Abstract
Background
Temporarily improved platelet reactivity may reduce the bleeding in patients on antiplatelet therapy who have ongoing bleeding or who are in need of acute surgery. Adrenaline can bind to adrenergic α2A‐receptors on platelets and potentially enhance platelet reactivity.
Objective
To assess if adrenaline can improve adenosine diphosphate (ADP)‐induced platelet aggregation and activation in blood samples from patients on dual antiplatelet therapy with acetylsalicylic acid (ASA) and the ADP‐receptor antagonist ticagrelor.
Methods
Blood samples were collected from a total of forty acute coronary syndrome patients on dual antiplatelet therapy with ASA and ticagrelor. ADP‐induced platelet aggregation (by impedance aggregometry) and activation (by flow cytometry) were assessed before and after supplementation with adrenaline and/or platelet concentrate.
Results
Adrenaline supplementation (770 nmol L−1) increased median ADP‐induced aggregation from 15 (25‐75th percentiles: 10‐20) to 26 (18‐38) aggregation units. The effect was independent of concomitant platelet supplementation. Adrenaline also increased ADP‐induced platelet activation: from 40% (36‐54%) to 83% (74‐88%) platelets with active fibrinogen receptor (binding PAC‐1) and from 13% (7‐21%) to 35% (18‐50%) P‐selectin‐expressing platelets.
Conclusions
Adrenaline potentiated ADP‐induced platelet aggregation and activation in blood samples from ticagrelor‐treated patients. Adrenaline infusion may be a new method to enhance platelet function in ticagrelor‐treated patients who are in need of acute surgery or have ongoing bleeding. In vivo studies are needed to confirm the present results.
Keywords: adrenaline, flow cytometry, platelet aggregation, platelet aggregation inhibitors, platelet function tests
Essentials.
Interventions to temporarily improve platelet function may reduce bleeding in ticagrelor‐treated patients.
Adrenaline was added to blood from patients on ticagrelor and platelet function was assessed.
Adrenaline supplementation potentiated ADP‐induced platelet aggregation and activation.
Adrenaline infusion may be used to enhance platelet function in patients on ticagrelor.
1. Introduction
Dual antiplatelet therapy (DAPT) with acetylsalicylic acid (ASA) and an adenosine diphosphate (ADP) receptor (P2Y12) antagonist such as clopidogrel, ticagrelor, or prasugrel reduces the risk of thrombotic events compared to ASA only in patients with acute coronary syndrome (ACS), but it is also associated with an increased risk of spontaneous and perioperative bleeding complications.1, 2, 3 Both the efficacy and the bleeding risk are higher when ticagrelor, a P2Y12‐receptor antagonist of the third generation, is used instead of clopidogrel.3 If bleeding occurs, or if acute surgery is unavoidable, the effective platelet inhibition in patients with ongoing ticagrelor treatment becomes a significant disadvantage. Accordingly, a high incidence of severe perioperative bleeding complications has been reported in ACS patients with ongoing or recently discontinued DAPT with ASA and ticagrelor.4, 5 The perioperative bleeding risk in cardiac surgery patients, with ongoing or recently discontinued treatment with a P2Y12‐receptor antagonist, correlates to the residual ADP‐dependent platelet aggregability.5, 6, 7 In two recent studies, even small differences in residual platelet aggregability were found to have an effect on the bleeding risk.5, 6
In patients on ticagrelor, platelet transfusion has little or no effect,8, 9, 10 and there is no currently available antidote to ticagrelor. New alternatives are therefore needed to reduce the risk of excessive bleeding in ticagrelor‐treated patients who have ongoing bleeding or who are in need of emergent surgery. One potential method would be to temporarily enhance platelet activation and aggregation during surgery or until the bleeding has stopped. Supplementation with adrenaline, which is commonly used in cardiac surgery during and after cardiopulmonary bypass to maintain blood pressure, has previously been shown to potentiate platelet aggregation induced by ADP in healthy subjects11, 12 and in clopidogrel‐treated patients.13 In the present study, we hypothesized that adrenaline would potentiate ADP‐induced platelet aggregation and activation in patients with ongoing ASA and ticagrelor treatment, since the platelet membrane express adrenergic α2A‐receptors.11, 14, 15, 16 To test this hypothesis, we performed an in vitro study using blood samples from ACS patients on ongoing DAPT with ASA and ticagrelor. In addition, we also tested the effect of concomitant supplementation with platelet concentrate and adrenaline.
2. Materials and methods
2.1. Ethics approval
The study was approved by the regional research ethics committee and was performed in accordance with the Declaration of Helsinki. All the patients gave written informed consent.
2.2. Patients
Forty in‐hospital acute coronary syndrome patients (mean age 64 [46‐84] years, 83% male) with ongoing ASA and ticagrelor treatment were included in the study. All patients had received loading doses (ASA loading dose: 300 mg, ticagrelor loading dose: 180 mg) and at least one additional dose of ASA (75 mg) and ticagrelor (90 mg). Blood samples were collected 2‐8 hours after the latest dose of platelet inhibitors. Patient characteristics are given in Table 1.
Table 1.
Patient characteristics. Values are presented as mean value (standard error of the mean) or number (frequency)
| Aggregation: Adrenaline | Aggregation: Adrenaline ± platelets | Aggregation: Adrenaline ± ADP | Activation: Adrenaline ± ADP | Aggregation: Pooled data | |
|---|---|---|---|---|---|
| N | 10 | 10 | 10 | 10 | 30 |
| Age (years) |
58 (1.9), range 50‐66 |
66 (3.3), range 51‐82 |
65 (3.7), range 46‐80 |
65 (5), range 38‐84 |
63 (1.9), range 46‐82 |
| Gender (male) | 10 (100%) | 7 (70%) | 8 (80%) | 8 (80%) | 25 (83.3%) |
| Platelet count (×109 L−1) | 227 (19.7) | 233 (19.4) | 280 (39.3) | 211 (34.3) | 247 (16.4) |
| Hemoglobin (g L−1) | 145 (3.9) | 142 (3.9) | 136 (7.6) | 145 (5.7) | 141 (3.1) |
| Serum creatinine (μmol L−1) | 93.8 (2.7) | 80.9 (6.2) | 98.4 (13.2) | 91.1 (5.9) | 91.4 (5.0) |
| BMI (kg m−2) | 27.6 (1.4) | 26.6 (1.4) | 27.4 (0.9) | 27.1 (1.1) | 27.2 (0.8) |
| Diabetes mellitus | 0 | 0 | 2 (20%) | 2 (20%) | 2 (6.7%) |
| Type of ACS | |||||
| STEMI | 7 (70%) | 1 (10%) | 5 (50%) | 5 (50%) | 13 (43.3%) |
| NSTEMI | 2 (20%) | 6 (60%) | 4 (40%) | 5 (50%) | 12 (40%) |
| Unstable angina | 1 (10%) | 3 (30%) | 1 (10%) | 0 | 5 (16.7%) |
| Medicationa | |||||
| Fondaparinux | 1 (10%) | 3 (30%) | 0 | 2 (20%) | 4 (13.3%) |
| Heparin | 0 | 2 (20%) | 0 | 0 | 2 (6.7%) |
| Simvastatin | 0 | 0 | 1 (10%) | 1 (10%) | 1 (3.3%) |
| Atorvastatin | 9 (90%) | 10 (100%) | 8 (80%) | 8 (80%) | 27 (90%) |
ACS, acute coronary syndrome; BMI, body mass index; STEMI, ST‐segment elevation myocardial infarction; NSTEMI, non‐ST‐segment elevation myocardial infarction.
Within 24 hours of sampling.
2.3. Study design
The blood samples were supplemented with adrenaline, platelet concentrates, and/or ADP. Impedance aggregometry was used to assess platelet aggregation and flow cytometry was used to evaluate platelet activation before and after supplementations. Firstly, the effect of five different added concentrations of adrenaline on platelet aggregation was assessed (n = 10). Next, platelet aggregation was measured after addition of adrenaline alone or in combination with platelet concentrate (n = 10). To evaluate whether adrenaline induces aggregation/activation by itself or whether it potentiates ADP‐induced aggregation/activation, it was added alone or in combination with ADP and platelet aggregation (n = 10), and activation (n = 10) was assessed. The order in which the different substances/concentrations were tested was alternated to avoid any systematic influence of time.
2.4. Adrenaline
The plasma concentrations of adrenaline that were tested were 6, 27, 82, 153, and 770 nmol L−1. Adrenaline was added to 1.5 mL blood, which corresponds approximately to 0.825 mL plasma (55%). Adrenaline (Mylan, Canonsburg, PA, USA) with a stock concentration of 5.5 mmol L−1 was used. The stock solution was diluted using saline (NaCl, 9 mg mL−1) (Fresenius Kabi, Bad Homburg, Germany) and added at volumes ranging between 12 and 118 μL to achieve the calculated concentrations in plasma.
2.5. Platelet concentrates
Apheresis platelet concentrates of blood group O stored for a median of 1 day (range: 1‐3) were used. The platelet concentrates were produced by the blood bank at Sahlgrenska University Hospital using an apheresis device (Trima Accel; Terumo BCT Europe, Zaventem, Belgium) in accordance with European guidelines.17 Autologous plasma was used as storage medium. The mean platelet count in the platelet concentrates was 1747 ± 41 × 109 L−1. In vitro addition of 120 × 106 platelets to a 1‐mL blood sample was tested. This corresponds to an increase in platelet count achieved by transfusion of approximately three apheresis units to a 70‐kg patient. By using the platelet concentration in the individual concentrate, the specific volume to add from the concentrate was calculated. The mean volume of platelet concentrate added was 69 ± 1.6 μL.
2.6. Analyses
2.6.1. Impedance aggregometry
Blood samples were collected in hirudin tubes (0.15 mg L−1; Roche Diagnostics GmbH, Mannheim, Germany). Whole blood impedance aggregometry (Multiplate; Roche Diagnostics, Basel, Switzerland) was used to study platelet aggregation as previously described.18 The tests used were the ADP HS test (ADP 6.3 μmol L−1 and prostaglandin E1 9.4 nmol L−1) which assesses the P2Y12 receptor‐dependent aggregation, and the ASPI test (arachidonic acid AA, 0.5 mmol L−1), which assesses cyclooxygenase‐dependent aggregation (i.e, is ASA‐sensitive). When the effect on aggregation of adrenaline alone was studied, no other agonist was added.
2.6.2. Flow cytometry
Flow cytometry was used to assess platelet activation by measuring the binding of PAC‐1 (which binds to the activated fibrinogen receptor glycoprotein IIb/IIIa)19 and expression of the platelet activation marker P‐selectin (CD62p). Blood samples were collected in hirudin tubes (0.15 mg L−1, Roche Diagnostics GmbH). The first blood sample tube (containing at least 2 mL blood) was discarded to avoid preactivation. Duplicate samples were prepared containing either no agonists, adrenaline (770 nmol L−1), ADP (6.5 μmol L−1), or a combination of adrenaline (770 nmol L−1) and ADP. NaCl (9 mg L−1; Fresenius Kabi) was added to samples with less than two agonists, to keep the volume constant. Antibodies for platelet activation markers were then added: anti‐GPIb‐PE (final concentration: 2.5 μg mL−1) (CD42, platelet surface marker; Dako, Agilent Technologies, Santa Clara, CA, USA), PAC‐1‐FITC (final concentration: 0.56 μg mL−1) (BD Biosciences, San José, CA, USA) and anti‐P‐selectin‐APC (final concentration: 0.17 μg mL−1) (BD Biosciences). For dilution and to prevent unspecific binding, HEPES ‐buffer (137 mmol L−1 NaCl, 2.7 mmol L−1 KCl, 1 mmol L−1 MgCl2, 5.6 mmol L−1 glucose, 20 mmol L−1 Hepes, and 0.1% BSA, pH 7.40) was added to the tubes.19 Isotype controls were prepared containing IgG‐APC (final concentration: 0.17 μg mL−1) (BD Biosciences) instead of anti‐P‐selectin‐APC to determine the boundary between positively and negatively stained platelets. One isotype control was diluted in HEPES ‐buffer for the setting of the anti‐P‐selectin‐APC gate, while the other isotype control was diluted in HEPES ‐buffer with 10 mmol L−1 ethylenediaminetetraacetic acid (EDTA) for the setting of the PAC‐1‐FITC gate. The gates were placed to obtain approximately 1.5% positive platelets in the isotype sample.20 Then, 3 μL of blood was added to each tube at 20‐seconds intervals, resulting in a final volume of 36 μL. After 10 minutes of incubation, 700 μL of HEPES ‐buffer was added at 20‐seconds intervals to stop the reaction. Flow cytometry (FACSCalibur flow cytometer; BD Biosciences) was performed within an hour and 10 000 GPIb‐positive platelet events were acquired and analyzed using CellQuest Pro software (BD Biosciences). The order in which the tubes were analyzed was alternated for each patient to avoid any systematic influence of time.
2.7. Statistics
Data are presented in boxplots with median and 25th‐75th percentiles. Platelet aggregation, before and after addition of adrenaline and/or platelet concentrate and/or ADP, was compared using the non‐parametric Friedman test for repeated measures. Expression of platelet activation markers before and after addition of adrenaline and/or ADP was also compared using the Friedman test. Any P value of <0.05 was considered statistically significant. To determine which pairs of samples were significantly different, the Wilcoxon signed‐rank test (matched pairs) was used for post hoc analysis with Bonferroni‐Holm correction to control for the family‐wise error rate.21 All statistical analyses were performed with SPSS version 22 (IBM Corporation, Armonk, NY, USA).
3. Results
3.1. Effects of adrenaline on platelet aggregation
Addition of adrenaline at plasma concentrations of 153 and 770 nmol L−1 significantly improved ADP‐induced aggregation compared to samples without adrenaline; from 12.5 (9‐19) U to 16 (13‐25) U (P = 0.009) at 153 nmol L−1 and to 20 (16‐28) U at 770 nmol L−1 (P = 0.007) (Figure 1). Addition of adrenaline at lower concentrations (6, 27, and 82 nmol L−1) did not significantly improve ADP‐induced aggregation; 12.5 (10‐16) U (P = 0.68) at 6 nmol L−1, 14.5 (9‐19) U (P = 0.2) at 27 nmol L−1, and 16.5 (12‐20) U at 82 nmol L−1 (P = 0.018, not significant with Bonferroni‐Holm correction).
Figure 1.
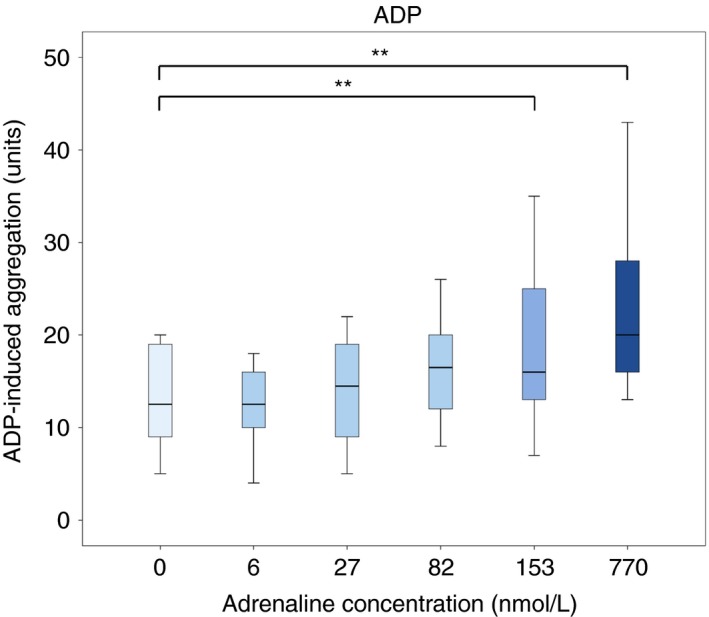
Effect of adrenaline on adenosine diphosphate (ADP)‐induced platelet aggregation in blood samples from patients on ongoing treatment with acetylsalicylic acid and ticagrelor (n = 10). The concentrations are resulting plasma concentration of adrenaline in the blood samples. Boxes indicate 25th and 75th percentiles while the median is indicated by the line across the inside of the box. Whiskers denote the minimum and maximum values. **P < 0.01
Addition of adrenaline at plasma concentrations of 153 and 770 nmol L−1 significantly improved AA‐induced aggregation compared to samples without adrenaline supplementation; from 14 (5‐16) U to 17.5 (10‐19) U (P = 0.012) at 153 nmol L−1 and to 20 (15‐23) U at 770 nmol L−1 (P = 0.007) (Figure S1). Addition of adrenaline at lower concentrations did not significantly improve AA‐induced aggregation; 15.5 (7‐16) U (P = 0.78) at 6 nmol L−1, 16 (6‐17) U (P = 0.072) at 27 nmol L−1, and 12 (6‐19) U at 82 nmol L−1 (P = 0.31).
Using pooled data from all aggregation experiments (n = 30 for ADP and n = 20 for AA), adrenaline supplementation increased ADP‐induced aggregation by 80% (51‐165%) with the highest adrenaline concentration; from median 15 (10‐20) U to 22.5 (14‐26) U (P < 0.001) at 153 nmol L−1 and to 26 (18‐38) U at 770 nmol L−1 (P < 0.001 vs either baseline or 153 nmol L−1). AA‐induced aggregation increased by 60% (180‐133%) with the highest adrenaline concentration, from median 14 (9.5‐16) U to 17.5 (12.5‐21) U (P = 0.001) at 153 nmol L−1 and to 21.5 (15.5‐23) U at 770 nmol L−1 (P < 0.001 vs either baseline or 153 nmol L−1)
3.2. Effects of adrenaline ± platelets on platelet aggregation
Addition of platelet concentrate alone did not significantly improve ADP‐induced aggregation; 19 (13‐22) U at baseline compared to 17 (14‐23) U (P = 0.57) (Figure 2). Addition of adrenaline (at 770 nmol L−1) increased ADP‐induced aggregation compared to platelet concentrate alone; from 17 (14‐23) U to 29 (22‐38) U (P = 0.005). The combination of adrenaline (770 nmol L−1) and platelet concentrate increased ADP‐induced aggregation more than platelet concentrate alone, from 17 (14‐23) U to 24 (21‐30) U (P = 0.005), but not significantly more than adrenaline alone (P = 0.074).
Figure 2.
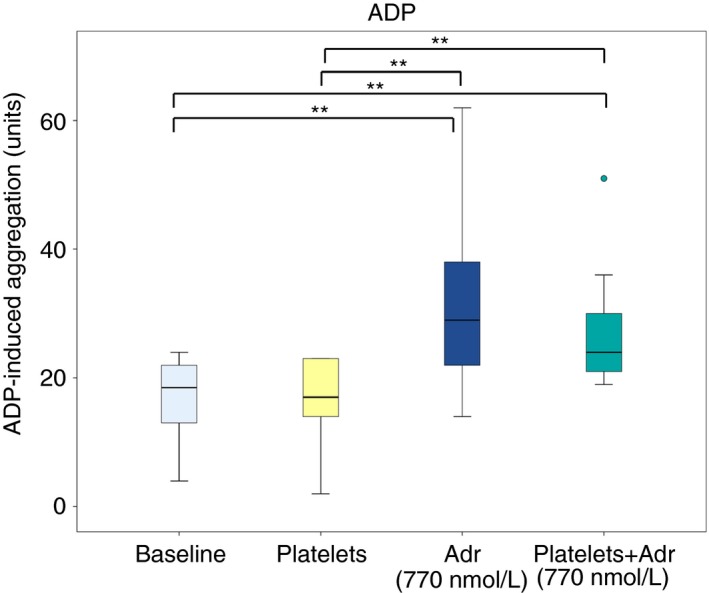
Effect of addition of adrenaline (Adr) alone or in combination with platelet concentrate on adenosine diphosphate (ADP)‐induced platelet aggregation in blood samples from patients on ongoing treatment with acetylsalicylic acid and ticagrelor (n = 10). Boxes indicate 25th and 75th percentiles while the median is indicated by the line across the inside of the box. Whiskers denote the minimum and maximum values excluding outliers (values more than 1.5 times the interquartile range) and extreme points (values more than 3 times the interquartile range). Outliers are presented as dots. **P < 0.01
Addition of platelet concentrate alone significantly increased AA‐induced aggregation compared to baseline; from 14 (12‐16) U to 71 (51‐85) U (P = 0.007) (Figure S2). Addition of adrenaline resulted in a lower AA‐induced aggregation compared to platelet concentrate alone; 22 (19‐23) U (P = 0.007). The combination of adrenaline (770 nmol L−1) and platelet concentrate increased AA‐induced aggregation more than platelet concentrate alone; from 71 (51‐85) U to 96 (85‐108) U (P = 0.007).
3.3. Effects of adrenaline ± ADP on platelet aggregation and platelet activation
Adrenaline alone (at 770 nmol L−1) increased platelet aggregation compared to baseline (no agonist); from 5 (1‐14) to 11 (4‐17) U (P = 0.011) (Figure 3). Addition of ADP alone increased aggregation more than adrenaline alone, to 17 (12‐21) U (P = 0.005 vs baseline and P = 0.013 vs adrenaline only). Addition of adrenaline (770 nmol L−1) together with ADP significantly increased aggregation compared to both adrenaline alone and ADP alone; from 11 (4‐17) U for adrenaline alone and 17 (12‐21) U for ADP alone to 29 (17‐42) U (P = 0.005 vs either adrenaline alone or ADP alone).
Figure 3.
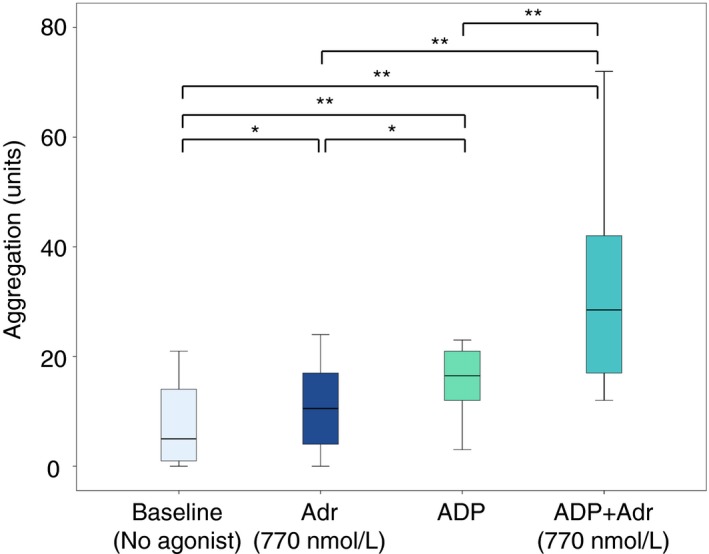
Effect of addition of adrenaline (Adr) alone, ADP HS test alone and combination of adrenaline with ADP HS test on platelet aggregation in blood samples from patients on ongoing treatment with acetylsalicylic acid and ticagrelor (n = 10). Boxes indicate 25th and 75th percentiles while the median is indicated by the line across the inside of the box. Whiskers denote the minimum and maximum values. *P < 0.05, **P < 0.01
Addition of adrenaline alone (at 770 nmol L−1) increased expression of active fibrinogen receptor (PAC‐1 binding); from 2% (2‐3%) when no agonist was added to 22% (11‐34%) (P = 0.005) (Figure 4). Addition of ADP alone increased PAC‐1 binding more than with adrenaline alone, to 40% (36‐54%) (P = 0.005 vs either no agonist or adrenaline alone). The combination of ADP and adrenaline (770 nmol L−1) resulted in the highest PAC‐1 binding; 83% (74‐88%) (P = 0.005 vs all other).
Figure 4.
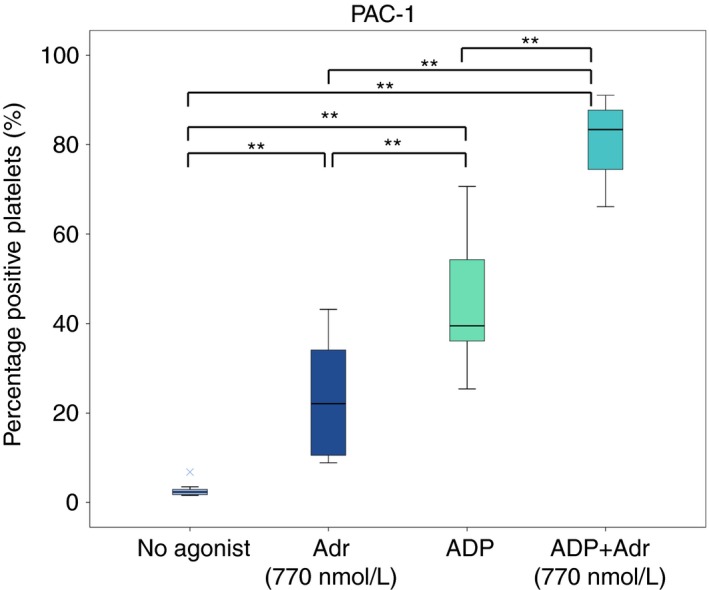
Effect of addition of adrenaline (Adr) alone, ADP alone and the combination of ADP and adrenaline on PAC‐1 binding (binds to activated fibrinogen receptor) in blood samples from patients on ongoing treatment with acetylsalicylic acid and ticagrelor (n = 10). Boxes indicate 25th and 75th percentiles while the median is indicated by the line across the inside of the box. Whiskers denote the minimum and maximum values excluding outliers (values more than 1.5 times the interquartile range) and extreme points (values more than three times the interquartile range). Extreme points are indicated by the symbol ×. **P < 0.01
Addition of adrenaline alone (at 770 nmol L−1) increased P‐selectin expression compared to when no agonist was added; from 4% (3‐6%) to 6% (4‐11%) (P = 0.013) (Figure 5). Addition of ADP alone increased P‐selectin expression more than with adrenaline alone, to 13% (7‐21%) (P = 0.005 vs either no agonist or adrenaline alone). The combination of ADP and adrenaline (770 nmol L−1) resulted in the highest expression of P‐selectin; 35% (18‐50%) (P = 0.005 vs all other).
Figure 5.
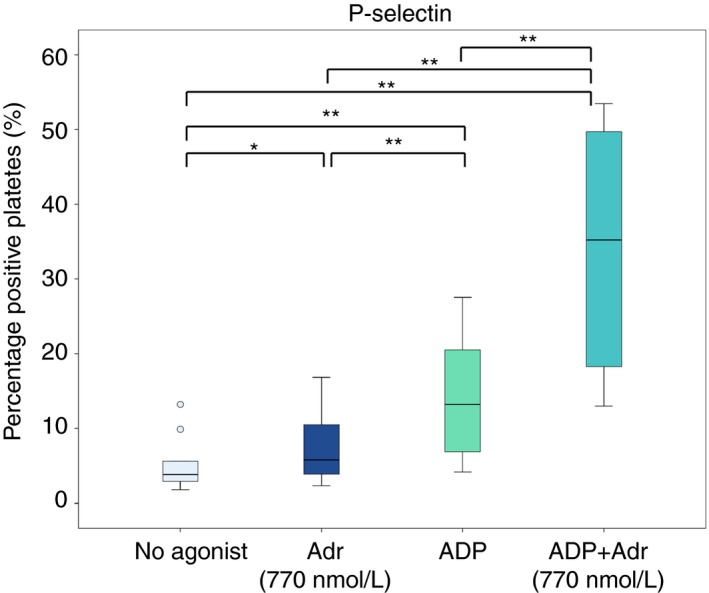
Effect of addition of adrenaline (Adr) alone, ADP alone and the combination of ADP and adrenaline on expression of platelet activation marker P‐selectin in blood samples from patients on ongoing treatment with acetylsalicylic acid and ticagrelor (n = 10). Boxes indicate 25th and 75th percentiles while the median is indicated by the line across the inside of the box. Whiskers denote the minimum and maximum values excluding outliers (values more than 1.5 times the interquartile range) and extreme points (values more than 3 times the interquartile range). Outliers are presented as dots. *P < 0.05, **P < 0.01
4. Discussion
The main finding of the present study was that addition of adrenaline to blood samples from patients who were on DAPT with ASA and ticagrelor potentiated ADP‐induced platelet aggregation and activation.
There is a need for new methods of reducing the risk of excessive bleeding in ticagrelor‐treated patients with ongoing bleeding or in need of acute surgery. One option would be to transiently enhance platelet function until bleeding has stopped or until the operation is completed. Platelets express adrenergic receptors, and supplementation with adrenaline has previously been shown to potentiate ADP‐induced platelet aggregation in healthy subjects11 and in clopidogrel‐treated patients.13 In a study conducted on platelet‐rich from healthy subjects, the P2Y12‐receptor antagonists ticagrelor, clopidogrel, prasugrel, and cangrelor were added in vitro and adrenaline supplementation (1 μmol L−1) was shown to restore ADP (5 μmol L−1)‐induced aggregation with either P2Y12‐receptor antagonist present.12 In the present study, we hypothesized that adrenaline would increase platelet activation and aggregation in blood samples from ACS patients on dual antiplatelet therapy with ASA and ticagrelor which, to our knowledge, has not previously been investigated. The results of the present study support our hypothesis. Adrenaline in itself had only a mild effect but potentiated ADP‐induced platelet activation and aggregation in the blood samples from ASA and ticagrelor‐treated patients in a concentration‐dependent way.
One could speculate that the potentiating effect of adrenaline may be due to the molecular actions of the substances. ADP binds to the G‐protein‐coupled receptors P2Y1 22 and P2Y12 23, 24 located on the platelet surface membrane. For a normal ADP‐induced activation and aggregation, responses from both pathways are required.25, 26, 27 When a P2Y12‐receptor antagonist such as ticagrelor has bound to the platelet receptor, the ADP‐induced activation of P2Y12 is inhibited. Adrenaline binds to the α2A‐receptor, activating a pathway which mimics the P2Y12‐receptor mediated pathway.26, 28, 29 This may explain that the combination of addition of ADP and adrenaline to blood samples from patients on ongoing treatment with ticagrelor results in a better aggregation response compared to either substance alone, as responses from both signaling pathways are achieved.
Although the results indicate that adrenaline improved ADP‐induced platelet aggregation in ticagrelor‐treated patients, it was by no means normalized. According to the impedance aggregometry manufacturer, the normal aggregation range with the ADP HS test in untreated individuals is 43‐100 U. In the present study, median aggregation increased with adrenaline from 15 to 26 U when pooled data from all aggregation experiments were included. This moderate improvement might at first sight be regarded as insufficient. However, in two recent observational studies on ACS patients undergoing CABG, small differences in preoperative platelet aggregation, determined with impedance aggregometry as in the present study, markedly influenced the risk of major bleeding complications. In fact, both studies suggested a cut‐off level of 22 U for when cardiac surgery may be performed without an increased risk of bleeding,5, 6 which is lower than what was achieved in the present study.
We observed that platelet supplementation alone did not influence ADP‐induced aggregation but markedly increased AA‐induced aggregation. This is in line with results from previous studies, which showed no effect or only a minor effect of platelet transfusion on ADP‐induced aggregation8, 9, 10 and an improvement in AA‐induced aggregation8 in patients treated with ASA and ticagrelor. Platelet transfusions may therefore still be of clinical importance in patients with ongoing DAPT, as they markedly increase AA‐induced aggregation and can therefore treat the platelet inhibition induced by ASA.
The present study had some important limitations. Although a clear concentration‐dependent effect of adrenaline on platelet aggregation was found, the effect was only statistically significant with the higher concentrations of adrenaline (153 and 770 nmol L−1). All tested concentrations, except the lowest, were higher than the plasma concentrations achieved by infusion of adrenaline at clinically relevant doses.30 Accordingly, the results of the present study support the concept that adrenaline enhances ADP‐dependent platelet aggregation, but in vivo studies with clinically acceptable adrenaline doses are necessary to validate the present findings. Furthermore, only adrenaline was tested in the present study. Other clinically used adrenergic agents such as noradrenaline also need to be evaluated, although in vitro data suggest that noradrenaline is not as potent as adrenaline in inducing aggregation of untreated platelets.11, 15, 16
In conclusion, the results demonstrate that adrenaline potentiates ADP‐induced activation and aggregation in blood samples from patients on ongoing treatment with ASA and ticagrelor. Adrenaline could therefore possibly be used to treat and prevent excessive bleeding in ticagrelor‐treated patients. In vivo studies are needed to confirm the present results.
Relationship Disclosure
The authors report nothing to disclose.
Author Contributions
S. Singh contributed to study design, patient recruitment, data collection and analysis, and drafting of the paper. C.J. Malm contributed to study design, patient recruitment, and revision of the manuscript. S. Ramström and C. Hesse interpreted data and revised the manuscript. A. Jeppsson contributed to study design, interpretation of data, and revision of the manuscript.
Supporting information
Acknowledgments
We are grateful to research nurses Eva Berg, Eva Lysell, and Maria Tellin for assistance with blood sampling.
Singh S, Malm CJ, Ramström S, Hesse C, Jeppsson A. Adrenaline enhances in vitro platelet activation and aggregation in blood samples from ticagrelor‐treated patients. Res Pract Thromb Haemost. 2018;2:718–725. 10.1002/rth2.12149
Funding information
This work was supported by the Swedish Heart and Lung Foundation (grant number 20150587 to A.J.) and by Västra Götaland Region (grant numbers ALFGBG‐146281 and ALFGBG‐725131 to A.J.).
References
- 1. Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST‐segment elevation. N Engl J Med. 2001;345:494‐502. [DOI] [PubMed] [Google Scholar]
- 2. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001‐15. [DOI] [PubMed] [Google Scholar]
- 3. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045‐57. [DOI] [PubMed] [Google Scholar]
- 4. Hansson EC, Jideus L, Aberg B, et al. Coronary artery bypass grafting‐related bleeding complications in patients treated with ticagrelor or clopidogrel: a nationwide study. Eur Heart J. 2016;37:189‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Malm CJ, Hansson EC, Akesson J, et al. Preoperative platelet function predicts perioperative bleeding complications in ticagrelor‐treated cardiac surgery patients: a prospective observational study. Br J Anaesth. 2016;117:309‐15. [DOI] [PubMed] [Google Scholar]
- 6. Ranucci M, Colella D, Baryshnikova E, Di Dedda U. Effect of preoperative P2Y12 and thrombin platelet receptor inhibition on bleeding after cardiac surgery. Br J Anaesth. 2014;113:970‐6. [DOI] [PubMed] [Google Scholar]
- 7. Mahla E, Prueller F, Farzi S, et al. Does platelet reactivity predict bleeding in patients needing urgent coronary artery bypass grafting during dual antiplatelet therapy? Ann Thorac Surg. 2016;102:2010‐17. [DOI] [PubMed] [Google Scholar]
- 8. Hansson EC, Shams Hakimi C, Åström‐Olsson K, et al. Effects of ex vivo platelet supplementation on platelet aggregability in blood samples from patients treated with acetylsalicylic acid, clopidogrel, or ticagrelor. Br J Anaesth. 2014;112:570‐5. [DOI] [PubMed] [Google Scholar]
- 9. O'Connor SA, Amour J, Mercadier A, et al. Efficacy of ex vivo autologous and in vivo platelet transfusion in the reversal of P2Y12 inhibition by clopidogrel, prasugrel, and ticagrelor: the APTITUDE study. Circ Cardiovasc Interv. 2015;8:e002786. [DOI] [PubMed] [Google Scholar]
- 10. Teng R, Carlson GF, Nylander S, Andersson TL. Effects of autologous platelet transfusion on platelet inhibition in ticagrelor‐treated and clopidogrel‐treated subjects. J Thromb Haemost. 2016;14:2342‐52. [DOI] [PubMed] [Google Scholar]
- 11. Mills DC, Roberts GC. Effects of adrenaline on human blood platelets. J Physiol. 1967;193:443‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scavone M, Femia EA, Caroppo V, Cattaneo M. Inhibition of the platelet P2Y12 receptor for adenosine diphosphate does not impair the capacity of platelet to synthesize thromboxane A2. Eur Heart J. 2016;37:3347‐56. [DOI] [PubMed] [Google Scholar]
- 13. Beres BJ, Toth‐Zsamboki E, Vargova K, et al. Analysis of platelet alpha2‐adrenergic receptor activity in stable coronary artery disease patients on dual antiplatelet therapy. Thromb Haemost. 2008;100:829‐38. [PubMed] [Google Scholar]
- 14. Spalding A, Vaitkevicius H, Dill S, MacKenzie S, Schmaier A, Lockette W. Mechanism of epinephrine‐induced platelet aggregation. Hypertension. 1998;31:603‐7. [DOI] [PubMed] [Google Scholar]
- 15. Lasch P, Jakobs KH. Agonistic and antagonistic effects of various alpha‐adrenergic agonists in human platelets. Naunyn Schmiedebergs Arch Pharmacol. 1979;306:119‐25. [DOI] [PubMed] [Google Scholar]
- 16. Jakobs KH, Saur W, Schultz G. Characterization of alpha‐ and beta‐adrenergic receptors linked to human platelet adenylate cyclase. Naunyn Schmiedebergs Arch Pharmacol. 1978;302:285‐91. [DOI] [PubMed] [Google Scholar]
- 17. European Directorate for the Quality of Medicines & Health Care . Guide to the preparation, use and quality assurance of blood components. 18th ed. Strasbourg: Council of Europe; 2015. [Google Scholar]
- 18. Toth O, Calatzis A, Penz S, Losonczy H, Siess W. Multiple electrode aggregometry: a new device to measure platelet aggregation in whole blood. Thromb Haemost. 2006;96:781‐8. [PubMed] [Google Scholar]
- 19. Shattil SJ, Hoxie JA, Cunningham M, Brass LF. Changes in the platelet membrane glycoprotein IIb.IIIa complex during platelet activation. J Biol Chem. 1985;260:11107‐14. [PubMed] [Google Scholar]
- 20. Schmitz G, Rothe G, Ruf A, et al. European Working Group on Clinical Cell Analysis: consensus protocol for the flow cytometric characterisation of platelet function. Thromb Haemost. 1998;79:885‐96. [PubMed] [Google Scholar]
- 21. Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65‐70. [Google Scholar]
- 22. Jin J, Daniel JL, Kunapuli SP. Molecular basis for ADP‐induced platelet activation. II. The P2Y1 receptor mediates ADP‐induced intracellular calcium mobilization and shape change in platelets. J Biol Chem. 1998;273:2030‐4. [DOI] [PubMed] [Google Scholar]
- 23. Hollopeter G, Jantzen HM, Vincent D, et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409:202‐7. [DOI] [PubMed] [Google Scholar]
- 24. Zhang FL, Luo L, Gustafson E, et al. ADP is the cognate ligand for the orphan G protein‐coupled receptor SP1999. J Biol Chem. 2001;276:8608‐15. [DOI] [PubMed] [Google Scholar]
- 25. Savi P, Beauverger P, Labouret C, et al. Role of P2Y1 purinoceptor in ADP‐induced platelet activation. FEBS Lett. 1998;422:291‐5. [DOI] [PubMed] [Google Scholar]
- 26. Jin J, Kunapuli SP. Coactivation of two different G protein‐coupled receptors is essential for ADP‐induced platelet aggregation. Proc Natl Acad Sci U S A. 1998;95:8070‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Foster CJ, Prosser DM, Agans JM, et al. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest. 2001;107:1591‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Woulfe D, Jiang H, Mortensen R, Yang J, Brass LF. Activation of Rap1B by G(i) family members in platelets. J Biol Chem. 2002;277:23382‐90. [DOI] [PubMed] [Google Scholar]
- 29. Daniel JL, Dangelmaier C, Jin J, Kim YB, Kunapuli SP. Role of intracellular signaling events in ADP‐induced platelet aggregation. Thromb Haemost. 1999;82:1322‐6. [PubMed] [Google Scholar]
- 30. Ensinger H, Lindner KH, Dirks B, Kilian J, Grunert A, Ahnefeld FW. Adrenaline: relationship between infusion rate, plasma concentration, metabolic and haemodynamic effects in volunteers. Eur J Anaesthesiol. 1992;9:435‐46. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


