Abstract
Background
In Parkinson's disease (PD), gait disorders lead to increased risk of falls and patients' reduced participation and independence. Several observations suggest that a single session of focal muscle vibration (fMV) applied to trunk or lower limb muscles during gait may improve several gait variables in patients with PD. The possible long‐term beneficial effects of repetitive sessions of fMV (r‐fMV) on gait of patients with PD have been investigated.
Methods
A randomized, controlled trial study has been conducted in an outpatient rehabilitation department. Twenty patients with PD diagnosis have been randomized in two groups: “real” or “sham” r‐fMV application to quadriceps and paraspinal muscles in patients with PD. Gait was evaluated with objective gait analysis, and a number of variables, including velocity, step length, stride length, percentage of stance, double support duration, cadence, swing velocity, and step width, have been measured. Gait analysis was performed before and 24 hours and 1 and 3 weeks after r‐fMV.
Results
After real, but not sham, r‐fMV, patients with PD had significant gait improvement as a result of increased walking velocity and stride length. The r‐fMV‐induced beneficial after effects lasted at least 1 week after the end of stimulation.
Conclusions
Data emerging from our pilot randomized, controlled trial study suggest that r‐fMV may improve gait disorders in patients with PD. r‐fMV might be a feasible, safe approach for possibly improving gait disorders in patients with PD and might enhance the impact of specific rehabilitation programs in PD.
Keywords: gait analysis, muscle vibration, Parkinson's disease, rehabilitation, sensorimotor integration
In Parkinson's disease (PD), motor symptoms include specific gait disorders that, in more advanced stages of the disease, progressively worsen and become poorly responsive to dopaminergic treatments, therefore representing a major clinical challenge.1 Experimental studies with gait analysis (GA) have demonstrated that patients with PD manifest slowness of walking,2 reduced stride length,3 and decreased cadence, often accompanied by the tendency toward a longer duration in the double‐support phase.4 In PD, gait disorders may also include freezing of gait (FOG), which is characterized by brief paroxysmal events during which a parkinsonian patient is unable to start or continue locomotion.5 In PD, gait disorders overall crucially lead to patients' reduced participation and independence and increased risk of falls.6
Several previous studies have tried to improve gait disorders in PD by applying various types of sensory cues during gait,7 following the well‐known observation that externally given sensory cues improve overall motor control in PD.7, 8, 9 Among the various experimental strategies, a previous study applied a brief period of focal muscle vibration (fMV), delivered during gait over specific trunk or lower‐limb muscles, and found that fMV induces short‐term improvement in step length and gait abnormalities in patients with PD.10 However, the previous studies overall have failed to induce long‐term improvement of gait disorders in patients with PD, thus making the previous strategies unhelpful for neurorehabilitative purposes in PD.
To induce long‐term improvement of gait disorders in PD, a new experimental approach might be to apply plasticity‐inducing protocols able to induce long‐term changes in central nervous system (CNS) function. A number of plasticity‐inducing protocols consisting of repetitive sensory stimulation11, 12, 13 are now available, including those involving repetitive fMV (r‐fMV).14, 15 In a double‐blind, randomized, controlled trial (RCT) in healthy subjects, rMV has been applied for 3 consecutive days and researchers demonstrated that r‐fMV elicits long‐term improvement of stance control and muscle performance.16.
No studies have previously investigated the possible r‐fMV‐induced long‐term beneficial changes on gait disorders in patients with PD, with an RCT designed to test a number of kinetic/kinematic gait parameters. In addition, none has clarified whether r‐fMV can be helpful for rehabilitative purposes in PD.
In this pilot RCT study in a small cohort of patients with PD, we investigated the effect of “real” or “sham” r‐fMV applied to both quadriceps and paraspinal muscles on a large number of kinetic/kinematic gait parameters (velocity, step length, stride length, stance, double support, cadence, step width, and swing velocity).
Materials and Methods
Subjects
The study was conducted on 20 patients with PD (8 men and 12 women; mean age: 64.85 ± 8.74 years) enrolled at San Raffaele Cassino Rehabilitation Institute (Cassino, Italy). PD diagnosis was made according to the UK Brain Bank Criteria.17 All patients were clinically evaluated before participating in the experimental session when in the on state of therapy. Motor signs were scored using the motor section of the UPDRS18 and the H & Y scale.19 Patients had no motor fluctuations and dyskinesias, and thus patients were considered “stable responders” to levodopa. Cognitive function was evaluated using the Mini–Mental State Evaluation (MMSE).20 The patients enrolled had no dementia or other neuropsychiatric disorders, including depression. None of the patients with PD had any type of FOG when in the off or on state of therapy. Clinical characteristics of patients are shown in Table 1. l‐dopa equivalent daily dose (mg; LEDD) was calculated for each patient according to the criteria of Hobson et al. (40).
Table 1.
Clinical features of PD patients
| Study Group (n = 9) | Control Group (n = 8) | P Value | |||
|---|---|---|---|---|---|
| Median | Range | Median | Range | ||
| Age (years) | 67 ± 7.96 | 53–74 | 65.5 ± 9.85 | 48–79 | 0.74 |
| Disease duration (years) | 8.0 ± 5.57 | 3.0–12.0 | 7.5 ± 3.70 | 4.0–12.0 | 0.69 |
| H & Y | 3 ± 0.45 | 2–3 | 2.5 ± 0.39 | 2–3 | 0.11 |
| UPDRS | 20 ± 5.54 | 15–31 | 22.5 ± 5.49 | 12–29 | 0.90 |
| MMSE | 27.5 ± 1.72 | 25–30 | 29.5 ± 1.61 | 26–30 | 0.14 |
| LEDD (mg/day) | 740 ± 53.75 | 580–750 | 690 ± 116.14 | 580–800 | 0.64 |
Data are expressed as median and range (min‐max). Note that LEDD (mg) was calculated for each patient according to the criteria of Hobson et al.40
The study was designed according to the Declaration of Helsinki (1964) and was approved by the local ethics committee. All study participants provided informed consent.
Intervention
r‐FMV was delivered by using a specific device consisting of an electromechanical transducer, a mechanical support, and an electronic control device (Cro System; Nemoco SRL, Rome, Italy). The mechanical support allowed the orientation, positioning, and rigid fixation of the transducer in every direction relative to the individual's body. The transducer was positioned bilaterally on the quadriceps tendon close to the rectus femoris insertion at approximately 2 cm from the medial edge of the patella and on the lumbar paraspinal muscles (Fig. 1). In a single experimental session, each participant received r‐FMV over the quadriceps muscles first to improve muscle performance16 and then also over the lumbar paraspinal muscles in order to orient the body tilt and the spine stability.21 For each muscle group (quadriceps and lumbar paraspinal muscles), r‐FMV was applied for three sessions of 10 minutes each, with an intersession interval of 1 minute (total time of r‐FMV application: 60 minutes). The same protocol was repeated for 3 consecutive days in order to elicit “cumulative after effects” and according to previously reported techniques.16, 22, 23, 24 The mechanical support for delivering r‐FMV allowed the compression of soft tissues overlying the muscle‐tendon complex with low amplitude (200–500 μm) and high frequency (100‐Hz) sinusoidal displacement and thus well below the threshold for perceiving illusory movements and for eliciting the tonic vibration reflex (TVR).14, 15, 25, 26.
Figure 1.
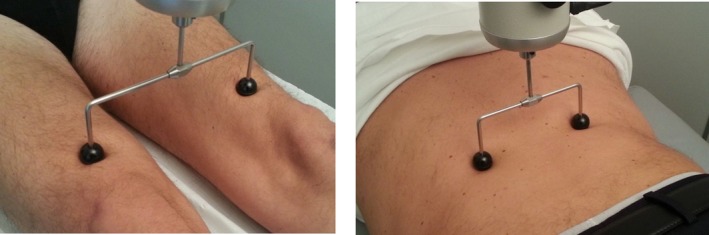
fMV device and its application setting to the quadriceps muscles and to the paraspinal lumbar muscles.
Experimental design
We used a single‐blind, parallel‐group study design. Patients were selected by block randomization (Random Allocation Software, a free share software) into two groups: the study group (SG; 10 patients) and the control group (CG; 10 patients). Patients in the SG received real r‐fMV, whereas the CG underwent sham r‐fMV. We evaluated all patients in the best clinical state in the morning, 1.5 hours after dopaminergic drug administration. During real or sham r‐fMV, the participants were first asked to lie supine to treat the quadriceps and then prone during the application of r‐fMV in the lumbar paraspinal muscles. During the application on the quadriceps, patients were asked to perform an isometric muscle contraction to keep the popliteal cavum in contact with the bed, while the assessors controlled the presence of muscle contraction throughout the r‐fMV application.16, 22 For the lumbar application, no muscle activation was required (real session).21 In the CG (sham r‐fMV), the vibrator was positioned close to the skin, but without touching the skin surface, in order to avoid the possible influence mediated by the cutaneous mechanoreceptor. In this condition, the patients did not refer any local sensation and were subjected only to the faint buzzing sound of the vibrator.
GA
In order to evaluate the effect of r‐fMV on gait in PD, a GA evaluation was done before r‐fMV (T0) and 24 hours (T1), 1 week (T2), and 3 weeks (T3) after the last session of r‐fMV. The complete evaluation consisted of clinical examination and three‐dimensional//3D GA. All patients were evaluated instrumentally using an optoelectronic system with passive markers (ELITE2002; BTS, Milan, Italy) with a sampling rate of 100 Hz and two television camera video systems (BTS, Italy) synchronized with the system and the platforms for video recording. To evaluate the kinematics of each body segment, passive markers were positioned on the participants' body, as described by Davis.27 Participants were asked to walk barefoot at their own natural pace (self‐selected and comfortable speed) along a walkway (10 m long) where the two force platforms were placed. At least six trials were collected for each individual in order to ensure the consistency of the data. All the acquisitions were acquired by the same operator with experience, to assure reproducibility of the acquisition technique and avoid the introduction of errors resulting from different operators. In addition, the investigator performing the GA evaluation was blind to the type of fMV (real or sham).
All graphs obtained from GA were normalized as percent of gait cycle, and kinetic data were normalized for individual body weight. In order to define the gait pattern of patients with PD before and after r‐fMV therapy in the SG and in the CG, the following spatiotemporal parameters were evaluated:
Velocity (m/s): mean velocity of progression;
Step length (m): longitudinal distance from one foot that moves forward in front of the other one;
Stride length (m): average distance between two successive placements of the same foot. It consists of two‐steps length, left and right;
% stance (as percent of the gait cycle): duration of the stance phase;
Double support (as percent of the gait cycle): period of the gait cycle when both feet are on the ground;
Cadence (step/min): number of steps for time
Step width (m): mediolateral distance between the two feet during double support;
Swing velocity (m/s): velocity during the swing phase.
Physiotherapy program
All patients with PD underwent a standardized physiotherapy program (1 hour session/day, 3 days/week, 12 weeks in total). The physiotherapy program was carefully designed to improve gait, balance, and posture, increase the patient physical capacity and, more specifically, the upper‐limb function, and finally, maximize the functional ability and minimize secondary complications in all patients. All the physical therapists contributing to the physiotherapy program received specific instructions to ensure uniformity in the treatment procedures and were blind to the type of fMV (real or sham). All patients received real or sham fMV in the early phase of the physiotherapy program (within 1 month from physiotherapy onset). Physiotherapy was stopped during the week in which patients underwent gait analysis and received real or sham fMV.
Statistical analysis
Normality assumption was tested using the skewness and kurtosis statistics and the normal probability plot. The statistical analysis of the continuous variables was conducted calculating median and range (min‐max), because these variables were not normally distributed.
Mann‐Whitney's U test was used to compare SG and CG at the T0 session.
Friedman's test for paired data was used to evaluate difference between T1 and T0, T2 and T0, and T3 to T0 in the SG and CG for all the spatiotemporal parameters, separately. Wilcoxon's signed‐rank test was used in the post‐hoc analysis. Statistical significance was set at P < 0.05. The analysis was carried out using SPSS software (version 19.0; SPSS, Inc., Chicago, IL). The presentation of the study is in accord with the CONSORT Statement.28.
Results
None of the patients showed any side effects. The two groups of patients with PD receiving real or sham r‐fMV respectively were comparable in terms of mean age and disease duration and had similar UDPRS, H & Y, and MMSE scores. None of the patients experienced side effects during or after the experimental intervention. Three patients dropped out at the follow‐up after 3 weeks for reasons not strictly related to the study (i.e., side effects) and therefore were not included in the analysis. The flow diagram of participants (trial profile) is shown in Figure 2.
Figure 2.
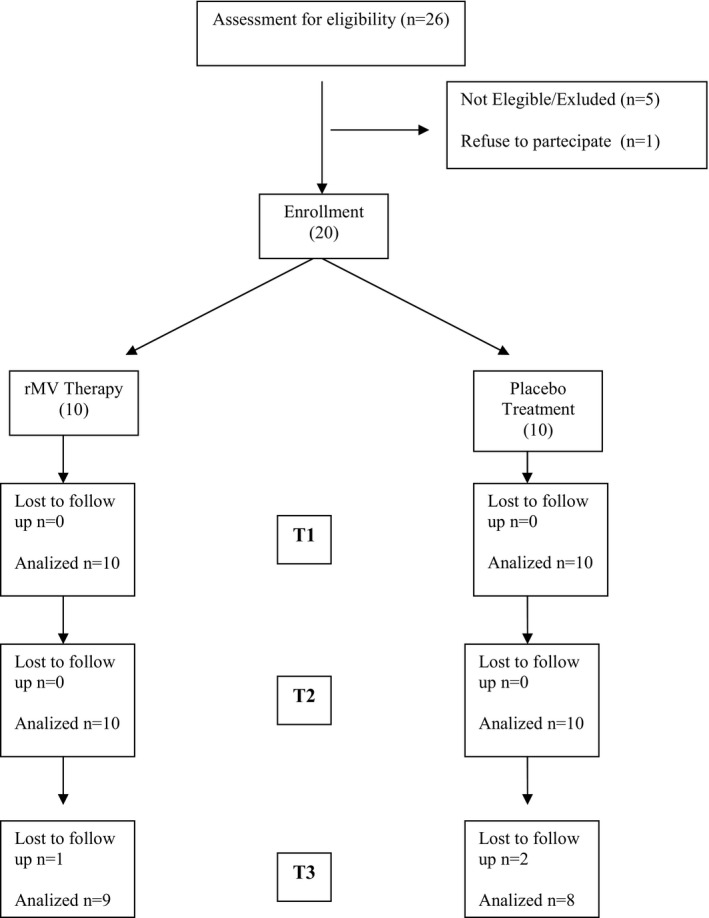
Trial profile.
In terms of the spatiotemporal parameters, no statistical differences were found at T0 session between the two groups of patients with PD (P > 0.05).
In the group receiving real r‐fMV, Friedman's test showed that velocity increases significantly at T1 (P = 0.036) and T2 (P = 0.02) and there was also a trend for increase velocity at T3 (P = 0.058; Fig. 3A). In addition, Friedman's test showed that stride length also increased at T1 (P = 0.021), T2 (P = 0.008), and T3 (P = 0.025; Fig. 3B). Finally, step length increased at T1 (P = 0.022), T2 (P = 0.001), and T3 (P = 0.025), and swing velocity improved at T1 (P = 0.006), T2 (P = 0.005), and T3 (P = 0.049). Conversely, the remaining gait variables (step width cadence, stance time, and double support) remained unchanged at the three time points considered (Table 2).
Figure 3.
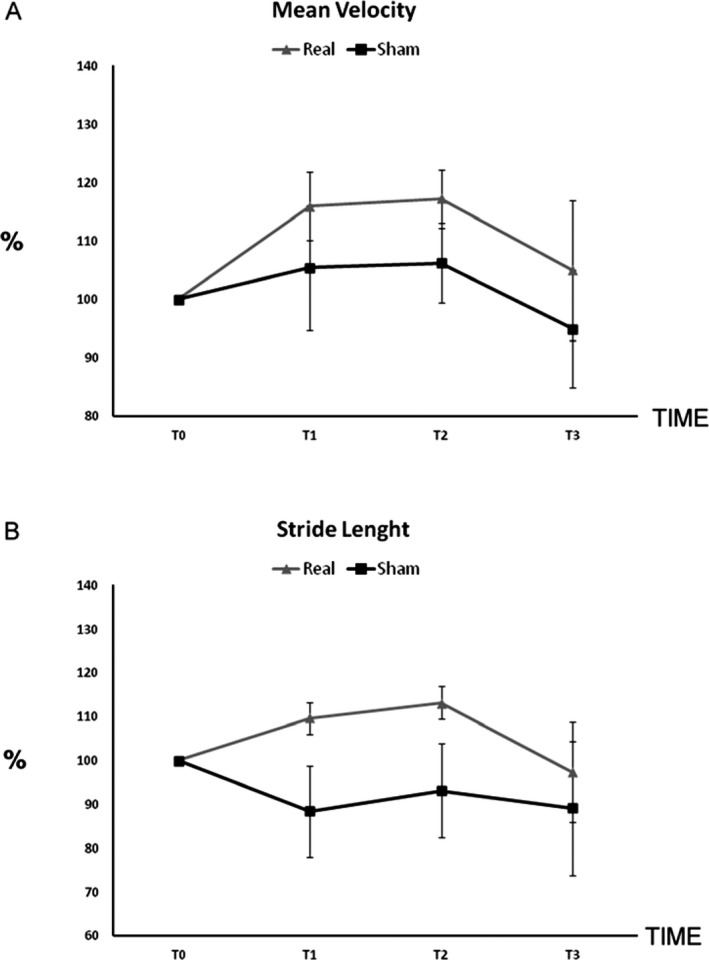
Percentage of variation of velocity (A) and stride length (B) at T1, T2, and T3 compared to the baseline T0 in the real and in the sham group (error base indicates the standard error).
Table 2.
Spatiotemporal parameters in the study and in the control group
| Spatiotemporal Parameters | Study Group (n = 9) Median (Range) | Control Group (n = 8) Median (Range) |
|---|---|---|
| Velocity (m/s) T0 | 0.63 (0.50–1.03)* | 0.88 (0.37–1.20) |
| Velocity (m/s) T1 | 0.80 (0.50–1.30)* | 0.90 (0.30–1.20) |
| Velocity (m/s) T2 | 0.83 (0.60–1.07) | 0.83 (0.50–1.17) |
| Velocity (m/s) T3 | 0.73 (0.50–1.17) | 0.90 (0.60–1.17) |
| Step length (m) T0 | 0.41 (0.28–0.58)* | 0.49 (0.31–0.61) |
| Step length (m) T1 | 0.44 (0.25–0.66)* | 0.50 (0.40–0.61) |
| Step length (m) T2 | 0.46 (0.38–0.65)* | 0.49 (0.33–0.58) |
| Step length (m) T3 | 0.44 (0.28–0.59) | 0.49 (0.36–0.57) |
| Stride length (m) T0 | 0.80 (0.58–1.15)* | 0.99 (0.91–1.08) |
| Stride length (m) T1 | 0.84 (0.66–1.30)* | 0.96 (0.89–1.13) |
| Stride length (m) T2 | 0.86 (0.78–1.26)* | 1.02 (0.86–1.16) |
| Stride length (m) T3 | 0.87 (0.64–1.17) | 0.96 (0.81–1.08) |
| Swing velocity (m/s) T0 | 1.79 (1.20–2.73)* | 2.14 (1.10–2.70) |
| Swing velocity (m/s) T1 | 1.95 (1.43–3.10)* | 2.20 (1.67–2.80) |
| Swing velocity (m/s) T2 | 1.97 (1.53–2.53)* | 2.21 (1.43–2.73) |
| Swing velocity (m/s) T3 | 1.90 (1.27–2.80) | 2.21 (1.53–2.73) |
| Step width (m) T0 | 0.17 (0.13–0.19) | 0.17 (0.14–0.22) |
| Step width (m) T1 | 0.17 (0.15–0.19) | 0.17 (0.14–0.25) |
| Step width (m) T2 | 0.16 (0.15–0.19) | 0.17 (0.14–0.22) |
| Step width (m) T3 | 0.17 (0.15–0.19) | 0.18 (0.14–0.21) |
| Cadence (step/min) T0 | 104.88 (82.20–114.67) | 106.00 (70.80–121.23) |
| Cadence (step/min) T1 | 103.61 (88.03–120.33) | 107.05 (75.37–118.57) |
| Cadence (step/min) T2 | 107.58 (90.40–124.40) | 107.53 (80.30–126.60) |
| Cadence (step/min) T3 | 107.63 (84.00–119.37) | 110.88 (90.83–122.97) |
| Stance time (%gc) T0 | 61.61 (56.03–65.17) | 61.25 (57.37–69.23) |
| Stance time (%gc) T1 | 60.67 (56.10–66.40) | 60.16 (57.57–62.77) |
| Stance time (%gc) T2 | 60.70 (57.13–65.63) | 60.65 (57.57–63.77) |
| Stance time (%gc) T3 | 61.13 (55.97–66.27) | 60.70 (56.87–64.07) |
| Double support (%gc) T0 | 11.92 (8.57–14.34) | 10.31 (9.05–17.32) |
| Double support (%gc) T1 | 10.41 (7.73–15.03) | 10.11 (8.98–12.65) |
| Double support (%gc) T2 | 11.22 (8.97–13.55) | 11.18 (8.90‐11.93) |
| Double support (%gc) T3 | 10.89 (8.73–16.6) | 11.08 (9.25–12.28) |
Data are expressed as median and range (min‐max).
*denotes P < 0.05.
%gc, percent gait cycle.
By contrast, in the group receiving sham r‐fMV, Friedman's test showed no significant differences in all the gait variables considered here (Table 2).
Discussion
In this pilot RCT study, we found that real, but not sham, r‐fMV leads to beneficial long‐term changes on gait disorders in patients with PD.
We excluded several confounding factors possibly affecting the responses to r‐fMV in patients with PD. Patients receiving sham r‐fMV had comparable clinical features compared to patients treated with real r‐fMV, thus excluding baseline differences in the two groups. All experimental sessions took place at a comparable daytime, and all patients were tested at their best clinical state in the morning. The experimental procedures used during GA lasted for around 30 minutes, thus unlikely leading to fatigue in patients with PD.29, 30 None of the patients had clinically evident motor fluctuations, dyskinesias, or any type of FOG, thus excluding confounding factors from motor fluctuations and FOG‐related interference on gait variables.6, 31 Given that fMV was delivered below the threshold for eliciting the TVR,14, 15, 25, 26 we also excluded possible confounding factors from TVR‐related mechanisms. After r‐fMV, gait disorders in PD might have improved merely reflecting a “placebo effect.” It is known that placebo effect is particularly relevant in patients with PD.32 This hypothesis, however, seems very unlikely because in our RCT study, the real and sham r‐fMV were applied randomly in patients with PD, thus excluding the placebo effect. The observation of comparable stride lengths in the real and sham groups at T0 make the hypothesis that differences in baseline stride length would have contributed to r‐fMV‐induced gait improvement in the real group very unlikely. Finally, because all physiotherapists contributing to the physiotherapy program were blind to the type of r‐fMV (real or sham), all patients received real or sham r‐fMV in the early phase of the physiotherapy program and, finally, physiotherapy was stopped during the week in which patients underwent GA and received real or sham r‐fMV, we can exclude confounding resulting from a possible interference of physiotherapy on the r‐fMV‐induced influence on gait.
The main finding in this study is that in PD, real r‐fMV led to significant improvement of gait owing to increased walking velocity and stride length. These findings are in line with the previous observation of De Nunzio et al.,10 who found that fMV applied during gait increased walking velocity and stride length in patients with PD.10 Differently from the previous study of De Nunzio et al.,10 we now report that r‐fMV induced gait improvement in PD after the end of intervention, possibly owing to differences in the specific fMV protocol. It is difficult to clarify through which physiological mechanisms r‐fMV improved gait in PD. A possible mechanism includes the ability of r‐fMV to modify sensory afferent inputs to the CNS. fMV is currently thought to operate by inducing sensory afferent inputs to the CNS through specific activation of primary muscle spindle endings.14, 16, 22 A first hypothesis possibly explaining the r‐fMV‐induced gait improvement in patients with PD concerns the possible beneficial effect of real r‐fMV on lower‐limb rigidity. An increased reciprocal inhibition between agonist and antagonist muscles and a reduction in Ib tendon inhibition of the H‐reflex are currently considered important mechanisms underlying the pathophysiology of rigidity in PD.33, 34 In the present study, however, patients had comparable UPDRS subscores for lower‐limb rigidity, before and after each r‐fMV session, thus making very unlikely the hypothesis that r‐fMV improves gait in PD only by decreasing lower‐limb rigidity. In patients with PD, increasing evidence suggests that motor symptoms, including gait disorders, reflect altered sensorimotor integration processes. Sensorimotor integration includes physiological processes linking sensory inputs to motor output to produce accurate motor control.35, 36, 37 Early movement studies have shown that motor performance worsens in PD, particularly when patients perform simultaneous and sequential movements. In addition, patients with PD have impaired motor execution that, compared to healthy subjects, worsen dramatically when no external sensory cues are provided.8, 9 Overall these findings may suggest the hypothesis of impaired sensorimotor integration as a pathophysiological mechanism contributing to motor symptoms in PD.8, 9 We therefore speculate that r‐fMV might improve gait by inducing sensory afferent inputs to the CNS able to improve the functional activation of neuronal generators responsible for locomotion in the CNS, through restored sensorimotor integration. This hypothesis agrees with recent experimental studies with epidural electrical stimulation of the spinal cord (SCS) in an animal model of PD. Fuentes et al.38 have demonstrated that SCS improves locomotion in 6‐OHDA animal models of PD.38 Given that SCS consists of a minimally invasive method able to activate superficial fibers of the dorsal columns, and, in turn, ascending pathways in the CNS, it is likely that, in animal models of PD, SCS improves locomotion through restored sensorimotor integration processes.38 Although SCS‐induced gait improvement has not been replicated in a previous pilot study in a small cohort of patients with PD,39 future studies in larger cohorts of patients will clarify whether SCS might help in improving locomotion in PD. Finally, although physiological mechanisms activated by SCS in a PD animal model differ from those responsible for r‐fMV‐induced improvement in gait in patients with PD, we conjecture that both techniques (SCS and r‐fMV) might operate by inducing changes in sensory afferent inputs to neuronal generators responsible for locomotion and posture in the CNS and thus through restored sensorimotor integration.
It is hard to explain which cortical or subcortical structure activated by r‐fMV is responsible for the gait improvement observed here in patients with PD. In healthy subjects, locomotion is thought to reflect the activation of a complex network including several neuronal structures at cortical (primary motor and nonprimary motor areas), basal ganglia, brainstem (including the pedunculopontine nucleus), and spinal level.1, 2, 5, 6, 7 Several lines of evidence suggest that r‐fMV induces changes in sensorimotor organization at multiple levels of the CNS, including sensorimotor cortical areas.14, 15, 16, 22 Although we speculate that in PD, the r‐fMV improves locomotion in PD by possibly restoring sensorimotor integration processes in several neuronal generators responsible for locomotion in the CNS, the specific CNS regions targeted by r‐fMV and responsible for r‐fMV‐induced improvement of locomotion in PD remain unclear.
Another important finding in this study was that the r‐fMV‐induced improvement in mean velocity and stride length was evident not only immediately after 3 days of r‐fMV, but rather lasted around 3 weeks from the intervention. To the best of our knowledge, the present study provides the first evidence of r‐fMV‐induced long‐term improvement of gait observed in PD. The observation that r‐fMV improved gait for at least 1 week might reflect neuroplastic changes in the excitability of neuronal generators responsible for locomotion in the CNS. This hypothesis fully agrees with previous studies demonstrating fMV‐induced long‐term changes in excitability of neuronal generators in several CNS regions, including the sensorimotor cortex and spine.14, 15, 16, 22.
When interpreting our findings in patients with PD, several limitations should be taken into account. The present findings come from a pilot RCT study performed in a relatively small cohort of PD patients with specific clinical features (stable responders to l‐dopa with no motor fluctuation, dyskinesias, and FOG). In addition, in this study, we have not compared gait variables, before and after f‐fMV, in patients with PD and age‐matched healthy controls. This methodological limitation does not allow to clarify whether r‐fMV may improve gait specifically in PD or rather may boost unspecifically physiological mechanisms contributing to gait also in healthy subjects. In our study, although the investigators performing the GA were blind to the type of intervention (real or sham fMV), participants received no effective muscle vibration during the sham fMV, thus not allowing us to fully exclude a placebo effect specifically related to the subjective sensation of skin vibration. We have not collected a standardized clinical scale of gait impairment possibly helpful to identify patients with different gait disorder severity. Finally, although we suggest that mechanisms of sensorimotor integration may contribute to the physiological basis of r‐fMV‐induced gait improvement in patients with PD, this hypothesis remains largely speculative. A final comment is that the relatively small impact of r‐fMV on gait reported here in parkinsonian patients is far from being considered a helpful therapeutic strategy to improve gait in PD. To this purpose, future studies with larger cohorts of healthy subjects and patients with PD and longer sessions of r‐fMV would clarify whether r‐fMV can be considered a useful nonpharmacological strategy for improving parkinsonian gait.
Conclusions
We suggest that r‐FMV is a feasible, safe approach for improving gait disorders in patients with PD. The simplicity of treatment, the lack of side effects, and the positive results observed here support the recommendation to further investigate the application of r‐FMV to enhance the impact of specific rehabilitation programs in PD. Given the relatively small number of patients investigated here and the limitation that researchers were not blinded to the group assignment, future double‐blind, placebo‐controlled, crossover studies in larger cohorts of patients with PD are warranted to confirm the beneficial effect of r‐FMV on gait disorders.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the First Draft, B. Review and Critique.
F.C.: 1A, 1B, 1C, 3A, 3B
C.C.: 1A, 1B, 3A, 3B
A.S.: 1A, 1B, 3A, 3B
M.G.: 1C, 2B, 2C
V.C.: 1C, 2B, 2C
G.M.F.: 1A, 3B
G.L.T.: 2A, 2B, 2C
G.A.: 1A
F.S.: 1A
M.F.D.P.:1B, 1C, 3B
Disclosures
Funding Sources and Conflicts of Interest: The authors report no sources of funding. The authors certify that G.M.F. has a relation of consultancy with the dealer of the device used in this study. None of the remaining researchers has a conflict of interest that could alter the primary interest of this study.
Financial Disclosures for previous 12 months: The authors declare that there are no disclosures to report.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Ebersbach G, Moreau C, Gandor F, Defebvre L, Devos D. Clinical syndromes: parkinsonian gait. Mov Disord 2013;28:1552–1559. [DOI] [PubMed] [Google Scholar]
- 2. Morris ME, Matyas TA, Iansek R, Summers JJ. Temporal stability of gait in Parkinson's disease. Phys Ther 1996;76:763–780. [DOI] [PubMed] [Google Scholar]
- 3. Blin O, Ferrandez AM, Serratrice G. Quantitative analysis of gait in Parkinson patients: increased variability of stride length. J Neurol Sci 1990;98:91–97. [DOI] [PubMed] [Google Scholar]
- 4. Morris ME, Iansek R, Matyas TA, Summers JJ. Stride length regulation in Parkinson's disease. Normalization strategies and underlying mechanism. Brain 1996;119:551–568. [DOI] [PubMed] [Google Scholar]
- 5. Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol 2011;10:734–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kerr GK, Worringham CJ, Cole MH, Lacherez PF, Wood JM, Silburn PA. Predictors of future falls in Parkinson disease. Neurology 2010;75:116–124. [DOI] [PubMed] [Google Scholar]
- 7. Grabli D, Karachi C, Welter ML, et al. Normal and pathological gait: what we learn from Parkinson's disease. J Neurol Neurosurg Psychiatry 2012;83:979–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berardelli A, Rothwell JC, Thompson PD, Hallett M. Pathophysiology of bradykinesia in Parkinson's disease. Brain 2001;124:2131–2146. [DOI] [PubMed] [Google Scholar]
- 9. Mazzoni P, Shabbott B, Cortés JC. Motor control abnormalities in Parkinson's disease. Cold Spring Harb Perspect Med 2012;2:a009282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Nunzio AM, Grasso M, Nardone A, Godi M, Schieppati M. Alternate rhythmic vibratory stimulation of trunk muscles affects walking cadence and velocity in Parkinson's disease. Clin Neurophysiol 2010;121:240–247. [DOI] [PubMed] [Google Scholar]
- 11. Kaelin‐Lang A, Luft AR, Sawaki L, Burstein AH, Sohn YH, Cohen LG. Modulation of human corticomotor excitability by somatosensory input. J Physiol 2002;540:623–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McKay DR, Ridding MC, Thompson PD, Miles TS. Induction of persistent changes in the organization of the human motor cortex. Exp Brain Res 2002;143:342–349. [DOI] [PubMed] [Google Scholar]
- 13. Charlton CS, Ridding MC, Thompson PD, Miles TS. Prolonged peripheral nerve stimulation induces persistent changes in excitability of human motor cortex. J Neurol Sci 2003;208:79–85. [DOI] [PubMed] [Google Scholar]
- 14. Rosenkranz K, Rothwell JC. Differential effect of muscle vibration on intracortical inhibitory circuits in humans. J Physiol 2003;551:649–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rosenkranz K, Rothwell JC. Modulation of proprioceptive integration in the motor cortex shapes human motor learning. J Neurosci 2012;32:9000–9006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Filippi GM, Brunetti O, Botti FM, et al. Improvement of stance control and muscle performance induced by focal muscle vibration in young‐elderly women: a randomized controlled trial. Arch Phys Med Rehabil 2009;90:2019–2025. [DOI] [PubMed] [Google Scholar]
- 17. Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry 1988;51:745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fahn S, Elton RL. UPDRS program members Parkinson's Disease rating scale In: Fahn S, Marsden CD, Goldstein M, Calne DB, eds. Recent Developments in Parkinson's Disease. vol. 2 Florham Park, NJ: Macmillan Healthcare Information; 1987:153–163. [Google Scholar]
- 19. Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427–442. [DOI] [PubMed] [Google Scholar]
- 20. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 21. Courtine G, De Nunzio AM, Schmid M, Beretta MV, Schieppati M. Stance‐ and locomotion‐dependent processing of vibration‐induced proprioceptive inflow from multiple muscles in humans. J Neurophysiol 2007;97:772–779. [DOI] [PubMed] [Google Scholar]
- 22. Marconi B, Filippi GM, Koch G, et al. Long‐term effects on motor cortical excitability induced by repeated muscle vibration during contraction in healthy subjects. J Neurol Sci 2008;275:51–59. [DOI] [PubMed] [Google Scholar]
- 23. Caliandro P, Celletti C, Padua L, et al. Focal muscle vibration in the treatment of upper limb spasticity: a pilot randomized controlled trial in patients with chronic stroke. Arch Phys Med Rehabil 2012;93:1656–1661. [DOI] [PubMed] [Google Scholar]
- 24. Celletti C, Fattorini L, Camerota F, et al. Focal muscle vibration as a possible intervention to prevent falls in elderly women: a pragmatic randomized controlled trial. Aging Clin Exp Res 2015;27:857–863. [DOI] [PubMed] [Google Scholar]
- 25. Hagbarth KE, Eklund G. The effects of muscle vibration in spasticity, rigidity, and cerebellar disorders. J Neurol Neurosurg Psychiatry 1968;31:207–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roll JP, Vedel JP, Ribot E. Alteration of proprioceptive messages induced by tendon vibration in man: a microneurographic study. Exp Brain Res 1989;76:213–222. [DOI] [PubMed] [Google Scholar]
- 27. Davis RB, Ounpuu S, Tyburski DJ, Gage JR. A gait analysis data collection and reduction technique. Hum Mov Sci 1991;10:575–587. [Google Scholar]
- 28. Moher D, Hopewell S, Schulz KF, et al. for the CONSORT Group . CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trial. BMJ. 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Berardelli A, Conte A, Fabbrini G, et al. Pathophysiology of pain and fatigue in Parkinson's disease. Parkinsonism Relat Disord 2012;18(Suppl 1):226–228. [DOI] [PubMed] [Google Scholar]
- 30. Fabbrini G, Latorre A, Suppa A, Bloise M, Frontoni M, Berardelli A. Fatigue in Parkinson's disease: motor or non‐motor symptom? Parkinsonism Relat Disord 2013;19:148–152. [DOI] [PubMed] [Google Scholar]
- 31. Fabbrini G, Defazio G, Colosimo C, Suppa A, Bloise M, Berardelli A. Onset and spread of dyskinesias and motor symptoms in Parkinson's disease. Mov Disord 2009;24:2091–2096. [DOI] [PubMed] [Google Scholar]
- 32. De la Fuente‐Fernández R, Schulzer M, Stoessl AJ. The placebo effect in neurological disorders. Lancet Neurol 2002;1:85–91. [DOI] [PubMed] [Google Scholar]
- 33. Meunier S, Pol S, Houeto JL, Vidailhet M. Abnormal reciprocal inhibition between antagonist muscles in Parkinson's disease. Brain 2000;123:1017–1026. [DOI] [PubMed] [Google Scholar]
- 34. Simonetta Moreau M, Meunier S, Vidailhet M, Pol S, Galitzky M, Rascol O. Transmission of group II heteronymous pathways is enhanced in rigid lower limb of de novo patients with Parkinson's disease. Brain. 2002;125:2125–2133. [DOI] [PubMed] [Google Scholar]
- 35. Abbruzzese G, Berardelli A. Sensorimotor integration in movement disorders. Mov Disord 2003;18:231–240. [DOI] [PubMed] [Google Scholar]
- 36. Suppa A, Biasiotta A, Belvisi D, et al. Heat‐evoked experimental pain induces long‐term potentiation‐like plasticity in human primary motor cortex. Cereb Cortex 2013;23:1942–1951. [DOI] [PubMed] [Google Scholar]
- 37. Suppa A, Li Voti P, Rocchi L, Papazachariadis O, Berardelli A. Early visuomotor integration processes induce LTP/LTD‐like plasticity in the human motor cortex. Cereb Cortex. 2013;25:703–712. [DOI] [PubMed] [Google Scholar]
- 38. Fuentes R, Petersson P, Nicolelis MA. Restoration of locomotive function in Parkinson's disease by spinal cord stimulation: mechanistic approach. Eur J Neurosci 2010;32:1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thevathasan W, Mazzone P, Jha A, et al. Spinal cord stimulation failed to relieve akinesia or restore locomotion in Parkinson disease. Neurology 2010;74:1325–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hobson DE, Lang AE, Martin WR, Razmy A, Rivest J, Fleming J. Excessive daytime sleepiness and sudden‐onset sleep in Parkinson disease: a survey by the Canadian Movement Disorders Group. JAMA 2002;287:455–463. [DOI] [PubMed] [Google Scholar]


