a significantly increased risk of Coronary Artery Disease (CAD) relative to individuals without diabetes.18,19 The underlying mechanisms increase the normal residence time of LDL-C in plasma from 3 days to 5 days for highly atherogenic LDL-C. In addition, damage to apoB100 by glycation influences the residence time.19,20 However, reductions in the antioxidative and anti-inflammatory effects of HDL-C have been reported in patients with diabetes, alongside an impaired ability to counteract the inhibition of endothelium-dependent vasorelaxation by oxidized LDL-C. Moreover, glycation has recently been shown to reduce the sphingosine-1-phosphate content of HDL-C, reducing its ability to activate protective intracellular survival pathways during oxidative stress, leading to further catabolism.20
A study by Shen and colleagues, investigated whether apolipoprotein A (apoA)-I glycation and paraoxonase (PON) activities are associated with the severity of CAD in patients with T2DM. It is well established that the main function of PON 1 and PON 3 is to prevent LDL-C from peroxidation, conferring antagonistic effects against atherosclerosis. Strikingly ApoA-1 is responsible for reverse cholesterol transport, stabilization of atherosclerotic plaque, and anti-inflammatory and antioxidant effects. The results of the study demonstrated that the relative intensity of apoA-1 glycation was higher but the activities of HDL-C-associated PON1 and PON3 were lower in diabetic patients with significant CAD than in those without.21
Experimentally, HDL-C can impede the glycative modification of LDL-C, and this property of HDL-C is more marked with HDL obtained from people with higher serum PON1 activity. A small amount of oxidation does accompany in vitro glycation, and the process is best regarded as glycoxidation. Lipid peroxidation of LDL-C accompanying in vitro glycation is impeded in the presence of HDL-C, and it is possible that adduction of lipid peroxidation products to the e amino groups of lysine residues of apoB in vivo renders these groups more susceptible to combinations with glucose.22
In vivo, oxygen-free radical exposure of LDL-C may predispose to glycation and explain the high levels of circulating GLDL-C. HDL-C, by impeding LDL-C oxidation, may thus in turn slow its glycation. Another study by Galland and colleagues on T2DM, used the oxidized LDL-C/LDL-C ratios, as an accurate estimation of in vivo LDL-C oxidation, which has been reported as elevated and associated with macrovascular disease. Lipid abnormalities play an important role in the development of atherosclerosis in T2DM. Thus, dyslipidemia observed in T2DM includes both quantitative and qualitative lipid abnormalities. Of the qualitative abnormalities is the glycation of apolipoproteins .23 Based on Galland and colleagues’ work we introduced in our study a highly novel ratio of glycated HDL-C (mmol/l)/HDL-C (mmol/l) and glycated LDL-C (mmol/l)//LDL-C (mmol/l), for their unprecedented diagnostic/prognostic utility as glycation indices in MetS and T2DM.
In recent years, the PLR and NLR ratios were also introduced as new inflammatory markers in different situations.24,25 The PLR ratio combines the predictive risk of PLT and lymphocyte counts.26 In Akdoğanand colleagues, HbA1c, which is a marker of long-term glycemic control, weakly correlated with MPV, Platelet Distribution Width (PDW), RDW, PLR, and NLR ratios and AIP (log10 TG/HDL-C).27 There is an evidence that hyperglycemia induces nonenzymatic glycation of proteins on the surface of the platelets and erythrocytes which decreases membrane fluidity and increases its reactivity.28 The link between hyperlipidemia and platelet hyperactivation is supported by the previous studies that lipid-lowering agents possess antithrombotic properties.29 Adak and colleagues showed that hyperglycemia-induced oxidative stress leads to structural functional alterations in red blood cells.30 In a recent study, Pérez31 compared the general adiposity index (BMI) with abdominal obesity indices in order to examine the best predictor of cardiometabolic risk factors. The study concluded that all obesity indices correlated significantly with blood pressure, HDL-C, TC/HDL-C, LDL-C, TG, FBG and HbA1c. Nevertheless, our findings could identify significant correlations of WC and WHtR with both biomarkers (p < 0.001). This may suggestively conclude that both GLDL-C and GHDL-C can be considered as predictive biomarkers of deranged metabolic status and its consequences.
Our results are in accordance with previous studies conducted by Bavbek and colleagues and Ozder and Eker, which showed association of increased MPV with diabetes.32,33 Demirtas and colleagues also found that MPV and PLR ratios were significantly higher in diabetic patients than in the control group.34 Moreover, MPV may be related to poor glycemic control, possibly due to the osmotic effect resulting from increased glucose levels and some of its metabolites in blood.35
Conclusion
Introducing the ratio of GHDL-C/HDL-C and GLDL-C/LDL-C as novel predictive indices of metabolic derangements alongside to adiposity, atherogenic and hematological indices may be of clinical relevance to metabolic anomalies. This is a cross-sectional study, thus, no causality relationship could be concluded. It cannot be precisely speculated whether the biomarker itself caused the metabolic disturbances of diabetes and MetS or the diabetes and MetS caused the level change of biomarker. Future prospective cohort studies are needed to establish a cause-effect relationship.
Acknowledgments
The Deanship of Academic Research and Quality Assurance at The University of Jordan is graciously thanked for supporting this research.
Footnotes
Author’s Note: Approval for the study was obtained from Institutional Review Board (IRB) affiliated with the National Center for Diabetes, Endocrinology, and Genetics (1151, 1152, 1153/9/SM). Author confirms that all patients have provided their signed consent forms.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Violet Kasabri  https://orcid.org/0000-0003-1927-0193
https://orcid.org/0000-0003-1927-0193
Contributor Information
Rawan Mohammad Al Saudi, School of Pharmacy, The University of Jordan, Queen Rania Street, Amman, Jordan.
Violet Kasabri, School of Pharmacy, The University of Jordan, Queen Rania Street, Amman 11942, Jordan.
Randa Naffa, School of Medicine, The University of Jordan, Queen Rania Street, Amman, Jordan.
Nailya Bulatova, School of Pharmacy, The University of Jordan, Queen Rania Street, Amman, Jordan.
Yasser Bustanji, School of Pharmacy, The University of Jordan, Queen Rania Street, Amman, Jordan.
References
- 1. American Diabetes Association. Standards of medical care in diabetes 2016. Diabetes Care 2016; 39: S1–S71.26696671 [Google Scholar]
- 2. International Diabetes Federation Atlas. The IDF consensus worldwide definition of the metabolic syndrome. Brussels, Belgium: International Diabetes Federation Atlas, 2006. [Google Scholar]
- 3. Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement: executive summary. Circulation 2005; 112: 285–290. [DOI] [PubMed] [Google Scholar]
- 4. Ruderman NB, Carling D, Prentki M, et al. AMPK, insulin resistance, and the metabolic syndrome. J Clin Invest 2013; 123: 2764–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou M, Zhu L, Cui X, et al. The triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio as a predictor of insulin resistance but not of β cell function in a Chinese population with different glucose tolerance status. Lipids Health Dis 2016; 15: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Singh VP, Bali A, Singh N, et al. Advanced glycation end products and diabetic complications. Korean J Phys Pharmacol 2014; 18: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Veiraiah A. Hyperglycemia, lipoprotein glycation, and vascular disease. Angiology 2005; 56: 431–438. [DOI] [PubMed] [Google Scholar]
- 8. Liu D, Ji L, Zhang D, et al. Nonenzymatic glycation of high-density lipoprotein impairs its anti-inflammatory effects in innate immunity. Diabetes Metab Res Rev 2012; 28: 186–195. [DOI] [PubMed] [Google Scholar]
- 9. Vergès B. Lipid modification in type 2 diabetes: the role of LDL and HDL. Fundament Clin Pharmacol 2009; 23: 681–685. [DOI] [PubMed] [Google Scholar]
- 10. Morsi H, Ismail M, Gaber H, et al. Macrophage migration inhibitory factor and malondialdehyde as potential predictors of vascular risk complications in type 2 diabetes mellitus: cross-sectional case control study in Saudi Arabia. Mediators Inflam 2016; 5797930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jiang H, Yan W, Li C, et al. Elevated white blood cell count is associated with higher risk of glucose metabolism disorders in middle-aged and elderly chinese people. Int J Env Res Pub Health 2014; 11: 5497–5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dubey I, Gaur BS, Singh R. A study to find correlation of platelet indices with HbA1c in diabetic patients with absence/presence of vascular complications. Int J Res Med Sci 2017; 5: 1042–1047. [Google Scholar]
- 13. Abbasian M, Delvarianzadeh M, Ebrahimi H, et al. Lipid ratio as a suitable tool to identify individuals with MetS risk: a case- control study. Diabetes Metab Syndr: Clin Res Rev 2016; 10: 132–136. [DOI] [PubMed] [Google Scholar]
- 14. Kajingulu MF, Lepira BF, Mbutiwi IN, et al. The atherogenic dyslipidemia ratio log (Tg)/Hdl-C was not associated with urinary albumin excretion rate (Uaer) and increased cardiovascular risk in black patients with type 2 diabetes. World J Cardiovasc Dis 2016; 6: 14–20. [Google Scholar]
- 15. Tonding SF, Silva FM, Antonio JP, et al. Adiposity markers and risk of coronary heart disease in patients with type 2 diabetes mellitus. Nutr J 2014; 13: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nadeem A, Naveed A, Hussain M, et al. Cut-off values of anthropometric indices to determine insulin resistance in Pakistani adults. J Pak Med Assoc 2013; 10: 1220–1225. [PubMed] [Google Scholar]
- 17. Geliebter A, Atalayer D, Flancbaum L, et al. Comparison of body adiposity index (BAI) and body mass index (BMI) with estimations of % body fat in clinically severe obese women. Obesity (Silver Spring) 2013; 3: 493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singh V, Bali A, Singh N, et al. Advanced glycation end products and diabetic complications. Korean J Phys Pharmacol 2014; 1: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sukino S, Kotani K, Nirengi S, et al. Dietary intake of vitamin D is related to blood levels of advanced glycation end products during a weight loss program in obese women. J Biomed 2016; 1: 1–14. [Google Scholar]
- 20. Vergès B. Pathophysiology of diabetic dyslipidaemia: where are we? Diabetologia 2015; 58: 886–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shen Y. Association of elevated apoA-I glycation and reduced HDL-associated paraoxonase1, 3 activity,and their interaction with angiographic severityof coronary artery disease in patients with type 2 diabetes malletus. Cardiovascul Diabetology 2015; 14: 52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Akhter F, Khan M, Alatar A, et al. Antigenic role of the adaptive immune response to D-ribose glycated LDL in diabetes, atherosclerosis and diabetes atherosclerotic patients. Life Sci 2016; 151: 139–146. [DOI] [PubMed] [Google Scholar]
- 23. Sorana H, Schofielda J, Liua Y, et al. How HDL protects LDL against atherogenic modification: paraoxonase 1 and other dramatis personae. Curr Opin Lipidol 2015; 26: 247–256. [DOI] [PubMed] [Google Scholar]
- 24. Galland F, Duvillard L, Petit J, et al. Effect of insulin treatment on plasma oxidized LDL/LDL-cholesterol ratio in type 2 diabetic patients. Diabetes Metab 2006; 32: 625–631. [DOI] [PubMed] [Google Scholar]
- 25. Kaya A, Kurt M, Tanboga IH, et al. Relation of neutrophil to lymphocyte ratio with the presence and severity of stable coronary artery disease. Clin Appl Thromb Hemost 2014; 20: 473–477. [DOI] [PubMed] [Google Scholar]
- 26. Wu Y, Chen Y, Yang X, et al. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) were associated with disease activity in patients with systemic lupus erythematosus. Int Immunopharmacol 2016; 36: 94–99. [DOI] [PubMed] [Google Scholar]
- 27. Akdoğan M, Ustundag-Budak Y, Huysal K. The association of hematologic inflammatory markers with atherogenic index in type 2 diabetic retinopathy patients. Clinical (Auckland, N.Z.) 2016; 10: 1797–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Winocour P, Watala C, Kinlough-Rathbone R. Membrane fluidity is related to the extent of glycation of proteins, but not to alterations in the cholesterol to phospholipid molar ratio in isolated platelet membranes from diabetic and control subjects. Thromb Haemost 1992; 67: 567–571. [PubMed] [Google Scholar]
- 29. Tehrani S, Mobarrez F, Antovic A, et al. Atorvastatin has antithrombotic effects in patients with type 1 diabetes and dyslipidemia. Thromb Res 2010; 126: e225–e231. [DOI] [PubMed] [Google Scholar]
- 30. Adak S, Chowdhury S, Bhattacharyya M. Dynamic and electrokinetic behavior of erythrocyte membrane in diabetes mellitus and diabetic cardiovascular disease. Biochim Biophys Acta 2008; 1780: 108–115. [DOI] [PubMed] [Google Scholar]
- 31. Palacios C, Pérez CM, Guzmán M, et al. Association between adiposity indices and cardiometabolic risk factors among adults living in Puerto Rico. Public Health Nutr 2012; 14: 1714–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bavbek N, Kargili A, Kaftan O, et al. Elevated concentrations of soluble adhesion molecules and large platelets in diabetic patients: are they markers of vascular disease and diabetic nephropathy? Clin Appl Thromb Hemost 2007; 13: 391–397. [DOI] [PubMed] [Google Scholar]
- 33. Ozder A, Eker H. Investigation of mean platelet volume in patients with type 2 diabetes mellitus and in subjects with impaired fasting glucose: a cost-effective tool in primary health care? Int J Clin Exp Med 2014; 7: 2292–2297. [PMC free article] [PubMed] [Google Scholar]
- 34. Demirtas L, Degirmenci H, Akbas E, et al. Association with hematological indices with diabetes, impaired glucose regulation and microvascular complications of diabetes. Int J Clin Exp Med 2015; 8: 11420–11427. [PMC free article] [PubMed] [Google Scholar]
- 35. Dalamaga M, Karmaniolas K, Lekka A, et al. Platelet markers correlate with glycemic indices in diabetic, but not diabetic-myelodysplastic patients with normal platelet count. Diabetes Markers 2010; 29: 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]