Abstract
Background
Few studies have been performed on palliative care in Parkinson's disease (PD). This study was undertaken to understand treatment preferences of PD patients toward end‐of‐life care.
Methods
A questionnaire modified from the Willingness to Accept Life‐Sustaining Treatment instrument was administered to participants. Four different scenarios based on the burden of care and outcome of the treatment were presented in detail to obtain decisions for end‐of‐life care. The responses in each scenario were compared between PD patients and controls. Further analyses were performed to identify factors that influenced treatment preferences among PD patients.
Results
In total, 136 PD patients and 60 controls were recruited. Parkinson's disease patients and controls were demographically similar, except that PD patients had more previous hospital admissions (P = 0.0195). Parkinson's disease patients were more likely to opt for high‐burden care with poor outcome than controls (odds ratio [OR] = 2.11, P = 0.04).
In the subgroup analysis for PD patients, the factors that influenced treatment preference toward end‐of‐life care were belief in religion (OR: 7.43, 95% confidence interval:1.97–28.07), higher Unified Parkinson's Disease Rating Scale (UPDRS) motor score (2.51, 1.14–5.50) in scenario B; belief in religion (6.93, 2.23–21.43), married patients (6.93, 2.23–21.43) in scenario C; and Chinese patients (0.29, 0.10–0.79), better PD knowledge (0.37, 0.17–0.80), and higher UPDRS motor scores (3.05, 1.35–6.9) in scenario D.
Conclusion
Parkinson's disease patients were more likely to agree to high‐burden care with a poor outcome compared to controls. Among PD patients, race, marital status, religious status, knowledge about PD, and severity of motor impairment significantly influenced their end‐of‐life treatment preferences.
Keywords: Parkinson's disease, end‐of‐life, treatment preferences
Parkinson's disease (PD) is a chronic, progressive, and debilitating disease that significantly impacts patients physically as well as mentally. Although PD is viewed as a life‐limiting condition rather than a terminal illness, studies have shown that the end‐of‐life suffering of PD patients are comparable to patients with end‐stage cancer.1, 2 However, end‐of‐life care for PD has not received as much attention when compared with other chronic conditions. As such, a greater awareness of the role of palliative care for patients with advanced PD is needed.
Advance care planning (ACP) is the cornerstone of palliative care. It allows patients to make treatment decisions for end‐of‐life care and enables clinicians to provide care in accordance with the patients' treatment preferences. Advance care planning has been widely used for patients with cancer, chronic kidney disease, heart failure, and dementia to communicate their end‐of‐life treatment preferences.3, 4, 5 Studies conducted in a range of healthcare settings have shown that ACP can improve patients' and their families' satisfaction with care, as well as reduce the stress, anxiety, and depression of surviving family members.6, 7, 8, 9, 10, 11 However, only a few studies on ACP for PD patients have been performed.
Previous studies of ACP in palliative care for PD patients have focused on symptom burden and assessment1, 12; desired information, timing and initiator for ACP discussion13; and proxy decision making.14 Little is known about PD patients' actual treatment preferences toward end‐of‐life care. We therefore undertook this study to understand treatment preferences of PD patients toward end‐of‐life care by comparing treatment preferences between PD patients and controls, and to identify factors that influence the treatment preferences of PD patients.
Methods
All patients with PD seen between January and November 2013 in the Movement Disorders Clinic of the National Neuroscience Institute, Singapore, who meet the inclusion and exclusion criteria were invited to participate in the study. Inclusion criteria were PD patients fulfilling National Institute of Neurological Disorders and Stroke diagnostic criteria for Parkinson's disease15; ability to understand English, Malay, or Chinese; as well as cognitive and physical ability to understand and answer questions. Patients were excluded if they had other parkinsonism disorders, any significant psychiatric problems that interfered with sound judgment, and significant cognitive impairment or dementia. Control participants who did not have any neurodegenerative condition or psychiatric problems that would interfere with sound judgment were invited from neuroscience clinics or the community. Control participants from the clinic included patients with hemifacial spasms, essential tremors, focal dystonia, and chronic headaches, whereas community participants were healthy volunteers. All participants provided informed consent. This study was approved by the institutional review board at Singapore Health Services.
A questionnaire modified from the Willingness to Accept Life‐Sustaining Treatment instrument16 (see Appendix S1) was administered by an in‐person interview to assess the treatment preference of participants to different clinical scenarios. Four different scenarios were presented in detail to participants to obtain their views on their treatment preference toward end‐of‐life care. In scenarios A and B, participants were asked if they were willing to receive low‐burden care that involved undergoing simple procedures such as X‐rays and blood draws, along with basic treatment such as intravenous antibiotics and oxygen therapy. Accepting low‐burden care would result in a good outcome with a return to the current state of health (scenario A), or a poor outcome with physical disability (bedbound state requiring assistance with all activities of daily living) but intact cognition (scenario B). In scenarios C and D, participants were asked if they were willing to receive high‐burden care that involved undergoing procedures such as computerized tomography and surgery, along with care in an intensive care unit with a mechanical ventilator. Accepting high‐burden care would result in a good outcome with a return to the current state of health (scenario C), or a poor outcome with physical dependency but intact cognition (scenario D). In each scenario, participants were informed that rejecting treatment would result in death, whereas accepting treatment will result either a good outcome (scenarios A and C) or poor outcome (scenarios B and D). All four interviewers for the study underwent a 1‐day training and standardization in administering the questionnaire.
Basic demographic data and health‐related profiles were obtained for each participant. Participants were also assessed on their knowledge of PD by answering six basic PD‐related questions (see Appendix S2). Parkinson's disease patients were evaluated using the Unified Parkinson's Disease Rating Scale (UPDRS) motor score, Hoehn and Yahr stage, Parkinson's Disease Questionnaire‐8 (PDQ‐8), Mini‐Mental Status Examination (MMSE),17 and Schwab and England Activities of Daily Living Scale.
Statistical analysis
Simple frequency was used to describe the study population's demographics and health‐related profiles. Parkinson's disease and control groups were compared to detect differences using Fisher's exact tests. Frequency was also used to summarize participants' treatment preferences for each scenario. Comparisons were made between PD patients and controls using Fisher's exact tests. Participants who were unable to decide on their choice of treatment in a particular scenario were excluded from that analysis. When differences in subjects' characteristics between the two groups were detected, the effects of PD status on treatment choices were further adjusted using multivariate logistic regression. The effects were represented as odds ratio (OR) and 95% confidence interval (CI). Subgroup analysis was performed to analyze the participant characteristics, as well as disease‐specific characteristics that influenced treatment preferences among PD patients. Multivariate logistic regression with stepwise variable selection was employed. Statistical analyses were performed using SAS 9.2 for Windows (SAS Institute Inc., Carey, NC). Statistical significance was set as P ≤ 0.05.
Results
A total of 196 participants were recruited into the study. Of these, 136 were PD patients and 60 were controls. The demographics and health characteristics of participants are summarized in Table 1. The characteristics between PD and control groups were similar, except that PD patients were significantly more likely to have had previous hospital admissions compared to controls.
Table 1.
Characteristics of the Study Participants
| Characteristics | PD n = 136(%) | Control n = 60(%) | P Valuea | |
|---|---|---|---|---|
| Age group | >65 years old | 61 (44.9%) | 29 (48.3%) | 0.756 |
| ≤65 years old | 75 (55.1%) | 31 (51.7%) | ||
| Gender | Male | 84 (61.8%) | 31 (51.7%) | 0.2696 |
| Female | 52 (38.2%) | 29 (48.3%) | ||
| Race | Chinese | 110 (80.9%) | 52 (86.7%) | 0.4144 |
| Non‐Chineseb | 26 (19.1%) | 8 (13.3%) | ||
| Religion | With Religionc | 115 (84.6%) | 53 (88.3%) | 0.6583 |
| Without religion | 21 (15.4%) | 7 (11.7%) | ||
| Employment | Employed | 50 (36.8%) | 26 (43.3%) | 0.4279 |
| Unemployed | 86 (63.2%) | 34 (56.7%) | ||
| Marital status | Married | 114 (83.8%) | 49 (81.7%) | 0.6849 |
| Othersd | 22 (16.2%) | 11 (18.3%) | ||
| Years of education | >10 years | 60 (44.1%) | 19 (31.7%) | 0.1156 |
| ≤10 years | 76 (55.9%) | 41 (68.3%) | ||
| Past hospital admissions | At least 1 | 71 (52.2%) | 20 (33.3%) | 0.0195 |
| 0 | 65 (47.8%) | 40 (66.7%) | ||
| Charlson weighted index | >2 | 72 (52.9%) | 27 (45.0%) | 0.3533 |
| ≤2 | 64 (47.1%) | 33 (55.0%) | ||
| Hoehn and Yahr stages | >2 | 29 (21.3%) | ||
| ≤2 | 107 (78.7%) | |||
| UPDRS motor scores | >17 | 64 (47.1%) | ||
| ≤17 | 72 (52.9%) | |||
| Duration of PD | >5 years | 71 (52.2%) | ||
| ≤5 years | 65 (47.8%) | |||
| Mini‐Mental Status Examination | ≥24 | 114 (83.8%) | ||
| <24 | 22 (16.2%) | |||
| Parkinson's Disease Questionnaire‐8 | >7.63 | 68 (50%) | ||
| ≤7.63 | 68 (50%) | |||
| Schwab and England Activities of Daily Living Scale | >90 | 36 (26.5%) | ||
| ≤90 | 100 (73.5%) | |||
From Fisher's exact test.
Includes Malay, Indian, and minority races.
Includes Buddhism/Taoism, Christianity, Islam, and Hinduism.
Includes single, divorce/separated, and widow.
PD, Parkinson's disease; UPDRS Motor scores, Unified Parkinson's Disease Rate Scale Motor Scores.
Participants' treatment preferences
Figure 1 shows treatment preferences of PD patients and controls according to the burden and outcome of treatment. The proportion of participants (73%) opting for treatment in the low‐burden scenarios (A + B) was higher than those (57%) in the high‐burden scenarios (C + D) (P < 0.0001). The proportion of participants (87%) opting for treatment with the good‐outcome scenarios (A + C) was higher than those (43%) with the poor‐outcome scenarios (B + D) (P < 0.0001).
Figure 1.
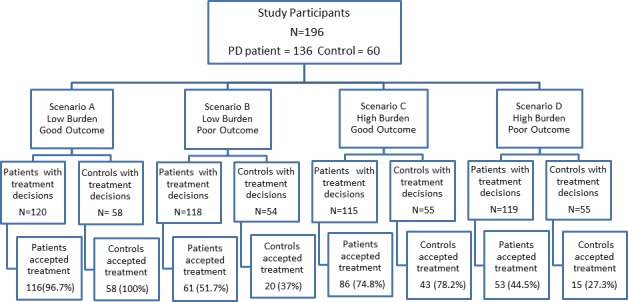
Treatment preference to each scenario. The number of participants who made treatment decisions and accepted treatment under each scenario are listed.
There was no statistically significant difference in the treatment preferences between the PD and control group for scenarios A, B, and C in the both unadjusted and adjusted analysis (adjusted for previous hospital admission). In scenario D, PD patients were significantly more (approximately 2 times) likely to opt for treatment than controls, even after adjustment for previous admission. The details are shown in Table 2.
Table 2.
Effect of Parkinson's Disease Status on Treatment Preferences According to Scenario
| Parameters | Scenario A Low Burden Good Outcome | Scenario B Low Burden Poor Outcome | Scenario C High Burden Good Outcome | Scenario D High Burden Poor Outcome | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | OR (95% CI) | P‐value | |
| Unadjusteda | ||||||||
| PD vs. control | 0.22(0.01–4.28) | 0.32 | 1.82(0.94–3.52) | 0.08 | 0.83(0.39–1.78) | 0.63 | 2.14(1.07–4.29) | 0.03 |
| Adjustedb | ||||||||
| PD vs. control | 0.17(0.01–3.16) | 0.24 | 1.68(0.86–3.29) | 0.13 | 0.84(0.39–1.82) | 0.65 | 2.11(1.04–4.27) | 0.04 |
Unadjusted: univariate analysis by using logistic regression.
Adjusted: multivariate analysis by using logistic analysis, adjusted for admission.
OR, odds ratio; CI, confidence interval; PD, Parkinson's disease.
Within the control group, male participants were more likely to agree to treatment (OR = 5.06, P = 0.028) in scenario C after multivariate analysis. This was the only significant factor that was associated with treatment preferences among controls.
Analysis of Parkinson′s disease patients
Among 136 patients with PD, 84 were males and 52 were female. Their mean age was 63 years old. The summary of PD‐associated features is shown in Table 1. Further analyses were performed to identify the factors that influenced the treatment preferences of PD patients in each scenario. These findings are summarized in Table 3. In scenario A, almost all (96.7%) patients opted for treatment and no factor was found that influenced their decisions. In scenario B, PD patients who had a religion and with higher UPDRS motor scores were significantly more likely to agree to treatment. After multivariate analysis, patients with a religion were 7.43 times more likely to agree to treatment compared to patients without a religion (P = 0.003). Parkinson's disease patients having higher UPDRS motor score (>17) were 2.51 times more likely to be agreeable to treatment compared to patients with less motor impairment (P = 0.022). In scenario C, patients who had a religion were about 7 times more likely to agree to treatment (P = 0.001). Married PD patients were similarly more likely to agree to treatment (OR = 6.93, P = 0.001) in scenario C. For scenario D, Chinese patients were about 70% less likely to agree to treatment compared to other races (P = 0.016). In the same scenario, PD patients who had better knowledge about the disease were also less likely agree to treatment (OR = 0.37, P = 0.012), whereas patients with higher UPDRS motor score were 3 times more likely to be agreeable to treatment (OR = 3.05, P = 0.008).
Table 3.
Predictors for Treatment Preferences of Parkinson's Disease Patients
| Scenarios | Parameters | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|---|
| Scenario Ba | ||||
|
Low burden Poor outcome |
Religion vs. free thinker | 7.43 | 1.97–28.07 | 0.003 |
| Motor score >17 vs. motor score ≤17 | 2.51 | 1.14–5.50 | 0.022 | |
| Scenario Ca | ||||
|
High burden Good outcome |
Religion vs. free thinker | 6.93 | 2.23–21.43 | 0.001 |
| Married vs. othersb | 6.93 | 2.23–21.43 | 0.001 | |
| Scenario Da | ||||
|
High burden Poor outcome |
Chinese vs. non‐Chinese | 0.29 | 0.10–0.79 | 0.016 |
| Knowledge about PD high vs. low | 0.37 | 0.17–0.80 | 0.012 | |
| Motor score >17 vs. motor score ≤17 | 3.05 | 1.35–6.90 | 0.008 | |
Multivariate logistic regression with stepwise selection. Variables entered: age, gender, race, religion, employment status, marital status, education level, admission status, Charlson's weighted index, knowledge about PD, Hoehn and Yahr stage, duration of PD, UPDRS Motor Score, Schwab and England Activities of Daily Living Scale, Parkinson's Disease Questionnaire‐8, Mini‐Mental Status Examination.
Includes single, divorced/separated, and widow.
PD, Parkinson's disease; UPDRS Motor Score, Unified Parkinson's Disease Rate Scale Motor Scores.
Discussion
There has been limited research performed on palliative care or end‐of‐life decisions among PD patients. Although there have been a few articles addressing advanced care planning in PD, to our best knowledge this is the first publication to evaluate the treatment preference of PD patients when faced with end‐of‐life decisions. In this study using a standardized, previously validated questionnaire,16 we found that the treatment preferences toward end‐of‐life care of PD patients differed from controls. Parkinson's disease patients were significantly more likely to agree to high‐burden care with poor outcomes compared to controls. Among PD patients, the factors that significantly influenced their end‐of‐life treatment preferences were race, marital status, religious status, knowledge about PD, and severity of motor impairment. All these predictors for treatment preferences found in PD group were not found in the control group.
The proportion of participants opting for treatment in the low‐burden care scenarios was significantly higher than those in the high‐burden care scenarios. Similarly, the proportion of participants opting for treatment in good‐outcome scenarios was significantly higher than those with the poor‐outcome scenarios. Our results are consistent with previous studies that showed the burden of care and treatment outcomes were important considerations in end‐of‐life treatment preferences.16 The participants' choices are understandable because minimal intervention and suffering, together with a good outcome and quality of life, are important considerations for many individuals when faced with an end‐of‐life decision.
When the treatment choices of PD patients were compared to controls, PD patients were more likely to agree to treatment in the high‐burden care scenario with a poor outcome. These results contrast with previous studies on patients with chronic disease,5 which showed that patients who had poorer health status tended to want less aggressive treatments.18 However, another study among cancer, congestive heart failure, and chronic obstructive pulmonary disease patients showed that these patients were willing to undergo high‐burden therapy despite a high likelihood of an undesirable outcome.19 The authors attributed these findings to their patients' uncertainty of their disease prognosis and their perception that death was not imminent. For the PD patients in this study, we believe that the chronic nature of the disease may have resulted in their ability to cope and adapt well to their disabilities.20, 21 As such, they were more willing to accept and tolerate further physical disability rather than face death when compared to controls.
The only PD‐specific feature found to bear an influence on end‐of‐life treatment preference was the UPDRS motor score. In this study, patients with greater motor impairment were more likely to opt for either low‐burden or high‐burden care despite poor outcomes (scenarios B and D). Such a finding may be counterintuitive because many would expect that patients with greater motor impairment would not be willing to accept care that would result in further physical disability. This result could be explained as follows: Firstly, these patients could have adapted to their disability over time and may be more willing to accept further disability, especially in the scenario in which their cognitive ability remains intact. Secondly, the majority of our participants were relatively young (mean age 63 years old) and at early stages of the disease (median disease duration of 5 years, 78% ≤ Hoehn and Yahr stage 2) and may not have perceived themselves to be near the end of life.
Religion has an important influence on an individual's treatment preferences at the end of life.22 Previous studies have found that cancer or terminally ill patients who had a religion were more likely to opt for life‐sustaining measures compared to similar patients who did not have a religion.23, 24, 25 This has been attributed to the role of religion in providing hope for patients to accept their condition and faith to believe that their condition may be healed. These same reasons likely explain why our patients who had a religious faith were more likely to opt for high‐burden care if the outcome was good and for low‐burden treatment even if the outcome was poor. Interestingly, religion was not a significant factor in the scenario of high‐burden care with poor outcome. Previous studies have revealed that by better addressing spiritual needs such as finding acceptance and spiritual peace in dying, coupled with quality‐of‐life discussions, aggressive treatment in terminally ill patients might be reduced.25 This acceptance of death could possibly explain why PD participants with religion did not opt for high‐burden treatment if the outcome was poor. However, further research is needed to confirm this.
This study was performed in a multiracial society. We found that Chinese PD patients were less likely to opt for high‐burden care if the outcome was poor when compared to non‐Chinese. The Chinese are thought to be more face‐conscious. Face is an important Chinese cultural concept that is embedded in every aspect of life. It represents the respect, pride, and dignity of an individual as a consequence of personal social achievement.26 It also relates to a person's image and status within a social structure.27 As such, it is hypothesized that physical disability arising from this scenario that would result in a “loss of face” or loss of dignity had influenced the decision to decline treatment so as to avoid an embarrassing, disabling physical condition. Our finding is consistent with that of a study among the Chinese in Macau who similarly declined aggressive medical treatment when faced with a terminal illness.28
In our study, PD patients who had better knowledge of PD were less likely to opt for high‐burden care if the outcome of treatment was poor. We believe that when equipped with adequate knowledge of the disease, PD patients are able to make better decisions. A previous Cochrane review of randomized trials of decisional aids showed that better knowledge of the disease and its treatment options led to improved patient–practitioner communication and increased medication adherence in the setting of various chronic diseases.29 Our study also highlights that a better knowledge of PD has an influence on the treatment preferences of patients relating to end‐of‐life issues.
In this study, married PD patients—when compared to singles, divorcees, or widows—were more likely to opt for high‐burden care if the outcome was good. This reflected the willingness of married patients to undergo intensive, high‐burden care in order to recover to their baseline function. This treatment decision is similar to the finding of a study done among geriatric inpatients, which found that married patients were more willing to undergo cardiopulmonary resuscitation in the event of a collapse than unmarried patients.30 Our finding highlights the attachment that patients have to their spouses, which results in their willingness to prioritize survival over other factors.
There is likely to be a significant cultural influence on end‐of‐life medical decisions because different societies and cultures hold different values and perceptions toward death. As such, our findings in an Asian context may not be applicable to a non‐Asian context. A second limitation of our study is that PD is not only a motor disorder; nonmotor symptoms such as cognition may contribute to the severity of PD. We only studied the poor‐outcome scenario in which patients were physically disabled without cognitive disability. We did not study other poor‐outcome scenarios, such as cognitive disability without physical disability or when both cognitive and physical disabilities were the outcomes. As such, our ability to interpret the participants' responses is limited. A third limitation of the study is that although we only recruited participants with no significant cognitive decline, no detailed psychometric testing was performed. There is a possibility that participants with some cognitive impairment may inevitably have been recruited into the study, which may affect their answers provided in the study. We have nevertheless corrected for MMSE scores in the final analysis. Fourthly, we recognize that our study participants may not be representative of the group of PD patients at their end of life. However, there would be many challenges if we restricted our study participants to those who were at their end of life; by then, many will have cognitive impairment or may be too ill to participate in such a study.
Conclusion
The results of our study show that different choices are made between PD patients and controls when faced with end‐of‐life decisions. Various demographic and disease‐related factors appear to influence some of these decisions. Although some of these associations make sense, others appear counterintuitive. Our study suggests that knowledge of the disease and cultural factors (religion, race, and marital status) have an important influence on end‐of‐life decisions. Another factor at play appears to be the adaptation of PD patients to their disabilities during the course of their illness. Given the many individual factors that contribute to end‐of‐life decisions, it is difficult for healthcare providers and family members to accurately predict a patient's end‐of‐life treatment preference. As such, a well facilitated ACP discussion is essential to empower patients to exert their autonomy to make well‐informed decisions. Patients and family members need to be equipped with the knowledge of PD, disease prognosis, treatment options, and treatment burden as well as possible outcomes. Healthcare professionals who facilitate these ACP sessions ought to sensitively blend together patients' values, sociocultural backgrounds, and wishes into the end‐of‐life discussion.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the First Draft, B. Review and Critique.
WEI L.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
H.L.N.: 1A, 1B, 1C, 2A, 2C, 3A, 3B
WENYUN L.: 2B, 2C, 3A, 3B
A.N.P.: 1A, 1B, 1C, 2A, 2C, 3A, 3B
S.A.K.: 1A, 1B, 1C, 2A, 2C, 3A, 3B
K.Y.T.: 1A, 2C, 3A, 3B
W.L.A. 1A, 2C, 3A, 3B
L.CS.T.: 1A, 1B, 2A, 2B, 2C, 3B
Disclosures
Funding Sources and Conflicts of Interest: This study was supported by the National Neuroscience Institution, Singapore, and the Singapore National Research Foundation under its Translational and Clinical Research Flagship Programme (TCR12dec010) and administered by the Singapore Ministry of Health's National Medical Research Council; and the Singapore Millennium Foundation.
Financial Disclosures for previous 12 months: Wei L. reports no disclosure. H.L.N. reports no disclosure. Wenyun L. reports no disclosure. A.N.P. has received speakers honorarium from: Medichem Pharmaceuticals, Sun Pharmaceutical, Abbott Philippines, Novartis Philippines, Natrapharm, Torrent Philippines, and has also received travel grants from Medichem Pharmaceuticals and Torrent Philippines. S.A.K. reports no disclosure. K.Y.T. reports no disclosure. W.L.A. reports no disclosure. L.CS.T. has received grant support from the Singapore National Research Foundation under its Translational and Clinical Research Flagship Programme (TCR12dec010) and administered by the Singapore Ministry of Health's National Medical Research Council; and the Singapore Millennium Foundation. He has also received honoraria and travel grants for speaking engagements from Novartis Pharmaceuticals.
Supporting information
Appendix S1: A questionnaire modified from the Willingness to Accept Life‐Sustaining Treatment (WALT) instrument
Appendix S2: Knowledge of Parkinson Disease Questionnaire
Acknowledgments
We would like to thank all participants who took part in this study for providing us valuable information. We also would like to thank Dr. Ng Peng Soon for inputs at the initial stages of the study.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Miyasaki JM, Long J, Mancini D, et al. Palliative care for advanced Parkinson disease: an interdisciplinary clinic and new scale, the ESAS‐PD. Parkinsonism Relat Disord 2012;18(suupl 3):S6–S9. [DOI] [PubMed] [Google Scholar]
- 2. Hudson PL, Toye C, Kristjanson LJ. Would people with Parkinson's disease benefit from palliative care? Palliat Med 2006;20:87–94. [DOI] [PubMed] [Google Scholar]
- 3. Houben CHM, Spruit MA, Groenen MTJ, Wouters EFM, Janssen DJA. Efficacy of advanced care planning: a systematic review and meta‐analysis. J Am Med Dir Assoc 2014;15:477–489. [DOI] [PubMed] [Google Scholar]
- 4. Kirchhoff KT, Hammes BJ, Kehl KA, Briggs LA, Brown RL. Effect of a disease‐specific advanced care planning intervention on end‐of‐life care. J Am Geriatr Soc 2012;60:946–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sanchez‐Tomero JA, Rodriguez‐Jornet A, Balda S, et al. Exploring the opinion of CKD patients on dialysis regarding end‐of‐life & advance care planning. Nefrologia 2011;31:449–456. [DOI] [PubMed] [Google Scholar]
- 6. Brinkman‐Stoppelenburg A, Rietjens JAC, Heide A. The effects of advance care planning on end‐of‐life care: a systematic review. Palliat Med 2014;28:1000–1025. [DOI] [PubMed] [Google Scholar]
- 7. Engelhardt JB, McClive Reed KP, Toseland RW, et al. Effects of a program for coordinated care of advanced illness on patients, surrogates and healthcare costs: a randomized trial. Am J Manag Care 2006;12:93–100. [PubMed] [Google Scholar]
- 8. Horne G, Seymour J, Shepherd K. Advance care planning for patients with inoperable lung cancer. Int J Palliat Nurs 2006;12:172–178. [DOI] [PubMed] [Google Scholar]
- 9. Rabow MW, Dibble SL, Pantilat SZ, et al. The comprehensive care team: a controlled trial of outpatient palliative medicine consultation. Arch Intern Med 2004;164:83–91. [DOI] [PubMed] [Google Scholar]
- 10. Pautex S, Hermann FR, Zulian GB. Role of advance directives in palliative care units. Palliat Med 2008;22:835–841. [DOI] [PubMed] [Google Scholar]
- 11. Detering KM, Hancock AD, Reade MC, et al. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. March 24, 2010. Available at: http://www.bmj.com/content/340/bmj.c1345. Accessed March 22, 2015. [DOI] [PMC free article] [PubMed]
- 12. Lee M, Prentice W, Hildreth A, Walker RW. Measuring symptom load in idiopathic Parkinson's disease. Parkinsonism Relat Disord 2007;13:284–289. [DOI] [PubMed] [Google Scholar]
- 13. Tuck KK, Brod L, Nutt J, et al. Preferences of patient with Parkinson's disease for communication about advanced care planning. Am J Hosp Palliat Care 2015;32:68–77. [DOI] [PubMed] [Google Scholar]
- 14. Kwak J, Wallendal MS, Fritsch T, et al. Advance care planning and proxy decision making for patients with advanced Parkinson's disease. South Med J 2014;107:178–185. [DOI] [PubMed] [Google Scholar]
- 15. Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson's disease. Arch Neurol 1999;56:33–39. [DOI] [PubMed] [Google Scholar]
- 16. Fried TR, Bradley EH, Towle VR. Assessment of patient preferences: integrating treatments and outcomes. J Gerontol B Psychol Sci Soc Sci 2002;57B:S348–S354. [DOI] [PubMed] [Google Scholar]
- 17. Ng TP, Niti M, Chiam PC, Kua EH. Ethnic and education differences in cognitive test performance on mini‐mental state examination in Asians. Am J Geriatri Psychiatry 2007;15:130–139. [DOI] [PubMed] [Google Scholar]
- 18. Schwartz CE, Merriman MP, Reed GW, et al. Measuring patient treatment preferences in end‐of‐life care research: applications for advance care planning interventions and response shift research. Palliat Med 2004;7:233–245. [DOI] [PubMed] [Google Scholar]
- 19. Fried TR, Bradley EH, Towle VR, Phil M, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med 2002;346:1061–1066. [DOI] [PubMed] [Google Scholar]
- 20. Hermanns M. Weathering the storm: living with Parkinson's disease. J Christ Nurs 2011;28:76–82. [DOI] [PubMed] [Google Scholar]
- 21. Frazier LD. Coping with disease‐related stressors in Parkinson's disease. Gerontologist 2000;40:53–63. [DOI] [PubMed] [Google Scholar]
- 22. Sharp S, Carr D, Macdonald C. Religion and end‐of‐life treatment preference: assessing the effects of religious denomination and beliefs. Soc Forces 2012;91:275–298. [Google Scholar]
- 23. Phelps AC, Maciejewski PK, Matthew Nilsson BS, et al. Association between religious coping and use of intensive life prolonging care near death among patients with advanced cancer. JAMA 2009;301:1140–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. True G, Phipps EJ, Braitman LE, Harralson T, Tester W. Treatment preferences and advance care planning at end of life: the role of ethnicity and spiritual coping in cancer patients. Ann Behav Med 2005;30:174–179. [DOI] [PubMed] [Google Scholar]
- 25. Balboni TA, Balboni M, Enzinger AC, et al. Provision of spiritual support to patients with advanced cancer by religious communities and associations with medical care at the end of life. JAMA Intern Med 2013;173:1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leung TKP, Chan RKK. Face, favour and positioning: a Chinese power game. Eur J Mark 2003;37:1575–1598. [Google Scholar]
- 27. Cardon PW, Scott JC. Chinese business face: communication behaviors and teaching approaches. Business Communication Quarterly 2003;66:9–22. [Google Scholar]
- 28. Ho SW, Sanders GF. Preferences on end‐of‐life decisions among older Chinese in Macau. J Transcult Nurs 2015;26:157–163. [DOI] [PubMed] [Google Scholar]
- 29. Stacey D, Legare F, Col NF. Decision aids for people facing health treatment or screening decisions review. Cochrane Database Syst Rev 2014;1:CD001431. doi: 10.1002/14651858.CD001431.pub4. [DOI] [PubMed] [Google Scholar]
- 30. Bruce‐Jones P, Roberts H, Bowker L, Cooney V. Resuscitating the elderly: what do the patients want? J Med Ethics 1996;22:154–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: A questionnaire modified from the Willingness to Accept Life‐Sustaining Treatment (WALT) instrument
Appendix S2: Knowledge of Parkinson Disease Questionnaire


