Abstract
Background and Objectives
Investigations of platelet function by light transmission aggregometry (LTA) using a dedicated aggregometer is time consuming and labor intensive. This multicenter study evaluated an automated LTA method using a coagulation analyzer to establish reference ranges and ideal testing regimen.
Methods
Sysmex CS‐2x00 series analyzers were used to measure aggregation using a range of agonists and concentrations: ADP (1‐20 μM); arachidonic acid (0.5‐1.5 mM); collagen (1.25‐5 μg/mL); ristocetin (0.5‐1.5 g/L); epinephrine (5‐10 μM); TRAP (1‐20 μM); U46619 (1 μM); and saline. Maximum and final aggregation, disaggregation, slope, and acquisition time were compared for each.
Results
For 42 normal subjects there was no significant difference in aggregation parameters for: 10 μM and 20 μm ADP; 2 and 2.5 μM ADP; 1 and 1.5 mM arachidonic acid; 2.5 and 5 μg/mL collagen; 1 and 1.25 μg/mL collagen; 1.25 and 1.5 g/L ristocetin; 5 and 10 μM epinephrine; 5 and 10 μM or 20 μM TRAP. Maximum aggregation was reached by 300 seconds with 20 and 10 μM ADP, 1 μM U46619, 1 and 1.25 μg/mL collagen, 1.5 g/L ristocetin and 5, 10, and 20 μM TRAP: all others agonists required 600s.
Conclusions
A standard panel of agonists can be used on the Sysmex CS‐2x00 series analyzers: ADP (10, 5, 2.5, and 1.25 μM); 1 mM arachidonic acid; 1 μM U46619; 2.5 and 1.25 μg/mL collagen; 1.25 and 0.5 g/L ristocetin; 5 μM epinephrine; 5 and 10 μM TRAP; and saline. Aggregation should be observed for 600 seconds for all agonists except TRAP and U46619, which require 300 seconds. If further studies confirm these concentrations detect platelet disorders then Sysmex CS‐series analyzers could replace dedicated aggregometers, or perform LTA where it is currently not available.
Keywords: blood platelets, hemostasis, laboratory diagnosis, platelet aggregation, standardization
Essentials.
Platelet function assays are poorly standardized between laboratories.
Three current guidelines give different advice on agonist concentrations and acquisition times.
Sysmex CS‐2x00 analyzer enabled standardization of platelet function assays in a multicenter study.
Agonist concentrations, acquisition times, and reference ranges were optimized for automated technique.
1. INTRODUCTION
Platelet aggregation is most commonly measured by light transmission aggregometry (LTA), in which the increase in light transmission through a stirred suspension of platelet‐rich plasma (PRP) is measured over time as platelets aggregate, and plotted graphically. The technique has changed little since being first described in the early 1960s,1, 2 but the assessment of platelet aggregation to a range of agonists is central to the investigation of platelet function disorders.3, 4, 5 It is only tested by a few specialized hemostasis laboratories,6 since the processes of sample preparation, analysis and the subtle interpretation of the curves produced require considerable expertise and familiarity to diagnose platelet function defects.
Recently, however, an automated LTA method has been described that offers potential standardization of measurement, and a less laborious analytical process. Lawrie et al7 first described the method, testing 14 individuals with no bleeding history or on anti‐platelet drugs. They established that the Sysmex CS‐2000i analyzer gave aggregation traces equivalent to those obtained using the Helena AggRam; Helena Biosciences Europe, Tyne and Wear, UK, using reagents from one manufacturer. More recently, Ling et al8 established that when using a Sysmex CS‐2100i analyzer, results from normal individuals are more precise than those obtained with a Chronolog‐700, and looked at the effect of platelet count on the results obtained. Lastly, Frère et al9 showed that in a population of 62 patients taking antiplatelet drugs the CS‐2000i analyzer gave equivalent results to those obtained with an APACT‐4004 aggregometer.
In this multicenter study, we have analyzed residual PRP from 64 patients being investigated for platelet function disorders and from 42 normal subjects in the hemophilia clinics of three centers with specialized laboratory facilities on three different analyzers of the Sysmex CS‐2x00 series using seven agonists over a wide range of agonist concentrations (26 tests per sample in total) to match the recommendations of different national and international guidelines.3, 4, 5 The aims of the preliminary study reported here were to: establish which concentrations of agonists should be recommended for use on these analyzers; to establish reference ranges for maximum aggregation (MA), final aggregation (FA), lag time, slope, and disaggregation; and to determine appropriate acquisition times. A subsequent study comparing results for patients with previously confirmed platelet disorders between the Sysmex CS‐2x00 series analyzers and two different dedicated platelet aggregometers will be reported separately.
2. MATERIALS AND METHODS
2.1. Sample preparation
As part of routine practice for patients being tested for platelet disorders, 20 mL whole blood was collected into 0.105‐0.109 M sodium citrate in a ratio of one part anticoagulant to 9 parts whole blood: two laboratories used samples collected using Vacutainers (Beckton‐Dickinson, Oxford, UK); one laboratory used samples collected using Vacuette (Greiner Bio‐One Ltd, Stonehouse, UK). Platelet‐rich plasma (PRP) and platelet‐poor plasma (PPP) were prepared in line with local laboratory standard operating procedures, which adhere to the guidelines set by the BSH.4 Platelet count on PRP was measured on local laboratory full blood count analyzers to ensure that there were enough platelets to perform aggregometry (i.e, >150 × 109/L in PRP). Although adjusting of the platelet count of PRP with autologous PPP is occasionally recommended,3, 8 it has been shown to cause abnormalities10, 11 and is currently considered unnecessary4, 5; no patient or control samples had their PRP adjusted by addition of either PPP or buffer.
Alongside patient samples, local practice at all participating laboratories is to bleed a volunteer member of staff with no history of bleeding and no recent nonsteroidal anti‐inflammatory drug use at the same time as the patient(s). Platelet rich plasma and PPP were prepared from this “normal control” at the same time as the patient samples.
Any residual PRP/PPP from the patients or controls was included in the study, and therefore under the auspices of the Human Tissue Act 200412 did not require ethics committee approval for inclusion in the study since it is a method evaluation.
Due to the impracticalities of transporting samples between laboratories, and testing limited volumes of residual PRP/PPP within 4 hours of samples collection as recommended in the guidelines, no direct comparison between different CS‐series analyzers was possible.
2.2. Analyzers
All samples were tested by LTA on a Sysmex CS‐2x00 series analyzer (Sysmex UK, Milton Keynes, UK) using standard protocols. Three different analyzers were used: a Sysmex CS‐2100i; a Sysmex CS‐2400; and a Sysmex CS‐2500. The Sysmex CS‐2400 (without cap‐piercing capability) and Sysmex CS‐2500 (with cap‐piercing capability) analyzers are updated versions of the Sysmex CS‐2100i analyzer with an updated exterior appearance and enhanced PC software. The analytical modules and analytical software are identical on the three analyzers.
Samples were concurrently assayed by LTA on existing dedicated platelet aggregometers in the participating laboratories. Two laboratories used a Biodata PAP‐8E (Alpha Laboratories, Hampshire, UK) and one used a Helena AggRam (Helena Biosciences Europe). Data obtained from these analyses will be used for future comparative studies between the different LTA techniques.
Tubes of PPP and PRP were placed on the Sysmex CS‐2x00 analyzer sampler unit, and PRP was mixed by multiple gentle inversions immediately before analysis. The analyzer aspirated PPP or PRP directly from the sample rack in micro mode, rather than taking a daughter specimen sufficient for all requested tests.
On the Sysmex CS‐2x00 series analyzers, 100% aggregation was defined by observing the absorbance of 140 μL PPP to which 20 μL normal saline had been added. For analysis of PRP, the analyzer pipetted 140 μL PRP into a plastic stir‐bar cuvette before addition of 20 μL of agonist (7:1 PRP to agonist), at which point 0% aggregation is defined by the instrument. Absorbance was monitored for 600 seconds whilst the contents of the cuvette were stirred at a constant 800 rpm. All assays were completed within 4 hours of sample collection.
At the end of the measuring period traces were saved as PDF files, and raw data were entered into a Microsoft Excel spreadsheet (Microsoft, Redmond, WA, USA).
Data were analyzed using GraphPad Prism 7.0 for Windows (GraphPad Software, La Jolla, CA, USA).
2.3. Reagents
All laboratories used the same reagents on the Sysmex CS‐2x00 analyzers. Revohem reagents (Sysmex UK) were used for ADP, arachidonic acid, collagen, ristocetin, and epinephrine. Reagents for TRAP‐6 and U46619 were obtained from Hart Biologicals (Hartlepool, UK). All reagents were reconstituted according to manufacturer's instructions to give stock reagent concentrations of 160 μM ADP, 12 g/L ristocetin, 12 mM arachidonic acid, 800 μg/mL collagen, 800 μM epinephrine, 80 μM U46619, and 800 μM TRAP. Further dilutions of all agonists except collagen were made in normal saline to their working concentrations: two laboratories made working concentrations fresh with each batch; one laboratory froze working reagents below ‐20°C (for up to 2 months) before being thawed for single use following local validation. All laboratories made fresh working concentrations of collagen diluted in kit buffer with each batch and these were discarded after use.
2.4. Rationale for choice of starting agonists and concentrations and acquisition times
Participating laboratories agreed agonists to be used for this study by referencing guidelines from Clinical and Laboratory Standards Institute (CLSI),3 British Society of Haematology (BSH),4 and International Society on Thrombosis and Haemostasis (ISTH),5 for suggested concentrations. In order to further evaluate results in comparison to existing techniques in the participating laboratories, agonists already in local panels were included for evaluation.
The ISTH5 guideline does not recommend testing ADP at concentrations lower than 2 μM or epinephrine below 5 μM, but CLSI3 and BSH4 guidelines recommend testing both at lower concentrations, although neither suggests when this could be clinically useful. A single lower concentration of 1 μM ADP was included in the study, which was a one‐half dilution of the 2μM agonist, selected for ease of preparation. A concentration of epinephrine below 5 μM was not included in the study.
To check for spontaneous aggregation, normal saline was used in place of an agonist with all assays.
The agonists selected and their final concentrations when mixed with PRP are summarized in Table 1.
Table 1.
Guideline recommended final concentrations of agonists and those used for study
| Agonist | ISTH3 | BSH4 | CLSI5 | Participating laboratories | Final concentrations selected for study | |||
|---|---|---|---|---|---|---|---|---|
| Start | Range | Start | Range | Start | Range | |||
| ADP (μM) | 2 | Higher if abnormal | 2.5 | 0.5‐20 | 5 | 0.5‐10 | 2.5/5/10 | 1/2/2.5/5/10/20 |
| Arachidonic acid (mM) | 1 | Higher if abnormal | 1 | 0.5‐1 | 0.5‐1.6 | — | 1/1.6 | 0.5/1/1.5 |
| Collagen (μg/mL) | 2 | Higher if abnormal | 1.25 | 1‐5 | 2 | 1‐5 | 1/1.25/2.5/5 | 1/1.25/2.5/5 |
| High ristocetin (g/L) | 1.2 | 2.0 if absent | 1.2‐1.5 | 1.2‐1.5 | — | 0.8‐1.5 | 1/1.25/1.5 | 1/1.25/1.5 |
| Low ristocetin (g/L) | — | 0.5‐0.7 | — | 0.5‐0.7 | ≤0.6 | — | 0.25/0.5/0.75 | 0.5/0.75 |
| Epinephrine (μM) | 5 | Higher if abnormal | 5 | 0.5‐10 | 5 | 0.5‐10 | 10 | 5/10 |
| U46619 (μM) | 1 | Higher if abnormal | 1 | — | — | 1‐2 | 1/10 000 | 1 |
| TRAP (PAR1) (μM) | 10 | Higher if abnormal | — | 10‐100 | — | — | 10/20 | 1/5/10/20 |
ADP, adenine diphosphate; BSH, British Society for Haematology; CLSI, Clinical and Laboratory Standards Institute; ISTH, International Society on Thrombosis and Haemostasis; TRAP, thrombin receptor activating peptide; U46619, endoperoxide analogue.
All concentrations quoted are the final concentrations of agonist after addition to platelet rich plasma.
3. RESULTS
Samples from 64 patients being investigated for bleeding and 42 volunteer controls were tested at the participating sites in a 14‐month period from September 2016 to November 2017. Only data obtained from volunteer controls were analyzed for numerical comparison in this preliminary study.
Data reported from the CS‐2x00 series analyzers include: the absorbance of PPP and PRP; MA as a percentage; FA as a percentage; lag time in seconds; area under the curve (%*s); and the maximum velocity (%/s), time of maximum velocity (s) and angle of maximum velocity (°) of the primary phase (and secondary phase if present) for each agonist.
CLSI, BSH, and ISTH guidelines3, 4, 5 all recommend that lag time, MA, FA, disaggregation, and slope be considered when interpreting results. Disaggregation is not reported by the analyzer so a figure for disaggregation was calculated for each agonist by subtracting the FA from the MA and relating this to the original MA, so that a sample that completely disaggregated showed 100% disaggregation, regardless of the original MA.
3.1. Reference ranges
CLSI guidelines3 recommend that a minimum of 20 normal subjects are used to establish reference intervals, whereas BSH guidelines4 state that more than 40 normal subjects are required. Other authors recommend a minimum of 39.13, 14
One‐way ANOVA analysis showed that there were no statistically significant differences between results for controls obtained at each site, despite the different blood collection tubes used and the different reagent handling. Therefore data was combined and reference ranges were established by finding the 2.5th to 97.5th percentile for each parameter from samples collected from 42 volunteers with no history of bleeding and no recent NSAID use. The results are summarized in Table 2.
Table 2.
Reference ranges
| Final concentration of agonist | |||||||
|---|---|---|---|---|---|---|---|
| Agonist | Parameter | 20 μM | 10 μM | 5 μM | 2.5 μM | 2 μM | 1 μM |
| ADP | MA (%) | 89 (76‐103) | 87 (48‐106) | 84 (44‐98) | 74 (22‐100) | 82 (42‐98) | 56 (18‐96) |
| FA (%) | 82 (68‐96) | 79 (25‐97) | 76 (1‐93) | 64 (0‐93) | 73 (0‐92) | 33 (0‐85) | |
| DA (%) | 9 (3‐22) | 9 (3‐48) | 9 (3‐97) | 12 (1‐100) | 10 (1‐100) | 14 (4‐100) | |
| Slope (%/s) | 114 (89‐164) | 117 (45‐158) | 102 (56‐154) | 84 (38‐142) | 105 (72‐140) | 28 (14‐93) | |
| Lag time (s) | 22 (16‐34) | 24 (16‐60) | 25 (17‐67) | 30 (18‐59) | 24 (18‐45) | 39 (23‐306) | |
| AA 1.5 mM | AA 1 mM | AA 0.5 mM | U46619 1 μM | ||
|---|---|---|---|---|---|
| AA and U46619 | MA (%) | 88 (59‐103) | 86 (70‐105) | 95 (87‐109) | 86 (60‐104) |
| FA (%) | 72 (0‐94) | 69 (0‐97) | 78 (0‐104) | 76 (0‐94) | |
| DA (%) | 10 (2‐100) | 10 (4‐100) | n/a | 11 (6‐40) | |
| Slope (%/s) | 97 (9‐180) | 59 (2‐159) | 73 (1‐162) | 100 (0‐170) | |
| Lag Time (s) | 50 (26‐91) | 73 (30‐340) | 81 (40‐126) | 35 (23‐74) |
| 5 μg/mL | 2.5 μg/mL | 1.25 μg/mL | 1 μg/mL | ||
|---|---|---|---|---|---|
| Collagen | MA (%) | 92 (81‐95) | 92 (78‐111) | 89 (54‐100) | 88 (77‐102) |
| FA (%) | 83 (67‐89) | 85 (71‐103) | 80 (48‐91) | 80 (70‐96) | |
| DA (%) | 9 (5‐14) | 7 (3‐13) | 9 (5‐23) | 9 (5‐17) | |
| Slope (%/s) | 120 (79‐144) | 121 (57‐144) | 116 (42‐137) | 122 (64‐143) | |
| Lag Time (s) | 58 (33‐80) | 64 (41‐95) | 76 (44‐151) | 76 (41‐100) |
| 1.5 g/L | 1.25 g/L | 1 g/L | 0.75 g/L | 0.5 g/L | ||
|---|---|---|---|---|---|---|
| Ristocetin | MA (%) | 88 (64‐100) | 92 (78‐99) | 86 (3‐94) | 9 (2‐97) | 3 (0‐15) |
| FA (%) | 83 (38‐96) | 89 (70‐98) | 78 (0‐92) | 4 (0‐93) | 0 (0‐13) | |
| DA (%) | 6 (1‐40) | 3 (1‐12) | n/a | n/a | n/a | |
| Slope (%/s) | 95 (37‐140) | 101 (37‐130) | 73 (2‐109) | 5 (0‐68) | 2 (1‐36) | |
| Lag time (s) | 30 (16‐64) | 33 (23‐191) | 51 (28‐244) | 105 (49‐170) | n/a |
| 10 μM | 5 μM | ||
|---|---|---|---|
| Epinephrine | MA (%) | 86 (14‐108) | 85 (3‐104) |
| FA (%) | 79 (11‐106) | 77 (0‐94) | |
| DA (%) | 8 (2‐17) | 10 (1‐63) | |
| Slope (%/s) | 39 (10‐75) | 40 (0‐74) | |
| Lag time (s) | 62 (25‐563) | 56 (24‐190) |
| 20 μM | 10 μM | 5 μM | 1 μM | ||
|---|---|---|---|---|---|
| TRAP | MA (%) | 91 (75‐104) | 92 (81‐105) | 90 (51‐100) | 12 (0‐104) |
| FA (%) | 84 (58‐98) | 85 (53‐99) | 81 (0‐96) | 1 (0‐101) | |
| DA (%) | 8 (3‐22) | 8 (6‐35) | 9 (4‐100) | n/a | |
| Slope (%/s) | 148 (93‐179) | 138 (100‐193) | 152 (73‐469) | 137 (72‐166) | |
| Lag time (s) | 22 (14‐35) | 24 (15‐39) | 25 (19‐52) | 28 (25‐45) |
AA, arachidonic acid; ADP, adenine diphosphate; DA, disaggregation; FA, final aggregation; MA, maximum aggregation; U46619, endoperoxide analogue; TRAP, thrombin receptor activating peptide.
Numbers in brackets denote the 2.5th to 97.5th percentiles. All concentrations quoted are the final concentrations of agonist after addition to platelet rich plasma.
3.2. Differences between agonists
Comparisons were made between the MA for each agonist concentration and also between FA for each agonist concentration for all samples tested. Analysis for normality by Shapiro‐Wilk test showed that only the MA for ADP at 20 and 2 μM, collagen at 1 μg/mL, TRAP at 20 and 10 μM, and ristocetin at 1.25 g/L were normally distributed. Only the FA for ADP at 20 μM, collagen at 5, 2.5, and 1 μg/mL, TRAP at 20 μM and ristocetin at 1.25 g/L were normally distributed. Therefore all comparisons were made by the non‐parametric Tukey test, summarized in Table 3.
Table 3.
Results of Tukey's test for comparison between different concentrations of each agonist
| Maximum aggregation (%) | Final aggregation (%) | Lag time (seconds) | Slope (%/s) | |||||
|---|---|---|---|---|---|---|---|---|
| Significant difference? | P Value | Significant difference? | P Value | Significant difference? | P Value | Significant difference? | P Value | |
| 20 vs 10 μM ADP | No | 0.96 | No | 0.79 | No | 0.80 | No | >0.99 |
| 10 vs 5 μM ADP | No | 0.75 | No | 0.35 | No | >0.99 | No | 0.36 |
| 5 vs 2.5 μM ADP | Yes | 0.001 | Yes | <0.001 | No | 0.99 | Yes | 0.006 |
| 2.5 vs 2 μM ADP | Yes | 0.003 | No | 0.99 | No | 0.96 | No | 0.94 |
| 2.5 vs 1 μM ADP | Yes | <0.001 | Yes | <0.001 | No | 0.99 | Yes | 0.028 |
| 2 vs 1 μM ADP | No | 0.59 | Yes | 0.002 | No | >0.99 | Yes | 0.004 |
| 1 μM ADP vs saline | Yes | <0.001 | Yes | 0.042 | n/a | Yes | <0.001 | |
| 1.5 mM vs 1 mM arachidonic acid | No | 0.96 | No | >0.99 | Yes | <0.001 | Yes | 0.005 |
| 1 mM vs 0.5 mM arachidonic acid | Yes | <0.001 | Yes | 0.001 | No | 0.81 | Yes | 0.034 |
| 0.5 mM arachidonic acid vs saline | Yes | <0.001 | Yes | 0.001 | n/a | Yes | 0.001 | |
| 5 μg/mL vs 2.5 μg/mL collagen | No | 0.99 | No | 0.99 | No | 0.059 | No | 0.86 |
| 2.5 μg/mL vs 1.25 μg/mL collagen | No | 0.32 | No | 0.29 | Yes | 0.039 | No | 0.56 |
| 1.25 μg/mL vs 1 μg/mL collagen | No | 0.64 | No | 0.70 | No | 0.79 | No | 0.93 |
| 1 μg/mL collagen vs saline | Yes | <0.001 | Yes | <0.001 | n/a | Yes | <0.001 | |
| 1.5 g/L vs 1.25 g/L ristocetin | No | >0.99 | No | 0.99 | No | 0.98 | No | 0.64 |
| 1.25 g/L vs 1 g/L ristocetin | Yes | <0.001 | Yes | <0.001 | Yes | 0.008 | Yes | <0.001 |
| 1 g/L vs 0.75 g/L ristocetin | Yes | <0.001 | Yes | <0.001 | No | 0.31 | Yes | <0.001 |
| 0.75 g/L vs 0.5 g/L ristocetin | Yes | <0.001 | Yes | 0.002 | n/a | No | 0.54 | |
| 0.5 g/L ristocetin vs saline | No | 0.99 | No | >0.99 | n/a | No | 0.99 | |
| 10 vs 5 μM epinephrine | No | 0.59 | No | 0.47 | No | 0.93 | No | 0.81 |
| 5 μM epinephrine vs saline | Yes | <0.001 | Yes | <0.001 | n/a | Yes | <0.001 | |
| 20 vs 10 μM TRAP | No | 0.99 | No | 0.99 | No | 0.64 | No | >0.99 |
| 10 vs 5 μM TRAP | No | 0.76 | No | 0.51 | No | 0.34 | No | >0.99 |
| 5 vs 1 μM TRAP | Yes | <0.001 | Yes | <0.001 | No | 0.7 | Yes | <0.001 |
| 1 μM TRAP vs saline | No | 0.39 | No | 0.86 | n/a | No | 0.25 | |
ADP, adenine diphosphate; TRAP, thrombin receptor activating peptide.
All concentrations quoted are the final concentrations of agonist after addition to platelet rich plasma.
The MA for each agonist and concentration in controls are shown in Figure 1.
Figure 1.

Box and whisker plot of maximum aggregation for control samples. Abbreviations. ADP, adenine diphosphate; U46619, endoperoxide analogue; TRAP, thrombin receptor activating peptide. All concentrations quoted are the final concentrations of agonist after addition to platelet rich plasma
3.3. Acquisition time
ISTH guidelines state that aggregation should be monitored for a minimum of 3 minutes after adding an agonist, or 5 minutes if MA for an agonist in most control samples is not achieved within 3 minutes, or 10 minutes if MA for an agonist in most controls samples is not achieved within 5 minutes.5
Time to MA in controls where the percentage MA exceeded 20% was assessed for each agonist, and is shown in Figure 2.
Figure 2.
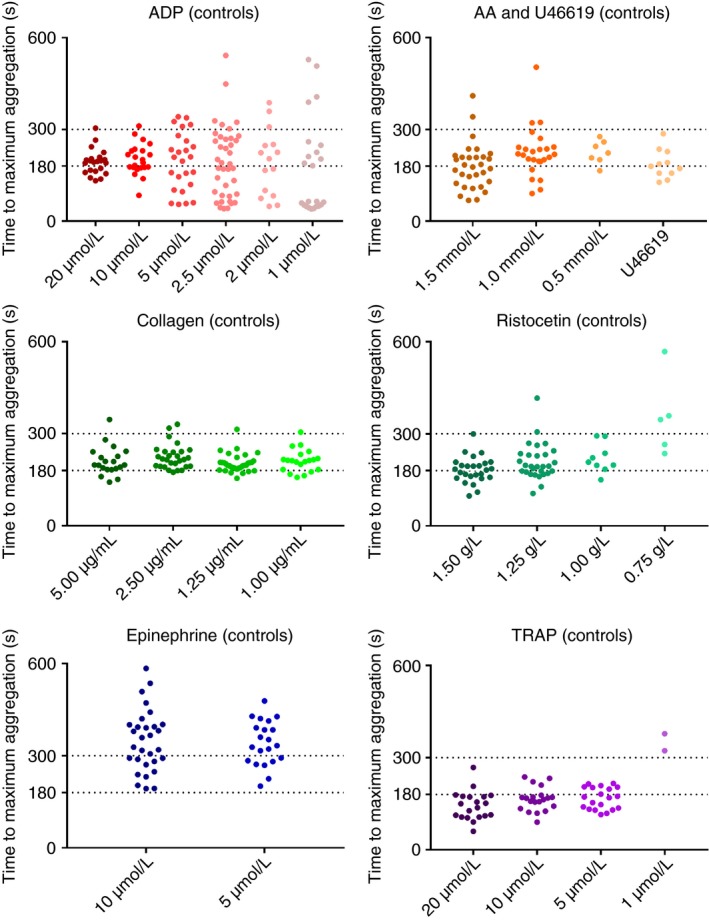
Scatter plot of time to maximum aggregation for control samples. Abbreviations. ADP, adenine diphosphate; AA, arachidonic acid; U46619, endoperoxide analogue; TRAP, thrombin receptor activating peptide. All concentrations quoted are the final concentrations of agonist after addition to platelet rich plasma
The 97.5th percentile was used to determine the time by which most control samples had achieved MA. This exceeded 180 seconds for all agonists tested, and was below 300 seconds only for TRAP at 20, 10, and 5 μM, U46619 at 1 μM, ADP at 20, and 10 μM, collagen at 1.25 and 1.00 μg/mL, and ristocetin at 1.50 g/L. For all other agonists and concentrations tested, the 97.5th percentile exceeded 300 seconds.
3.4. Patients with platelet function disorders
In patients being investigated for platelet disorders, the platelet count of PRP ranged from 61‐402 × 109/L, with three patient samples having platelet count below 100 × 109/L and four with platelet count between 100 and 150 × 109/L. Of the remaining 57 patient samples included in the analysis there was one patient with genetically confirmed Glanzmann's Thrombasthenia (tested three times) and one patient with a genetically confirmed COX‐1 variant.
The patient with genetically confirmed Glanzmann's Thrombasthenia showed no reaction to any agonist except ristocetin, and this was seen on the Helena AggRam and the Sysmex CS‐2x00 analyzer. This was the expected response and the traces from both methods are shown in Figure 3.
Figure 3.
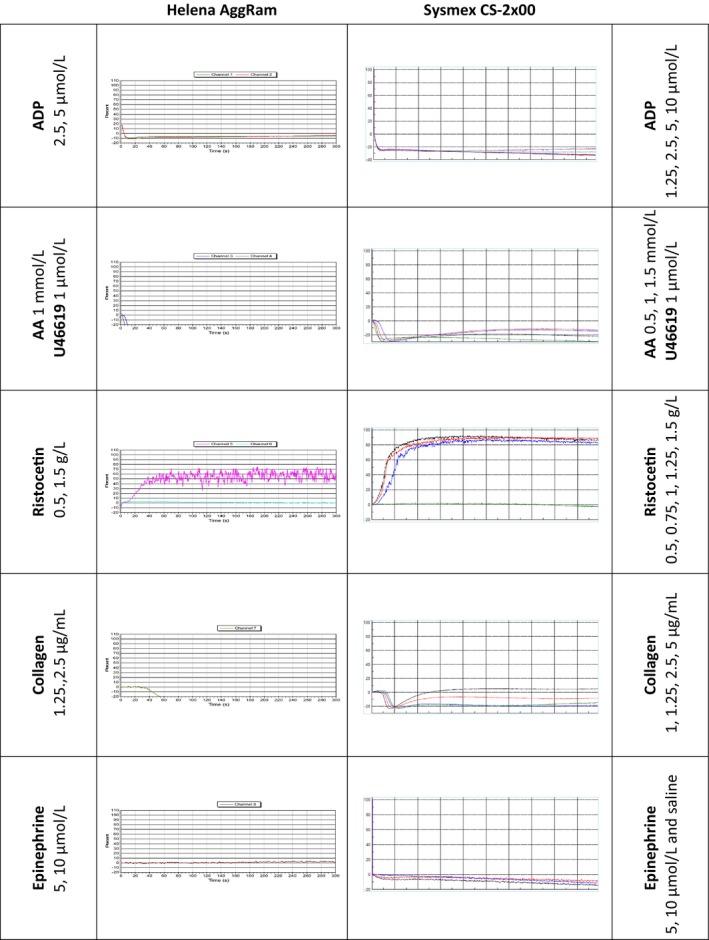
Aggregation traces from a patient with Glanzmann's Thrombasthenia. Abbreviations. ADP, adenine diphosphate; AA, arachidonic acid; U46619, endoperoxide analogue. All concentrations quoted are the final concentrations of agonist after addition to platelet rich plasma
The patient with genetically confirmed COX‐1 deficiency showed absent response to arachidonic acid with normal response to the thromboxane analogue U46619, with disaggregation with low doses of ADP and poor response to collagen, and this was seen on the Helena AggRam and the Sysmex CS‐2x00 analyzer. This was the expected response and the traces from both methods are shown in Figure 4.
Figure 4.
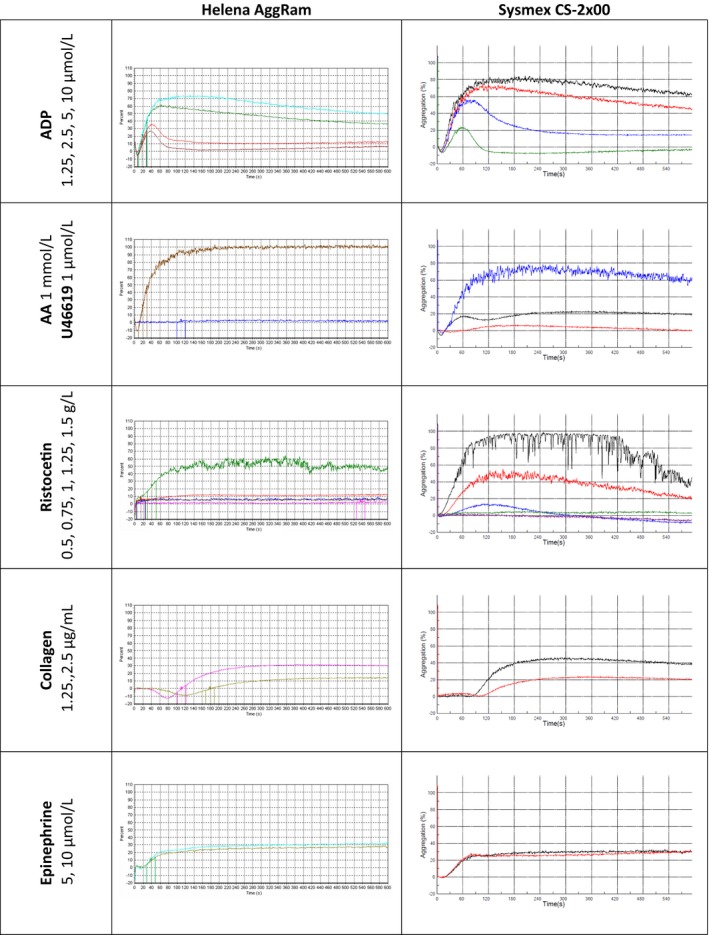
Aggregation traces from a patient with COX‐1 defect. Abbreviations. ADP, adenine diphosphate; AA, arachidonic acid; U46619, endoperoxide analogue. All concentrations quoted are the final concentrations of agonist after addition to platelet rich plasma
The remaining samples included two patients with aspirin‐like defects, one patient with storage pool disease, 40 with reduced response to at least one agonist, and 10 with normal responses to all agonists. The data from these analyses in comparison to dedicated aggregometers will be reported separately.
4. DISCUSSION
The preliminary results from normal subjects in this multisite study show that a standardized protocol for platelet aggregation studies can be applied using these analyzers. We have generated reference ranges for MA, FA, lag time, slope, and disaggregation, against which local laboratories can verify this method.
These preliminary results show that there was no significant difference in the results for MA, FA, lag time, or slope obtained using ADP at 20 or 10 μM in the normal donors, and therefore it is not necessary to test ADP at concentrations above 10 μM for the investigation of heritable platelet disorders, since using a higher concentration risks over‐activating abnormal platelets and generating potentially misleading normal responses. This is in contrast to the recommendations of BSH.4 There was no significant difference for FA, lag time or slope between the starting concentrations of 2 μM recommended by ISTH5 or 2.5 μM recommended by BSH,4 although the difference in MA reached statistical significance (P = 0.003): either concentration could be used. Using 1 μM ADP, where the platelets aggregated to a MA of more than 20%, 19 out of 20 patients and 7 out of 15 controls disaggregated, and thus a lower concentration is unlikely to be of diagnostic utility for the investigation of a bleeding disorder. Maximum aggregation was reached within 300 seconds using 20 or 10 μM ADP, but more than 300 seconds for all other concentrations. We recommend that laboratories using the CS‐2x00 analyzers use concentrations of 10, 5, 2.5, and 1.25 μM, so that doubling dilutions of a 10‐μM stock solution can be made, with an acquisition time of 600 seconds in line with BSH recommendations.4 A shorter acquisition time of 300 seconds could be used for 10 μM ADP in line with ISTH recommendations.5
There was no significant difference in the results for MA or FA obtained using arachidonic acid at 1.5 mM or 1 mM, and therefore it does not appear to be necessary to test arachidonic acid above a concentration of 1 mM since using a higher concentration risks over‐activating abnormal platelets and generating potentially misleading normal responses. ISTH guidelines recommend testing at a higher concentration of the results at 1 mM are abnormal, but we did not observe any difference in MA or FA in controls or patients, which included those with known storage pool disorder, COX‐1 variant or an aspirin‐like defect. Using 0.5 mM arachidonic acid only 7 out of 15 controls and 6 out of 31 patients reached a MA of more than 20%, and this concentration is unlikely to be of diagnostic utility for the investigation of a bleeding disorder. The endoperoxide analogue U46619 showed aggregation at 1 μM concentration as recommended by the various guidelines.3, 4, 5 Maximum aggregation was reached within 300 seconds for U46619 but more than 300 seconds for all concentrations of arachidonic acid tested. We recommend that laboratories using the CS‐2x00 analyzers use a concentration of 1 mM arachidonic acid with an acquisition time of 600 seconds in line with BSH recommendations,4 but a shorter acquisition time of 300 seconds could be used for 1 μM U46619 in line with ISTH recommendations.5
There was no significant difference in the results obtained for MA, FA, lag time, or slope between any of the concentrations of collagen that were analyzed, except for a statistical difference in lag time between 2.5 and 1.25 μg/mL (P = 0.039). Maximum aggregation was reached within 300 seconds using 1 and 1.25 μg/mL collagen but slightly more than 300 seconds for 2.5 and 5 μg/mL collagen. It does not appear to be necessary to test collagen above a concentration of 2.5 μg/mL since using a higher concentration risks over‐activating abnormal platelets and generating potentially misleading normal responses. We recommend that laboratories using the CS‐2x00 analyzers use concentrations of collagen of 1.25 and 2.5 μg/mL with an acquisition time of 600 seconds.
There was no significant difference in the results for MA, FA, lag time, or slope obtained using ristocetin at 1.5 or 1.25 g/L, and therefore it does not appear to be necessary to test response to ristocetin above a concentration above 1.25 g/L. However, it should be noted that no patients with von Willebrand disease (VWD) or Bernard Soulier Syndrome were tested during this study: ISTH guidelines recommend testing ristocetin at 2 g/L if there is absent agglutination at 1.2 g/L which will be explored in the planned subsequent study on patients with characterized primary hemostatic disorders. There were significant differences for MA and FA at all other concentrations of ristocetin tested. The results using 0.75 and 1 g/L ristocetin were highly variable, with a wide reference range, highlighting that these concentrations may be useful for testing ristocetin induced platelet aggregation when testing for VWD but of limited diagnostic use as a screening test. There was no difference between 0.5 g/L ristocetin and saline in any of the controls or patients that were tested, although no patients with type 2B or pseudo VWD were included in the study. Maximum aggregation was reached within 300 seconds using 1.5 g/L ristocetin but more than 300 seconds with all other concentrations of ristocetin. We recommend that laboratories using the CS‐2x00 analyzers use concentrations of ristocetin of 1.25 and 0.5 g/L with an acquisition time of 600 seconds.
There was no significant difference in the results for MA, FA, lag time, or slope obtained using epinephrine at 10 μM or 5 μm epinephrine. All guidelines3, 4, 5 recommend the use of an initial concentration of 5 μM epinephrine and using a higher dose if abnormal. However, there is a wide reference range for both doses of epinephrine tested. This suggests that there may be little diagnostic value in repeating testing with higher doses of epinephrine, especially if this is the only abnormality noted. Low doses of epinephrine can be used to detect the minimum dose that induces secondary aggregation but although the CLSI3 or BSH4 guidelines suggest testing using lower doses if the response to 5 μM epinephrine is normal, neither state how this is useful in the diagnosis of a bleeding disorder: the ISTH guidelines5 state only that higher doses need to be tested if the response to 5 μM epinephrine is abnormal. Maximum aggregation was reached within 600 seconds for both doses of epinephrine used in this study. We recommend that laboratories using the CS‐2x00 analyzers use a concentration of epinephrine of 5 μM with an acquisition time of 600 seconds.
There was no significant difference in the results for MA, FA, lag time, or slope obtained using TRAP at 20, 10 or 5 μM: there was also no difference for MA, FA, lag time, or slope using TRAP at 1 μM or using saline as agonist (although two controls did respond to this dose of TRAP). BSH4 and ISTH5 guidelines recommend using concentrations of TRAP of at least 10 μM, but we saw good response at 5 μM in most cases. The dose of TRAP that is needed to discriminate between normal and abnormal platelet aggregation is likely to be between 5 and 1 μM. Maximum aggregation was reached within 300 seconds for all doses of TRAP except 1 μM. We recommend that laboratories using the CS‐2x00 analyzers use a concentration of TRAP of 5 μM with an acquisition time of 300 seconds. A minimum dose of TRAP required for platelet aggregation is yet to be elucidated.
As a result of these experiments, our recommendations for agonist concentrations and acquisition times mapped to the guidelines are presented in Table 4.
Table 4.
Recommended concentrations of agonists and acquisition times for Sysmex CS‐2x00 analyzers
| Agonist | Concentrations | Acquisition time (s) |
|---|---|---|
| Arachidonic acid (mM) | 1 | 600 |
| Collagen (μg/mL) | 1.25/2.5 | 600 |
| High ristocetin (g/L) | 1.25 | 600 |
| Low ristocetin (g/L) | 0.5 | 600 |
| Epinephrine (μM) | 5 | 600 |
| U46619 (μM) | 1 | 300 |
| TRAP (PAR1) (μM) | 5/10 | 300 |
| Saline | 0.9% (w/v) | 600 |
ADP, adenine diphosphate; TRAP, thrombin receptor activating peptide; U46619, endoperoxide analogue.
All concentrations quoted are the final concentrations of agonist after addition to platelet rich plasma.
Using these agonist concentrations and acquisition times on the CS‐2x00 analyzers, we obtained the expected results for a patient with a genetically confirmed diagnosis of Glanzmann's Thromboasthenia, and for a patient with a genetically confirmed COX‐1 deficiency. Two patients who had been previously characterized with aspirin‐like defects and one who had been previously diagnosed with a storage pool disease also gave the expected results, and all patients with normal platelet aggregation by dedicated‐analyzer LTA had normal platelet aggregation with the CS‐2x00 analyzers (data not shown). The findings from this preliminary optimization study will form the basis of the next phase of the study in which a wider group of patients with characterized disorders will be tested, including some with Bernard Soulier Syndrome and von Willebrand disease.
It should be noted that this study used only one manufacturer as a source for each agonist, and that reagents from other manufacturers may give different results. Only Sysmex CS‐2x00 analyzers were included in the study, but as the Sysmex CS‐5100 analyzer uses the same analytical software these findings may be applicable to that analyzer. The Sysmex CS‐5100 has more detectors available for platelet function and therefore potentially higher throughput. As analyses are performed in micro mode, there is a risk that platelets will settle out of suspension while waiting to be analyzed by the analyzer: we have not tested this and it should be noted as a limitation of our study.
In conclusion, this method allows for greater standardization of the analysis of platelet function, although pre‐analytical preparation of platelet poor plasma and platelet rich plasma is still a time‐consuming process that may also be a source of inter‐laboratory variation. The Sysmex CS‐2x00 analyzers allow for platelet aggregometry to be performed by staff with less experience of performing manual techniques, although interpretation of results and correlation with clinical phenotype still needs to be undertaken by those with experience. Further studies comparing dedicated aggregometer LTA with Sysmex CS‐series LTA are planned using samples from patients with known platelet function disorders and VWD. If these studies show equivalent results then Sysmex CS‐series analyzers could be used for routine platelet function analyses where budgetary restraint or insufficient numbers of suitably trained staff prevent testing currently.
RELATIONSHIP DISCLOSURE
I. Holding is an employee of Sysmex UK. All other authors declared no conflicts of interest.
AUTHOR CONTRIBUTIONS
All authors designed the research study. M. Bukht, D. Gurney, A. McCormick, and S. Platton performed the research. A. McCormick and S. Platton analyzed the data. S. Platton wrote the first draft of the paper. All authors reviewed and critically edited the manuscript.
ACKNOWLEDGMENTS
The reagents and consumables used in these experiments were donated by Sysmex UK.
Platton S, McCormick Á, Bukht M, Gurney D, Holding I, Moore GW. A multicenter study to evaluate automated platelet aggregometry on Sysmex CS‐series coagulation analyzers—preliminary findings. Res Pract Thromb Haemost. 2018;2:778–789. 10.1002/rth2.12140
REFERENCES
- 1. Born G, Cross M. The aggregation of blood platelets. J Physiol. 1963;168:178–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. O'Brien J. Platelet aggregation: part II. Some results from a new method of study. J Clin Pathol. 1962;15:452–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Christie D, Carrington L, Cohen E, et al. Platelet function testing by aggregometry; approved guideline: document H58‐A. Clin Lab Standards Inst. 2008;38:31. [Google Scholar]
- 4. Harrison P, Mackie I, Mumford A, et al. Guidelines for the laboratory investigation of heritable disorders of platelet function. Br J Haematol. 2011;155:30–44. [DOI] [PubMed] [Google Scholar]
- 5. Cattaneo M, Cerletti C, Harrison P, et al. Recommendations for the standardization of light transmission aggregometry: a consensus of the working party from the platelet physiology subcommittee of SSC/ISTH. J Thromb Haemost. 2013;11:1183–9. [DOI] [PubMed] [Google Scholar]
- 6. Jennings I, Woods T, Kitchen S, Walker I. Platelet function testing: practice among UK National External Quality Assessment Scheme for Blood Coagulation participants. J Clin Pathol. 2008;61:950–4. [DOI] [PubMed] [Google Scholar]
- 7. Lawrie A, Kobayashi K, Lane P, Mackie I, Machin S. The automation of routine light transmission platelet aggregation. Int J Lab Hematol. 2014;36:431–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ling LQ, Liao J, Niu Q, et al. Evaluation of an automated light transmission aggregometry. Platelets. 2017;28(7):712–9. [DOI] [PubMed] [Google Scholar]
- 9. Frère C, Kobayashi K, Dunois C, Amiral J, Morange P‐E, Alessi M‐C. Assessment of platelet function on the routine coagulation analyzer Sysmex CS‐2000i. Platelets. 2018;29:95–7. [DOI] [PubMed] [Google Scholar]
- 10. Cattaneo M, Lechi A, Zighetti M, Lussana F. Platelet aggregation studies: autologous platelet‐poor plasma inhibits platelet aggregation when added to platelet‐rich plasma to normalize platelet count. Haematologica. 2007;92:694–7. [DOI] [PubMed] [Google Scholar]
- 11. Castilloux J, Moffat K, Liu Y, Seecharan J, Pai M, Hayward C. A prospective cohort study of light transmission platelet aggregometry for bleeding disorders: is testing native platelet‐rich plasma non‐inferior to testing platelet count adjusted samples? Thromb Haemost. 2011;106:675–82. [DOI] [PubMed] [Google Scholar]
- 12. Human Tissue Act 2005. [Accessed 2018 February 19] Available from http://www.legislation.gov.uk/ukpga/2004/30/contents
- 13. Hayward C, Moffat K, Pai M, et al. An evaluation of methods for determining reference intervals for light transmission platelet aggregation tests on samples with normal or reduced platelet counts. Thromb Haemost. 2008;100:134–45. [PubMed] [Google Scholar]
- 14. Horn P, Pesce A. Reference intervals: an update. Clin Chem Acta. 2003;334:5–23. [DOI] [PubMed] [Google Scholar]


