Abstract
Background
The aim of this work was to utilize meta‐analysis in examining the effects of memantine on neuropsychological functioning in patients with Parkinson's disease dementia (PDD) and dementia with Lewy bodies (DLB).
Methods
Included studies fulfilled these criteria: included objective cognitive measures, a comparison group of participants not taking memantine, and provided sufficient data for calculation of effect size. We examined effect sizes across global cognition and five specific neuropsychological domains. Moderator variables examined included neuropsychological domain, diagnostic cohort (PDD, DLB, or mixed PDD‐DLB cohort), study design (open label or placebo‐controlled), and trial length.
Results
Six publications met inclusion criteria totaling 57 effects. Homogeneity analysis across cognitive domains revealed a small overall effect size (d = 0.25) that was significantly heterogeneous (P < 0.001). Specific neuropsychological domains did not moderate effect size, though. Moderator analysis studies using a mix of DLB and PDD subjects showed larger effects than those that included DLB or PDD patients only. Additionally, open‐label trials had significantly (P < 0.001) larger effect sizes (d = 1.32) than placebo‐controlled trials (d = 0.12).
Conclusions
The present study indicates that effect‐size heterogeneity across studies of memantine in DLB and PDD patients is moderated by diagnostic makeup of the cohort and study design. The small overall effect size noted in placebo‐controlled trials versus open‐label trials indicates that memantine has minimal effects on cognition in PDD and DLB and is unlikely to demonstrate clinically significant improvements in cognition.
Keywords: memantine, dementia with Lewy bodies, Parkinson's disease dementia, meta‐analysis, neuropsychology, cognitive
Parkinson's disease (PD) dementia (PDD) and dementia with Lewy bodies (DLB) are common degenerative causes of dementia. Although prevalence and incidence estimates vary because of methodological differences across studies, several systematic reviews of dementia in PD including studies that examined PDD or DLB indicate a point prevalence of 25% to 30% and incidence rates 4 to 6 times that of healthy controls.1, 2 DLB prevalence estimates indicate it is the second‐most common neurodegenerative dementia in the Western world after Alzheimer's disease (AD).3 Importantly, cumulative prevalence estimates indicate that approximately 75% of PD patients who survive more than 10 years will develop dementia, highlighting the need for efficacious treatment for cognitive deficits.2
PDD and DLB are similar in clinical presentation, including parkinsonism, profile of cognitive impairment (i.e., executive dysfunction), and the presence of psychiatric symptoms (e.g., psychosis). However, a PDD diagnosis requires that parkinsonism precedes cognitive impairment for more than 1 year, and cognitive impairment preceding or occurring within 1 year of developing parkinsonism suggests the clinical syndrome of DLB,4, 5 indicating that these diagnoses may represent a spectrum of disease. Cognitive changes in PDD and DLB have been linked to dopaminergic, cholinergic, and, possibly, noradrenergic and glutamatergic activity.6, 7 There is particular support for cholinergic dysfunction.8 Cholinesterase inhibitors have been shown to be beneficial in the treatment of PDD and DLB,9 and one (rivastigmine) is U.S. Food and Drug Administration approved and recommended for use to treat dementia in PD by an International Parkinson and Movement Disorder Society (MDS) task force on the treatment of nonmotor symptoms in PD. However, the MDS task force concluded that there was insufficient evidence for recommendation of other cholinesterase inhibitors, as well as memantine, which is thought to improve memory through other mechanisms.10
With regard to glutamatergic dysfunction, research has demonstrated that glutamate transmission becomes hyperactive after dopamine depletion. Parkinsonian animal models have demonstrated that overactivity of glutamate has a role in the development of parkinsonian syndrome in rats.11 Research has also shown that abnormal alpha‐synuclein affects cortical glutamatergic synapses and is associated with abnormal glutamate binding in patients with DLB versus age‐matched controls at autopsy.12 Glutamate antagonists may alter glutamatergic transmission involving the striatum, subthalamic projections, and thalamocortical pathways.13 Considering the evidence for glutamatergic dysfunction in DLB patients and in parkinsonian animal models, overactivity of glutamate may contribute to development of PDD and DLB. This suggests that memantine, a partial glutamate receptor antagonist, may be beneficial in decreasing glutamate neurotransmission and preventing excitotoxicity.
Results regarding memantine's effects in PDD and DLB across placebo‐controlled trials have varied; however, there were some significant benefits in all studies, and memantine was well tolerated.14, 15, 16, 17 Several meta‐analytic reviews assessing safety and efficacy of memantine in PDD and DLB have recently been published.18, 19 Primary efficacy outcome measures relevant to cognition included the Clinical Global Impression of Change (CGIC) and global cognition as assessed by the Mini–Mental State Examination20 (MMSE). Results of both meta‐analyses revealed that memantine slightly improved overall global impression (i.e., CGIC), but had no significant effects on MMSE scores.
There are several potential explanations for these findings. Although the MMSE has been recommended for use in detecting dementia in PD in the past,21 the MMSE was designed for AD and may not account for the variable neurocognitive phenotypes observed in PDD and DLB. Although other brief cognitive assessments that cover more cognitive domains have been used in clinical trials and cohort studies (i.e., Montreal Cognitive Assessment22; Alzheimer's Disease Assessment Scale–Cognitive Subscale [ADAS‐cog]23; and Dementia Rating Scale‐2 [DRS‐2]24), these are screening tools for cognitive abilities. A comprehensive neuropsychological evaluation is often desired to increase sensitivity and further describe the phenotype of cognitive impairment. It is possible that memantine has effects on differential neuropsychological domains that may not be adequately assessed on global cognitive measures. Characterization of memantine's potential effects on discrete neurocognitive domains, in addition to global cognition would be useful, and build upon recently published meta‐analyses.
Wang et al.19 meta‐analysis examined the effects of memantine in PDD and DLB on global clinical impression, a measure of global cognition, and safety outcomes such as dropouts and adverse events; however, the researchers examined only randomized, placebo‐controlled trials. Matsunaga et al.18 included both open‐label and placebo‐controlled trials, but did not examine the potential moderating effect of trial type. The design of the trial may moderate potential efficacy of memantine and warrants investigation. Also, whereas the sample composition was reported in these studies, potential moderating effects of sample composition were not investigated. Considering the temporal onset differences and neuropathological variability in PDD versus DLB, sample composition may influence effects observed across studies. Finally, whereas the length of the trial was reported in the previous meta‐analyses, potential moderating effects of trial length were not investigated.
In the current study, we conduct a quantitative meta‐analysis of existing studies examining memantine treatment in PDD and DLB. Our analysis included all available studies to evaluate the cognitive effects of memantine in PDD and DLB. A meta‐analytic approach allows for the combination of results across studies and provides a more powerful estimate of true population differences. We examined both global cognition and neuropsychological changes across five commonly accepted domains (reaction time, executive functioning, memory, language, and visuospatial functions) in PDD and DLB compared to control groups (either placebo‐controlled or open‐label trials with a group not taking memantine). Furthermore, we sought to identify the impact of various potential clinical moderator variables, including potential effects of sample composition (i.e., PDD only, DLB only, or mixed cohorts), type of trial (i.e., open‐label vs. placebo‐controlled), and trial length (i.e. number of weeks), on memantine's efficacy.
Materials and Methods
Literature Search Strategy
Articles were identified through a computerized literature search using PubMed, PsychINFO, and MEDLINE Web of Science databases to find relevant studies with the search terms “memantine, dementia with Lewy bodies, Parkinson's disease dementia, neuropsychology, cognitive, attention, executive, memory, and visual perceptual.” The search was limited to English articles published between 1990 and July 2014 that included human subjects. Additionally, a thorough manual review of articles was performed utilizing cross‐references from identified original articles and reviews. Studies eligible for inclusion focused on examining the effects of memantine on cognition in patients with PDD or DLB. This search procedure yielded 11 articles. Studies to be included in the meta‐analysis were reviewed by three of the authors (L.B., A.P., and P.J.M.) and had to meet three criteria: (1) inclusion of objective measures of global cognition or measures assessing specific neuropsychological domains; (2) included a comparison group of participants not taking memantine (placebo‐controlled or open‐label trials); and (3) provided sufficient data or statistical information to allow for the calculation of an effect size.
After review, five articles of the original 11 were excluded, resulting in six publications14, 15, 16, 25, 26, 27 totaling 57 effects for analysis that had reported results of neurocognitive testing. Reasons for exclusion included: (1) lack of cognitive outcome measures (N = 4) and (2) extension of a study already identified and included in analysis (N = 1).
Methodological Variables
We sought to define neurocognitive functioning broadly, looking at effect size across six domains including: (1) global cognition; (2) executive functioning; (3) memory; (4) language; (5) reaction time; and (6) visuospatial abilities. Assignment of neurocognitive tests to selected domains was guided by the classifications made in source articles and consensus of the authors. In the absence of assignment in source articles, tests were assigned to a domain based on discussions between authors (L.B. and P.J.M.), neuropsychologists with expertise in PD. Table 1 presents the measures included in each cognitive domain.
Table 1.
Measures included in each Neuropsychological Domain
| Neuropsychological Domain | Measure | References |
|---|---|---|
| Global Cognition | Mini‐Mental State Exam (MMSE) |
Aarsland et al. (2009)15
Litvinenko et al. (2008)25 Leroi et al. (2009)37 Levin et al. (2009)26 |
| Dementia Rating Scale‐2 (DRS‐2) |
Leroi et al. (2009)37
Levin et al. (2009)26 |
|
| Alzheimer's Disease Assessment Scale – Cognitive Subscale | Litvinenko et al. (2008)25 | |
| Reaction Time/Attention | CDRa Simple Reaction Time | Wesnes et al. (2014)27 |
| CDR Choice Reaction Time | Wesnes et al. (2014)27 | |
| CDR Choice Reaction Time Accuracy | Wesnes et al. (2014)27 | |
| CDR Immediate Word Recognition Reaction Time | Wesnes et al. (2014)27 | |
| CDR Delayed Word Recognition Reaction Time | Wesnes et al. (2014)27 | |
| AQTa Color | Aarsland et al. (2009)15 | |
| AQT Form | Aarsland et al. (2009)15 | |
| AQT Color‐Form | Aarsland et al. (2009)15 | |
| Stroop Interference Test –Congruent | Emre et al. (2010)16 | |
| Executive Functioning | Digit Ordering Test | Emre et al. (2010)16 |
| Alternating Categories Test | Emre et al. (2010)16 | |
| Trail Making Test A | Emre et al. (2010)16 | |
| Trail Making Test B | Emre et al. (2010)16 | |
| Controlled Oral Word Association Test (COWAT) | Emre et al. (2010)16 | |
| CogState Set Shifting Task | Emre et al. (2010)16 | |
| Stroop Interference Test – Incongruent | Emre et al. (2010)16 | |
| Frontal Dysfunction Assessment Battery | Litvinenko et al. (2008)25 | |
| DKEFS Verbal Fluency | Litvinenko et al. (2008)25 | |
| Boston Naming Test | Emre et al. (2010)16 | |
| Language | Category Fluency Test | Emre et al. (2010)16 |
| CDR Immediate Word Recognition | Wesnes et al. (2014)27 | |
| Memory | CDR Delayed Word Recognition | Wesnes et al. (2014)27 |
| Verbal Paired Associates Learning Test | Emre et al. (2010)16 | |
| Alzheimer's Disease Assessment Scale – Cognitive Subscale, Orientation | Emre et al. (2010)16 | |
| Verbal Recall Test Delayed | Emre et al. (2010)16 | |
| Verbal Recall Test Immediate | Emre et al. (2010)16 | |
| Benton Facial Recognition Test | Emre et al. (2010)16 | |
| Visuospatial and Visuoperceptual | Benton Judgment of Line Orientation | Emre et al. (2010)16 |
| Clock Drawing Test |
Litvinenko et al. (2008)25
Levin et al. (2009)26 Emre et al. (2010)16 |
Stroop Tasks utilized here were adapted versions of the traditional Stroop task
CDR, CDR Systems Scale; AQT, A Quick Test of Cognition.
Moderator Variables
In the event of significant effect heterogeneity over the studies examined, moderator analysis of the six specific neurocognitive domains was undertaken. Within the patient population, the following clinical moderator variables were coded: (1) study diagnostic cohort (i.e., PDD only, DLB only, or mixed cohort); (2) study design (i.e., open‐label or placebo‐controlled); and (3) trial length. The included articles were searched for additional demographic characteristics, including ethnic background and education level of participants; however, these data were not sufficiently reported to be included in formal analyses.
Statistical Analyses
Meta‐analyses were conducted with Comprehensive Meta‐analysis Version 2.0 software.28 The mean difference in cognitive test scores from baseline to follow‐up between studies contrasting patients taking memantine versus the comparison group was standardized by calculating Cohen's d. Effect sizes were calculated based on the difference between the two raw means (i.e., baseline mean and follow‐up mean) divided by the pooled standard deviation (SD) and were categorized as small (d ≥ 0.2), medium (d ≥ 0.5), or large (d ≥ 0.8) consistent with Cohen's metric (Cohen, 1988). When means and SDs were not available, d was calculated from reported univariate F tests, t statistics, or P values. The confidence intervals (CIs) and z transformations of the effect size were used to determine whether mean effect sizes were statistically significant. To assess homogeneity of the effect sizes across studies for each neuropsychological domain, the Cochran Q statistic was used (Hedges, 1985). If analysis of the Q statistic revealed significant within‐group heterogeneity, we used a random‐effects model to compute the significance level of the mean effect sizes. In addition to visual/graphic examination of the funnel plot, mathematical methods for the evaluation of possible publication bias included those recommended by Begg and Mazumdar (1994), Egger, Smith, Schneider, and Minder (1997), and Duvall and Tweedy (2000).
In categorical domains with significant heterogeneity, potential effect‐size moderators were assessed with the Q statistic. Analysis was performed on all neuropsychological performance differences for eligible studies. Effects of continuous moderator variables were analyzed with metaregression methods. Further analysis comprised comparison of studies by pertinent study and clinical characteristics.
Results
Overall Meta‐Analysis Results
Analysis of effect sizes across neurocognitive domains for the entire sample revealed a small overall effect size (N = 57 effects; d = 0.25; 95% CI = 0.14 < δ < 0.36) that was significantly heterogeneous (Q B[56] = 187.2; P < 0.001). Given that the variability in effect sizes between subjects taking memantine and the comparison groups differed more than would be expected from sampling error alone, analysis of potential moderator variables was undertaken.
Publication Bias
Analysis for the presence of possible publication bias revealed an asymmetric funnel plot and significant Begg (P < 0.001, one‐tailed) and Egger (P < 0.001, one‐tailed) tests, suggesting a potential “file drawer” or publication bias in this literature. To address the latter possibility, we imputed the potentially missing studies using the Duval and Tweedie (2000) “trim and fill” method. This procedure indicated that no studies were missing from analysis and generated an identical imputed point estimate (d = 0.25; 95% CI = 0.14 < δ < 0.36) to the original Cohen's d of 0.25. In addition, calculation of a fail‐safe N revealed that 790 “null” studies would need to be found and included in the current analysis to negate the presented effect. As such, these methods converge in supporting the notion that the current data accurately represent the extant literature concerning neurocognitive function in PDD and DLB patients taking memantine.
Moderator Analyses
Neuropsychological Domains
Whereas moderator analysis across the six specific domains of neuropsychological function revealed a trend toward greater effect size in studies that utilized a global measure of cognitive function (e.g., MMSE or DRS‐2; Q B[6] = 10.6; P = 0.059), variability in effect sizes across studies were not fully accounted for by the assessment of discrete neuropsychological domains (Fig. 1).
Figure 1.
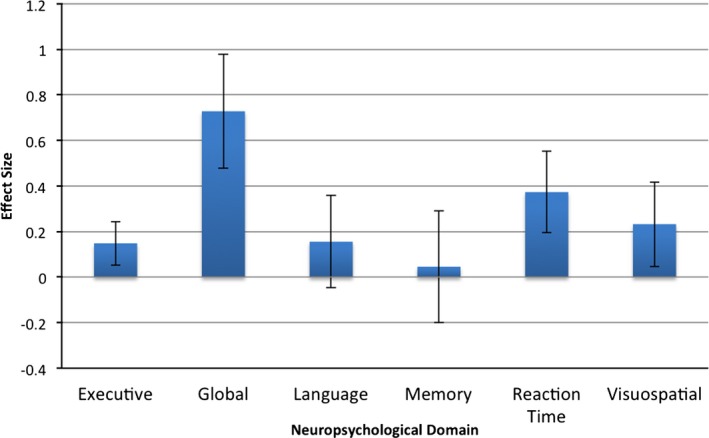
Effect sizes for individual neuropsychological domains across all studies (mean and 95% confidence interval).
Sample Composition
Moderator analysis across the three diagnostic cohorts included in the meta‐analysis (i.e., PDD only [k = 25], DLB only [k = 21], or mixed cohort [k = 11]) revealed significant heterogeneity among effects (Q B[2] = 9.06; P = 0.011). A small‐to‐moderate effect size was noted in the mixed group (d = 0.42; 95% CI = 0.25 < δ < 0.58), which was significantly larger than the effect noted in the DLB only group (d = 0.11; 95% CI = 0.004 < δ < 0.23; Q B[1] = 8.53; P = 0.003), but not the PDD group (d = 0.30; 95% CI = 0.09 < δ < 0.50; Q B[1] = 0.84; P = 0.36). Effects sizes between the PDD and DLB groups did not significantly differ.
Study Design
Moderator analysis examining whether the type of study design (i.e., open‐label [k = 8] vs. placebo‐controlled [k = 49]) had an impact on the observed heterogeneity revealed that open‐label trials had significantly larger effect sizes (d = 1.34; 95% CI = 0.93 < δ < 1.72) compared with placebo‐controlled trials (d = 0.11; 95% CI = 0.04 < δ < 0.18; Q B[1] = 35.18; P < 0.001).
Trial Length
Metaregression revealed no significant relationship between increasing trial length and cognitive outcomes (Z = −1.44; P = 0.15).
Discussion
The current meta‐analysis builds and expands upon recent quantitative reviews of memantine's effects in PDD or DLB,18, 19 in that a broader range of neurocognitive functions was examined and the possible contributions of sample composition, trial type, and trial length on the impact/effect size of the treatment were also explored. Although the meta‐analysis indicated significant heterogeneity across studies, when cognition was viewed broadly, specific neuropsychological domains did not account for variability in effect sizes across studies. There was, however, a trend toward larger effects being observed on tests tapping global cognitive functions.
Although the effects on cognition are small, there are examples in the literature showing positive outcomes from treatment with memantine. For example, memantine may be considered as a reasonable treatment option, particularly considering evidence for its good safety and tolerability profile.19 Additionally, several recent studies have demonstrated memantine's benefits on quality of life29 and rapid eye movement behavior disorder30 in these populations. A recent open‐label extension31 of a placebo‐controlled trial included in the present meta‐analysis15 evaluated the effects of memantine on survival. After 36‐month follow‐up, patients in the memantine group had a longer survival compared to patients in the placebo group. Within the active treatment group, survival analysis 36 months from baseline showed that responders during the controlled phase of the study had higher rates of survival compared with nonresponders. Although longer survival may not always be a useful indicator of therapeutic benefit in a chronic dementing illness, this finding suggests a potential disease‐modifying effect of memantine in PDD and DLB and merits long‐term evaluation in a larger study sample.
The trend for larger effects regarding global cognitive functions may have its basis in the heterogeneity of the illness itself. That is, PD, PDD, and DLB are heterogeneous disorders with regard to individual symptom presentation as well as cognitive dysfunction observed.6, 32 It seems possible that global measures “cast a wider net” and are able to capture this individual variability or heterogeneity better than a focal neuropsychological test. With the press toward a more person‐centered or “precision medicine” approach, measures that are able to detail subtle changes in an individual's cognition may be better able to capture the efficacy of any pharmacological intervention. For example, “intraindividual variability” or IIV has been shown to confer unique predictive information about cognitive functioning beyond mean performance33 and is specific to an individual. Preliminary studies have suggested that elevated IIV is a characteristic of PD34, 35 and may be predictive of the development of dementia. Considering evidence that memantine leads to improvements in quality of life as well as evidence for a strong association between quality of life and cognition in demented and nondemented PD patients,36, 37, 38 it is reasonable to hypothesize that improvements in quality of life are likely to be accompanied by improvements in cognition. Future studies of cognitive function in PD that utilize this type of person‐centered methodology may help to resolve the conflicting findings between improved quality of life after memantine treatment and the lack of observable cognitive improvement.
Moderator analysis revealed significant moderating effects of sample composition and trial type. In previous meta‐analyses, Wang et al.19 detected substantial heterogeneity in observed effects when PDD and DLB were evaluated together, but potential moderating effects of sample composition were not examined. In the current study, effect sizes for mixed cohorts were significantly larger than DLB‐only cohorts; however, effect sizes for mixed cohorts were not significantly larger than PDD‐only cohorts. There were no significant differences in effect sizes for PDD‐only versus DLB‐only cohorts. These findings suggest that using mixed cohorts may influence effect sizes and efficacy. Our results indicate that in the context of the significant moderating effect of sample composition, future trials of memantine may benefit from inclusion of only PDD or DLB subjects. If both PDD and DLB subjects are included in future trials, independent analyses are suggested for PDD and DLB subjects.
With regard to trial type, Matsunaga et al.18 meta‐analysis included both open‐label and placebo‐controlled trials, but did not examine the potential moderating effect of trial type. Placebo‐controlled trials have typically been the gold standard of pharmacological research; however, this rigorous research design often excludes subjects with substantial medical comorbidity and does not allow concomitant medication treatments.39 Because medical comorbidity and collateral medication use are common in clinical practice, placebo‐controlled studies have been critiqued as lacking external validity. Along this same line, open‐label trials in PD have been shown to be influenced by physician biases and expectation of benefit owing to knowledge of receiving active medication.40 In reference to the latter controversies, the finding of larger effect sizes in open‐label studies likely reflect the contributions of both drug and placebo effects and reinforce the contention that randomized, placebo‐controlled trials are recommended when possible.
Limitations of the current meta‐analysis include the number of eligible studies for inclusion, the variety of neuropsychological measures used across studies, and inability to examine level of education as a potential moderator variable owing to inconsistent reporting of education level across studies. Education level should be recorded in future trials to allow for this analysis. Overall, memantine may be a feasible treatment option, considering the beneficial effects observed in trials examining safety profile, quality of life, and sleep disturbances. Although the effectiveness of memantine on improvement in cognition is quite small, larger prospective, multicenter studies may further assess potential benefits of memantine on cognition in PDD and DLB utilizing person‐centered approaches to neuropsychological assessment and data analysis. If a comprehensive neuropsychological battery is used in a trial, the present results suggest that assessment of global cognition, rather than discrete neuropsychological domains, may better capture effects of memantine on cognition because of the heterogeneity of cognitive deficits across PDD and DLB patients.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
L.B.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
A.P.: 1A, 1B, 1C, 2B, 2C, 3B
J.E.D.: 1A, 1B, 2C, 3B
J.F.M.: 2C, 3B
D.W.: 2C, 3B
J.R.W.: 2C, 3B
P.J.M.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
Disclosures
Funding Sources and Conflicts of Interest: The authors report no sources of funding and no conflicts of interest.
Financial Disclosures for previous 12 months: J.E.D. receives research support from the Department of Veterans Affairs, the National Institutes of Health (NIH), and the Michael J. Fox Foundation for Parkinson's Research (MJFF). J.F.M receives research support from the Department of Veterans Affairs and GE Healthcare. D.W. has received research funding from MJFF, NIH, Novartis Pharmaceuticals, Department of Veterans Affairs, and Alzheimer's Disease Cooperative Study; honoraria from Biotie, Teva Pharmaceuticals, Lundbeck Inc., Pfizer, Avanir Pharmaceuticals, Acadia Pharmaceuticals, Merck & Co., UCB, Bristol‐Myers Squibb Company, Novartis Pharmaceuticals, Clintrex LLC, Theravance, Medivation, CHDI Foundation, and the Weston Foundation; license fee payments from the University of Pennsylvania for the QUIP and QUIP‐RS; royalties from Wolters Kluwel; and fees for testifying in two court cases related to impulse controls disorders in Parkinson's disease (March, 2013–April, 2014, payments by Eversheds and Roach, Brown, McCarthy & Gruber, P.C.).
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Philadelphia VA Medical Center. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the U.S. government.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Aarsland D, Zaccai J, Brayne C. A systematic review of prevalence studies of dementia in Parkinson's disease. Mov Disord 2005;20:1255–1263. [DOI] [PubMed] [Google Scholar]
- 2. Aarsland D, Kurz MW. The epidemiology of dementia associated with Parkinson disease. J Neurol Sci 2010;289:18–22. [DOI] [PubMed] [Google Scholar]
- 3. Aarsland D, Rongve A, Piepenstock N, et al. Frequency and case identification of dementia with lewy bodies using the revised consensus criteria. Dement Geriatr Cogn Disord 2008;26:445–452. [DOI] [PubMed] [Google Scholar]
- 4. Mckeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB consortium. Neurology 2005;65:1863–1872. [DOI] [PubMed] [Google Scholar]
- 5. Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord 2007;22:1689–1707. [DOI] [PubMed] [Google Scholar]
- 6. Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson's disease. Lancet Neurol 2010;9:1200–1213. [DOI] [PubMed] [Google Scholar]
- 7. Klein JC, Eggers C, Kalbe E, et al. Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology 2010;74:885–892. [DOI] [PubMed] [Google Scholar]
- 8. Tiraboschi P, Hansen LA, Alford M, et al. Cholinergic dysfunction in diseases with Lewy bodies. Neurology 2000;54:407–411. [DOI] [PubMed] [Google Scholar]
- 9. Rolinski M, Fox C, Maidment I, Mcshane R. Cholinesterase inhibitors for dementia with Lewy bodies, Parkinson's disease dementia and cognitive impairment in Parkinson's disease (Review). Cochrane Database Syst Rev 2012;3:CD006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seppi K, Weintraub D, Coelho M, et al. The Movement Disorder Society evidenced‐based medicine review update: treatments for non‐motor symptoms of Parkinson's disease. Mov Disord 2011;26(Suppl 3):S42–S80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kucheryanu VG, Kryzhanovskii GN. Effect of glutamate and antagonists of N‐methyl‐D‐Aspartate receptors on experimental parkinsonian syndrome in rats. Bull Exp Biol Med 2000;130:629–632. [DOI] [PubMed] [Google Scholar]
- 12. Dalfó E, Albasanz JL, Martín M, Ferrer I. Abnormal metabotropic glutamate receptor expression and signaling in the cerebral cortex in diffuse Lewy Body disease is associated with irregular α‐synuclein/phospholipase C (PLCβ1) interactions. Brain Pathol 2004;14:388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Starr MS. Glutamate/dopamine D1/D2 balance in the basal ganglia and its relevance to Parkinson's disease. Synapse 1995;19:264–293. [DOI] [PubMed] [Google Scholar]
- 14. Leroi I, Overshott R, Byrne EJ, Daniel E, Burns A. Randomized controlled trial of memantine in dementia associated with Parkinson's disease. Mov Disord 2009;24:1217–1221. [DOI] [PubMed] [Google Scholar]
- 15. Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson's disease dementia or dementia with Lewy bodies: a double‐blind, placebo‐controlled, multicentre trial. Lancet Neurol 2009;8:613–618. [DOI] [PubMed] [Google Scholar]
- 16. Emre M, Tsolaki M, Bonuccelli U, et al. Memantine for patients with Parkinson's disease dementia or dementia with Lewy bodies: a randomised, double‐blind, placebo‐controlled trial. Lancet Neurol 2010;9:969–977. [DOI] [PubMed] [Google Scholar]
- 17. Ondo WG, Shinawi L, Davidson A, Lai D. Memantine for non‐motor features of Parkinson's disease: a double‐blind placebo controlled exploratory pilot trial. Parkinsonism Relat Disord 2011;17:156–159. [DOI] [PubMed] [Google Scholar]
- 18. Matsunaga S, Kishi T, Iwata N. Memantine for Lewy body disorders: systematic review and meta‐analysis. Am J Geriatr Psychiatry 2015;23:373–383. [DOI] [PubMed] [Google Scholar]
- 19. Wang HF, Yu JT, Tang SW, et al. Efficacy and safety of cholinesterase inhibitors and memantine in cognitive impairment in Parkinson's disease, Parkinson's disease dementia, and dementia with Lewy bodies: systematic review with meta‐analysis and trial sequential analysis. J Neurol Neurosurg Psychiatry 2015;86:135–143. [DOI] [PubMed] [Google Scholar]
- 20. Folstein M, Folstein S, McHugh P. Mini‐Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 21. Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson's disease dementia: recommendations from the Movement Disorder Society Task Force. Mov Disord 2007;22:2314–2324. [DOI] [PubMed] [Google Scholar]
- 22. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–696. [DOI] [PubMed] [Google Scholar]
- 23. Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry 1984;141:1356–1364. [DOI] [PubMed] [Google Scholar]
- 24. Mattis S. Dementia Rating Scale. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- 25. Litvinenko I, Odinak M, Mogil'naya V, Perstnev S. Use of memantine (akatinol) for the correction of cognitive impairments in Parkinson's disease complicated by dementia. Neurosci Behav Physiol 2010;40:37–42. [DOI] [PubMed] [Google Scholar]
- 26. Levin OS, Batukaeva LA, Smolentseva IG, Amosova NA. Efficacy and safety of memantine in Lewy body dementia. Neurosci Behav Physiol 2009;39:597–604. [DOI] [PubMed] [Google Scholar]
- 27. Wesnes KA, Aarsland D, Ballard C, Londos E. Memantine improves attention and episodic memory in Parkinson's disease dementia and dementia with Lewy bodies. Int J Geriatr Psychiatry 2015;30:46–54. [DOI] [PubMed] [Google Scholar]
- 28. Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta Analysis, Version 2. Englewood, NJ: Biostat; 2005. [Google Scholar]
- 29. Larsson V, Engedal K, Aarsland D, Wattmo C, Minthon L, Londos E. Quality of life and the effect of memantine in dementia with lewy bodies and Parkinson's disease dementia. Dement Geriatr Cogn Disord 2011;32:227–234. [DOI] [PubMed] [Google Scholar]
- 30. Larsson V, Aarsland D, Ballard C, Minthon L, Londos E. The effect of memantine on sleep behaviour in dementia with Lewy bodies and Parkinson's disease dementia. Int J Geriatr Psychiatry 2010;25:1030–1038. [DOI] [PubMed] [Google Scholar]
- 31. Stubendorff K, Larsson V, Ballard C, Minthon L, Aarsland D, Londos E. Treatment effect of memantine on survival in dementia with Lewy bodies and Parkinson's disease with dementia: a prospective study. BMJ Open 2014;4:e005158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dujardin K, Leentjens AF, Langlois C, et al. The spectrum of cognitive disorders in Parkinson's disease: a data‐driven approach. Mov Disord 2013;28:183–189. [DOI] [PubMed] [Google Scholar]
- 33. MacDonald SW, Li SC, Bäckman L. Neural underpinnings of within‐person variability in cognitive functioning. Psychol Aging 2009;24:792–808. [DOI] [PubMed] [Google Scholar]
- 34. De Frias CM, Dixon RA, Camicioli R. Neurocognitive speed and inconsistency in Parkinson's disease with and without incipient dementia: an 18‐month prospective cohort study. J Int Neuropsychol Soc 2012;18:764–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. De Frias CM, Dixon RA, Fisher N, Camicioli R. Intraindividual variability in neurocognitive speed: a comparison of Parkinson's disease and normal older adults. Neuropsychologia 2007;45:2499–2507. [DOI] [PubMed] [Google Scholar]
- 36. Klepac N, Trkulja V, Relja M, Babić T. Is quality of life in non‐demented Parkinson's disease patients related to cognitive performance? A clinic‐based cross‐sectional study. Eur J Neurol 2008;15:128–133. [DOI] [PubMed] [Google Scholar]
- 37. Leroi I, McDonald K, Pantula H, Harbishettar V. Cognitive impairment in Parkinson disease: impact on quality of life, disability, and caregiver burden. J Geriatr Psychiatry Neurol 2012;25:208–214. [DOI] [PubMed] [Google Scholar]
- 38. Duncan GW, Khoo TK, Yarnall AJ, et al. Health‐related quality of life in early Parkinson's disease: the impact of nonmotor symptoms. Mov Disord 2014;29:195–202. [DOI] [PubMed] [Google Scholar]
- 39. Nierenberg AA, Husain MM, Trivedi MH, et al. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: a STAR*D report. Psychol Med 2010;40:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Freed C, Greene P, Breeze R, et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med 2001;344:710–719. [DOI] [PubMed] [Google Scholar]


