Abstract
Large trees in the tropics are reportedly more vulnerable to droughts than their smaller neighbours. This pattern is of interest due to what it portends for forest structure, timber production, carbon sequestration and multiple other values given that intensified El Niño Southern Oscillation (ENSO) events are expected to increase the frequency and intensity of droughts in the Amazon region. What remains unclear is what characteristics of large trees render them especially vulnerable to drought-induced mortality and how this vulnerability changes with forest degradation. Using a large-scale, long-term silvicultural experiment in a transitional Amazonian forest in Bolivia, we disentangle the effects of stem diameter, tree height, crown exposure and logging-induced degradation on risks of drought-induced mortality during the 2004/2005 ENSO event. Overall, tree mortality increased in response to drought in both logged and unlogged plots. Tree height was a much stronger predictor of mortality than stem diameter. In unlogged plots, tree height but not crown exposure was positively associated with drought-induced mortality, whereas in logged plots, neither tree height nor crown exposure was associated with drought-induced mortality. Our results suggest that, at the scale of a site, hydraulic factors related to tree height, not air humidity, are a cause of elevated drought-induced mortality of large trees in unlogged plots.
This article is part of a discussion meeting issue ‘The impact of the 2015/2016 El Niño on the terrestrial tropical carbon cycle: patterns, mechanisms and implications'.
Keywords: drought, tree mortality, climate change, tropical forest, logging, resilience
1. Introduction
The Amazon region is predicted to experience hotter and more frequent droughts as a result of elevated sea surface temperatures that intensify El Niño Southern Oscillation (ENSO) events and affect the intertropical convergence zone [1,2]. As a result, forests there may face increased tree mortality rates due to water stress [3,4]. At the level of individual trees, drought-induced mortality is likely to vary with interactions among microenvironmental conditions and tree characteristics. Understanding of these interactions and their underlying mechanisms is needed to predict the fates of trees and forests in a changing climate. Here, we examine the effects of the 2005 ENSO event on tree mortality in a selectively logged transitional Amazonian forest in Bolivia.
Predicting individual tree responses to drought remains challenging but a number of characteristics reportedly contribute to drought tolerance and avoidance, including narrow vessels, high wood density and high leaf mass per area as well as various hydraulic properties of leaves and wood. One reported pantropical pattern is that large trees suffer elevated risks of drought-related mortality ([5–9], but see [10–12]). This relationship is of particular concern due to its implications for forest structure, timber production and carbon sequestration as the climate changes.
The two principal mechanisms proposed for large tree vulnerability to drought focus on the decreased soil water potential that droughts cause. First, as soil water potential decreases, so do plant water potentials. To overcome gravity, tall trees must maintain higher xylem tensions than short trees, and with increased xylem tension comes increased cavitation risk [5,9]. Xylem cavitation decreases hydraulic conductivity, leading to more negative leaf water potentials, often reduced stomatal conductance and reduced net photosynthesis. Second, given that metabolic maintenance costs increase with tree size, large trees are more likely to suffer carbon deficits than smaller trees if photosynthesis is constrained by cavitation or stomatal closure [4]. Both of these mechanisms result in increased mortality risk either directly as a result of carbon deficits, inability of the leaf's anti-oxidant system to scavenge reactive oxygen species [13], or indirectly via increased susceptibility to pests, pathogens and other causes of mortality [14]. Despite abundant circumstantial evidence, these proposed mechanisms for large tree vulnerability to drought have not been explicitly tested.
While droughts affect both soil water potential and air humidity, the former has been the primary factor invoked to explain large tree vulnerability to drought (but see [11]). Increased vapour pressure deficit (VPD) during droughts due to reduced air humidity and increased air temperature likely contribute to large trees' vulnerability to drought, and may better explain drought-induced mortality than tree size. Trees with exposed crowns, regardless of their height, require more water for transpirational cooling and experience relatively high VPD. High VPD leads to high transpiration rates per unit of carbon fixed. Therefore, when soil water potential is low, trees with exposed crowns may fix less carbon (in the case of isohydry or facultative deciduousness) and/or suffer xylem cavitation (in the case of anisohydry), and hence be particularly susceptible to drought-induced mortality. For this reason, we hypothesize here that canopy exposure is a better predictor of tree mortality than tree size (i.e. stem diameter (diameter at breast height, DBH) or height).
2. Forest management and drought
Trees in a stand compete for soil water and consequently, when stands are thinned, remnant trees may access a larger share of that water via expanded root systems and increased soil water availability [15,16]. Thinning may also increase total available soil water by increasing the total amount of precipitation reaching the forest floor due to a reduction in leaf area index and hence interception of precipitation [17,18] and transpiration [19]. Ground water levels may also rise in treefall gaps [19], potentially allowing trees to access ground water that previously relied on soil water, and potentially benefiting neighbouring trees via hydraulic lift [20].
Numerous studies in temperate forests have shown that thinning reduces drought stress and increases resistance and resilience to drought across a broad range of forest types [21–27], with a few exceptions [28,29]. The drought-related benefits conferred by thinning may be limited by soil water storage capacity [15] and may decline over time [23]. For example, trees may adapt their architecture and physiology in response to thinning-induced increases in water availability by decreasing their Huber values (sapwood area : leaf area) and increasing stomatal conductance, rendering them more vulnerable to future droughts [17]. Emerging understory vegetation may also compete with remnant trees for soil water [15]. To maintain the drought resistance benefit of thinning, repeated treatments are recommended [30,31]. Finally, the extent to which thinning protects remnant trees from drought may depend on abiotic conditions and vary across gradients such as elevation [21,22,32,33].
Managing temperate forests for resistance and resilience to climate change, typically via influencing the species mix, size distribution and stand basal area, is a topic of current discussion [34–38]. In comparison, the same discussions about tropical forests are relatively data-deficient and lack direct tests [39]. Caution in extending results from temperate to tropical forests is warranted given their myriad differences and the fact that temperate forests vary in their responses to thinning [23]. The few available studies on drought effects on tropical trees hint that they respond similarly to their temperate counterparts. In particular, Leighton & Wirawan [5] reported that during a major drought in Borneo, large trees benefitted from low-intensity understory fires that killed many small trees, possibly due to reduced competition for water. Similarly, Shenkin et al. [40] showed that logging-damaged trees suffered from droughts less than undamaged trees, likely due to their proximity to logging gaps.
Key to understanding how logging affects tropical forest responses to drought is whether it renders large trees more or less vulnerable, and how this response varies with size and crown exposure. For example, reduced belowground competition and increased throughfall in logged plots may confer drought-resistance to trees of all sizes. By contrast, if large trees live especially close to their drought stress limit, logging may confer on them a particularly large advantage. Alternatively, logging may allow small trees to build carbon reserves and therefore benefit more than large trees. On the other hand, logging may expose small trees to high VPD, thereby increasing their vulnerability to drought. Sparse evidence exists to support differential responses to drought across size and exposure classes: Kolb et al. [41] and Erickson & Waring [42] found that older, and presumably larger, pines in Arizona fared better during droughts in logged than unlogged plots, and Trouvé et al. [43] found that understory trees suffered more from drought in dense but not in open stands.
To address these questions, we compared the powers of tree size and crown exposure to explain drought-induced mortality, examined how logging changes those relationships and test whether logging buffered large trees against drought. In particular, we test our expectation that logging conferred drought resistance to large trees in our study system. Finally, we reflected on the implications of these results for future research and our understanding of the futures of undisturbed and managed tropical forests.
3. Material and methods
This study was conducted in the Long-Term Silvicultural Research Plots (LTSRPs) of the Instituto Boliviano de Investigación Forestal (IBIF) within the forestry concession held by Agroindustria Forestal La Chonta, 30 km east of Ascención de Guarayos, Bolivia (15°47′ S, 62°55′ W). This semi-deciduous forest (hereafter ‘La Chonta’) receives an annual average of 1580 mm of precipitation, with four months (May–September) that each receive less than 100 mm [44]. The forest contains tree species from both wet Amazonian lowland forests to the north and dry Chiquitano forests to the south and falls within WWF's Global 200 Southwestern Amazonian Moist Forest Region. Located on the southern edge of the Amazon Basin, approximately 30% of the tree species that grow to be greater than 10 cm DBH are deciduous and liana densities are very high [45]. The soils of La Chonta are a mosaic of what have been described as nutrient-rich sedimentary ultisols [46] and poorer soils derived from the Brazilian Precambrian Shield [47]. The concession's terrain is undulating with some granitic outcrops (i.e. inselbergs), none of which are in the permanent sample plots.
The LTSRPs established in 2000/2001 include three blocks of four 27 ha treatments: control (no logging); normal logging; improved logging; and improved logging with intensive silvicultural treatments [45]. All logging was selective, planned and carried out by trained crews according to reduced-impact logging (RIL) guidelines. Pre-felling of lianas in to-be-felled trees was carried out approximately six months prior to logging, and lianas were cut from future crop trees in the improved and intensive treatments. Trees overtopping future crop trees of commercial species were girdled in the improved and intensive plots, and the soil was scarified in gaps in the intensive plots to encourage pioneer tree establishment.
Within each plot, all trees greater than 40 cm DBH were censused semi-annually, with trees greater than 20 cm and greater than 10 cm DBH censused in half of the main plot and in four 1 ha plots, respectively. Censuses often took more than a year to complete due to the size of the experiment and logistical challenges. After the initial pre-logging census in 2000–2001, five more censuses were conducted (2001–2002; 2002–2003; 2004–2005; 2006–2007; 2009). In total, 46 194 individual trees were measured across the entire study, 9854 of which were excluded due to being in a burned area or suffering silviculture-induced mortality. On average, 29 744 individuals trees were encountered during each census, 7713 of which were greater than 40 cm DBH.
Each tree in every census was assigned a crown exposure class with the system of Clark & Clark [48]: 1 (no direct light), 2 (some lateral light), 3 (10–90% overhead light), 4 (greater than 90% overhead light) and 5 (full overhead and later light). During the first census in 2000–2001, tree heights were visually estimated by experienced crews. Heights for the following censuses were predicted with a height allometry model to predict tree heights from DBH for all trees across all census intervals. If a tree was present in the first census, and hence had a height measurement, we maintained its offset from the general allometry such that if a tree was 1 m taller than the allometry would predict in the first census, we predicted it would be 1 m taller than the mean prediction every subsequent census based on its DBH. Coefficients from models based on height may be very different from those based on DBH for two reasons: because the allometric relationship between height and DBH is nonlinear, and because we included the individual offsets in height prediction described above. See electronic supplementary material for details.
We used precipitation records from the nearest long-term weather station in Ascención de Guarayos, accessed via the Bolivian National Meterological and Hydrological Service database (http://www.senamhi.gob.bo/). Over the course of the study (2000–2011), annual precipitation averaged 1318 mm, with a maximum of 1798 mm in 2007 and a minimum of 994 mm in 2010.
We calculated soil water deficits with the Climatological Water Deficit (CWD) model, a simple bucket model that fills with precipitation and assumes evapotranspiration of 3.33 mm per day [49–51]. We started calculations of CWD on 1 January 1970 and then ran them forward day-by-day, adding daily precipitation and subtracting 3.33 mm day−1 (electronic supplementary material, figures S2 and S3), capping CWD at a maximum of 0 (saturated soil). For each census interval, the Maximum Climatological Water Deficit (MCWD) is the most negative value of CWD observed. Logging and other factors affect the soil moisture environment experienced by trees; thus, we used MCWD here as a general indicator of climatological drought conditions.
(a). Model formulation and selection
We constructed models that include the direct effects and interactions of variables representing factors pertinent to our questions: drought (MCWD), tree size (DBH, height), crown exposure (crown position; CP) and logging. A subset of models (Model 11, Model 12, Model 21 and Model 22) contain just size and not crown exposure predictors. These simpler models addressed the direct question of whether large trees are more vulnerable to drought than small ones, and left the question of the roles of size versus exposure for the more complex models.
We distinguished between classes of models based on different metrics of tree size (DBH, height or both DBH + height) and whether they distinguished between logged and unlogged areas (electronic supplementary material, table S1). We tested the effects of logging with two further classes of models: those run on the complete dataset that include a variable (logged) indicating whether the tree is in a logged (1) or unlogged (−1) plot; and those run on data from logged and unlogged plots separately. We did not compare across models fit to separate datasets as this would introduce unnecessary complexity; instead, we used these models to reduce complexity and to aid interpretation of differences between logged and unlogged plots.
Our models integrated, instead of separating out, the effects of species in order to reduce complexity, aid interpretation and promote generality of results. Logging shifts species composition to more acquisitive recruits [52] and drought-tolerant seedlings [53]. Functional composition of recruits and regeneration may or may not have a strong effect on drought-induced mortality of larger trees. Either way, the species-agnostic approach integrates the effect of this compositional shift instead of separating it out.
We ranked classes of models with corrected-AIC scores to gain insight into the most important predictors of mortality. For hypothesis testing, we examined predictor direction and significance in the higher ranking models that contained our variables of interest. To avoid data dredging, we confined our models' formulations to those we deemed to contain ecologically informative interactions instead of testing a complete set of possible permutations. Because height was derived in part from DBH when direct estimates were not available, we limited our use of models that include both predictors.
(b). Statistical models
Unless otherwise indicated, models were fit using generalized linear mixed models (GLMMs; [54]) in the R statistical environment [55] with the lme4 package [56]. When GLMM fits indicated potential convergence issues, we verified parameter estimates by fitting Bayesian models via the R INLA package [57] with the same structure as the GLMM model. GLMM parameter confidence interval estimates were unreliable in these cases and are thus not reported. Instead, we rely on Bayesian 95% credible intervals as determined by the 0.025 and 0.975 quantile values shown in the coefficient plots (electronic supplementary material). To test for a nonlinear relationship between mortality and tree size, we examined scaled model residuals [58]. Confidence intervals in prediction plots were determined using Wald estimates, as models typically took hours to converge, and hence bootstrap methods were unavailable. We used bootstrap techniques applied to GLMs fit to data from the driest intervals to test for absolute differences in mortality rates of the largest trees between logged and unlogged plots.
Data were coded such that mortality (coded as 1) and survival (coded as 0) were associated with an individual census interval. Census intervals varied in length, and this variation was accounted for by including offsets in our models (see the electronic supplementary material). We used a binomial error structure with a complementary log–log link function. An ‘event’ consists of one observation of one individual from one census to the next. For example, if an individual survived through all four census intervals, there are four ‘survival events’ for that tree.
We included random effects in our models to account for block effects 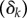 and repeated measures
and repeated measures 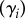 . To account for block effects, we assign a random variable for all 12 plots since four treatments nested within three blocks would be too few levels. When models were run on just the data from the unlogged plots, blocks were included as fixed instead of random effects, as there were too few levels to treat them as random effects. To aid interpretation, we present just the structure of the linear predictor
. To account for block effects, we assign a random variable for all 12 plots since four treatments nested within three blocks would be too few levels. When models were run on just the data from the unlogged plots, blocks were included as fixed instead of random effects, as there were too few levels to treat them as random effects. To aid interpretation, we present just the structure of the linear predictor 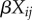 and omit other covariates when listing models.
and omit other covariates when listing models.
In all models, independent variables were scaled such that s.d. = 1 and centred on 0 where appropriate to render outputs interpretable [59]. Unless otherwise indicated, trees that died due to felling, collateral logging damage or silvicultural treatments were removed, as were trees in areas that burned in 2004.
(c). Interpreting coefficients
Coefficients of the linear predictor 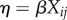 in GLMMs with complementary log–log links, when exponentiated, can be interpreted as hazard ratios (HRs). Specifically, HR = 1 − exp(coefficient). If exp(coefficient) = 1, then that characteristic has no influence on HRs. In our case, a hazard ratio is the increase in annual probability of mortality compared to the control or mean group due to a unit increase in a characteristic. Thus, if the expected mortality rate of a tree with DBH = mean(DBHij) is 2% and the DBH coefficient is −0.7, then
in GLMMs with complementary log–log links, when exponentiated, can be interpreted as hazard ratios (HRs). Specifically, HR = 1 − exp(coefficient). If exp(coefficient) = 1, then that characteristic has no influence on HRs. In our case, a hazard ratio is the increase in annual probability of mortality compared to the control or mean group due to a unit increase in a characteristic. Thus, if the expected mortality rate of a tree with DBH = mean(DBHij) is 2% and the DBH coefficient is −0.7, then 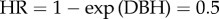 . Hence a unit increase in DBH corresponds to a 50% decrease in expected annual mortality relative to the mean expected annual mortality rate of 2%, resulting in an expected 1% annual mortality rate. As another example, if an HR of 1.05 is reported, this indicates that a unit increase of that variable raises a base mortality rate of 2% by 5%, to an expected 2.1%. A potential source of confusion when reporting HRs is that, when reported as percentages, they might erroneously be interpreted as additive terms to the base mortality rate; in fact, they should be interpreted as multiplicative. For example, if an HR of 1.05 (5%) is reported, the resulting mortality rate due to a unit increase of the variable is not 2% + 5% = 7%, but rather 2% × 105% = 2.1%.
. Hence a unit increase in DBH corresponds to a 50% decrease in expected annual mortality relative to the mean expected annual mortality rate of 2%, resulting in an expected 1% annual mortality rate. As another example, if an HR of 1.05 is reported, this indicates that a unit increase of that variable raises a base mortality rate of 2% by 5%, to an expected 2.1%. A potential source of confusion when reporting HRs is that, when reported as percentages, they might erroneously be interpreted as additive terms to the base mortality rate; in fact, they should be interpreted as multiplicative. For example, if an HR of 1.05 (5%) is reported, the resulting mortality rate due to a unit increase of the variable is not 2% + 5% = 7%, but rather 2% × 105% = 2.1%.
Because our predictors were scaled, the unit of change corresponds to one standard deviation of that characteristic in our observed data. The logged plot dummy variable, logged, was coded with contrasts −1 (unlogged) and 1 (logged). Therefore, HRs including the logged variable should be doubled.
4. Results
(a). 2005 ENSO in La Chonta
La Chonta experienced a severe drought in 2004/2005 (electronic supplementary material, figure S2). CWD typically reaches zero during the wet season, but failed to do so in those years. MCWD reached its lowest point of −635 mm H2O since 1970 in the 2004 dry season, surpassed only by the 2010 dry season that is not addressed here (electronic supplementary material, figure S3).
(b). Model comparison
Our interest in the effects of logging and drought led us to examine whether the inclusion of those factors improved the models. Models that included interactions between MCWD and height, and MCWD and crown exposure, had similar AIC scores to those without them, which indicated that these interactions lent enough explanatory power to warrant their inclusion (electronic supplementary material, table S4). Height-based models were not more parsimonious when logging was included, though all height-based models were similar enough to warrant examination of the factors of interest in this study.
The models we tested grouped principally according to whether they included height or DBH. Height-based models were better predictors of mortality than DBH-based models in general (ΔAICcModel 15−Model 5 = −260; electronic supplementary material, table S4), and all height-based models fell within a relatively small range of ΔAICc (ΔAICcModel 8−Model 24 = −6.2), while DBH-based models varied substantially (ΔAICcModel 15−Model 12 = −24.5). As such, we focus our discussion on height-based models, though we note results from DBH-based models where appropriate.
Lagged MCWD predictors were weak and thus discarded. Scaled residuals did not indicate a nonlinear response of mortality to tree size (electronic supplementary material, figure S15), so we maintained a linear modelling framework. An expanded explication of model results can be found in the electronic supplementary material.
(c). Roles of tree size and crown exposure in overall mortality
We first present non-drought-related factors that influence tree mortality, and then address factors affecting drought-induced mortality below. Height and DBH were strongly associated with overall reductions in mortality risk. Crown exposure was, unexpectedly, associated with increased mortality risk in height-based models (electronic supplementary material, figure S12), and strong decreases in mortality risk in DBH-based models (figure 3; electronic supplementary material, figure S9a).
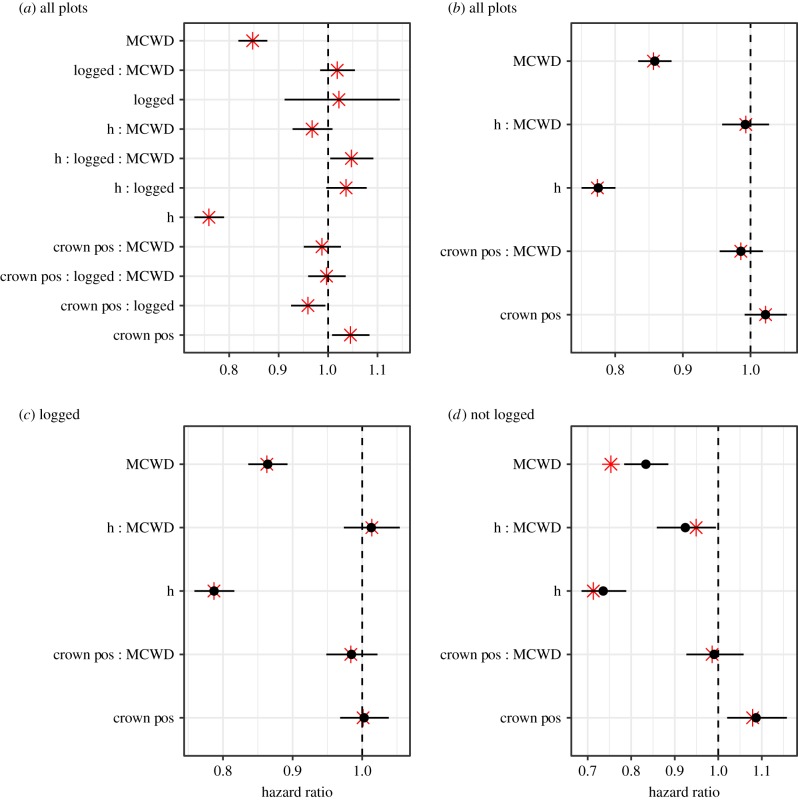
Figure 3.
Hazard ratios (exponentiated coefficients) derived from selected height-based models: (a) full height logging model (Model 29), (b) full height model (Model 5), and the same full height model parameterized with (c) logged (Model 19) and (d) unlogged (Model 20) data. Black points are the 0.5 quantiles and horizontal bars are the 95% credible intervals from a Bayesian Integrated Nested Laplace Approximations (INLA) algorithm, and red stars are the mean parameter estimates of the corresponding GLMM fit. Intercept and random effects are removed for readability. (Online version in colour.)
(d). Does logging affect the roles of tree size and crown exposure in tree mortality?
The two modes of inference employed here conflicted: height-based models were not improved by adding logging interactions, but the three-way interaction h:logged:MCWD was positive and significant, indicating that the role of height in drought-induced mortality was significantly different between logging treatments. Furthermore, consistent shifts in the roles of size and crown exposure in drought-induced mortality were observed when fitting models to separate logged and unlogged datasets (see below). Finally, a number of terms that included the logging predictor were significant in our models (e.g. figure 3). Predictions indicated that, while the coefficients of the interactive terms are not large, they exert important influences on tree mortality (figures 1 and 2; electronic supplementary material figures S11b and S14). We therefore concluded that logging does affect the roles of size and crown exposure in drought-induced mortality.
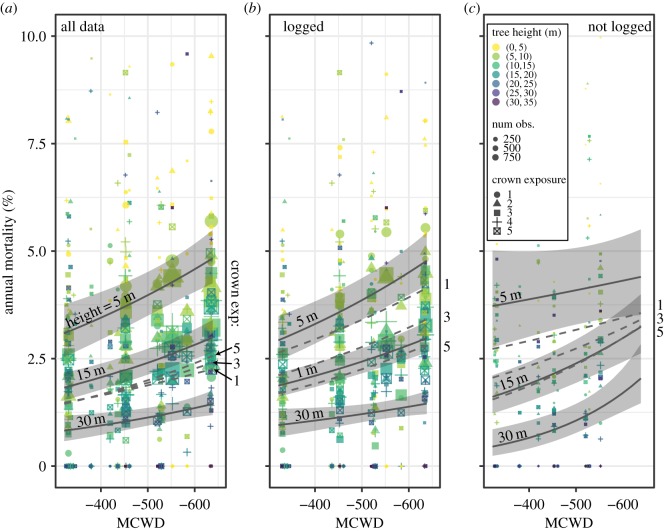
Figure 1.
Mortality rates as a function of MCWD, tree height and crown exposure in observed data (points) and modelled predictions (lines and 95% mean confidence intervals). (a) The effects of height averaged across crown positions 1–5, and crown position averaged across heights 5–35 m, across all data (full height model, Model 5), (b) the effects of height (Model 22) and crown position (Model 23) in logged plots and (c) in unlogged plots. The height and crown exposure effects in (b,c) are not controlled for each other, as reflected in the respective model formulae. Conditions go from wet to dry along the x-axis from left to right. Crown position 1 is in the shaded understory, while crown position 5 is fully illuminated. (Online version in colour.)
Figure 2.

Annual mortality rates and 95% mean Wald confidence intervals for tree heights not corrected for crown exposure (Model 22). (Online version in colour.)
(e). Roles of tree size and crown exposure in drought-induced mortality
Overall, mortality rates increased during drier intervals (electronic supplementary material, figure S5) as expected, which was confirmed by the negative coefficients for the MCWD term in all our models (figure 3; electronic supplementary material, figures S9, S10 and table S7).
Across all plots (figure 1a) and within logging treatments (figure 1b,c), tree height was associated with greater shifts in mortality risk during droughts than was crown exposure. Tree height was associated with increased drought-induced mortality risk in unlogged plots, but not so in logged plots (figure 1a,b; electronic supplementary material, figure S12a, figure 3c,d). This difference was confirmed by the positive and significant three-way interaction between height, logging and MCWD (h : logged : MCWD = 1.047 in Model 29, figure 3a; electronic supplementary material figure S9a and table S7). The result is that, in unlogged plots, mortality increased more quickly as a result of drought in taller trees than in shorter trees (figure 1c).
In the case of DBH-based models, DBH was associated with reductions in drought-induced mortality (i.e. drought tolerance) in logged plots, and was not associated with a directional effect in unlogged plots (electronic supplementary material, figure S9e,f).
The effect of crown exposure on drought-induced mortality was consistent between logged and unlogged plots, and varied primarily as a result of whether tree height was included in the model or not. Crown exposure was not an important predictor of drought-induced mortality in height-based models, and was associated with an increased risk of drought-induced mortality in DBH models.
(f). Relative importance of tree size and crown exposure in drought-induced mortality
Estimating predictor importance by comparing models, we found that models that included crown exposure's influence on drought-induced mortality fit the data slightly better than those including height (electronic supplementary material, table S4). Specifically, Model 7 (h + CP × MCWD) had a lower AICc score (ΔAICc = 0.59) than Model 6 (CP + h × MCWD). Conversely, tree height's mean influence on drought-induced mortality was stronger than that of crown exposure in the unlogged plots (figure 3a; Model 20 in electronic supplementary material, table S7, h : MCWD = −0.077, CP:MCWD = −0.010). Neither factor played a strong role in predicting drought-induced mortality in logged plots (figure 3c).
Predictions showed that the difference in mortality between height classes changes more than that between crown exposure classes across the range of MCWD we observed when examining effects both controlling (figure 1a) and not controlling (figure 1b,c) for each other. Electronic supplementary material, figure S12 further shows that in unlogged plots, when controlling for other effects, the difference in mortality between height classes changed more as a function of drought than the difference in mortality between crown exposure classes.
The weight of evidence when comparing the roles of tree height and crown exposure led us to conclude that tree height was a more important determinant of drought-induced mortality than crown exposure, especially in unlogged plots.
In DBH-based models, DBH was slightly more important than crown exposure in logged plots, but acted in a direction opposite to expectation (i.e. drought-induced mortality decreased with tree size; electronic supplementary material, figure S9e,f). Neither variable was a significant predictor of drought-induced mortality in unlogged plots, so we did not evaluate relative importance in that case.
(g). The effect of logging on drought-induced mortality of large trees
Removing the crown exposure predictor from height-based models allowed for a direct test of the effect of tree height across logging treatments, regardless of the effect that logging may have had on crown exposure. These models showed that mortality rates of a 35 m-tall tree in a logged plot had a 1.1% chance of dying during a drought year with −619 mm H2O MCWD, whereas the same tree in an unlogged plot had a 1.6% chance. Owing to the rarity of large-tree mortality events, non-parametric boostrap tests found that these differences were not significant.
5. Discussion
The influences of tree height, stem diameter and crown exposure on drought-induced mortality are complex and varied according to other covariates and logging. In general, tree height was the predictor most strongly correlated with drought-induced mortality; it increased the risk of drought-induced mortality in unlogged plots. Stem diameter had the opposite effect, tending to decrease the risk of drought-induced mortality when it had an influence. Crown exposure seemed to render trees vulnerable to risk during droughts, but when height was added to models, this effect was reduced; the correlation between crown exposure and tree height (Pearson r = 0.57) may partially explain this reduction.
Our results suggest that hydraulic factors associated with tree height, such as decreased hydraulic conductance, greater embolism risk or decreased leaf water potentials were more likely causal factors of the observed pattern of large-tree vulnerability to drought than local VPD variation or other dimensions of tree size. The hypothesis that crown exposure plays a larger role than tree size in drought-induced mortality was not supported by our data. While crown exposure was more likely to be associated with increased drought risk than stem diameter across conditions and models, tree height seemed to be a more important predictor, particularly in unlogged areas.
The magnitude of the interactions between tree height, diameter, crown exposure and MCWD often ranged from 20 to 35% of the magnitude of the direct effects. This constitutes an important indicator of how trees and forests respond to drought, and could have important implications for future forest structure.
While previous studies associated increased stem diameter with increased drought risk ([5–8], but see [10,11]), our results indicate that tree height, not stem diameter, is the likely cause of this pattern. Indeed, diameter was actually more likely to confer drought tolerance than predispose trees to drought risk. We show here how studies that do not control for crown exposure or tree height could come to the conclusion that stem diameter is positively associated with drought-induced mortality. Crown exposure and tree height, which both tend to increase with stem diameter, predisposed trees to drought-induced mortality more strongly than diameter buffered them. This means that when these covariates are not included, models may find stem diameter positively associated with drought-induced mortality.
(a). Does logging increase drought resistance in this tropical forest?
While we found that the relationship between tree size and drought risk differed as a function of logging, we did not find that trees across all size classes were better off in logged plots during droughts. This finding is in contrast to studies in temperate forests showing that thinning may assist trees of all sizes to resist drought. Studies across gradients of environments and forest types in the tropics are needed to determine if the drought-tolerance conferred by logging in temperate forests is truly absent in tropical forests.
(b). Does logging protect large trees from drought?
Taller trees suffered larger increases in drought-induced mortality than shorter trees in unlogged plots. This pattern was diminished or absent in logged plots. Predictions indicated that this trend, in droughts deeper than measured here, may lead to elevated rates of drought-induced mortality of large trees in unlogged plots when compared to logged plots. Nonetheless, because large trees are relatively rare, and mortality of those trees even more so, we did not find a significant difference in the mortality rates of large trees between logged and unlogged plots during the deepest drought examined in this study. Thus, further study, examining deeper droughts or larger sample sizes, is necessary to bring clarity to this question.
Increased tree height and crown exposure did not leave trees vulnerable to drought in logged plots, which contradicts our hypothesis. Indeed, the relationship between crown exposure and drought response differed little between logged and unlogged plots. We expected that rapid exposure of many trees to high VPDs and insolation due to logging would render them vulnerable to drought-induced mortality. Increased soil water availability in logged plots may counteract these potentially damaging conditions given that the trees most likely to suffer post-logging shocks are on the edges of logging gaps where they benefit from increased soil water availability.
Tests of relationships between dimensions of tree size, neighbourhood metrics such as canopy position, and drought-induced mortality across rainfall and disturbance gradients in the tropics are needed to elucidate the determinants of drought vulnerability. Key questions for future research include: how does thinning via selective logging affect stand-level resistance and resilience to drought across tropical forests? How do edaphic factors alter the interactive roles of tree structural and functional characteristics in determining drought vulnerability? And how do different logging intensities and silvicultural treatments interact with tree characteristics to affect drought vulnerability?
(c). Why do trees grow tall?
One finding from this study that contradicted our expectations about overall (not drought-induced) mortality stood out: crown exposure was associated with higher mortality rates when controlling for tree height, especially in unlogged plots (crown pos = 1.087 in figure 3d; electronic supplementary material, figure S12b). The assumed primary function for trees growing tall is to out-compete neighbours in a contest for the limiting resource of light. We would therefore expect crown exposure to be associated with lower mortality rates when controlling for tree height, and tree height to be associated with higher mortality rates when controlling for crown exposure. Instead, our results suggest the opposite: that at least in terms of mortality, advantage lies in a tree growing tall, and that crown exposure is a negative consequence of this growth in this regard rather than its purpose.
While unexpected, this result should be seen in its proper context. That is, mortality is just one facet of fitness, and growth, reproduction, viability and other fitness-related traits may have different relationships with height and exposure. Furthermore, unbalanced species compositions across treatments and collinearity between height and crown exposure may underlie this pattern. Nonetheless, the assumption that gaining an exposed crown is the ultimate goal of growing tall could serve as an interesting topic for further research.
(d). Implications for future forests
Phillips et al. [60] suggest that large tree vulnerability to drought may place an upper limit on tropical tree sizes in the future. Our findings corroborate this conclusion in undisturbed forests to a certain degree, but not in disturbed areas.
Much of our knowledge about how tropical forests respond to drought is derived from studies in plots specifically chosen for their lack of disturbance. Since at least half of extant tropical forests are managed or disturbed [61], it is equally important to understand the response of these ‘degraded’ forests.
Our results support the view that tall trees are particularly vulnerable to drought in undisturbed forests. However, after a certain proportion of large trees die due to drought, and if logging approximates that process of drought-culling of large trees, then the remnant large trees may no longer be particularly vulnerable. In fact, their size, and stem diameter, in particular, may serve to buffer them against future droughts.
If our finding that, in logged forests, drought-induced mortality does not depend on tree height holds across the tropics, then instead of a limit on tree size as previously suggested, we may see an initial reduction in the density of large trees in drought-stressed but unlogged areas, which could then level off to lower densities in a more drought-affected world.
Recent studies (e.g. [3]) suggest that forests will experience ever increasing drought-induced mortality rates as a result of contemporary changes, including a changing climate and anthropogenic disturbance. What these studies often fail to consider is how vulnerability to drought may change as forests degrade. Here we found that the reduced competition that logging affords during droughts seems to counteract the size signal in drought-induced mortality found in unlogged areas. Thus, while pristine forests are likely to feel the full brunt of droughts in the near term, they may change to a different, degraded, steady state of lower basal area that might weather the future changes bearing down on them [62]. These lower density forests may be better able to persist as climate change progresses.
(e). Implications for policy and research
Our results indicate the importance of distinguishing between undisturbed and selectively logged forests when making projections regarding how forests will respond to climate change-induced changes. Specifically, projections of future carbon and timber stocks should take into account that in terms of mortality, large trees may suffer disproportionately in closed canopy forests, but such a size signal may reduce or disappear as stands thin due to drought-induced mortality or selective logging.
Stand thinning has been suggested as a potential protective measure to pre-adapt temperate forests to climate change-related increases in drought frequency and intensity. This study does not support such a treatment for conferring tolerance across all stem size classes. Thinning in order to protect large trees, however, remains an open question. Further studies of deeper droughts in tropical forests are necessary to answer this question. Population modelling studies are necessary to project the implications of altered mortality regimes into the future. These questions are especially pertinent in regions where droughts are expected to increase in depth and frequency.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank the Instituto Boliviano de Investigacion Forestal (http://www.ibifbolivia.org.bo), and in particular Bonifacio Mostacedo, Zulma Villegas and Marisol Toledo, without whose perseverance over the years this work, and the work of many others, would not be possible. We would also thank the many field assistants, and Angel ´Don Wichó Mendez and Don Ricardo Mendez, in particular, who made this study possible. José Miguel Ponciano guided the development of models to account for varying census interval lengths. We thank Tobias Jackson, four anonymous reviewers and two editors for their time and helpful comments that improved this manuscript.
Data accessibility
Data are available as part of the electronic supplementary material. Other data available upon request to al@shenkin.org.
Authors' contributions
M.P.-C., A.S., J.C.L., F.E.P. and N.A. carried out the fieldwork and participated in data analysis, F.E.P. and M.P.-C. participated in the design of the experiment; A.S. and B.B. carried out the statistical analysis; A.S. and F.E.P. drafted the manuscript; F.E.P., A.S. and M.P.-C. conceived of the study. All the authors gave their final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This work was supported in part by the WWF Kathryn S. Fuller Science for Nature Fund.
References
- 1.Marengo JA, Nobre CA, Tomasella J, Oyama MD, Oliveira GSD, Oliveira RD, Camargo H, Alves LM, Brown IF. 2008. The drought of Amazonia in 2005. J. Clim. 21, 495–516. ( 10.1175/2007jcli1600.1) [DOI] [Google Scholar]
- 2.Malhi Y, Roberts JT, Betts RA, Killeen TJ, Li W, Nobre CA. 2008. Climate change, deforestation, and the fate of the Amazon. Science 319, 169–172. ( 10.1126/science.1146961) [DOI] [PubMed] [Google Scholar]
- 3.Allen CD, Breshears DD, McDowell NG. 2015. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene. Ecosphere 6, 1–55. ( 10.1890/ES15-00203.1) [DOI] [Google Scholar]
- 4.McDowell NG, Allen CD. 2015. Darcy's law predicts widespread forest mortality under climate warming. Nature Clim. Change 5, 669–672. ( 10.1038/nclimate2641) [DOI] [Google Scholar]
- 5.Leighton M, Wirawan N. 1986. Catastrophic drought and fire in Borneo tropical rain forest associated with the 1982–1983 El Nino Southern Oscillation event. In Tropical rain forests and the world atmosphere (ed. Prance GT.), pp. 75–102. Boulder, CO: Westview Press. [Google Scholar]
- 6.Nepstad DC, Tohver IM, Ray D, Moutinho P, Cardinot G. 2007. Mortality of large trees and lianas following experimental drought in an Amazon forest. Ecology 88, 2259–2269. ( 10.1890/06-1046.1) [DOI] [PubMed] [Google Scholar]
- 7.Van Nieuwstadt MGL, Sheil D. 2005. Drought, fire and tree survival in a Borneo rain forest, East Kalimantan, Indonesia. J. Ecol. 93, 191–201. ( 10.1111/j.1365-2745.2004.00954.x) [DOI] [Google Scholar]
- 8.da Costa AC, et al. 2010. Effect of 7 yr of experimental drought on vegetation dynamics and biomass storage of an eastern Amazonian rainforest. New Phytol. 187, 579–591. ( 10.1111/j.1469-8137.2010.03309.x) [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y-J, et al. 2009. Size-dependent mortality in a Neotropical savanna tree: the role of height-related adjustments in hydraulic architecture and carbon allocation. Plant Cell Environ. 32, 1456–1466. ( 10.1111/j.1365-3040.2009.02012.x) [DOI] [PubMed] [Google Scholar]
- 10.Williamson GB, Laurance WF, Oliveira AA, Delamônica P, Gascon C, Lovejoy TE, Pohl L. 2000. Amazonian tree mortality during the 1997 El Niño drought. Conserv. Biol. 14, 1538–1542. ( 10.1046/j.1523-1739.2000.99298.x) [DOI] [Google Scholar]
- 11.Bennett AC, McDowell NG, Allen CD, Anderson-Teixeira KJ. 2015. Larger trees suffer most during drought in forests worldwide. Nat. Plants 1, 15139 ( 10.1038/nplants.2015.139) [DOI] [PubMed] [Google Scholar]
- 12.Nakagawa M, et al. 2000. Impact of severe drought associated with the 1997–1998 El Niño in a tropical forest in Sarawak. J. Trop. Ecol. 16, 355–367. ( 10.1017/S0266467400001450) [DOI] [Google Scholar]
- 13.Cruz de Carvalho MH. 2008. Drought stress and reactive oxygen species: production, scavenging and signaling. Plant Signal. Behav. 3, 156–165. ( 10.4161/psb.3.3.5536) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDowell NG, Beerling DJ, Breshears DD, Fisher RA, Raffa KF, Stitt M. 2011. The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends Ecol. Evol. 26, 523–532. ( 10.1016/j.tree.2011.06.003) [DOI] [PubMed] [Google Scholar]
- 15.Gebhardt T, Häberle K.-H., Matyssek R, Schulz C, Ammer C. 2014. The more, the better? Water relations of Norway spruce stands after progressive thinning. Agric. For. Meteorol. 197, 235–243. ( 10.1016/j.agrformet.2014.05.013) [DOI] [Google Scholar]
- 16.Zou CB, Breshears DD, Newman BD, Wilcox BP, Gard MO, Rich PM. 2008. Soil water dynamics under low- versus high-ponderosa pine tree density: ecohydrological functioning and restoration implications. Ecohydrology 1, 309–315. ( 10.1002/eco.17) [DOI] [Google Scholar]
- 17.McDowell NG, Adams HD, Bailey JD, Hess M, Kolb TE. 2006. Homeostatic maintenance of ponderosa pine gas exchange in response to stand density changes. Ecol. Appl. 16, 1164–1182. ( 10.1890/1051-0761(2006)016%5B1164:HMOPPG%5D2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 18.Stogsdili WR, Wittwer RF, Hennessey TC, Dougherty PM. 1992. Water use in thinned loblolly pine plantations. For. Ecol. Manage. 50, 233–245. ( 10.1016/0378-1127(92)90338-A) [DOI] [Google Scholar]
- 19.Aussenac G. 2000. Interactions between forest stands and microclimate: ecophysiological aspects and consequences for silviculture. Ann. For. Sci. 57, 287–301. ( 10.1051/forest:2000119) [DOI] [Google Scholar]
- 20.Dawson TE. 1993. Hydraulic lift and water use by plants: implications for water balance, performance and plant–plant interactions. Oecologia 95, 565–574. ( 10.1007/BF00317442) [DOI] [PubMed] [Google Scholar]
- 21.Elkin C, Giuggiola A, Rigling A, Bugmann H. 2015. Short- and long-term efficacy of forest thinning to mitigate drought impacts in mountain forests in the European Alps. Ecol. Appl. 25, 1083–1098. ( 10.1890/14-0690.1) [DOI] [PubMed] [Google Scholar]
- 22.Young DJN, Stevens JT, Earles JM, Moore J, Ellis A, Jirka AL, Latimer AM. 2017. Long-term climate and competition explain forest mortality patterns under extreme drought. Ecol. Lett. 20, 78–86. ( 10.1111/ele.12711) [DOI] [PubMed] [Google Scholar]
- 23.Sohn JA, Saha S, Bauhus J. 2016. Potential of forest thinning to mitigate drought stress: a meta-analysis. For. Ecol. Manage. 380, 261–273. ( 10.1016/j.foreco.2016.07.046) [DOI] [Google Scholar]
- 24.Bottero A, D'Amato AW, Palik BJ, Bradford JB, Fraver S, Battaglia MA, Asherin LA. 2017. Density-dependent vulnerability of forest ecosystems to drought. J. Appl. Ecol. 54, 1605–1614. ( 10.1111/1365-2664.12847) [DOI] [Google Scholar]
- 25.Bradford JB, Bell DM. 2017. A window of opportunity for climate-change adaptation: easing tree mortality by reducing forest basal area. Front. Ecol. Environ. 15, 11–17. ( 10.1002/fee.1445) [DOI] [Google Scholar]
- 26.Aldea J, Bravo F, Bravo-Oviedo A, Ruiz-Peinado R, Rodríguez F, del Río M. 2017. Thinning enhances the species-specific radial increment response to drought in Mediterranean pine-oak stands. Agric. For. Meteorol. 237, 371–383. ( 10.1016/j.agrformet.2017.02.009) [DOI] [Google Scholar]
- 27.Gleason KE, et al. 2017. Competition amplifies drought stress in forests across broad climatic and compositional gradients. Ecosphere 8, e01849 ( 10.1002/ecs2.1849) [DOI] [Google Scholar]
- 28.Camarero JJ. 2017. The multiple factors explaining decline in mountain forests: historical logging and warming-related drought stress is causing silver-fir dieback in the Aragón Pyrenees. In High mountain conservation in a changing world (eds Catalan J, Ninot JM, Aniz MM), pp. 131–154. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- 29.Dorman M, Svoray T, Perevolotsky A, Moshe Y, Sarris D. 2015. What determines tree mortality in dry environments? A multi-perspective approach. Ecol. Appl. 25, 1054–1071. ( 10.1890/14-0698.1) [DOI] [PubMed] [Google Scholar]
- 30.D'Amato AW, Bradford JB, Fraver S, Palik BJ. 2013. Effects of thinning on drought vulnerability and climate response in north temperate forest ecosystems. Ecol. Appl. 23, 1735–1742. ( 10.1890/13-0677.1) [DOI] [PubMed] [Google Scholar]
- 31.Sohn JA, Hartig F, Kohler M, Huss J, Bauhus J. 2016. Heavy and frequent thinning promotes drought adaptation in Pinus sylvestris forests. Ecol. Appl. 26, 2190–2205. ( 10.1002/eap.1373) [DOI] [PubMed] [Google Scholar]
- 32.Ruzicka KJ, Puettmann KJ, Brooks JR. 2017. Cross-scale interactions affect tree growth and intrinsic water use efficiency and highlight the importance of spatial context in managing forests under global change. J. Ecol. 105, 1425–1436. ( 10.1111/1365-2745.12749) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ameztegui A, Cabon A, De Cáceres M, Coll L. 2017. Managing stand density to enhance the adaptability of Scots pine stands to climate change: a modelling approach. Ecol. Model 356, 141–150. ( 10.1016/j.ecolmodel.2017.04.006) [DOI] [Google Scholar]
- 34.Puettmann KJ. 2011. Silvicultural challenges and options in the context of global change: ’Simple’ fixes and opportunities for new management approaches. J. For. 109, 321–331. [Google Scholar]
- 35.Nagel LM, et al. 2017. Adaptive silviculture for climate change: a national experiment in manager–scientist partnerships to apply an adaptation framework. J. For. 115, 167–178. ( 10.5849/jof.16-039) [DOI] [Google Scholar]
- 36.Grant GE, Tague CL, Allen CD. 2013. Watering the forest for the trees: an emerging priority for managing water in forest landscapes. Front. Ecol. Environ. 11, 314–321. ( 10.1890/120209) [DOI] [Google Scholar]
- 37.Gustafson EJ, Shinneman DJ. 2015. Approaches to modeling landscape-scale drought-induced forest mortality. In Simulation modeling of forest landscape disturbances (eds Perera AH, Sturtevant BR, Buse LJ), pp. 45–71. Cham, Switzerland: Springer International Publishing. [Google Scholar]
- 38.Linder M. 2000. Developing adaptive forest management strategies to cope with climate change. Tree Physiol. 20, 299–307. ( 10.1093/treephys/20.5-6.299) [DOI] [PubMed] [Google Scholar]
- 39.Hérault B, Gourlet-Fleury S. 2016. Will tropical rainforests survive climate change? In Climate change and agriculture worldwide (ed. Torquebiau E.), pp. 183–196. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 40.Shenkin A, Bolker B, Peña-Claros M, Licona JC, Putz FE. 2015. Fates of trees damaged by logging in Amazonian Bolivia. For. Ecol. Manage. 357, 50–59. ( 10.1016/j.foreco.2015.08.009) [DOI] [Google Scholar]
- 41.Kolb TE, Agee JK, Fulé P. Z., McDowell NG, Pearson K, Sala A, Waring RH. 2007. Perpetuating old ponderosa pine. For. Ecol. Manage. 249, 141–157. ( 10.1016/j.foreco.2007.06.002) [DOI] [Google Scholar]
- 42.Erickson CC, Waring KM. 2014. Old Pinus ponderosa growth responses to restoration treatments, climate and drought in a southwestern US landscape. Appl. Vegetation Sci. 17, 97–108. ( 10.1111/avsc.12056) [DOI] [Google Scholar]
- 43.Trouvé R, Bontemps J-D, Collet C, Seynave I, Lebourgeois F. 2017. Radial growth resilience of sessile oak after drought is affected by site water status, stand density, and social status. Trees 31, 517–529. ( 10.1007/s00468-016-1479-1) [DOI] [Google Scholar]
- 44.Peña-Claros M, et al. 2012. Soil effects on forest structure and diversity in a moist and a dry tropical forest. Biotropica 44, 276–283. ( 10.1111/j.1744-7429.2011.00813.x) [DOI] [Google Scholar]
- 45.Peña-Claros M, et al. 2008. Beyond reduced-impact logging: silvicultural treatments to increase growth rates of tropical trees. For. Ecol. Manage. 256, 1458–1467. ( 10.1016/j.foreco.2007.11.013) [DOI] [Google Scholar]
- 46.Paz-Rivera C, Putz FE. 2009. Anthropogenic soils and tree distributions in a lowland forest in Bolivia. Biotropica 41, 665–675. ( 10.1111/j.1744-7429.2009.00521.x) [DOI] [Google Scholar]
- 47.Quintero-Vallejo E, Peña-Claros M, Bongers F, Toledo M, Poorter L. 2015. Effects of Amazonian dark earths on growth and leaf nutrient balance of tropical tree seedlings. Plant Soil 396, 241–255. ( 10.1007/s11104-015-2558-6) [DOI] [Google Scholar]
- 48.Clark D, Clark D. 1992. Life history diversity of canopy and emergent trees in a neotropical rain forest. Ecol. Monogr. 62, 315–344. ( 10.2307/2937114) [DOI] [Google Scholar]
- 49.Aragão L, Malhi Y, Roman-Cuesta RM, Saatchi S, Anderson LO, Shimabukuro YE. 2007. Spatial patterns and fire response of recent Amazonian droughts. Geophys. Res. Lett 34, L07701 ( 10.1029/2006GL028946) [DOI] [Google Scholar]
- 50.Phillips OL, et al. 2009. Drought sensitivity of the Amazon rainforest. Science 323, 1344–1347. ( 10.1126/science.1164033) [DOI] [PubMed] [Google Scholar]
- 51.Malhi Y, Aragão L, Galbraith D, Huntingford C, Fisher R, Zelazowski P, Sitch S, McSweeney C, Meir P. 2009. Exploring the likelihood and mechanism of a climate-change-induced dieback of the Amazon rainforest. Proc. Natl Acad. Sci. USA 106, 20 610–20 615. ( 10.1073/pnas.0804619106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carreño-Rocabado G, Peña-Claros M, Bongers F, Alarcón A, Licona J.-C, Poorter L. 2012. Effects of disturbance intensity on species and functional diversity in a tropical forest. J. Ecol. 100, 1453–1463. ( 10.1111/j.1365-2745.2012.02015.x) [DOI] [Google Scholar]
- 53.Shenkin A. 2014. Fates of trees and forests in Boliva subjected to selective logging, fire, and climate change. Doctoral dissertation, University of Florida, Gainesville, FL [Google Scholar]
- 54.Bates D, Maechler M, Bolker B. 2011. lme4: Linear mixed-effects models using S4 classes. R package version 0.999375–42.
- 55.R Core Team. 2016. R: A language and environment for statistical computing. 3.3.2 ed. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 56.Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 57.Rue H, Martino S, Chopin N. 2009. Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. J. R. Stat. Soc. B 71, 319–392. ( 10.1111/j.1467-9868.2008.00700.x) [DOI] [Google Scholar]
- 58.Hartig F. 2018. DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.2.0 ed. [Google Scholar]
- 59.Schielzeth H. 2010. Simple means to improve the interpretability of regression coefficients. Methods Ecol. Evol. 1, 103–113. ( 10.1111/j.2041-210X.2010.00012.x) [DOI] [Google Scholar]
- 60.Phillips OL, et al. 2010. Drought–mortality relationships for tropical forests. New Phytol. 187, 631–646. ( 10.1111/j.1469-8137.2010.03359.x) [DOI] [PubMed] [Google Scholar]
- 61.Blaser J, Sarre A, Poore D, Johnson S. 2011. Technical series 38: Status of tropical forest management 2011. In ITTO technical series (ed. ITT Organization). Yokohama, Japan: International Tropical Timber Organization. [Google Scholar]
- 62.da Costa ACL, et al. 2017. Stand dynamics modulate water cycling and mortality risk in droughted tropical forest. Glob. Change Biol. 24, 249–258. ( 10.1111/gcb.13851) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available as part of the electronic supplementary material. Other data available upon request to al@shenkin.org.