Abstract
Drought-induced wildfires have increased in frequency and extent over the tropics. Yet, the long-term (greater than 10 years) responses of Amazonian lowland forests to fire disturbance are poorly known. To understand post-fire forest biomass dynamics, and to assess the time required for fire-affected forests to recover to pre-disturbance levels, we combined 16 single with 182 multiple forest census into a unique large-scale and long-term dataset across the Brazilian Amazonia. We quantified biomass, mortality and wood productivity of burned plots along a chronosequence of up to 31 years post-fire and compared to surrounding unburned plots measured simultaneously. Stem mortality and growth were assessed among functional groups. At the plot level, we found that fire-affected forests have biomass levels 24.8 ± 6.9% below the biomass value of unburned control plots after 31 years. This lower biomass state results from the elevated levels of biomass loss through mortality, which is not sufficiently compensated for by wood productivity (incremental growth + recruitment). At the stem level, we found major changes in mortality and growth rates up to 11 years post-fire. The post-fire stem mortality rates exceeded unburned control plots by 680% (i.e. greater than 40 cm diameter at breast height (DBH); 5–8 years since last fire) and 315% (i.e. greater than 0.7 g cm−3 wood density; 0.75–4 years since last fire). Our findings indicate that wildfires in humid tropical forests can significantly reduce forest biomass for decades by enhancing mortality rates of all trees, including large and high wood density trees, which store the largest amount of biomass in old-growth forests. This assessment of stem dynamics, therefore, demonstrates that wildfires slow down or stall the post-fire recovery of Amazonian forests.
This article is part of a discussion meeting issue ‘The impact of the 2015/2016 El Niño on the terrestrial tropical carbon cycle: patterns, mechanisms and implications’.
Keywords: post-fire dynamics, stem mortality, wood productivity, long-term recovery, fire disturbance, drought
1. Introduction
The successful reduction of the deforestation rate in the Brazilian Amazon between 2004 and 2017 has not been sufficient to reduce disturbance in the remaining forests [1]. Recent studies demonstrate that human-induced disturbances (e.g. wildfires and selective logging) can halve the conservation value and significantly decrease the carbon stocks of remaining Amazonian forests [2–4]. Moreover, Amazonian forests affected by wildfires are estimated to contribute on average 31 ± 21% of the gross emission values from deforestation, with contributions beyond 50% during drought years [5]. Yet, there is a critical knowledge gap regarding the long-term recovery of carbon stocks in forests affected by anthropogenic disturbances such as fire [2,3,6].
Humid tropical forests are not a fire-adapted ecosystem [7,8]. Previous studies suggested that wildfires in the Amazon basin have been rare since the start of the Holocene, with fire-return intervals exceeding centuries or millennia [8,9]. However, over the past three to four decades, wildfires have become increasingly prevalent across humid tropical forests, including Amazonia [10]. These tropical fires generally require an anthropogenic source to ignite, which generally comes from agricultural practices [11]. The likelihood of wildfires occurrence is also increased by forest disturbance, such as selective logging [12], and by deforestation that exposes remaining forests to edge effects [13] and reduces rainfall [14,15]. In addition, wildfires can be greatly exacerbated by extreme drought events [5,12,16–19]. For example, during the 2015 El Niño-induced extreme drought, 799 293 km2 of the Brazilian Amazon experienced positive active fire anomalies [5]. Given that extreme droughts are predicted to occur at a greater frequency in the Amazon Basin [20], wildfires are likely to become even more pervasive [21].
These wildfires have a major impact on forest carbon stocks, accounting for the mortality of up to 36% of tree stems and 67% of the biomass loss in central Amazonian forests 3 years after fires [22,23]. Fire-affected forests consequently become a globally important carbon source: based on the 2010 fire season, it was estimated that 27 555 km2 of old-growth forests burned in the whole Brazilian Legal Amazon, contributing to 14.8 Tg of C emissions to the atmosphere from direct combustion of organic material [19]. Immediately combustible carbon stocks—such as leaf litter and fine woody debris—make up only a very small proportion of forests' above-ground carbon stock [2] and most emissions are committed (0.001 to 0.165 Pg of C), which means they are likely to occur years after wildfires as a result of vegetation mortality and its subsequent decomposition [16].
Despite the growing prevalence and importance of wildfires in humid tropical forests, our knowledge of their ecological consequences is constrained by the lack of data in three key areas. First, the longer-term effects of wildfires on forest biomass are not known as most studies to date have focussed on relatively short-term responses of vegetation to fire [24–27]. For example, a pan-tropical assessment suggests there is no recovery of forest carbon stocks within at least 5 years [28], while a study on flooded Amazonian forests highlights the potential for fires to impede forest succession in the first 15 years after fire [29]. Second, most assessments are one-off inventories, meaning ecological processes and stem dynamics in fire-affected forests are very poorly understood. Extensive field assessments in undisturbed Amazonian forests show the importance of repeat surveys, which have enabled researchers to link the spatial variation of forest biomass to stem dynamics such as mortality and recruitment [30,31]. Finally, there are no data linking post-fire long-term forest dynamics with functional traits. Plant traits such as bark thickness and wood density provide important insights into post-fire changes and the susceptibility of forest ecosystems [32–36], especially as they are directly related to carbon storage function [37]. Recently, an assessment of the impacts of fire and other forest disturbances has shown that wood density remains below baseline conditions for at least 25 years following disturbance, indicating a slow recovery or impeded succession [38]. Longer-term assessments of forest dynamics could provide additional insights into the successional trajectories of burned forests, and their ability to recompose carbon stocks.
We address these knowledge gaps by using a unique large-scale and long-term assessment of forest dynamics, which is based on a set of chronosequences and re-census data from burned and unburned forests in five distinct regions of the Brazilian Amazon. We ask two main research questions:
(i) What are the longer-term effects of wildfires on forest biomass (i.e. up to 31 years after the fires)? We address this question by comparing, at the plot level, the total aboveground biomass (TAGB), and forest dynamics represented by mortality and wood productivity, between burned and unburned forests. The balance between tree mortality and productivity defines the ability of these fire-affected forests to recover to pre-disturbance carbon levels and offset carbon emissions.
(ii) How do wildfires affect forest growth, recruitment and mortality at stem level, and what insights do key structural traits such as wood density and stem size (diameter at breast height (DBH)) provide into the mechanisms underpinning the changes in biomass? We focus on wood density and size because both are important predictors of short-term fire-induced mortality [32,33] and both are linked to stem growth rates and carbon storage in undisturbed forests [39,40]. We divided stems into three classes of wood density and size to examine the changes in the probability density functions of growth, recruitment and mortality over time since fire degradation.
Finally, we combine results from both questions to discuss to what extent Amazon forests are recovering from fires.
2. Material and methods
(a). Experimental design for field data collection
We used tree inventory data collected as part of the Fire-Associated Transient Emissions in Amazonia (FATE) network. Since 2009, the FATE network has been monitoring permanent forest plots established in burned forests with different times since wildfire occurrence. Here, we collected and analysed field data from 64 permanent plots across Amazonia, from which we revisited and re-measured 55. All plots are located on old-growth non-flooded forests (Terra Firme) with 269.3 m median distance from the edge. We examined the terrain elevation and slope within 100 m buffer of each plot using a high-resolution (12.5 m) digital elevation model (ALOS PALSAR RTC). There is a very small slope across the plots (range: 2.8°–9.4°). Plots ranged from 0.25 to 1 ha. From a total of 64 plots, 29 are in unburned and 35 plots are in burned forests (electronic supplementary material, table S1).
We selected burned forest sites based on the inspection of Landsat images (1984–2016) followed by on-the-ground field confirmation. When we did not find evidence of fire in the satellite image for a specific site, but there was charcoal in the ground, we assumed the fire event occurred at the time of the earliest image (i.e. 1984). Because of the high intensity of the 1982–83 El Niño event, when 3.6 million ha were burned in East Kalimantan [41], it is likely that several forested areas elsewhere were affected by wildfires during this period. To enable pairwise comparisons between burned and unburned control sites, both were selected to avoid other anthropogenic disturbances such as selective logging. The unburned control plots, moreover, were carefully chosen to encompass a similar range and heterogeneity of both soils and topography to the burned sites. Independent proxies of fire intensity, such as char height, are not available for plots assessed a long time after fires when many of the affected trees will have died and decomposed. Without this additional information, we assume that all plots were subjected to low-intensity understorey wildfires that are the norm in previously undisturbed forests.
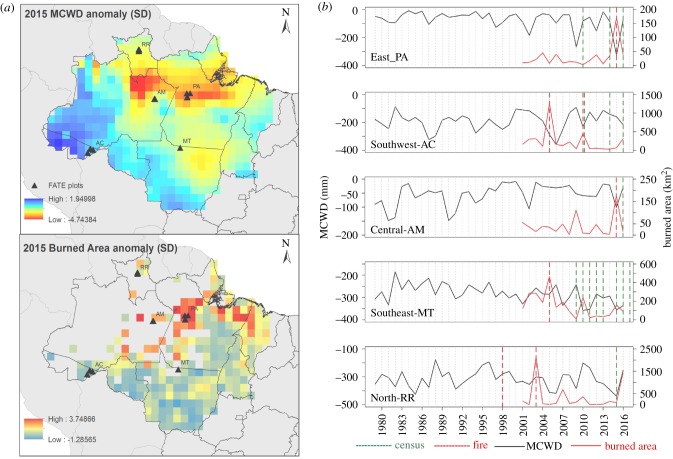
Our 31 years chronosequence dataset captures the effect of wildfires driven by El Niño events and North tropical Atlantic warming since the 1980s. The distribution of the FATE plots reflects the spatial occurrence of these major wildfire events (e.g. figure 1a) and accessibility. In order to link drought intensity over the last 40 years with wildfire extent, we used re-analysis derived data to calculate Maximum Climatological Water Deficit (MCWD) and satellite-derived products of burned area (BA) (please see detailed methods in electronic supplementary material, method S1). The data extracted from each plot location, along the BA and MCWD time series, show the association between MCWD and BA in each plots region (East, Southwest, Central, Southeast and North; figure 1b). Figure 1b also demonstrates when each site was sampled relative to the last fire event.
Figure 1.
Tree inventory plots and overlap of maximum cumulative water deficit (MCWD) and burned area (BA) anomalies (s.d.) over the Brazilian Amazon region. MCWD was derived from ERA-Interim and BA derived from MODIS (detailed methods in electronic Supplementary material, method S1). (a) MCWD red values representing extreme drought, or negative anomalies (s.d.) in relation to 1979–2016 period; BA red values representing extreme large affected areas, or positive anomalies (s.d.) in relation to the 2001–2016 period. (b) MCWD and BA variation over time extracted from each plots region located in the Brazilian states Pará (PA), Acre (AC), Amazonas (AM), Mato Grosso (MT) and Roraima (RR), the year of the tree inventory and the year of fire.
(b). Field inventory and total above-ground biomass
The inventory was conducted following the RAINFOR network protocol for the establishment of permanent sample plots [42]. We estimated above-ground biomass (AGB) of 9836 live trees, palms and lianas with DBH ≥ 10 cm. For both burned and unburned forests, TAGB represents the sum of all trees, palms and lianas AGB, and was estimated using a specific allometric equation for each group, following [37] for trees, [43] for palms and [44] for lianas. The AGB estimates for palms and lianas were based solely on their diameter, while for trees DBH and specific wood density values were used as input variables. We used the global wood density database [45,46] to match specific wood density to each species. For individuals not identified to the species level (approx. 5%), we used the mean value for the species belonging to that genus. Similarly, we used the mean specific wood density of the family for trees not identified at the genus level [30]. When an accurate identification was not achieved, the plot mean specific wood density was used.
(c). Plot-level assessment of long-term effects of wildfires on forest biomass
(i). Quantification of plot-level forest dynamics
To understand the response of old-growth forests to wildfires, we evaluated the long-term shifts in forest dynamics at the plot level. We quantified for all burned and unburned plots the net biomass change (Net TAGB), which is a function of wood productivity (Wp) and mortality (M) of all stems in the plot (equation (2.1)).
| 2.1 |
The term ΣM corresponds to plot mortality (Mg ha−1 yr−1), which was calculated as the amount of the biomass of all stems recorded as dead within a given census interval. The term ΣWp corresponds to the sum of the values of Wp for all measured stems in the plot and can be decomposed as (equation (2.2)).
| 2.2 |
where Wp (Mg ha−1 yr−1) was calculated as the sum of the biomass of stems that recruited during each census interval (ΣRecruits) and the sum of the growth in biomass of each stem present in the plot (ΣGrowth) during this same census interval.
Because census interval varied among plots, rates were weighted by the census interval length. In order to account for trees that both recruited and died during the census interval and also to correct for tree growth prior their death, M and Wp values were corrected at a tree-by-tree basis, following methods of [47].
(ii). Quantification of differences between burned and unburned forests
To assess if TAGB and dynamics from burned forests recovered to pre-disturbance levels, we quantified the per cent of the difference between burned and unburned forests. For TAGB and each dynamic parameter, the proportional difference between each burned plot and the mean of unburned plots were calculated as described in equation (2.3):
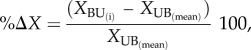 |
2.3 |
where X represents the variable of interest (TAGB, M, Wp and Net TAGB), BU(i) is each of the burned plots, and UB(mean) is the local mean of all unburned plots sampled in the same region at the same time as the burned plots. The error is presented as standard error of the mean (s.e.).
(iii). Long-term trajectories of burned forests' total aboveground biomass and dynamics
We used generalized additive mixed models (GAMM) to assess the trajectories of TAGB, M, Wp and Net TAGB over the time since last fire chronosequence. We used each individual plot measured repeatedly as a random effect. To assess the direction of the difference (%) in each variable in relation to the control-unburned forests, we used the local polynomial regression fit (LOESS), choosing the span values based on the minimum residual standard error obtained. All statistical analyses were performed in R v. 3.3.3 using gamm4 [48] R and lme4 [49] packages.
(d). Stem-level assessment of growth, recruitment and mortality
To explore the structural and successional mechanisms driving the long-term changes on TAGB of burned forests, we assessed the empirical probability density function of stem mortality rate and stem growth in three DBH (cm) classes: 10.0 to 19.9, 20 to 39.9 and greater than 40.0; and three specific wood density (g cm−3) classes: 0.1 to 0.49, 0.5 to 0.69 and greater than 0.7, for both burned and unburned plots. Including all plots from all regions, we divided the dataset into four categories considering the years since last fire (YSLF): 0.75–4; 5–8; 9–11; 12–31 years. For each plot, we calculated stem mortality as the exponential mortality coefficient (% yr−1) [50], mean stem growth as the annual mean growth (cm yr−1) of all living individuals and stem recruitment as the percentage rate of stems recruited relative to live stems in each census (% yr−1). Stem mortality and stem growth from each plot were stratified by classes of diameter, wood density and YSLF. Stem recruitment by plot was stratified by YSLF class, but we only used a grouping based on wood density class, as all recruitment falls into the smallest DBH class. The probability density functions of the unburned and burned plots were compared using the Wilcoxon test for two samples.
3. Results
(a). The long-term effects of wildfires on forest biomass at plot level
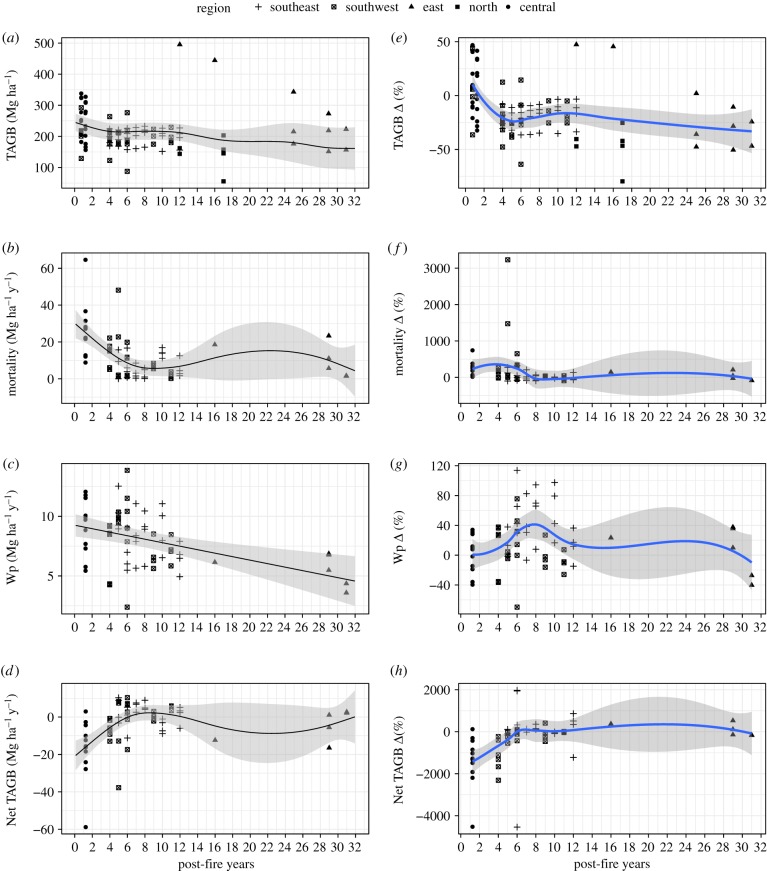
During the monitoring period, the biomass of unburned forest plots remained generally unchanged, with the exception of forest plots from southeast and east Amazonia that have experienced high mortality in the drought years of 2015 (15.2 Mg ha−1 yr−1; n = 4) and 2016 (9.9 Mg ha−1 yr−1; n = 20) respectively (electronic supplementary material, table S2). By contrast, the biomass of burned forest plots changed greatly with time since fire (table 1). Immediate fire effects on TAGB were smaller, with reduction of −2.1 ± 3.9% up to 4 years post-fire. From 5 to 8 years since fire, we found a much greater difference in TAGB, with reduction of −22.1 ± 2.9% in burned plots compared to unburned controls. The significantly lower biomass persisted up to 31 years post-fire, when burned plots remained 24.8 ± 6.9% below the baseline value of the control plots (figure 2a,e).
Table 1.
Mean difference (Δ) in % (±s.e.) between each burned plot and unburned mean values of TAGB, mortality, wood productivity (increment and recruitment values in electronic supplementary material, table S3) and Net TAGB.
| TAGB stock |
TAGB dynamics |
||||||
|---|---|---|---|---|---|---|---|
| YSLF categories | census year | TAGB △% | N | mortality △% | wood productivity △% | Net TAGB △% | N |
| (0.75–4) | 2009; 2011; 2014; 2015; 2016 | −2.1 (3.9) | 42 | 199.2 (43.5) | 4.0 (6.9) | −1308.4 (263.1) | 17 |
| (5–8) | 2010; 2011; 2012; 2013; 2016 | −22.1 (2.9) | 26 | 247.4 (135.6) | 30.0 (7.8) | −26.8 (212.1) | 26 |
| (9–11) | 2014; 2015; 2016 | −17.1 (2.9) | 12 | −8.6 (10.8) | 16.7 (11.2) | −45.5 (57.2) | 12 |
| (12–31) | 2010; 2014; 2016; 2017 | −24.8 (6.9) | 20 | 20.7 (33.7) | 8.9 (8.7) | 105.0 (183.3) | 10 |
Figure 2.
GAMM fitted models of burned forest pathways by dependent variables: (a) total above-ground biomass (TAGB), (b) mortality, (c) wood productivity (Wp) and (d) Net TAGB; and LOESS fit for per cent difference (Δ%) of each variable in relation to unburned forest (e–h).
The reduction in TAGB observed in burned forests reflects the imbalance between wood productivity and mortality. Although post-fire mortality declined during the first 8 years of the chronosequence (figure 2b), its negative influence on burned forests biomass is evident (figure 2a). The maximum difference in mortality between unburned and burned forests was at 4–5 years post-fire (247.4 ± 135.6%, table 1), with higher mortality values for burned forests. There was no difference in mortality between burned and unburned forests from 8 until 31 years (figure 2f). Wood productivity in burned forests followed a linear decline along the chronosequence (figure 2c). However, when compared to unburned forests, Wp rates in burned forests remained higher and the difference increased to its maximum value (30 ± 7.8%, table 1) until 8 years post-fire. The difference in Wp between burned and unburned forests then decreased and remained in a near-steady state until 31 years post-fire (figure 2g). Moreover, Net TAGB in burned forests increased during the first 8 years, shifting from a strong negative sign (source) to a neutral state (figure 2d). Compared to unburned forests, Net TAGB rates in burned forests were lower but increased until 6 years of the chronosequence, and then remained steady and equivalent to unburned forests levels (figure 2h).
(b). Uncertainties
Across pools, the largest uncertainties (table 1) are associated with mortality, due to the large influence exerted by the death of a single large tree. Temporally, and for all variables, there were large uncertainties from 16 to 27 years after fire, where data were lacking (figure 2a–h). It is reassuring that the trajectories predicted along the chronosequence using the GAMM model and LOESS fit agree. All GAMM fitted models' intercept and smooth component (YSLF) are statistically significant (table 2). While all models are significant (electronic supplementary material, figure S1), residual variability may be associated with the ‘random’ deviations from the predicted values that are not due to plots' specificities and/or YSLF, suggesting a possible association with fire intensity and environment conditions. Accordingly, the large TAGB and mortality variability observed across the plots explains the higher s.e. found in the intercept and slope of TAGB and mortality models. The fitted model's effective degrees of freedom values consistently show that burned forests' TAGB, mortality and Net TAGB response to time are nonlinear, while Wp is linear. For Wp, the effective degrees of freedom is equal to 1, meaning linearity for Wp in relation to time.
Table 2.
GAMM models' output by fixed term for intercept and the smooth term YSLF.
| TAGB | mortality | Wp | Net TAGB | |
|---|---|---|---|---|
| Intercept | ||||
| estimate | 216.2 | 11.4 | 8.1 | −3.3 |
| s.e. | 12.5 | 1.2 | 0.3 | 1.3 |
| s.d. | 72.8 | 0 | 1.3 | 0 |
| Pr(>|t|) | <2 × 10−16 | 2.71 × 10−13 | <2 × 10−16 | 0.01 |
| Smooth term (YSLF) | ||||
| estimate | −34.2 | −21.4 | −1.0 | 19.7 |
| s.e. | 17.9 | 9.6 | 0.3 | 9.7 |
| s.d. | 102.7 | 22.5 | 0 | 22.6 |
| edfa | 5.2 | 3.5 | 1 | 3.5 |
| p-value | 0.000463 | 2.05 × 10−15 | 0.00064 | 0.000119 |
| Residuals | ||||
| s.d | 12.3 | 9.9 | 1.8 | 10.2 |
aEffective degrees of freedom.
(c). Mortality, recruitment and growth rates at stem level
Wildfires had persistent effects on burned forest dynamics at stem level: from a total of 48 comparisons between burned and unburned forests of stem mortality and growth, 16 were significant (p < 0.05) and another 5 were marginally significant at p < 0.10 (figures 3 and 4). These significant results were distributed across all classes of time since last fire disturbance, and all classes of tree size and wood density.
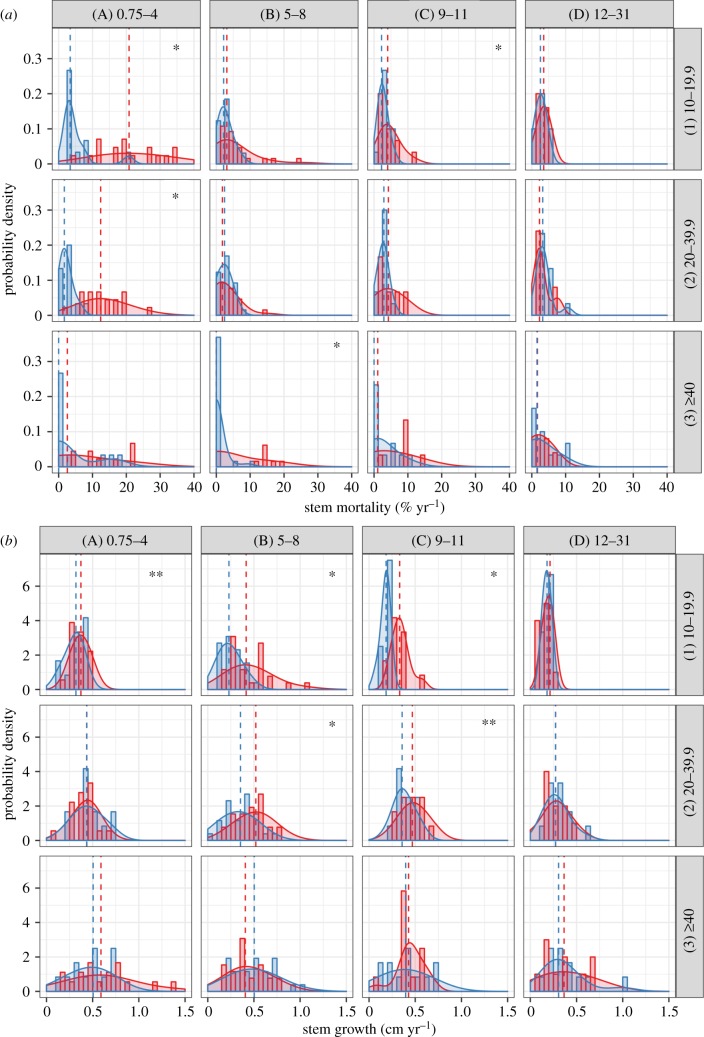
Figure 3.
Probability density function of: (a) stem mortality (% yr−1) and (b) stem growth (cm yr−1) by size classes (DBH: 10.0–19.9; 20.0–39.9; greater than 40.0 cm) in lines and years since last fire (YSLF) classes (0.75–4; 5–8; 9–11; 12–31 years) in columns. Dashed lines represent median, red colour for burned and blue for unburned forests. Significance of Wilcoxon text is represented by: *p < 0.05 and **p < 0.10.
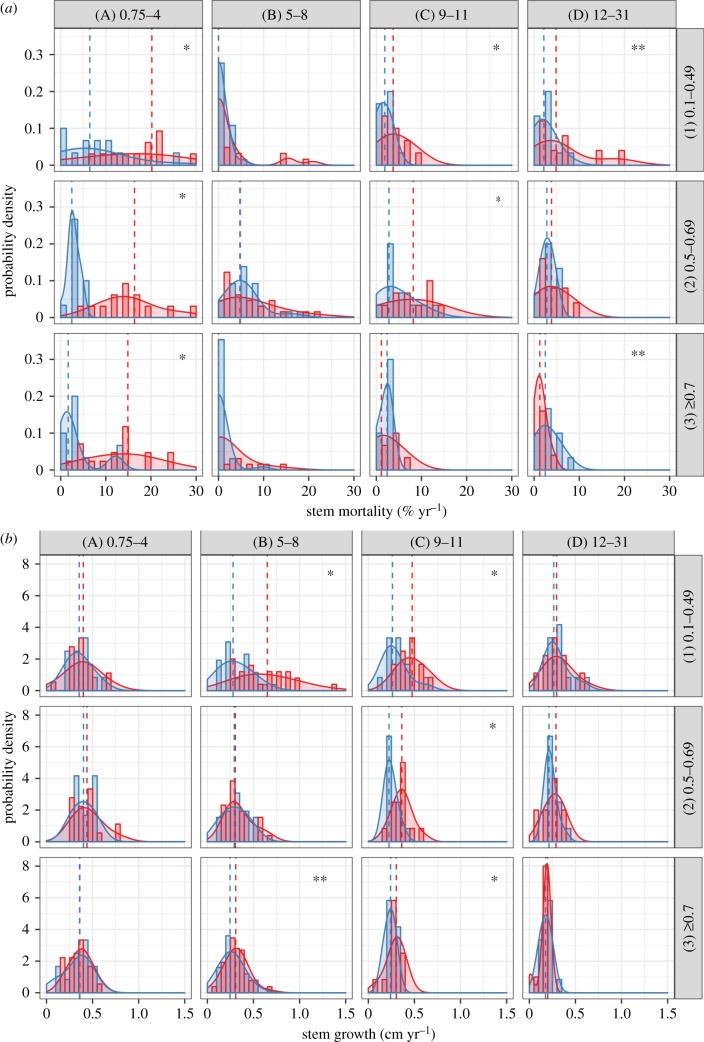
Figure 4.
Probability density function classes of (a) stem mortality in (% yr−1) and (b) stem growth (cm yr−1) by wood density (WD: 0.1–0.49; 0.5–0.69; greater than 0.7 g cm−3) in lines and YSLF classes (0.75–4; 5–8; 9–11; 12–31 years) in columns. Dashed lines represent median, red colour for burned and blue for unburned forests. Significance of Wilcoxon text is represented by: *p < 0.05 and **p < 0.10.
Stem mortality was skewed towards zero, but still higher in burned forests when compared to unburned forests. The significantly higher stem mortality was observed across all tree size and wood density classes—but not in all YSLF categories (figures 3a and 4a). The largest stem mortality differences between burned and unburned forests were observed at 0.75–4 YSF. On average, 22.8 ± 2.4% of trees from small classes of size (i.e. 10–19.9 cm DBH) and 23.8 ± 5.0% of trees with the lightest wood density (i.e. 0.1–0.49 g cm−3) died during 0.75–4 YSLF—these mortality rates were 341 and 239% higher than the equivalent size and wood density classes in unburned controls, respectively. However, the denser wood stems (i.e. greater than 40 cm DBH; 5–8 YSLF) and higher wood density classes (i.e. greater than 0.7 g cm−3; 0.75–4 YSLF) were also significantly affected in the burned forest, with mortality rates being 680% and 315% higher than unburned controls, respectively. Between 9 and 11 years since the wildfires, small size stems (i.e. 10–19.9 cm DBH) and stems from small (i.e. 0.1–0.49 g cm−3) and medium (i.e. 0.5–0.69 g cm−3) classes of wood density experienced significantly higher mortality in burned forests—these mortality rates were 74%, 173% and 69% higher than unburned controls, respectively.
Stem growth followed a normal distribution, and the mean values of burned forests were generally higher than those in unburned forests (figure 3b and 4b). The greatest difference in stem growth was observed in the small and medium size classes: when compared to unburned controls, mean stem growth was 94.1% (5–8 YSLF) and 96.6% (9–11 YSFL) higher in burned forests for small size class in the 5–8 and 9–11 YSLF categories, respectively, and 54.2% (5–8 YSLF) and 27.0% (9–11 YSFL) higher in burned forests for the medium size class at 5–8 and 9–11 YSLF categories, respectively. Similarly, for the class of low wood density, mean stem growth was 121.1% (5–8 YSLF) and 62.1% (9–11 YSFL) higher in burned forests than in unburned forests in the 5–8 and 9–11 YSLF categories, respectively. For medium wood density stems, mean stem growth was 50.0% higher in burned forests than in unburned forests at the 9–11 YSLF category. Finally, for high wood density stems, growth was 24.0% and 26.0% higher in burned forests than in unburned forests at 5–8 and 9–11 YSF, respectively.
Stem recruitment was skewed towards zero (electronic supplementary material, figure S2). Overall, mean stem recruitment values were generally higher in burned than unburned forests up to 12 years since last fire (electronic supplementary material, figure S3). There were no significant differences between recruitment in burned and unburned forests when separated by wood density classes.
4. Discussion
We provide one of the longest post-fire chronossequence assessements of fire-affected Amazonian forests, analysing the most extensive dataset to date. Our findings reveal that burned Amazonian forests persist in a reduced biomass state for at least 31 years since the last fire, at which point they store approximately 25% less TAGB than equivalent unburned forests. This decrease in biomass is driven by increases in mortality that are not fully compensated for by the relatively small changes in recruitment and growth rates (table 1). The high mortality in burned forests was not exclusively limited to small diameter and light wood trees, but also includes the large-stemmed and hardwood trees that contribute most to the carbon stock [30,51,52]. In contrast, the positive post-fire growth response was predominantly associated with small–medium-sized trees and lighter or intermediate classes of wood density—groups that contribute relatively little to overall above-ground carbon stocks. We examine these findings in more detail to understand how the post-fire changes in dynamics rates influence forest biomass in the long-term, and how this is underpinned by mortality, recruitment and growth among functional groups. Finally, we discuss the prospects of long-term slow recovery of Amazonian fire-affected forests and the future of tropical humid forests under the risk of wildfires.
(a). Post-fire changes in forest dynamics and consequences for the long-term recovery of biomass stocks
Our data show that long-term reduction on TAGB after fire is persistent, but the uncertainties inherent in space-for-time comparisons and delayed mortality of large trees mean it only became fully evident after 5 years of the fire events. After the initial fire-induced mortality, wood productivity rates in burned forests were higher than in unburned controls probably because of the increase in light and nutrients available to the surviving trees. However, this initial short-term increase in wood productivity (plot-level biomass gain) does not exceed mortality (plot-level biomass loss) and is insufficient to counteract the total biomass losses through mortality along the whole chronosequence. Previous studies have raised the question of whether enhanced forest growth, promoted by low-intensity fires, offsets carbon emissions due to post-fire tree mortality [53]. Our assessment refutes that suggestion: although burned forests were no longer a net carbon source 6 years after fires, the lack of biomass accumulation from 6 to 31 years shows they will not recover to pre-fire conditions on decadal time scales. Our findings also emphasize the importance of longer-term and larger-scale studies to monitor carbon dynamics in burned forests, which are particularly important for incorporating the variation of mortality and growth rates in C emission models.
(b). Post-fire mortality among functional groups with high contribution to biomass stocks
Wildfires affected the stem mortality rates of small–medium sized trees and all wood density classes in the first YSLF category (0.75–4) of the chronosequence. An initial increase in the mortality of high wood density trees (315%) compared to unburned forests, combined with a late increase in the mortality of large-sized trees (680%), has important impacts upon overall AGB loss. A burned forest that has lost its large size (figure 3a) and high wood density stems (figure 4a) will inevitably store less biomass that it did prior to disturbance (figure 2a,e). As well as corroborating previous studies on the late increase in mortality of large trees [23], we also show for the first time that this process can continue for up to 8 years after fire—suggesting that almost all previous studies will have underestimated total biomass loss from fires.
Although previous findings show tree mortality decreased as a function of increasing wood density [33], we show that all wood density classes are at risk of fire-induced mortality, especially in the first 4 years after the burn. It is important to note that our results do not show higher susceptibility of high wood density trees compared to lower wood density trees to post-fire mortality; instead we show higher stem mortality of high wood density trees in burned forests compared to unburned controls. One explanation for this high post-fire mortality across wood density classes reflects the fact that the full range of wood densities can be found in the small (i.e.10.0–19.9 cm DBH) and medium (i.e. 20.0–39.9 cm DBH) size classes, which are the fire-susceptible groups. Smaller trees are shown to have thinner bark, which in turn are at more risk of heat stress and fire-induced mortality [11,32].
(c). Post-fire stem gowth and recruitment
The significant loss of large size and emergent trees is likely to have triggered the increase in the growth of light-dependent and fast-growing species. As expected, this increase in wood productivity is associated with the stem growth responses of small and medium size trees from all wood density classes, and to a lesser extent to stem recruitment. Although light availability is expected to also benefit new recruits [54], stem recruitment is less evident and not significantly higher than in undisturbed forest in each individual wood density class. However, an ongoing successional process may be occurring within burned forests, as components of wood productivity (recruitment + growth) were higher compared to unburned (electronic supplementary material, table S3, figure S3). Our results suggest that pioneer species are colonizing and growing after fire, maintaining a natural forest succession process after disturbance. For instance, the late stem mortality of small trees (i.e. 10–19.9 cm DBH; 9–11 YSLF) and stem growth at mid–long-term (i.e. 5–8 and 9–11 YSLF) observed supports the expected post-disturbance forest succession. However, it is expected that recruitment of old growth species is limited after fire disturbance, which negatively affects the forest's ability to recover to its pre-disturbance functional state [29,38]. Consequently, fire disturbances are likely to shift forest composition and dynamics for much longer than 30 years.
(d). Prospects for forest recovery beyond the time-scale of our data
Although our data extend to 31 years post-fire, there are reasons to expect a slow recovery for many decades beyond this time-frame. First, the Net TAGB in burned forests was close to unburned forests' equilibrium in the long-term of the chronosequence and did not provide any signs of continued recovery. For the recovery to occur gains would need to surpass loss during this stage. Second, the fires killed many large size and high wood density trees, which will take the longest to recover; perhaps unsurprisingly we also found that their re-establishment will take longer than 31 years, and many could take centuries to recover, given the large trees' age (200–1400 years) in undisturbed Amazonian forests [55]. However, other unassessed factors could be important and are worthy of further investigation. For example, the destruction of the seedbank by fire and a low seedling survival may act to limit stem recruitment, as previously found in Amazonian flooded forest affected by fire [29]. In addition, any surviving seeds from shade-tolerant species are less likely to germinate in larger canopy gaps [56]. Finally, the reduced biomass stock may be exacerbated by early successional species inhibiting emergent and shade-tolerant species on decadal time scales [54].
(e). Post-fire forest recovery limitations and the future of tropical humid forests under the risk of wildfires
Forest disturbance from fires may interact with a changing climate. For example, burned forests have a more open canopy that allows solar radiation to penetrate. The increasing temperature in the interior of burned forests results in the increase of vapour pressure deficit and evapotranspiration, further exacerbating soil drying [7,57]. At the same time, the Amazon has seen an increase in drought conditions, limiting water availability [58] and potentially limiting the recruitment of trees [59]. Although Amazonian forests seem to be resilient to dry conditions, it is likely that water limitation can limit their recovery from fire disturbances [58,60]. Whether post-fire succession is permanently arrested or is just occurring at a very slow rate is difficult to ascertain based on the temporal scale of our dataset. As we only assessed individuals ≥10 cm DBH within 31 years since the last fires, we are unlikely to detect longer-term recovery or the re-establishment of slow-growing (high wood density) species. However, it is notable that assessments of saplings and seed banks in disturbed Amazonian forests indicate a slowdown or stalled forest recovery [29,38], and the stabilization of recovery after wildfires is in marked contrast to the consistent increases in forest biomass observed in the first decades after the disturbance in selectively logged or secondary forests [61–63].
Considering the increase in frequency and intensity of extreme events—such as the 2015/2016 El Niño—associated with increasing fire incidence [5], our findings highlight the urgent need to avoid fires in humid tropical forests. Our study also provides the largest ground-based assessment on patterns of post-fire forest recovery, which is particularly important considering the role of the Amazon in the global carbon cycle. Moreover, in our effort to cover the heterogeneity of once-burned forests subjected to similar fire intensities, our estimates describe a general response of Amazonian old-growth Terra Firme forests to fire disturbance. However, it is important to state that we investigated the effect of a single fire event on forest dynamics and biomass stocks through time. Recurrent fires are still somewhat rare in the Amazon—in 2010, they only accounted for 16% of all wildfires [64]. However, recurrent fires are likely to become increasingly prevalent across the Amazon, given the synergies between a drier and hotter climate, the pervasive use of fire in agriculture [65] and the human-induced disturbances such as selective logging that render forests more vulnerable to fires due to changes in the microclimate [2,11]. The combination of these factors will also affect the ability of forests to recover from fire disturbance.
Supplementary Material
Acknowledgements
We thank Paulo Brando and one anonymous reviewer for their valuable comments on an earlier version of this manuscript. We would like to thank Brazilian National Institute for Space Research (INPE) that with the collaboration of several Brazilian governmental institutes, universities and international partners, gave support to field data collection and processing. We thank the Large-Scale Biosphere-Atmosphere Program (LBA) for logistical and infrastructure support during field measurements. We thank the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBIO) for granting permits to access the study areas. We are sincerely grateful to field assistants and land holders who supported us in the field.
Data accessibility
All relevant data are within the paper and its electronic supplementary material information files.
Authors' contribution
L.E.O.C.A conceived the study. L.E.O.C.A., J.B. and L.O.A. designed the study. C.S., A.P.L., M.A.S. and B.C. processed the data. C.S. performed the analyses with support from L.E.O.C.A., F.E.S. and J.B., C.S., L.E.O.C.A., I.B., H.A.M.X., E.B., P.M.L.A.G., M.S., B.C., L.K. and A.P.L. carried out the FATE field data collection. C.S., L.E.O.C.A. and J.B. wrote the paper with contributions from all co-authors.
Competing interests
We declare we have no competing interests.
Funding
This work was financially supported by the Brazilian National Council for Scientific and Technological Development (CNPQ: grant no. 458022/2013-6). C.S. was supported by a Scholarship from Lancaster University Faculty of Science and Technology. J.B., F.E.S. and F.F. were supported by Natural Environment Research Council (NERC) (‘AFIRE’ NE/P004512/1). J.B. and F.E.S. also received support from grants (BIO-RED’ NE/N012542/1) and F.E.S. was also supported by Newton Fund (‘The UK Academies/FAPESP Proc. N°: 2015/50392-8). E.B., J.F. and J.B. were supported by Instituto Nacional de Ciência e Tecnologia—Biodiversidade e Uso da Terra na Amazônia (CNPq 574008/2008-0), Empresa Brasileira de Pesquisa Agropecuária—Embrapa (SEG: 02.08.06.005.00), the UK government Darwin Initiative (17-023), The Nature Conservancy, UK Natural Environment Research Council (NERC; NE/F01614X/1, NE/G000816/1, and NE/K016431/1) and H2020-MSCA-RISE (691053-ODYSSEA). FF is CNPq-funded (PELD-RAS 441659/2016-0). L.O.A. acknowledges CNPq productivity scholarship (process no. 309247/2016-0).
References
- 1.Aguiar APD, et al. 2016. Land use change emission scenarios: anticipating a forest transition process in the Brazilian Amazon. Glob. Chang. Biol. 22, 1821–1840. ( 10.1111/gcb.13134) [DOI] [PubMed] [Google Scholar]
- 2.Berenguer E, et al. 2014. A large-scale field assessment of carbon stocks in human-modified tropical forests. Glob. Chang. Biol. 20, 3713–3726. ( 10.1111/gcb.12627) [DOI] [PubMed] [Google Scholar]
- 3.Barlow J, et al. 2016. Anthropogenic disturbance in tropical forests can double biodiversity loss from deforestation. Nature 535, 144–147. ( 10.1038/nature18326) [DOI] [PubMed] [Google Scholar]
- 4.Longo M, et al. 2016. Aboveground biomass variability across intact and degraded forests in the Brazilian Amazon. Global Biogeochem. Cycles 30, 1639–1660. ( 10.1002/2016GB005465) [DOI] [Google Scholar]
- 5.Aragão LEOC, et al. 2018. 21st Century drought-related fires counteract the decline of Amazon deforestation carbon emissions. Nat. Commun. 9, 536 ( 10.1038/s41467-017-02771-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aragão LEOC, Poulter B, Barlow JB, Anderson LO, Malhi Y, Saatchi S, Phillips OL, Gloor E. 2014. Environmental change and the carbon balance of Amazonian forests. Biol. Rev. 89, 913–931. ( 10.1111/brv.12088) [DOI] [PubMed] [Google Scholar]
- 7.Cochrane MA. 2003. Fire science for rainforests. Nature 421, 913–919. ( 10.1038/nature01437) [DOI] [PubMed] [Google Scholar]
- 8.Power MJ, et al. 2008. Changes in fire regimes since the last glacial maximum: an assessment based on a global synthesis and analysis of charcoal data. Clim. Dyn. 30, 887–907. ( 10.1007/s00382-007-0334-x) [DOI] [Google Scholar]
- 9.McMichael CH, Piperno DR, Bush MB, Silman MR, Zimmerman AR, Raczka MF, Lobato LC. 2012. Sparse pre-Columbian human habitation in Western Amazonia. Science 336, 1429–1431. ( 10.1126/science.1219982) [DOI] [PubMed] [Google Scholar]
- 10.Jolly WM, Cochrane MA, Freeborn PH, Holden ZA, Brown TJ, Williamson GJ, Bowman DMJS. 2015. Climate-induced variations in global wildfire danger from 1979 to 2013. Nat. Commun. 6, 7537 ( 10.1038/ncomms8537) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uhl C, Kauffman JB. 1990. Deforestation, fire susceptibility, and potential tree responses to fire in the Eastern Amazon. Ecology 71, 437–449. ( 10.2307/1940299) [DOI] [Google Scholar]
- 12.Uhl C, Buschbacher R. 1985. A disturbing synergism between cattle ranch burning practices and selective tree harvesting in the Eastern Amazon. Biotropica 17, 265 ( 10.2307/2388588) [DOI] [Google Scholar]
- 13.Alencar AA, Brando PM, Asner GP, Putz FE. 2015. Landscape fragmentation, severe drought, and the new Amazon forest fire regime. Ecol. Appl. 25, 1493–1505. ( 10.1890/14-1528.1) [DOI] [PubMed] [Google Scholar]
- 14.Spracklen DV, Arnold SR, Taylor CM. 2012. Observations of increased tropical rainfall preceded by air passage over forests. Nature 489, 282–285. ( 10.1038/nature11390) [DOI] [PubMed] [Google Scholar]
- 15.Aragão LEOC. 2012. The rainforest's water pump. Nature 489, 217–218. ( 10.1038/nature11485) [DOI] [PubMed] [Google Scholar]
- 16.Alencar A, Nepstad D, Diaz MCV, Alencar A, Nepstad D, Diaz MCV. 2006. Forest understory fire in the Brazilian Amazon in ENSO and non-ENSO years: area burned and committed carbon emissions. Earth Interact. 10, 1–17. ( 10.1175/EI150.1) [DOI] [Google Scholar]
- 17.Aragão LEOC, Malhi Y, Barbier N, Lima A, Shimabukuro Y, Anderson L, Saatchi S. 2008. Interactions between rainfall, deforestation and fires during recent years in the Brazilian Amazonia. Phil. Trans. R. Soc. B 363, 1779–1785. ( 10.1098/rstb.2007.0026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gatti LV, et al. 2014. Drought sensitivity of Amazonian carbon balance revealed by atmospheric measurements. Nature 506, 76–80. ( 10.1038/nature12957) [DOI] [PubMed] [Google Scholar]
- 19.Anderson LO, et al. 2015. Disentangling the contribution of multiple land covers to fire-mediated carbon emissions in Amazonia during the 2010 drought. Global Biogeochem. Cycles 29, 1739–1753. ( 10.1002/2014GB005008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malhi Y, Wright J. 2004. Spatial patterns and recent trends in the climate of tropical rainforest regions. Phil. Trans. R. Soc. B 359, 311–329. ( 10.1098/rstb.2003.1433) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silvestrini RA, Soares-Filho BS, Nepstad D, Coe M, Rodrigues H, Assunção R. 2011. Simulating fire regimes in the Amazon in response to climate change and deforestation. Ecol. Appl. 21, 1573–1590. ( 10.1890/10-0827.1) [DOI] [PubMed] [Google Scholar]
- 22.Barlow J, Peres CA. 2006. Effects of single and recurrent wildfires on fruit production and large vertebrate abundance in a central Amazonian forest. Biodivers. Conserv. 15, 985–1012. ( 10.1007/s10531-004-3952-1) [DOI] [Google Scholar]
- 23.Barlow J, Peres CA, Lagan BO, Haugaasen T. 2002. Large tree mortality and the decline of forest biomass following Amazonian wildfires. Ecol. Lett. 6, 6–8. ( 10.1046/j.1461-0248.2003.00394.x) [DOI] [Google Scholar]
- 24.Rappaport DI, Morton DC, Longo M, Keller M, Dubayah R, dos-Santos MN. 2018. Quantifying long-term changes in carbon stocks and forest structure from Amazon forest degradation. Environ. Res. Lett. 13, 65013 ( 10.1088/1748-9326/aac331) [DOI] [Google Scholar]
- 25.Barlow J, et al. 2012. Wildfires in bamboo-dominated Amazonian forest: impacts on above-ground biomass and biodiversity. PLoS ONE 7, e33373 ( 10.1371/journal.pone.0033373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Numata I, Silva SS, Cochrane MA, d'Oliveira MVN. 2017. Fire and edge effects in a fragmented tropical forest landscape in the southwestern Amazon. For. Ecol. Manage. 401, 135–146. ( 10.1016/j.foreco.2017.07.010) [DOI] [Google Scholar]
- 27.Sato L, Gomes V, Shimabukuro Y, Keller M, Arai E, dos-Santos M, Brown I, Aragão L. 2016. Post-fire changes in forest biomass retrieved by airborne LiDAR in Amazonia. Remote Sens. 8, 839 ( 10.3390/rs8100839) [DOI] [Google Scholar]
- 28.de Andrade RB, Balch JK, Parsons AL, Armenteras D, Roman-Cuesta RM, Bulkan J. 2017. Scenarios in tropical forest degradation: carbon stock trajectories for REDD+. Carbon Balance Manag. 12, 6 ( 10.1186/s13021-017-0074-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flores BM, Fagoaga R, Nelson BW, Holmgren M. 2016. Repeated fires trap Amazonian blackwater floodplains in an open vegetation state. J. Appl. Ecol. 53, 1597–1603. ( 10.1111/1365-2664.12687) [DOI] [Google Scholar]
- 30.Baker TR, et al. 2004. Variation in wood density determines spatial patterns in Amazonian forest biomass. Glob. Chang. Biol. 10, 545–562. ( 10.1111/j.1365-2486.2004.00751.x) [DOI] [Google Scholar]
- 31.Johnson MO, et al. 2016. Variation in stem mortality rates determines patterns of above-ground biomass in Amazonian forests: implications for dynamic global vegetation models. Glob. Chang. Biol. 22, 3996–4013. ( 10.1111/gcb.13315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barlow J, Lagan BO, Peres CA. 2003. Morphological correlates of fire-induced tree mortality in a central Amazonian forest. J. Trop. Ecol. 19, 291–299. ( 10.1017/S0266467403003328) [DOI] [Google Scholar]
- 33.Brando PM, Nepstad DC, Balch JK, Bolker B, Christman MC, Coe M, Putz FE. 2012. Fire-induced tree mortality in a neotropical forest: the roles of bark traits, tree size, wood density and fire behavior. Glob. Chang. Biol. 18, 630–641. ( 10.1111/j.1365-2486.2011.02533.x) [DOI] [Google Scholar]
- 34.Pinard MA, Huffman J. 1997. Fire resistance and bark properties of trees in a seasonally dry forest in eastern Bolivia. J. Trop. Ecol. 13, 727–740. ( 10.1017/S0266467400010890) [DOI] [Google Scholar]
- 35.Pausas JG. 2015. Bark thickness and fire regime. Funct. Ecol. 29, 315–327. ( 10.1111/1365-2435.12372) [DOI] [Google Scholar]
- 36.Midgley JJ, Kruger LM, Skelton R. 2011. How do fires kill plants? The hydraulic death hypothesis and Cape Proteaceae ‘fire-resisters’. South African J. Bot. 77, 381–386. ( 10.1016/j.sajb.2010.10.001) [DOI] [Google Scholar]
- 37.Chave J, et al. 2014. Improved allometric models to estimate the aboveground biomass of tropical trees. Glob. Chang. Biol. 20, 3177–3190. ( 10.1111/gcb.12629) [DOI] [PubMed] [Google Scholar]
- 38.Berenguer E, Gardner TA, Ferreira J, Aragão LEOC, Mac Nally R, Thomson JR, Vieira ICG, Barlow J. 2018. Seeing the woods through the saplings: using wood density to assess the recovery of human-modified Amazonian forests. J. Ecol. 1–14. ( 10.1111/1365-2745.12991) [DOI] [Google Scholar]
- 39.Baker TR, Swaine MD, Burslem DFRP. 2003. Variation in tropical forest growth rates: combined effects of functional group composition and resource availability. Perspect. Plant Ecol. Evol. Syst. 6, 21–36. ( 10.1078/1433-8319-00040) [DOI] [Google Scholar]
- 40.Fauset S, et al. 2015. Hyperdominance in Amazonian forest carbon cycling. Nat. Commun. 6, 6857 ( 10.1038/ncomms7857) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kinnaird MF, O'Brien TG. 1998. Ecological effects of wildfire on lowland rainforest in Sumatra. Conserv. Biol. 12, 954–956. ( 10.1046/j.1523-1739.1998.00005.x) [DOI] [Google Scholar]
- 42.Phillips O, Baker T, Feldpausch T, Brienen R.2009. Field manual for establishment and remeasurement (RAINFOR), 22. See http://www.rainfor.org/upload/ManualsEnglish/RAINFOR_field_manual_version_June_2009_ENG.pdf .
- 43.Goodman RC, Phillips OL, del Castillo Torres D, Freitas L, Cortese ST, Monteagudo A, Baker TR. 2013. Amazon palm biomass and allometry. For. Ecol. Manage. 310, 994–1004. ( 10.1016/j.foreco.2013.09.045) [DOI] [Google Scholar]
- 44.Gerwing JJ, Farias DL. 2000. Integrating Liana abundance and forest stature into an estimate of total aboveground biomass for an Eastern Amazonian forest. J. Trop. Ecol. 16, 327–335. ( 10.1017/S0266467400001437) [DOI] [Google Scholar]
- 45.Chave J, Coomes D, Jansen S, Lewis SL, Swenson NG, Zanne AE. 2009. Towards a worldwide wood economics spectrum. Ecol. Lett. 12, 351–366. ( 10.1111/j.1461-0248.2009.01285.x) [DOI] [PubMed] [Google Scholar]
- 46.Zanne AE, Lopez-Gonzalez G, Coomes DA, Ilic J, Jansen S, Lewis SL, Miller RB, Swenson NG, Wiemann MC, Chave J. 2009. Data from: Towards a worldwide wood economics spectrum. Dryad Digital Repository ( 10.5061/dryad.234) [DOI] [PubMed] [Google Scholar]
- 47.Talbot J, et al. 2014. Methods to estimate aboveground wood productivity from long-term forest inventory plots. For. Ecol. Manage. 320, 30–38. ( 10.1016/J.FORECO.2014.02.021) [DOI] [Google Scholar]
- 48.Wood S, Scheipl F.. 2017. Package 'gamm4': Generalized Additive Mixed Models using 'mgcv' and 'lme4'. Version 0.2-5. Published 25/07/2017. [Google Scholar]
- 49.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effect models using 'lme4'. J. Stat. Soft. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 50.Sheil D, May RM. 1996. Mortality and recruitment rate evaluations in heterogeneous tropical forests. J. Ecol. 84, 91 ( 10.2307/2261703) [DOI] [Google Scholar]
- 51.Sist P, Mazzei L, Blanc L, Rutishauser E. 2014. Large trees as key elements of carbon storage and dynamics after selective logging in the Eastern Amazon. For. Ecol. Manage. 318, 103–109. ( 10.1016/j.foreco.2014.01.005) [DOI] [Google Scholar]
- 52.Slik JWF, et al. 2013. Large trees drive forest aboveground biomass variation in moist lowland forests across the tropics. Glob. Ecol. Biogeogr. 22, 1261–1271. ( 10.1111/geb.12092) [DOI] [Google Scholar]
- 53.Brando PM, Oliveria-Santos C, Rocha W, Cury R, Coe MT. 2016. Effects of experimental fuel additions on fire intensity and severity: unexpected carbon resilience of a neotropical forest. Glob. Chang. Biol. 22, 2516–2525. ( 10.1111/gcb.13172) [DOI] [PubMed] [Google Scholar]
- 54.Walker LR, del Moral R. 2003. Primary succession and ecosystem rehabilitation. Cambridge, UK: Cambridge University Press; See http://www.cambridge.org/us/academic/subjects/life-sciences/ecology-and-conservation/primary-succession-and-ecosystem-rehabilitation?format=HB&isbn=9780521800761#ATgsCqejlQYUCYk8.97. [Google Scholar]
- 55.Chambers JQ, Higuchi N, Schimel JP. 1998. Ancient trees in Amazonia. Nature 391, 135–136. ( 10.1038/34325) [DOI] [Google Scholar]
- 56.Denslow JS. 1987. Tropical rainforest gaps and tree species diversity. Annu. Rev. Ecol. Syst. 18, 431–451. ( 10.1146/annurev.es.18.110187.002243) [DOI] [Google Scholar]
- 57.Balch JK, Nepstad DC, Brando PM, Curran LM, Portela O, de Carvalho O, Lefebvre P. 2008. Negative fire feedback in a transitional forest of southeastern Amazonia. Glob. Chang. Biol. 14, 2276–2287. ( 10.1111/j.1365-2486.2008.01655.x) [DOI] [Google Scholar]
- 58.Malhi Y, Aragão LEOC, Galbraith D, Huntingford C, Fisher R, Zelazowski P, Sitch S, McSweeney C, Meir P. 2009. Exploring the likelihood and mechanism of a climate-change-induced dieback of the Amazon rainforest. Proc. Natl Acad. Sci. USA 106, 20610–20615. ( 10.1073/pnas.0804619106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Phillips OL, et al. 2009. Drought sensitivity of the Amazon rainforest. Science 323, 1344–1347. ( 10.1126/science.1164033) [DOI] [PubMed] [Google Scholar]
- 60.Bush MB. 2017. The resilience of Amazonian forests. Nature 541, 167–168. ( 10.1038/541167a) [DOI] [PubMed] [Google Scholar]
- 61.Rutishauser E, et al. 2015. Rapid tree carbon stock recovery in managed Amazonian forests. Curr. Biol. 25, R787–R788. ( 10.1016/J.CUB.2015.07.034) [DOI] [PubMed] [Google Scholar]
- 62.Poorter L, et al. 2016. Biomass resilience of Neotropical secondary forests. Nature 530, 211–214. ( 10.1038/nature16512) [DOI] [PubMed] [Google Scholar]
- 63.Bonner MTL, Schmidt S, Shoo LP. 2013. A meta-analytical global comparison of aboveground biomass accumulation between tropical secondary forests and monoculture plantations. For. Ecol. Manage. 291, 73–86. ( 10.1016/j.foreco.2012.11.024) [DOI] [Google Scholar]
- 64.Morton DC, Le Page Y, DeFries R, Collatz GJ, Hurtt GC. 2013. Understorey fire frequency and the fate of burned forests in southern Amazonia. Phil. Trans. R. Soc. B 368, 20120163 ( 10.1098/rstb.2012.0163) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carmenta R, Vermeylen S, Parry L, Barlow J. 2013. Shifting cultivation and fire policy: insights from the Brazilian Amazon. Hum. Ecol. 41, 603–614. ( 10.1007/s10745-013-9600-1) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the paper and its electronic supplementary material information files.