Abstract
Human-modified forests are an ever-increasing feature across the Amazon Basin, but little is known about how stem growth is influenced by extreme climatic events and the resulting wildfires. Here we assess for the first time the impacts of human-driven disturbance in combination with El Niño–mediated droughts and fires on tree growth and carbon accumulation. We found that after 2.5 years of continuous measurements, there was no difference in stem carbon accumulation between undisturbed and human-modified forests. Furthermore, the extreme drought caused by the El Niño did not affect carbon accumulation rates in surviving trees. In recently burned forests, trees grew significantly more than in unburned ones, regardless of their history of previous human disturbance. Wood density was the only significant factor that helped explain the difference in growth between trees in burned and unburned forests, with low wood–density trees growing significantly more in burned sites. Our results suggest stem carbon accumulation is resistant to human disturbance and one-off extreme drought events, and it is stimulated immediately after wildfires. However, these results should be seen with caution—without accounting for carbon losses, recruitment and longer-term changes in species composition, we cannot fully understand the impacts of drought and fire in the carbon balance of human-modified forests.
This article is part of a discussion meeting issue ‘The impact of the 2015/2016 El Nino on the terrestrial tropical carbon cycle: patterns, mechanisms and implications’.
Keywords: drought, wildfire, tree growth, tropical forests, degradation, ENSO
1. Introduction
The Amazon stores c. 86Pg of carbon [1], an amount equivalent to almost 10 years of combined global emissions from fossil fuels and the cement industry [2]. This large carbon reservoir has historically been threatened by deforestation, with large NGO-led campaigns bringing the issue to the public and pressuring governments for measures to effectively stop forest loss [3]. However, wildfires, i.e. fires that escape agricultural lands and invade forests, have been an often neglected although significant threat to Amazonian forests, substantially decreasing carbon stocks [4] and biodiversity [5,6]. In past decades, forest fires were directly linked to deforestation rates [7]; however, this is not the case anymore—although deforestation in the Brazilian Amazon has remained somewhat stable since 2009 [8], forest fires are increasing in number [9]. This surge in wildfire occurrence is a consequence of a combination of factors: greater frequency of extreme droughts [10], the indirect impacts of deforestation that creates flammable edges [11] and reduces regional rainfall [12], the spread of selective logging that increases forest flammability [13], and the prevalence of ignition sources used in Amazonian agricultural systems [14]. As a result, wildfires have become the new norm in the parts of the Amazon Basin most affected by human disturbance, especially during extreme dry years [15,16].
More frequent and more intense droughts are expected across Amazonia in this century [17–19]. Extreme droughts are known to double tree mortality rates in tropical rainforests, reverting them from carbon sinks to sources [20,21]. Drought-affected rainforest trees die either because they cannot move water from their roots to their leaves, known as hydraulic failure [22], or because they close their stomata in order not to lose water but, as a consequence, do not have enough sugars to keep their metabolism, a process known as carbon starvation [23]. This increase in tree mortality rates leads to more openings in the forest canopy, turning drought-affected forests more flammable due to the accumulation of fuel (i.e. branches and leaves) on the forest floor and the higher incidence of sun and wind on the understorey [13]. When drought-affected tropical rainforests catch fire, they experience even higher rates of tree mortality, sometimes close to 50% [24]. This large-scale mortality is then followed by severe structural and compositional changes [25] and significant reductions of their carbon stocks [4].
However, the influence of drought or wildfires on the growth of the surviving trees remains poorly understood. Results from drought experiments on undisturbed forests showed that radial tree growth was negatively impacted only after several years of continuous rainfall exclusion [26,27]. This has been corroborated by results from field monitoring, which showed that radial tree growth was not affected by a one-off extreme drought [28]. When evaluating the impacts of wildfires on tree growth, studies in Amazonia have focused solely on re-sprouting dynamics (e.g. [29,30]), and have not examined whether radial growth of the few surviving trees is altered. The one exception is a study conducted in the Amazon–savannah boundary [29], which found that low-severity fires increased post-fire tree growth. Notably, no studies to date have investigated the impacts of either extreme droughts or wildfires on trees growing in human-modified forests. For example, it is unclear whether droughts and wildfires affect tree growth and carbon accumulation in similar ways between undisturbed primary forests and those that have been human modified, or whether radial growth is inhibited in the years following drought and wildfires. It seems therefore crucial that we develop a better understanding of tree growth and stem carbon uptake in these altered systems, given the high rates of human-driven forest disturbance across the Amazon [30], the increasing ubiquity of forest fires and the paucity of studies examining the responses of surviving trees.
The 2015 El Niño event provided a valuable opportunity to address these knowledge gaps. The region of Santarém, in the Brazilian Amazon, was particularly affected by drought during this El Niño [31] and millions of hectares of forests burned. Prior to the El Niño, we had established 18 permanent forest plots in the region, where we had been measuring tree growth monthly in c.900 individuals. These plots were distributed along a gradient of human disturbance, from undisturbed primary forests, to logged primary forests, logged-and-burned primary forests and secondary forests (i.e. those regrowing on land previously cleared for agriculture). All our plots were severely affected by the El Niño drought, and some were also affected by the extensive wildfires that affected the region [32]. We draw on this unique dataset to investigate the responses of human-modified forests to El Niño–mediated droughts and fires, asking four questions: (i) How does tree growth and stem carbon accumulation compare between forest disturbance classes? (ii) How does stem growth and carbon accumulation in the El Niño–affected dry season compare to those of the following years? (iii) Is the post-El Niño growth and carbon accumulation of trees affected by drought different from those affected by both drought and fire? and (iv) What factors can influence differences in growth and carbon accumulation between trees located in drought-affected plots from those located in plots affected by both drought and fire?
2. Material and methods
(a). Study area
The study was conducted in three municipalities of the eastern part of the Amazon Basin: Santarém, Belterra and Mojuí-dos-Campos (hereafter Santarém region). The climate in the region is hot and humid, with an annual average of 25°C, 86% relative humidity and 1920 mm of rain [33]. The region has a marked dry season that usually lasts for four months, from August to November, when precipitation is less than 100 mm per month (electronic supplementary material, figure S1). Soils are rich in clay, but nutrient poor [34]. Data were collected in 18 permanent plots (250 × 10 m) distributed along a gradient of pre-El Niño human disturbance: undisturbed forests (n = 5), logged forests (n = 5), logged-and-burned forests (n = 4) and secondary forests (n = 4). Plots were located in terra firme forests situated between 1.5 and 97 km apart (electronic supplementary material, figure S2). In December 2015, seven of our study plots burned, including three of previously undisturbed forests, four of previously logged forests and one of previously logged-and-burned forest (electronic supplementary material, table S1).
(b). Tree growth and stem carbon accumulation
In all plots, trees were measured and identified to species level in 2014. We then installed 50 dendrometer bands in each plot, stratifying by tree size class: 10–19.9 cm diameter at breast height (DBH, 1.3 m from the forest floor), 20–29.9 cm DBH, 30–39.9 cm DBH, 40–49.9 cm DBH and ≥50 cm DBH. When a plot did not have 10 trees in a given size class, we distributed the remaining dendrometers evenly across the other size classes. Between July 2015 and December 2017, tree growth was measured monthly with digital callipers. In the case of a dendrometer been found damaged or a tree having suddenly died, the band would be removed immediately and promptly reinstalled. In the burned plots, the heat overstretched the metal springs and all dendrometers were reinstalled within four weeks of the fires. Monthly tree growth was converted into stem carbon accumulation by using a biomass equation for tropical trees [35] and assuming carbon content to represent 50% of biomass. The equation used takes into consideration the tree-measured growth, height and wood density. We allowed negative growth values, even though these reflect water loss from the bark and not a true decrease in tree size [36]. This was because some of the positive growth values are due to water accumulation in the bark, and the keeping of negative values is, therefore, necessary to balance out the fluctuating water content over the year [36].
(c). Factors influencing tree growth and carbon accumulation
Based on the literature, we selected six factors that could possibly influence post-fire tree growth and the consequent carbon accumulation on the stem: DBH, height, wood density, fire intensity and two measures reflecting the degree of competitive release from fires—the change in liana load and the change in basal area in the surrounding forest. The DBH and the height of each tree were assessed during a re-census of all plots in 2016. Wood density was derived from the Global Wood Density database [37], based on the species identification and filtering by South American tropical regions. We measured the maximum char height on all burned stems as a proxy for fire intensity. Liana loads were determined during both the 2014 and the 2016 censuses. This is an estimate of how much of the crown of a given tree is infested by liana leaves, ranging between 0, 1–25, 26–50, 51–75 and 76–100% [38]. Finally, the basal area of live stems was calculated in a 10 × 10 m plot surrounding each tree in both 2014 and 2016. Changes in both liana load and surrounding basal area were calculated as the difference between the 2014 and the 2016 values for each tree. We expected that the high mortality of lianas [39] and trees [40] immediately after wildfires would result in less competition for light and water among the surviving trees, thus probably influencing tree growth [41].
(d). Data analysis
To investigate whether there were any differences in radial growth and stem carbon accumulation between trees of different forest disturbance classes, we considered only individuals that were continuously measured over a 2.5 year period from July 2015 until December 2017 (n = 385), therefore excluding from this analysis all stems located in burned plots. We used ANOVAs followed by post hoc Tukey tests to examine whether there were any differences in the mean cumulative growth and carbon between the forest disturbance classes. The tests were run using both the absolute and normalized (growth/DBH) growth of each stem. For each test, we calculated the eta-square (h2), which is a measure of effect size and corresponds to the proportion of the total variation in the data that can be attributed to the explanatory variable.
We used a temporal comparison to assess the impacts of the El Niño from this analysis induced extreme drought. For this, we conducted two analyses. First, we compared the total dry-season growth and carbon accumulation of trees measured continuously during the 2015 El Niño from this analysis mediated drought with the two following dry seasons, 2016 and 2017 (n = 385). We built generalized linear mixed-effects models (GLMM) to assess whether dry-season growth and carbon accumulation were influenced by forest disturbance, year or an interaction between both. In these models, tree and plot identities were set as random effects. Second, we investigated whether relative growth and carbon accumulation rates were influenced by dry-season intensity, measured by the climatological water deficit (CWD). To calculate the relative growth and stem carbon accumulation rates, we used the interval growth between months. CWD was defined as precipitation in a given month (millimetres), minus evapotranspiration (100 mm), minus the previous month CWD; following [42]. Precipitation data were obtained from CHIRPS [43]. We built two sets of GLMMs, using either the relative growth or carbon accumulation rates as response variables. CWD was the explanatory variable in these models, while random effects included tree identity, study plot and year.
To compare the annual growth and carbon accumulation of trees located in drought-affected plots with those of trees located in plots affected by both drought and fire, we used data of individuals with continuous measurements throughout 2017 (n = 545), which was the only comparable period given that fires damaged the dendrometers. We then ran three two-way ANOVAs: on the first we used cumulative tree growth at the end of 2017 as the response variable, on the second we used the normalized growth (growth/DBH), while on the third we used the annual carbon accumulation. All ANOVAs used pre-El Niño forest disturbance class and fire (burned or unburned in 2015) as explanatory variables. After each test, we calculated their eta-square (h2).
Finally, we used a matching approach commonly used in landscape ecology (e.g. [44]) to investigate which factors predict post-fire tree growth and carbon accumulation. The matching approach linked individual trees in drought-and-fire–affected forests with functionally comparable stems in drought-affected forests. This was essential to answer our research question, as fire potentially imposes a non-random mortality, killing more small-stemmed and low wood–density trees [45]. As such, an unmatched comparison would not be able to fully distinguish differences in tree growth due to the newly altered functional characteristics of a forest (for example, if only large stems survived) or due to post-fire changes in forest conditions that may alter the growth of individual stems (e.g. decrease in liana infestation due to fire-induced mortality). For trees to be matched, they had to belong to the same pre-El Niño disturbance class and the matched stem had to be within a 10% margin of both the DBH and wood density of the burned forest stem. When more than one tree in unburned forests met the matching criteria, we favoured the one with the closest DBH to the tree in the burned forest. This choice was based on the fact that DBH is quadratic in the biomass equation used [35], as opposed to wood density, which is only elevated to the power of one. In total, 128 trees could be matched (i.e. 64 pairs).
After the matching, we ran linear models between the matched trees in each disturbance class to examine if either the growth or the carbon accumulation of trees in unburned forests could predict that of trees in burned forests. For each pair, we then calculated the difference in both total growth and carbon accumulated by the end of 2017. We ran generalized linear models to investigate which stem and forest structure factors could be influencing these differences in radial growth and stem carbon accumulation between matched trees. Models included forest disturbance class, DBH, height, wood density, char height, Δ liana load (i.e. 2016–2014) and Δ basal area of surrounding live stems (i.e. 2016–2014) of the fire-affected tree as explanatory variables. Prior to running the models, we checked for collinearity between explanatory variables and none was found (electronic supplementary material, figure S3). To facilitate our understanding of the effect size of each explanatory variable, they were all standardized between 0 and 1. All analyses were performed in R v. 3.4.0 using the BBmisc, corrplot, MASS and sjstats packages [46–49].
3. Results
(a). Tree growth and stem carbon accumulation across human-modified forests
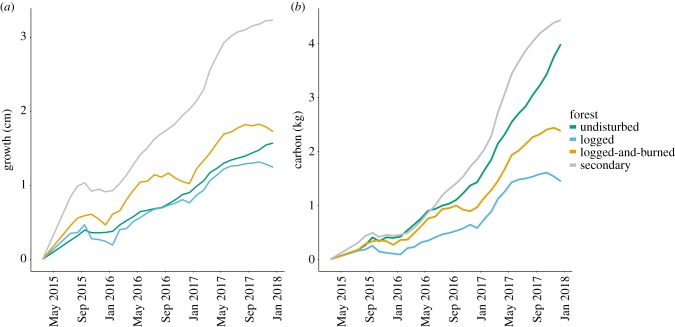
After 2.5 years of continuous measurements, thus focusing only on trees located in unburned sites, the mean individual growth was significantly higher in trees located in secondary forests (figure 1; electronic supplementary material, figure S4) than when compared with trees in all other forest classes (F3,381 = 14.27, p < 0.001, h2 = 0.10; Tukey tests involving secondary forests, all p < 0.001). However, there was no significant difference in carbon accumulation between any of the forest classes. The higher growth of trees in secondary forests was consistent across DBH size classes (electronic supplementary material, figure S5). These results were also consistent whether using absolute or normalized tree growth.
Figure 1.
Mean individual growth (a) and carbon (b) accumulated over 3 years in undisturbed primary forests (green), logged forests (blue), logged-and-burned forests (orange) and secondary forests (red).
(b). El Niño impacts on dry-season growth and carbon accumulation
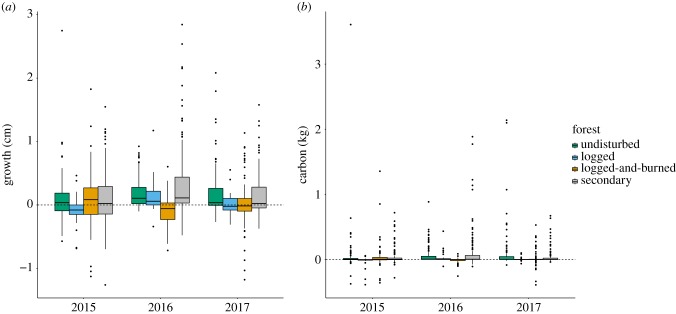
While dry-season growth was significantly higher in the post-El Niño years (figure 2a; both p < 0.05); dry-season carbon accumulation was not significantly influenced by the El Niño–mediated drought (figure 2b). Regardless of the year, trees in logged forests grew significantly less and accumulated significantly less carbon (both p < 0.05). In trees situated in undisturbed, logged and secondary forests (electronic supplementary material, figure S6), there was a weak but significant relationship between growth rates during the dry season and the climatological water deficit (all p < 0.001)—the more negative the deficit, the lower the growth. However, monthly carbon accumulation rates were only significantly affected by CWD in logged and secondary forests (electronic supplementary material, figure S7).
Figure 2.
Cumulative tree (a) growth and (b) carbon accumulation during the dry seasons of 2015, 2016 and 2017 in undisturbed, logged, logged-and-burned and secondary forests.
(c). Growth and stem carbon accumulation between trees in burned and unburned forests
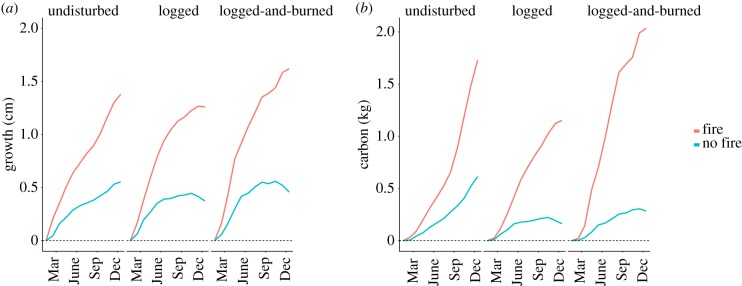
When analysing data from all surviving stems (n = 545) in forests affected by drought and those affected by drought and fire during the 2015 El Niño, both growth and carbon accumulation in the end of 2017 were significantly higher in trees located in burned plots (F1,389 = 41.64, 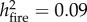 and F1,389 = 22.68
and F1,389 = 22.68 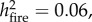 respectively; both p < 0.0001, figure 3). This pattern was maintained regardless of tree size or pre-El Niño forest disturbance class (electronic supplementary material, figure S8–S10). Results were consistent whether using absolute or normalized tree growth.
respectively; both p < 0.0001, figure 3). This pattern was maintained regardless of tree size or pre-El Niño forest disturbance class (electronic supplementary material, figure S8–S10). Results were consistent whether using absolute or normalized tree growth.
Figure 3.
Mean individual tree (a) growth and (b) carbon accumulated along 2017 in trees situated in forests that burned (red) or were only affected by drought (blue) during the 2015 El Niño. Forest classes correspond to forest condition prior the onset of the El Niño–mediated fires.
(d). Factors influencing differences in tree growth and stem carbon accumulation
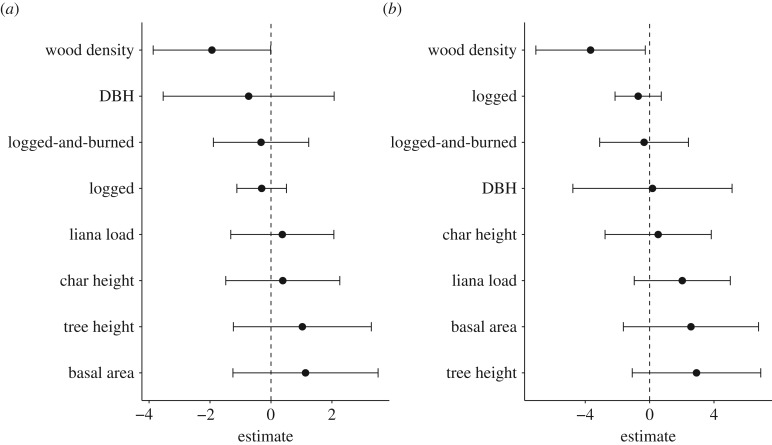
When focusing only on the matched trees (n = 128 trees, 64 pairs), neither the growth nor carbon accumulated in trees located in forests that burned during the 2015 El Niño could be predicted by their matched pairs in forests only affected by drought (all R2 ≤ 0.28, p > 0.05; electronic supplementary material, figure S11). Of all the factors examined with a generalized linear model to possibly explain differences in growth and carbon accumulation between matched trees, only wood density was significant (p = 0.05, β = −1.94; and p < 0.05, β = −3.67, respectively). Wood density had a negative relationship with the differences in growth and carbon accumulation between burned and unburned trees, thus the lighter the wood density, the greater the increase in growth in stems in recently burned forests (figure 4).
Figure 4.
Coefficient plots of the factors affecting the difference in (a) growth and (b) carbon accumulation between trees in burned and unburned forests.
4. Discussion
Our novel results provide important insights into tree growth and carbon accumulation in human-modified Amazonian forests, and the interaction between forest disturbance and extreme drought and fire events. Surprisingly, there was no significant difference in overall carbon accumulation between trees in undisturbed and human-modified forests. Furthermore, the extreme El Niño–mediated drought did not seem to inhibit carbon accumulation in surviving trees. We were also able to assess the impacts of wildfires on the few surviving trees and the factors affecting post-fire growth, something never done before in humid tropical forests. We found that trees situated in forests that burned during the 2015 El Niño presented a significantly higher radial growth and stem carbon accumulation than trees in forests only affected by drought, and that this difference was more pronounced in lighter wood density stems. We discuss these results in light of the increasing ubiquity of human-modified Amazonian forests and of the increased frequency of drought and fire events.
(a). The importance of human-modified forests for carbon accumulation
Over a 2.5-year period of continuous monitoring, trees in secondary forests grew significantly faster than those in undisturbed and disturbed primary Amazonian forests, a result that is consistent with others from elsewhere in the Neotropics [50]. However, these higher levels of individual growth did not lead to more carbon accumulation, with trees in undisturbed, disturbed and secondary forests accumulating comparable amounts of carbon. The apparent discrepancy between the results of radial growth and stem carbon accumulation can be explained by the dominance of lower wood density species in secondary forests [51]. For example, when we consider a 20-cm DBH and 15-m tall stem of a low wood density species commonly found in secondary forests, Jacaranda copaia, a 2-cm growth results in an increment of 0.66 kg of C. However, in a hyper-abundant primary forest species, Eschweilera coriacea [52], a stem of the same size and height experiencing the same growth will incorporate 1.57 kg of C, a difference of 236%. To achieve a similar amount of carbon accumulation, this hypothetical individual of Jacaranda copaia would have to grow 3.1 cm; i.e. it would have to grow 1.6 times more than the Eschweilera coriacea to accumulate the same amount of carbon. Therefore, although trees in secondary forests are showing higher rates of radial growth, this is compensated by their lower wood density, resulting in similar levels of carbon accumulation across all forest classes.
(b). Drought effects on tree growth and carbon accumulation
The El Niño–mediated drought negatively affected tree growth, but had no significant impact on overall stem carbon accumulation. This appears to indicate that low wood density trees, i.e. those that contribute less to carbon accumulation, were the most affected by the 2015 drought. In Amazonian forests, low wood density tree species tend to be less resistant to extreme droughts [20], as they present high turgor loss points and high osmotic potential [53]. In order words, when there is less water available, the leaves of low wood density trees are more likely to wilt, impacting photosynthesis [54] and, as a consequence, growth rates. However, the effects of the El Niño–mediated drought appeared to be transient, given that growth rates remained uninhibited in the following dry seasons. Furthermore, the weak relationships between climatological water deficit and both dry-season growth and carbon accumulation rates suggest that trees in both undisturbed and human-modified forests are adapted to seasonal droughts. This result is to be expected, as the distribution of Amazonian tree species follows a dry-tolerance pattern, which consists in more drought-tolerant taxa occurring in the parts of the basin that every year experience some months of little rainfall [55], such as the Santarém region. It is important to note however, that the dry seasons of 2015 and 2017 were stronger than those between 1970 and 1999—even in 1997, the year of the strongest El Niño on record [56], the maximum climatological water deficit in eastern Amazonia was approximately −200 mm [42], while in 2015 and 2017 it was of −368 mm and −316 mm, respectively. So far, eastern Amazonian trees seem resistant to the current drier climate, continually accumulating carbon despite more intense dry seasons than in the previous 30 years.
(c). Wildfire effects on tree growth and carbon accumulation
Trees in burned forests both grew more and accumulated more carbon than trees located in plots that only experienced drought during the El Niño. This is a completely novel finding from humid tropical rainforests. In other ecosystems, fire effects on tree growth lead to conflicting results: while low-intensity fires can increase tree growth in savannahs [57], it can supress radial growth in temperate forests [58]. The mechanisms behind these changes in growth rates remain unclear. In our sites, changes in post-fire tree growth were not explained by tree size, tree height, forest disturbance class, or proxies of fire intensity and competitive release (from lianas and other trees). Wood density was the only significant factor explaining differences in tree growth and carbon accumulation between stems located in burned plots and those located in drought-affected plots, with lower wood density trees in burned forests growing more than their counterparts in unburned forests. Given that our measures of competitive release were not important predictors of differences in tree growth between burned and unburned trees, it is unlikely that low wood–density trees experienced an enhanced growth due to greater light or water availability. Most probably, low wood density trees were benefitting from the large pulse of nutrients released by the combustion of organic matter. In general, low wood density trees have acquisitive life strategies, heavily investing in rapid growth [59]; while high wood density tree species are more conservative, with considerably slower growth rates [60]. The sudden input of nutrients has probably led to a disproportional investment in growth by low wood density trees.
(d). Amazonian forests in the Anthropocene
Tropical ecosystems face growing pressure from a combination of both global and local stressors [61]. Across Amazonia, a global stressor, climate change, is predicted to increase the frequency of two local stressors—extreme droughts and associated fires [9,17]. Other local stressors, such as selective logging, newly created forest edges and large infrastructure projects, are increasing the prevalence of human-modified forests [62,63]. Understanding ecosystem-level responses to these growing anthropogenic pressures can help predict their consequences, and opens up opportunities to mitigate their worst effects. Our study shows the relative resilience of tree growth and subsequent carbon accumulation to one-off droughts, and suggests that growth rates can even increase after wildfires. Still, stem growth is just one part of a forest's carbon balance: despite the spike in stem carbon accumulation, the carbon balance in burned forests is still largely negative—tree mortality following fires is extremely high [24,64] and cannot be compensated by the growth of the few surviving trees. Previous studies in Amazonia have shown that three years after fires, forests can lose c. 50% of its individuals and 75 Mg C ha−1. This can hardly be compensated by the remaining trees accumulating an extra 1 kg C, and demonstrate the importance of avoiding wildfires in humid tropical forests.
Supplementary Material
Acknowledgements
We thank the Large Scale Biosphere-Atmosphere Program (LBA) for logistical and infrastructure support during field measurements. We are deeply grateful to our parabotanists Nelson Rosa and Jair Freitas, as well as our field and laboratory assistants: Gilson Oliveira, Josué Oliveira, Renílson Freitas, Marcos Oliveira and Josiane Oliveira. We also thank all collaborating private land owners for their support and access to their land. This paper is number 70 in the Rede Amazônia Sustentável publication series.
Data accessibility
The data used in this paper is available as part of the electronic supplementary material.
Authors' contributions
E.B., Y.M. and J.B. designed the study. E.B. and J.F. were responsible for plot selection and subsequent authorizations from landowners. E.B., P.B., A.C.N.C., F.F., L.C.R. and M.M.M.S. performed data collection. E.B. conducted all statistical analyses. E.B. and J.B. wrote the manuscript with critical inputs from all authors.
Competing interests
We declare we have no competing interests.
Funding
We are grateful to the following for financial support: Instituto Nacional de Ciência e Tecnologia – Biodiversidade e Uso da Terra na Amazônia (CNPq 574008/2008-0), Empresa Brasileira de Pesquisa Agropecuária – Embrapa (SEG: 02.08.06.005.00), the UK government Darwin Initiative (17-023), The Nature Conservancy and the UK Natural Environment Research Council (NERC; NE/F01614X/1, NE/G000816/1, NE/K016431/1 and NE/P004512/1). E.B. and J.B. were also funded by H2020-MSCA-RISE (691053-ODYSSEA). F.F. was funded by Conselho Nacional de Pesquisa (PELD-RAS 441659/2016-0).
References
- 1.Saatchi SS, Houghton RA, Dos Santos Alvalá RC, Soares JV, Yu Y. 2007. Distribution of aboveground live biomass in the Amazon basin. Glob. Change Biol. 13, 816–837. ( 10.1111/j.1365-2486.2007.01323.x) [DOI] [Google Scholar]
- 2.Houghton RA, Nassikas AA. 2018. Negative emissions from stopping deforestation and forest degradation, globally. Glob. Change Biol. 24, 350–359. ( 10.1111/gcb.13876) [DOI] [PubMed] [Google Scholar]
- 3.Lambin EF, et al. 2018. The role of supply-chain initiatives in reducing deforestation. Nat. Clim. Change 8, 109–116. ( 10.1038/s41558-017-0061-1) [DOI] [Google Scholar]
- 4.Berenguer E, et al. 2014. A large-scale field assessment of carbon stocks in human-modified tropical forests. Glob. Change Biol. 20, 3713–3726. ( 10.1111/gcb.12627) [DOI] [PubMed] [Google Scholar]
- 5.Moura NG, Lees AC, Aleixo A, Barlow J, Berenguer E, Ferreira J, Mac Nally R, Thomson JR, Gardner TA. 2015. Idiosyncratic responses of Amazonian birds to primary forest disturbance. Oecologia 180, 1–14. ( 10.1007/s00442-015-3495-z) [DOI] [PubMed] [Google Scholar]
- 6.Flores BM, Fagoaga R, Nelson BW, Holmgren M. 2016. Repeated fires trap Amazonian blackwater floodplains in an open vegetation state. J. Appl. Ecol. 53, 1597–1603. ( 10.1111/1365-2664.12687) [DOI] [Google Scholar]
- 7.Aragão LEOC, Malhi Y, Barbier N, Lima A, Shimabukuro Y, Anderson L, Saatchi S. 2008. Interactions between rainfall, deforestation and fires during recent years in the Brazilian Amazonia. Phil. Trans. R. Soc. B. 363, 1779–1785. ( 10.1098/rstb.2007.0026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.INPE. 2016. Prodes. See http://www.obt.inpe.br/prodes/index.php.
- 9.Aragão LEOC, et al. 2018. 21st century drought-related fires counteract the decline of Amazon deforestation carbon emissions. Nat. Commun. 9, 536 ( 10.1038/s41467-017-02771-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erfanian A, Wang G, Fomenko L. 2017. Unprecedented drought over tropical South America in 2016: significantly under-predicted by tropical SST. Sci. Rep. 7, 5811 ( 10.1038/s41598-017-05373-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cochrane MA. 2001. Synergistic interactions between habitat fragmentation and fire in evergreen tropical forests. Conserv. Biol. 15, 1515–1521. ( 10.1046/j.1523-1739.2001.01091.x) [DOI] [Google Scholar]
- 12.Spracklen DV, Garcia-Carreras L. 2015. The impact of Amazonian deforestation on Amazon basin rainfall. Geophys. Res. Lett. 42, 9546–9552. ( 10.1002/2015GL066063) [DOI] [Google Scholar]
- 13.Uhl C, Kauffman J. 1990. Deforestation, fire susceptibility, and potential tree responses to fire in the eastern Amazon. Ecology 71, 437–449. [Google Scholar]
- 14.Carmenta R, Vermeylen S, Parry L, Barlow J. 2013. Shifting cultivation and fire policy: insights from the Brazilian Amazon. Hum. Ecol. 41, 603–614. ( 10.1007/s10745-013-9600-1) [DOI] [Google Scholar]
- 15.Alencar A, Nepstad D, Del Carmen Vera Diaz M. 2006. Forest understory fire in the Brazilian Amazon in ENSO and non-ENSO years: area burned and committed carbon emissions. Earth Interact. 10, 1–17. ( 10.1175/EI150.1) [DOI] [Google Scholar]
- 16.Le Page Y, Morton D, Hartin C, Bond-Lamberty B, Pereira MC, Hurtt G, Asrar G. 2017. Synergy between land use and climate change increases future fire risk in Amazon forests. Earth Syst. Dyn. 85194, 1237–1246. ( 10.5194/esd-8-1237-2017) [DOI] [Google Scholar]
- 17.Malhi Y, Wright J. 2004. Spatial patterns and recent trends in the climate of tropical rainforest regions. Phil. Trans. R. Soc. Lond. B 359, 311–329. ( 10.1098/rstb.2003.1433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu R, et al. 2013. Increased dry-season length over southern Amazonia in recent decades and its implication for future climate projection. Proc. Natl Acad. Sci. USA 110, 18 110–18 115. ( 10.1073/pnas.1302584110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duffy PB, Brando P, Asner GP, Field CB. 2015. Projections of future meteorological drought and wet periods in the Amazon. Proc. Natl Acad. Sci. 112, 13 172–13 177. ( 10.1073/pnas.1421010112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips OL, et al. 2009. Drought Sensitivity of the Amazon Rainforest. Science 323, 1344–1347. ( 10.1126/science.1164033) [DOI] [PubMed] [Google Scholar]
- 21.Lewis SL, Brando PM, Phillips OL, van der Heijden GMF, Nepstad D. 2011. The 2010 Amazon drought. Science 331, 554 ( 10.1126/science.1200807) [DOI] [PubMed] [Google Scholar]
- 22.Rowland L, et al. 2015. Death from drought in tropical forests is triggered by hydraulics not carbon starvation. Nature 528, 119 ( 10.1038/nature15539) [DOI] [PubMed] [Google Scholar]
- 23.Adams HD, et al. 2017. A multi-species synthesis of physiological mechanisms in drought-induced tree mortality. Nat. Ecol. Evol. 1, 1285–1291. ( 10.1038/s41559-017-0248-x) [DOI] [PubMed] [Google Scholar]
- 24.Barlow J, Peres C, Lagan B, Haugaasen T. 2003. Large tree mortality and the decline of forest biomass following Amazonian wildfires. Ecol. Lett. 6, 6–8. [Google Scholar]
- 25.Barlow J, Peres CA. 2008. Fire-mediated dieback and compositional cascade in an Amazonian forest. Phil. Trans. R. Soc. B 363, 1787–1794. ( 10.1098/rstb.2007.0013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brando PM, Nepstad DC, Davidson EA, Trumbore SE, Ray D, Camargo P. 2008. Drought effects on litterfall, wood production and belowground carbon cycling in an Amazon forest: results of a throughfall reduction experiment. Phil. Trans. R. Soc. B 363, 1839–1848. ( 10.1098/rstb.2007.0031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costa ACL, et al. 2010. Effect of 7 yr of experimental drought on vegetation dynamics and biomass storage of an eastern Amazonian rainforest. New Phytol. 187, 579–591. ( 10.1111/j.1469-8137.2010.03309.x) [DOI] [PubMed] [Google Scholar]
- 28.Doughty CE, et al. 2015. Drought impact on forest carbon dynamics and fluxes in Amazonia. Nature 519, 78–82. ( 10.1038/nature14213) [DOI] [PubMed] [Google Scholar]
- 29.Brando PM, Oliveria-Santos C, Rocha W, Cury R, Coe MT. 2016. Effects of experimental fuel additions on fire intensity and severity: unexpected carbon resilience of a neotropical forest. Glob. Change Biol. 1–9. ( 10.1111/gcb.13172) [DOI] [PubMed] [Google Scholar]
- 30.INPE. 2015. DEGRAD. See http://www.obt.inpe.br/degrad/
- 31.Jiménez-Muñoz JC, Mattar C, Barichivich J, Santamaría-Artigas A, Takahashi K, Malhi Y, Sobrino JA, Schrier G. 2016. Record-breaking warming and extreme drought in the Amazon rainforest during the course of El Niño 2015–2016. Sci. Rep. 6, 33130 ( 10.1038/srep33130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Withey K, et al. 2018. Quantifying the immediate carbon emissions from El Niño mediated wildfires in humid tropical forests. Phil. Trans. R. Soc. B 373, 20170312 ( 10.1098/rstb.2017.0312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parrotta JA, Francis JK, Almeida RR. 1995. Trees of tapajós. Río Piedras, Puerto Rico, USA: United States Department of Agriculture. [Google Scholar]
- 34.Silver WL, Neff J, McGroddy M, Veldkamp E, Keller M, Cosme R. 2000. Effects of soil texture on belowground carbon and nutrient storage in a lowland Amazonian forest ecosystem. Ecosystems 3, 193–209. ( 10.1007/s100210000019) [DOI] [Google Scholar]
- 35.Chave J, et al. 2014. Improved allometric models to estimate the aboveground biomass of tropical trees. Glob. Change Biol. 20, 3177–3190. ( 10.1111/gcb.12629) [DOI] [PubMed] [Google Scholar]
- 36.Sheil D. 2003. Growth assessment in tropical trees: large daily diameter fluctuations and their concealment by dendrometer bands. Can. J. For. Res. 33, 2027–2035. [Google Scholar]
- 37.Zanne AE, et al. 2009. Global wood density database. Dryad. 235. [Google Scholar]
- 38.Ingwell LL, Joseph Wright S, Becklund KK, Hubbell SP, Schnitzer SA. 2010. The impact of lianas on 10 years of tree growth and mortality on Barro Colorado Island, Panama. J. Ecol. 98, 879–887. ( 10.1111/j.1365-2745.2010.01676.x) [DOI] [Google Scholar]
- 39.Balch JK, Nepstad DC, Curran LM, Brando PM, Portela O, Guilherme P, Reuning-scherer JD, Carvalho O. 2011. Size, species, and fire behavior predict tree and liana mortality from experimental burns in the Brazilian Amazon. For. Ecol. Manage. 261, 68–77. ( 10.1016/j.foreco.2010.09.029) [DOI] [Google Scholar]
- 40.Brando PM, et al. 2014. Abrupt increases in Amazonian tree mortality due to drought-fire interactions. Proc. Natl Acad. Sci. USA 111, 6347–6352. ( 10.1073/pnas.1305499111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Heijden GMF, Phillips OL. 2009. Liana infestation impacts tree growth in a lowland tropical moist forest. Biogeosciences 6, 2217–2226. ( 10.5194/bg-6-2217-2009) [DOI] [Google Scholar]
- 42.Malhi Y, Aragão LEOC, Galbraith D, Huntingford C, Fisher R, Zelazowski P, Sitch S, McSweeney C, Meir P. 2009. Exploring the likelihood and mechanism of a climate-change-induced dieback of the Amazon rainforest. Proc. Natl Acad. Sci. USA 106, 20 610–20 615. ( 10.1073/pnas.0804619106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Funk C, et al. 2015. The climate hazards infrared precipitation with stations—a new environmental record for monitoring extremes. Sci. Data 2, 150066 ( 10.1038/sdata.2015.66) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soares-Filho B, et al. 2010. Role of Brazilian Amazon protected areas in climate change mitigation. Proc. Natl Acad. Sci. USA 107, 10 821–10 826. ( 10.1073/pnas.0913048107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brando PM, Nepstad DC, Balch JK, Bolker B, Christman MC, Coe M, Putz FE. 2012. Fire-induced tree mortality in a neotropical forest: the roles of bark traits, tree size, wood density and fire behavior. Glob. Change Biol. 18, 630–641. ( 10.1111/j.1365-2486.2011.02533.x) [DOI] [Google Scholar]
- 46.Bischl B. 2017. BBmisc: Miscellaneous Helper Functions for B. Bischl.
- 47.Wei T, Simko V. 2017. R package ‘corrplot’: Visualization of a Correlation Matrix (Version 0.84).
- 48.Venables WN, Ripley BD. 2002. Modern applied statistics with S. Fourth. New York, NY: Springer; See http://www.stats.ox.ac.uk/pub/MASS4. [Google Scholar]
- 49.Lüdecke D. 2018. sjstats: Statistical Functions for Regression Models ( 10.5281/zenodo.1284472) [DOI]
- 50.Rozendaal DMA, Chazdon RL. 2015. Demographic drivers of tree biomass change during secondary succession in northeastern Costa Rica. Ecol. Appl. 25, 506–516. ( 10.1890/14-0054.1) [DOI] [PubMed] [Google Scholar]
- 51.Berenguer E, Gardner T, Ferreira J, Aragão L, Mac Nally R, Thomson JR, Vieira I, Barlow J. 2018. Seeing the woods through the saplings: using wood density to assess the recovery of human-modified Amazonian forests.
- 52.ter Steege H, et al. 2013. Hyperdominance in the Amazonian tree flora. Science 342, 1243092 ( 10.1126/science.1243092) [DOI] [PubMed] [Google Scholar]
- 53.Santiago LS, De Guzman ME, Baraloto C, Vogenberg JE, Brodie M, Hérault B, Fortunel C, Bonal D. 2018. Coordination and trade-offs among hydraulic safety, efficiency and drought avoidance traits in Amazonian rainforest canopy tree species. New Phytol. 218, 1015–1024. ( 10.1111/nph.15058) [DOI] [PubMed] [Google Scholar]
- 54.Santos VAHF, Ferreira MJ, Rodrigues JVFC, Garcia MN, Ceron JVB, Nelson BW, Saleska SR. 2018. Causes of reduced leaf-level photosynthesis during strong El Niño drought in a Central Amazon forest. Glob. Change Biol. 24, 4266–4279. ( 10.1111/gcb.14293) [DOI] [PubMed] [Google Scholar]
- 55.Esquivel-Muelbert A, et al. 2017. Seasonal drought limits tree species across the Neotropics. Ecography 40, 618–629. ( 10.1111/ecog.01904) [DOI] [Google Scholar]
- 56.Takahashi K, Dewitte B. 2016. Strong and moderate nonlinear El Niño regimes. Clim. Dyn. 46, 1627–1645. ( 10.1007/s00382-015-2665-3) [DOI] [Google Scholar]
- 57.Werner PA. 2005. Impact of feral water buffalo and fire on growth and survival of mature savanna trees: an experimental field study in Kakadu National Park, northern Australia. Austral Ecol. 30, 625–647. ( 10.1111/j.1442-9993.2005.01491.x) [DOI] [Google Scholar]
- 58.Busse MD, Simon SA, Riegel GM. 2000. Tree-growth and understory responses to low-severity prescribed burning in thinned Pinus ponderosa forests of Central Oregon. For. Sci. 46, 258–268. ( 10.1093/forestscience/46.2.258) [DOI] [Google Scholar]
- 59.Díaz S, et al. 2016. The global spectrum of plant form and function. Nature 529, 167–171. ( 10.1038/nature16489) [DOI] [PubMed] [Google Scholar]
- 60.Wright SJ, et al. 2010. Functional traits and the growth–mortality trade-off in tropical trees. Ecology 91, 3664–3674. ( 10.1890/09-2335.1) [DOI] [PubMed] [Google Scholar]
- 61.Barlow J, et al. 2018. The future of hyperdiverse tropical ecosystems. Nature 559, 517–526. ( 10.1038/s41586-018-0301-1) [DOI] [PubMed] [Google Scholar]
- 62.Baccini A, Walker W, Carvalho L, Farina M, Sulla-Menashe D, Houghton RA. 2017. Tropical forests are a net carbon source based on aboveground measurements of gain and loss. Science 358, 230–234. ( 10.1126/science.aam5962) [DOI] [PubMed] [Google Scholar]
- 63.Sonter LJ, Herrera D, Barrett DJ, Galford GL, Moran CJ, Soares-Filho BS. 2017. Mining drives extensive deforestation in the Brazilian Amazon. Nat. Commun. 8, 1013 ( 10.1038/s41467-017-00557-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barlow J, et al. 2012. The critical importance of considering fire in REDD+ programs. Biol. Conserv. 154, 1–8. ( 10.1016/j.biocon.2012.03.034) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in this paper is available as part of the electronic supplementary material.