Abstract
Stroke is an acute disease with extremely high mortality and disability, including ischemic stroke and hemorrhagic stroke. Currently only limited drugs and treatments have been shown to have neuroprotective effects in stroke. As a medical gas, xenon has been proven to have neuroprotective effect in considerable amount of previous study. Its unique properties are different from other neuroprotective agents, making it is promising to play a special therapeutic role in stroke, either alone or in combination with other treatments. In this article, we aim to review the role of xenon in the treatment of stroke, and summarize the mechanism of using xenon to produce therapeutic effects after stroke according to the existing research. Moreover, we intend to explore and demonstrate the feasibility and safety of xenon for clinical treatment of stroke. Despite the disadvantages of difficulty in obtaining and being expensive, as long as the use of reasonable methods, xenon can play an important role in the treatment of stroke.
Keywords: stroke, xenon, ischemia, neuroprotective effects, brain diseases
INTRODUCTION
Stroke, a sudden neurological deficit caused by vessel dysfunction, is a clinical syndrome rather than a single disease.1 Usually, there are two types of stroke, ischemia stroke (55–90%) and hemorrhagic stroke (12–35%).2 Subarachnoid hemorrhage is a special type of hemorrhagic stroke because of its significant morbidity and mortality rate,3 although it accounts for only 5% of stroke.4 According to the report, stroke is the fifth cause of death in America and become the leading medical cause of acquired adult disability worldwide.5,6,7 Especially in low and middle income countries, the incidence of stroke continues to increase–accounting for 85% of global stroke burden.8 In China, it was reported that 837,300 urban residents and 1,023,400 rural residents died from stroke in 2014 alone.9 So far, plenty of studies have been conducted, intend to improve the prognosis of stroke and many substances as well as therapies have been discovered and applied.10 Among them, medical gas has received the attention of researchers because of its special physical properties.11,12
Recently, many studies suggest that a variety of medical gases may serve as neuroprotective agents against stroke.13 Xenon is anodorless and colorless noble gas,14 which was firstly used in surgical anesthesia by Stuart C. Cullen and Gross in 1951.15,16 As a kind of volatile anesthetics, xenon has been studied decades because of its potential value in neuroprotective area. Plenty of previous research has shown that xenon can play a protective role in models of hypoxic-ischemic insults, as well as subarachnoid hemorrhage.17,18,19,20 In this article, we intend to summarize the role of xenon in stroke and its mechanisms as well as the possible clinical application forms based on current research.
MECHANISM OF PROTECTIVE EFFECTS OF XENON IN STROKE
The most widely studied and well-known mechanism of xenon in neuroprotective area is N-methyl-d-aspartate (NMDA) receptor glycine site antagonism.21 By interacting with the aromatic ring of phenylalanine 758,22 xenon can binds to the NMDA receptor as other anesthetic gas such as isoflurane. Unlike isoflurane, helium has a non-competitive inhibition in addition to competitive inhibition.23
NMDA receptor is one of the two subtypes of ionotropic glutamate receptors, the another one is α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor. After stroke, multiple factors include lack of oxygen and nutrients, protein function damage lead to the accumulation of excitatory glutamate in extracellular space,24 which result in the over-activation of NMDA receptor. This process was called excitotoxicity and has been considered as one of the most important mechanisms of nerve injury after stroke.25,26
Once the NMDA receptor was activated, excessive Na+ and Ca2+ can flux into the cell, activate a series of downstream pathways. Such as the activation of calpain,27 nitric oxide (NO) production,28 generation of reactive oxygen species (ROS).29,30 Further causes of neuronal cell dysfunction and death are diverse, including but not limited to mitochondrial damage, DNA damage, cell membrane destruction.
However, several NMDA receptor antagonists has failed to show efficacy in clinical trials.31 The main possible factors affecting the efficacy are the following two points. According to the receptor location hypothesis, activation of NMDA receptors can trigger pro-survival or pro-death signaling depending on the subcellular locations or subtypes of NMDA receptors.24 Most NMDA antagonists could not target on the extrasynaptic NMDA receptor, which result in these antagonists produce adverse side effects or even neurotoxicity at neuroprotective concentrations.32 Moreover, for these agents, it is too hard to achieve the brain damage area immediately because of the existence of the blood-brain barrier (BBB). This makes the peak of drug concentration could not appear simultaneously with the peak of glutamate in the extracellular space, while it has been proved that delayed and prolonged blockade of NMDA receptors might be harmful.33,34 As a low-affinity use-dependent NMDA receptor antagonist, xenon has low blood-gas partition coefficient which is 0.115.35 Moreover, xenon can quickly pass the BBB after inhalation. These characteristics allow xenon could rapidly accumulate in the stroke area and be rapidly metabolized by the body when the administration is stopped. For the same reasons, xenon was also used in anesthesia.
In addition to blockade of NMDA receptors, another mechanism by which xenon plays a therapeutic role in stroke is the activation of two-pore domain potassium channel TWIK-related K+ channel-1 (TREK-1).36 The study which was held by Gruss et al.36 showed that at the concentration of 60% for xenon, 25% of TREK-1 was activated. Formal experiments have shown that TREK-1 channel has neuroprotective properties in ischemia.37
TREK-1 is a background potassium channel existed throughout the central nervous system,38,39,40 and is a member of two-pore-domain potassium channels family. The open of TREK-1 in the physiological range lets potassium ions out of the cell membrane, increases the negative charge in neurons and therefore contributes to the background as well as opposes depolarizing influences. By opening TREK-1 channel, xenon is able to inhibit the activation of voltage-dependent Ca2+ channels on the presynaptic membrane, which usually induce the release of glutamate after stroke, as well as enhance the blocking of NMDA receptors. By this way, xenon further suppressed the development of excitotoxicity (Figure 1).
Figure 1.
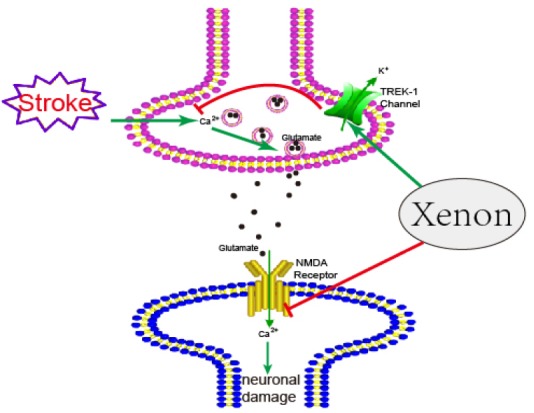
The mechanism of protective effects of xenon in stroke.
Note: NMDA: N-methyl-d-aspartate; TREK-1: TWIK-related K+ channel 1.
Another possible mechanism found by Bante et al.41 and his colleague is the activation of adenosine triphosphate-sensitive potassium (KATP) channel, which was considered play a key role in neuroprotective pathways. The pore-forming subunit Kir6.2 of KATP channel was found has a protective effect in ischemic brain injury.42 Traditional KATP channel openers including nicorandil and diazoxide could not penetrate through the BBB, which limits their implication in neuroprotective area. Compared with other KATP channel openers, xenon can diffuse through the BBB easily and is able to activate the KATP channel without the help of Mg2+. Moreover, xenon enhanced the KATP current by highly specific activate the Kir6.2 subunit, which means the effect of xenon is direct and precise.
In subarachnoid hemorrhage (SAH), it was found that xenon-treated animals presented with a milder damage, especially in the hippocampus, CA3 as well as dentate gyrus (DG).43 The possible mechanism postulated by Veldeman et al.43 is that xenon can reduce microglial activation by some still unknown immunomodulatory pathway. It has been proved that microglia activation inflicts delayed brain injury after subarachnoid hemorrhage. The NMDA receptor on microglial was presumed to be the trigger of this pathway and there is some evidence to support this hypothesis.44,45
There are also some other biological effects of xenon in the central nervous system that have not been proven in stroke. Shichino et al.46 showed that in early stage, xenon induced an initial increase in acetylcholine release while it was a gradual decrease later, which means that xenon can act on not only receptors but also the neurotransmitter. Franks et al.47 found that xenon inhibited calcium ATPase pump activity in rat brain synaptic plasma membranes, resulting in an increase in neuronal Ca2+ concentration and a decrease in neuronal excitability. It was also reported that xenon reduced whole-brain metabolic rate of glucose as well as regional cerebral blood flow, which may have help in the reduction of Intracranial pressure and enhance the brain protection effect.
EXPERIMENTAL STUDIES OF NITROUS OXIDE IN STROKE
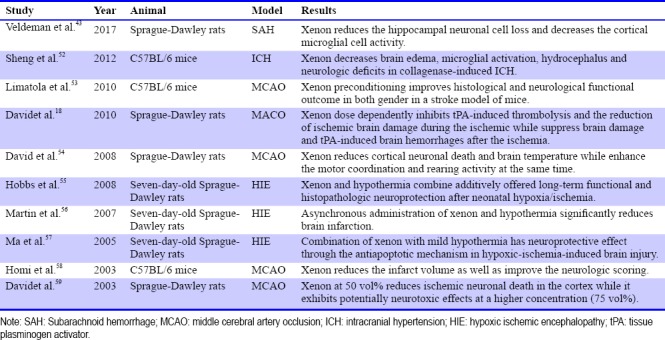
Since xenon is proved to be an NMDA antagonist, a considerable amount of study has been brought out to find out whether xenon has neuroprotective function or not. As the most important and unique disease in neurological diseases, the role of xenon in stroke has attracted growing attention of researchers. Most experiments have demonstrated the protective role of xenon in stroke, whether in ischemia or hemorrhage. But there are also some experimental results that are contradictory. It was discovered that xenon can inhibit the catalytic efficiency of tissue plasminogenactivator (tPA), which is widely used in the treatment of ischemia stroke.18,48 Because of this mechanism, xenon has been proven to be unsuitable to be used with tPA at the same time in the treatment of ischemic stroke. However, tPA also has side effects such as the BBB disruption in the acute stroke.49 It was also shown that xenon can help prevent from the hemorrhage as well as BBB dysfunction induced by tPA when it was used after ischemic.50 As other anesthetic gases do, xenon has the function of lowering body temperature. As it was proved that therapeutic hypothermia is a promising treatment of stroke,51 some researchers intend to find out whether xenon can exert a synergistic effect with hypothermia in stroke (Table 1).
Table 1.
Study on the role of xenon in stroke
CLINICAL STUDIES
Despite the lack of direct clinical trials of xenon in the treatment of stroke patients, several related clinical studies have yielded encouraging results. Laitio et al.60 from Turku University Hospital have conducted a randomized clinical trial to figure out the role of xenon in brain ischemia damage in cardiac arrest patients. 110 patients who had experienced out-of-hospital cardiac arrest were randomly assigned to receive hypothermia treatment alone or either inhaled xenon combined with hypothermia for 24 hours.60 According to this research, patients who inhaled xenon combined with hypothermia have less damage in white matter compared with to those patients who received hypothermia treat alone. It was also proved that xenon combined with hypothermia is feasible in the treatment of brain ischemia and has favorable cardiac features.61 Another common condition of cerebral ischemia and hypoxia is in newborns. Investigators from University of Bristol and St Michael's Hospital have conducted a clinical trial to examine the treat effect of inhaled xenon in the newborn infants with hypoxic-ischemic encephalopathy in combination with cooling (ClinicalTrials.gov, NCT02071394). It was assumed that the xenon plus cooling will produce better neuroprotection than the standard treatment of cooling alone in newborn infants. Yet there are no clinical studies to prove this, in vitro experiments from rats and newborn pigs have confirmed this hypothesis.55,62,63 The combination of xenon and therapeutic hypothermia is a promising therapy in brain ischemia and hypoxia.
GAS DELIVERY METHODS
As a kind of trace element in atmosphere, it is extremely expensive to separate xenon from air and it is still impossible for Industrial synthesis for now. As the result, the cost of xenon is around 10 $/L64 and the price of inhaling xenon is 150$/h,65 far higher than other anesthetic gases or neuroprotective agents. High prices and low yields are two important reasons hindering the clinical application of xenon. Dr. Dingley et al.66 from Swansea University has built up a closed-circuit xenon delivery system which can recirculate gases, remove CO2 and add O2 at the same time. Since xenon can be recycled in this system, with the help of this gas transmission route, the cost of applying xenon is greatly reduced. It was also reported that standard anesthesia workstation can be used to delivery xenon in a closed-circuit,67 although its pharmacokinetics still needs further exploration.68
Another promising method to deliver xenon is the use of echogenic liposomes (ELIP). Xenon can be encapsulated into ELIP with the help of pressurization-freeze method.69 After Intravenous injection of Xenon-ELIP, ultrasound can be used to trigger Xenon release into the internal carotid artery. The use of this technology can effectively transmit xenon into the central nervous system with higher efficiency and better targeting. It has been reported that in ischemic stroke, use of Xenon-ELIP in 5 hours can significantly reduce the infract size, the dose range of the maximum therapeutic effect is of 7–14 mg/kg.70 Similar, Xenon-ELIP also exerts a great neuroprotective effect in the model of subarachnoid hemorrhage.71
CONCLUSION AND FURTHER STUDIES
It has been proved that xenon has a bright future in the treatment of stroke. Despite the lack of sufficient clinical evidence, due to its unique chemistry properties and excellent performance in various experiments, we have reason to believe that xenon can play an important role in stroke therapy. However, there are still many difficulties that need to be further overcame. Firstly, the production process of xenon is too complicated, as well as the method of clinical use. Secondly, the exact mechanism of action and the molecular biology pathway of xenon in stroke and neuroprotection, remains to be further studied. Last but not least, a large number of clinical trials and data are still needed to demonstrate the effectiveness and safety of xenon in stroke.
Footnotes
Conflicts of interest
None.
Financial support
None.
Copyright license agreement
The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
C-Editor: Yang LJ, Zhao M; S-Editor: Yu J; L-Editor: Wang L; T-Editor: Jia Y
REFERENCES
- 1.Bath PM, Lees KR. ABC of arterial and venous disease. Acute stroke. BMJ. 2000;320:920–923. doi: 10.1136/bmj.320.7239.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8:355–369. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 3.Hanel RA, Xavier AR, Mohammad Y, Kirmani JF, Yahia AM, Qureshi AI. Outcome following intracerebral hemorrhage and subarachnoid hemorrhage. Neurol Res. 2002;24(Suppl 1):S58–62. doi: 10.1179/016164102101200041. [DOI] [PubMed] [Google Scholar]
- 4.Zacharia BE, Hickman ZL, Grobelny BT, et al. Epidemiology of aneurysmal subarachnoid hemorrhage. Neurosurg Clin N Am. 2010;21:221–233. doi: 10.1016/j.nec.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 6.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 7.Chai Q, Yuan Z, Jin Y, Zhang Q. Factors influencing acceptance of disability among stroke patients in Tianjin, China: A cross-sectional study. NeuroRehabilitation. 2016;38:37–44. doi: 10.3233/NRE-151293. [DOI] [PubMed] [Google Scholar]
- 8.O’Donnell MJ, Xavier D, Liu L, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376:112–123. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- 9.Chen WW, Gao RL, Liu LS, et al. China cardiovascular diseases report 2015: a summary. J Geriatr Cardiol. 2017;14:1–10. doi: 10.11909/j.issn.1671-5411.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kraglund KL, Mortensen JK, Grove EL, Johnsen SP, Andersen G. TALOS: a multicenter, randomized, double-blind, placebo-controlled trial to test the effects of citalopram in patients with acute stroke. Int J Stroke. 2015;10:985–987. doi: 10.1111/ijs.12485. [DOI] [PubMed] [Google Scholar]
- 11.Roffe C, Nevatte T, Crome P, et al. The Stroke Oxygen Study (SO2S) - a multi-center, study to assess whether routine oxygen treatment in the first 72 hours after a stroke improves long-term outcome: study protocol for a randomized controlled trial. Trials. 2014;15:99. doi: 10.1186/1745-6215-15-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li YS, Shemmer B, Stone E, A Nardi M, Jonas S, Quartermain D. Neuroprotection by inhaled nitric oxide in a murine stroke model is concentration and duration dependent. Brain Res. 2013;1507:134–145. doi: 10.1016/j.brainres.2013.02.031. [DOI] [PubMed] [Google Scholar]
- 13.Deng J, Lei C, Chen Y, et al. Neuroprotective gases--fantasy or reality for clinical use? Prog Neurobiol. 2014 Apr;115:210–245. doi: 10.1016/j.pneurobio.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Ballentine CJ, Barry PH. Noble gases. In: White W, editor. Encyclopedia of Geochemistry Encyclopedia of Earth Sciences Series. Cham: Springer; 2017. [Google Scholar]
- 15.Cullen SC, Gross EG. The anesthetic properties of xenon in animals and human beings, with additional observations on krypton. Science. 1951;113:580–582. doi: 10.1126/science.113.2942.580. [DOI] [PubMed] [Google Scholar]
- 16.Cullen SC, Eger EI 2nd, Cullen BF, Gregory P. Observations on the anesthetic effect of the combination of xenon and halothane. Anesthesiology. 1969;31:305–309. doi: 10.1097/00000542-196910000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Haelewyn B, David HN, Blatteau JE, et al. Modulation by the noble gas helium of tissue plasminogen activator: effects in a rat model of thromboembolic stroke. Crit Care Med. 2016;44:e383–389. doi: 10.1097/CCM.0000000000001424. [DOI] [PubMed] [Google Scholar]
- 18.David HN, Haelewyn B, Risso JJ, Colloc’ HN, Abraini JH. Xenon is an inhibitor of tissue-plasminogen activator: adverse and beneficial effects in a rat model of thromboembolic stroke. J Cereb Blood Flow Metab. 2010;30:718–728. doi: 10.1038/jcbfm.2009.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan Y, Zhang H, Acharya AB, Cruz-Flores S, Panneton WM. The effect of heliox treatment in a rat model of focal transient cerebral ischemia. Neurosci Lett. 2011;497:144–147. doi: 10.1016/j.neulet.2011.04.048. [DOI] [PubMed] [Google Scholar]
- 20.Miao YF, Peng T, Moody MR, et al. Delivery of xenon-containing echogenic liposomes inhibits early brain injury following subarachnoid hemorrhage. Sci Rep. 2018;8:450. doi: 10.1038/s41598-017-18914-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franks NP, Dickinson R, de Sousa SL, Hall AC, Lieb WR. How does xenon produce anaesthesia? Nature. 1998;396:324. doi: 10.1038/24525. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong SP, Banks PJ, McKitrick TJ, et al. Identification of two mutations (F758W and F758Y) in the N-methyl-D-aspartate receptor glycine-binding site that selectively prevent competitive inhibition by xenon without affecting glycine binding. Anesthesiology. 2012;117:38–47. doi: 10.1097/ALN.0b013e31825ada2e. [DOI] [PubMed] [Google Scholar]
- 23.Dickinson R, Peterson BK, Banks P, et al. Competitive inhibition at the glycine site of the N-methyl-D-aspartate receptor by the anesthetics xenon and isoflurane: evidence from molecular modeling and electrophysiology. Anesthesiology. 2007;107:756–767. doi: 10.1097/01.anes.0000287061.77674.71. [DOI] [PubMed] [Google Scholar]
- 24.Wu QJ, Tymianski M. Targeting NMDA receptors in stroke: new hope in neuroprotection. Mol Brai. 2018;11:15. doi: 10.1186/s13041-018-0357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- 26.Lai TW, Shyu WC, Wang YT. Stroke intervention pathways: NMDA receptors and beyond. Trends Mol Med. 2011;17:266–275. doi: 10.1016/j.molmed.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Xu J, Kurup P, Zhang Y, et al. Extrasynaptic NMDA receptors couple preferentially to excitotoxicity via calpain-mediated cleavage of STEP. J Neurosci. 2009;29:9330–9343. doi: 10.1523/JNEUROSCI.2212-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solaroglu I, Solaroglu A, Kaptanoglu E, et al. Erythropoietin prevents ischemia-reperfusion from inducing oxidative damage in fetal rat brain. Childs Nerv Syst. 2003;19:19–22. doi: 10.1007/s00381-002-0680-2. [DOI] [PubMed] [Google Scholar]
- 29.Lau A, Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch. 2010;460:525–542. doi: 10.1007/s00424-010-0809-1. [DOI] [PubMed] [Google Scholar]
- 30.Kristian T, Siesjo BK. Calcium in ischemic cell death. Stroke. 1998;29:705–718. doi: 10.1161/01.str.29.3.705. [DOI] [PubMed] [Google Scholar]
- 31.Ikonomidou C, Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002;1(6):383–386. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 32.Olney JW, Labruyere J, Wang G, Wozniak DF, Price MT, Sesma MA. NMDA antagonist neurotoxicity: mechanism and prevention. Science. 1991;254:1515–1518. doi: 10.1126/science.1835799. [DOI] [PubMed] [Google Scholar]
- 33.Hoyte L, Barber PA, Buchan AM, Hill MD. The rise and fall of NMDA antagonists for ischemic stroke. Curr Mol Med. 2004;4:131–136. doi: 10.2174/1566524043479248. [DOI] [PubMed] [Google Scholar]
- 34.Ovbiagele B, Kidwell CS, Starkman S, Saver JL. Neuroprotective agents for the treatment of acute ischemic stroke. Curr Neurol Neurosci Rep. 2003;3:9–20. doi: 10.1007/s11910-003-0031-z. [DOI] [PubMed] [Google Scholar]
- 35.Sanders RD, Maze M. Xenon: from stranger to guardian. Curr Opin Anaesthesiol. 2005;18:405–411. doi: 10.1097/01.aco.0000174957.97759.f6. [DOI] [PubMed] [Google Scholar]
- 36.Gruss M, Bushell TJ, Bright DP, Lieb WR, Mathie A, Franks NP. Two-pore-domain K+ channels are a novel target for the anesthetic gases xenon, nitrous oxide, and cyclopropane. Mol Pharmacol. 2004;65:443–452. doi: 10.1124/mol.65.2.443. [DOI] [PubMed] [Google Scholar]
- 37.Heurteaux C, Guy N, Laigle C, et al. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 2004;23:2684–2695. doi: 10.1038/sj.emboj.7600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fink M, Duprat F, Lesage F, et al. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J. 1996;15:6854–6862. [PMC free article] [PubMed] [Google Scholar]
- 39.Talley EM, Solorzano G, Lei Q, Kim D, Bayliss DA. Cns distribution of members of the two-pore-domain (KCNK) potassium channel family. J Neurosci. 2001;21:7491–7505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hervieu GJ, Cluderay JE, Gray CW, et al. Distribution and expression of TREK-1, a two-pore-domain potassium channel, in the adult rat CNS. Neuroscience. 2001;103:899–919. doi: 10.1016/s0306-4522(01)00030-6. [DOI] [PubMed] [Google Scholar]
- 41.Bantel C, Maze M, Trapp S. Noble gas xenon is a novel adenosine triphosphate-sensitive potassium channel opener. Anesthesiology. 2010;112:623–630. doi: 10.1097/ALN.0b013e3181cf894a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamada K, Ji JJ, Yuan H, et al. Protective role of ATP-sensitive potassium channels in hypoxia-induced generalized seizure. Science. 2001;292:1543–1546. doi: 10.1126/science.1059829. [DOI] [PubMed] [Google Scholar]
- 43.Veldeman M, Coburn M, Rossaint R, et al. Xenon reduces neuronal hippocampal damage and alters the pattern of microglial activation after experimental subarachnoid hemorrhage: a randomized controlled animal trial. Front Neurol. 2017;8:511. doi: 10.3389/fneur.2017.00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaindl AM, Degos V, Peineau S, et al. Activation of microglial N-methyl-D-aspartate receptors triggers inflammation and neuronal cell death in the developing and mature brain. Ann Neurol. 2012;72:536–549. doi: 10.1002/ana.23626. [DOI] [PubMed] [Google Scholar]
- 45.Yang S, Jin H, Zhu Y, et al. Diverse functions and mechanisms of pericytes in ischemic stroke. Curr Neuropharmacol. 2017;15:892–905. doi: 10.2174/1570159X15666170112170226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shichino T, Murakawa M, Adachi T, et al. Effects of xenon on acetylcholine release in the rat cerebral cortex in vivo. Br J Anaesth. 2002;88:866–868. doi: 10.1093/bja/88.6.866. [DOI] [PubMed] [Google Scholar]
- 47.Franks JJ, Horn JL, Janicki PK, Singh G. Halothane, isoflurane, xenon, and nitrous oxide inhibit calcium ATPase pump activity in rat brain synaptic plasma membranes. Anesthesiology. 1995;82:108–117. doi: 10.1097/00000542-199501000-00015. [DOI] [PubMed] [Google Scholar]
- 48.Haelewyn B, David HN, Colloc’h N, Colomb DG Jr, Risso JJ, Abraini JH. Interactions between nitrous oxide and tissue plasminogen activator in a rat model of thromboembolic stroke. Anesthesiology. 2011;115:1044–1053. doi: 10.1097/ALN.0b013e3182342860. [DOI] [PubMed] [Google Scholar]
- 49.Ding G, Zhang Z, Chopp M, et al. MRI evaluation of BBB disruption after adjuvant AcSDKP treatment of stroke with tPA in rat. Neuroscience. 2014;271:1–8. doi: 10.1016/j.neuroscience.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.David HN, Haelewyn B, Blatteau JE, Risso JJ, Vallee N, Abraini JH. Xenon-helium gas mixture at equimolar concentration of 37.5% protects against oxygen and glucose deprivation-induced injury and inhibits tissue plasminogen activator. Med Gas Res. 2017;7:181–185. doi: 10.4103/2045-9912.215747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ovesen C, Brizzi M, Pott FC, et al. Feasibility of endovascular and surface cooling strategies in acute stroke. Acta Neurol Scand. 2013;127:399–405. doi: 10.1111/ane.12059. [DOI] [PubMed] [Google Scholar]
- 52.Sheng SP, Lei B, James ML, et al. Xenon neuroprotection in experimental stroke: interactions with hypothermia and intracerebral hemorrhage. Anesthesiology. 2012;117:1262–1275. doi: 10.1097/ALN.0b013e3182746b81. [DOI] [PubMed] [Google Scholar]
- 53.Limatola V, Ward P, Cattano D, et al. Xenon preconditioning confers neuroprotection regardless of gender in a mouse model of transient middle cerebral artery occlusion. Neuroscience. 2010;165:874–881. doi: 10.1016/j.neuroscience.2009.10.063. [DOI] [PubMed] [Google Scholar]
- 54.David HN, Haelewyn B, Rouillon C, et al. Neuroprotective effects of xenon: a therapeutic window of opportunity in rats subjected to transient cerebral ischemia. FASEB J. 2008;22:1275–1286. doi: 10.1096/fj.07-9420com. [DOI] [PubMed] [Google Scholar]
- 55.Hobbs C, Thoresen M, Tucker A, Aquilina K, Chakkarapani E, Dingley J. Xenon and hypothermia combine additively, offering long-term functional and histopathologic neuroprotection after neonatal hypoxia/ischemia. Stroke. 2008;39:1307–1313. doi: 10.1161/STROKEAHA.107.499822. [DOI] [PubMed] [Google Scholar]
- 56.Martin JL, Ma D, Hossain M, et al. Asynchronous administration of xenon and hypothermia significantly reduces brain infarction in the neonatal rat. Br J Anaesth. 2007;98:236–240. doi: 10.1093/bja/ael340. [DOI] [PubMed] [Google Scholar]
- 57.Ma D, Hossain M, Chow A, et al. Xenon and hypothermia combine to provide neuroprotection from neonatal asphyxia. Ann Neurol. 2005;58:182–193. doi: 10.1002/ana.20547. [DOI] [PubMed] [Google Scholar]
- 58.Homi HM, Yokoo N, Ma D, et al. The neuroprotective effect of xenon administration during transient middle cerebral artery occlusion in mice. Anesthesiology. 2003;99:876–881. doi: 10.1097/00000542-200310000-00020. [DOI] [PubMed] [Google Scholar]
- 59.David HN, Leveille F, Chazalviel L, et al. Reduction of ischemic brain damage by nitrous oxide and xenon. J Cereb Blood Flow Metab. 2003;23:1168–1173. doi: 10.1097/01.WCB.0000087342.31689.18. [DOI] [PubMed] [Google Scholar]
- 60.Laitio R, Hynninen M, Arola O, et al. Effect of inhaled xenon on cerebral white matter damage in comatose survivors of out-of-hospital cardiac arrest: a randomized clinical trial. JAMA. 2016;315:1120–1128. doi: 10.1001/jama.2016.1933. [DOI] [PubMed] [Google Scholar]
- 61.Arola OJ, Laitio RM, Roine RO, et al. Feasibility and cardiac safety of inhaled xenon in combination with therapeutic hypothermia following out-of-hospital cardiac arrest. Crit Care Med. 2013;41:2116–2124. doi: 10.1097/CCM.0b013e31828a4337. [DOI] [PubMed] [Google Scholar]
- 62.Thoresen M, Hobbs CE, Wood T, Chakkarapani E, Dingley J. Cooling combined with immediate or delayed xenon inhalation provides equivalent long-term neuroprotection after neonatal hypoxia-ischemia. J Cereb Blood Flow Metab. 2009;29:707–714. doi: 10.1038/jcbfm.2008.163. [DOI] [PubMed] [Google Scholar]
- 63.Chakkarapani E, Dingley J, Liu X, et al. Xenon enhances hypothermic neuroprotection in asphyxiated newborn pigs. Ann Neurol. 2010;68:330–341. doi: 10.1002/ana.22016. [DOI] [PubMed] [Google Scholar]
- 64.Lynch C 3rd, Baum J, Tenbrinck R. Xenon anesthesia. Anesthesiology. 2000;92:865–868. doi: 10.1097/00000542-200003000-00031. [DOI] [PubMed] [Google Scholar]
- 65.Baumert JH. Xenon-based anesthesia: theory and practice. Open Access Surg. 2009;2:5–13. [Google Scholar]
- 66.Dingley J, Tooley J, Liu X, et al. Xenon ventilation during therapeutic hypothermia in neonatal encephalopathy: a feasibility study. Pediatrics. 2014;133:809–818. doi: 10.1542/peds.2013-0787. [DOI] [PubMed] [Google Scholar]
- 67.Rawat S, Dingley J. Closed-circuit xenon delivery using a standard anesthesia workstation. Anesth Analg. 2010;110:101–109. doi: 10.1213/ANE.0b013e3181be0e17. [DOI] [PubMed] [Google Scholar]
- 68.Ye ZH, Sun XJ. What's new in Medical Gas Research: Highlights for 2015. Med Gas Res. 2016;6:167–168. doi: 10.4103/2045-9912.191363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Britton GL, Kim H, Kee PH, et al. In vivo therapeutic gas delivery for neuroprotection with echogenic liposomes. Circulation. 2010;122:1578–1587. doi: 10.1161/CIRCULATIONAHA.109.879338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peng T, Britton GL, Kim H, et al. Therapeutic time window and dose dependence of xenon delivered via echogenic liposomes for neuroprotection in stroke. CNS Neurosci Ther. 2013;19:773–784. doi: 10.1111/cns.12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miao YF, Peng T, Moody MR, et al. Delivery of xenon-containing echogenic liposomes inhibits early brain injury following subarachnoid hemorrhage. Sci Rep. 2018;8:450. doi: 10.1038/s41598-017-18914-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


