The worldwide prevalence of late onset ataxia is approximately 10.2 of 100,000 people.1 Several scales have been developed to rate ataxias. The International Cerebellar Ataxia Rating Scale (ICARS) and the Scale for Assessment and Rating of Ataxia (SARA) are the two best‐known, validated, and broadly used rating scales. There is apparently no clear advantage of one in relation to the other.2 ICARS and SARA are long and time‐consuming, and they become impractical in a wider clinical‐practice context. Schmahmann et al.3 developed the Brief Ataxia Rating Scale (BARS), a shorter scale created from a MICARS, a modified ICARS that includes seven more tests in kinetic function, speech disorders, and oculomotor function. The objectives of this study were to translate MICARS and BARS in Brazilian Portuguese and to validate them by correlating with SARA in a heterogeneous group of patients with cerebellar ataxia.
Methods
The first step of this study was to translate both MICARS and BARS into Brazilian Portuguese. SARA has been previously validated in this language.4 Both MICARS and BARS were translated by two movement disorders specialists, who were native speakers of Brazilian Portuguese and were fluent in English. One consensus translation was performed based on the two previous independent translations (S.C., R.A.). The consensus translation was submitted to a back translation (R.M.). Consensus translation and back translation were compared with the original text and the necessary adjustments were made to create a comprehensive and verisimilar text (see Appendix S1). We then enrolled 35 consecutive ataxia patients from the UFMG Movement Disorders Clinic. All MICARS, SARA, and BARS tests were video recorded and two independent raters scored all patients. Statistical analyses were performed using IBM SPSS Statistics software package (version 23.0). Pearson correlation was used to measure the correlation between scales. As described by Schmahmann et al.,3 we also tested whether the five items of BARS correlated with MICARS minus those five items. Cronbach's alpha was used to test the internal consistency of the scores. Intraclass correlation coefficient (ICC) was used to evaluate the inter‐rater reliability of MICARS and BARS. Bland‐Altman plots were used to calculate the mean bias and the 95% limits of agreement between raters in both scales. The ethics committee of the institution approved the study, and all patients signed the informed consent.
Results
Of 35 patients, 18 were female (51%). Age at assessment varied from 16 to 73 years (44.8 ± 15.7 years) and age at onset was 5 to 72 years (35.9 ± 17.1 years). Disease duration ranged from 1 to 43 years (8.9 ± 8.6 years). A total of 65.7% were diagnosed with hereditary ataxia (SCA2 and 3, mitochondrial ataxia, Friedreich ataxia, and unknown recessive ataxia), 25.7% were diagnosed with autoimmune disorders, vasculopathies, multiple system atrophy, vitamin deficiency, and alcoholic cerebellar degeneration. Undetermined causes accounted for 8.6% of patients. Scores from [MICARS] correlated with [BARS] (r = 0.93; P = 0.0001) and [MICARS minus BARS] highly correlated with [BARS] (r = 0.90; P < 0.0001). SARA correlated with both BARS (r = 0.91) and MICARS (r = 0.97). Internal consistency (Cronbach's alpha) was 0.934 for MICARS and 0.972 for BARS. ICC was 0.98 for MICARS and 0.94 for BARS. The Bland‐Altman plots of inter‐rater agreement for MICARS and BARS are shown in Figures 1 and 2. Scores tended to distribute symmetrically around the mean difference of scores, and only two cases were outside the limits of agreement for each scale.
Figure 1.
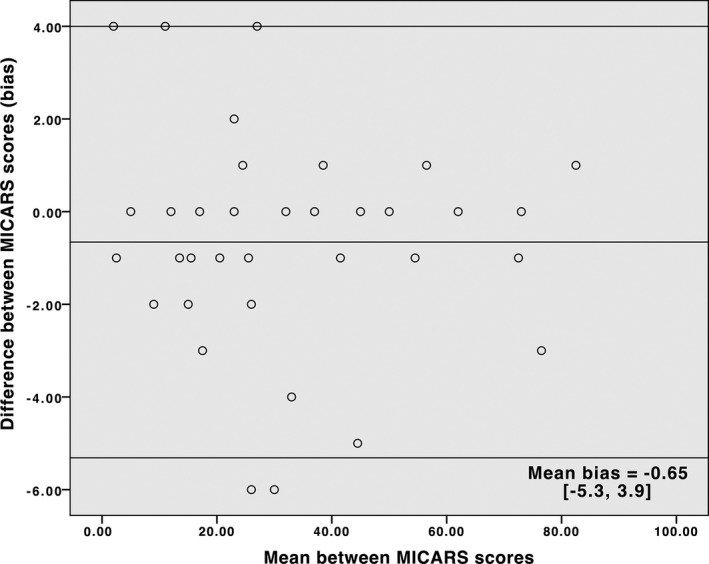
Bland‐Altman Plot for MICARS. The lines represent the mean bias and the 95% limits of agreement.
Figure 2.
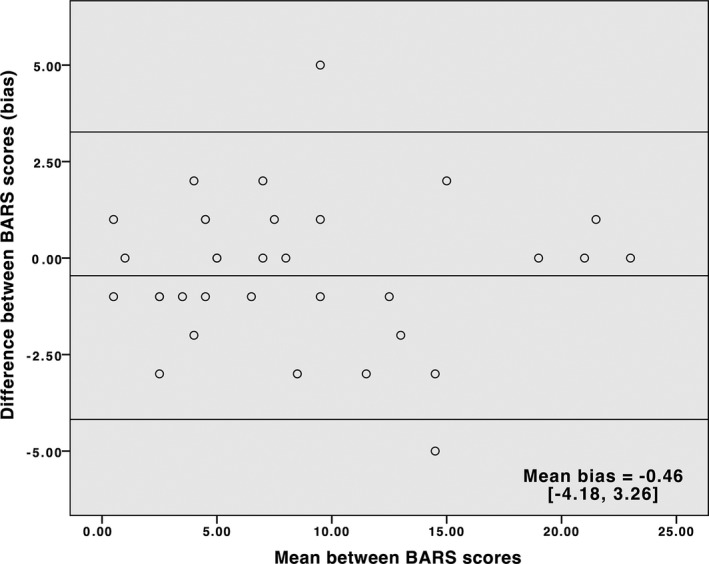
Bland‐Altman Plot for BARS. The lines represent the mean bias and the 95% limits of agreement.
Discussion
Following scale translation into Brazilian Portuguese, we were able to demonstrate that the brief scale (BARS) highly correlates with MICARS and SARA with an elevated internal consistency and optimal reliability. BARS has some advantages: it is easier to learn and the score can estimate the overall cerebellar motor function quickly. We estimate that whereas it takes 15 to 20 minutes to perform MICARS or SARA, BARS can be performed in 3 to 5 minutes. One theoretical disadvantage of BARS, however, is that for research purposes, it can be less sensitive to detect minimal differences in scores, because it is less detailed than MICARS.
The relatively small sample is a limitation of this study. However, BARS inter‐rater reliability and validity have been demonstrated in other studies with SARA or ICARS totaling 146 patients (50 with SCA7, 44 children with brain tumor, and 52 healthy children).5, 6, 7 BARS has also been used as the sole scale in few other studies so far, totaling 176 patients (17 with Niemann‐Pick, 12 with SCA2, and 148 with multiple sclerosis).8, 9, 10
We recommend BARS for use in clinical practice as a reliable tool to evaluate ataxia effectively and rapidly.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
S.C.: 1A, 1B, 2B, 3A
F.C.: 1A, 2B, 3B
R.M.:1B, 1C, 3B
L.H.: 1C, 2A
T.R.S.: 1C
V.G.C.: 1C
R.A.: 1C
Disclosures
Funding Sources and Conflict of Interest: The authors report no conflicts of interest.
Financial disclosures for previous 12 months: The authors declare that there are no disclosures to report.
Supporting information
Appendix S1. Brief Ataxia Rating Scale Translation to Brazilian Portuguese.
Additional Supporting Information may be found online in the supporting information tab for this article.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Muzaimi MB, Thomas J, Palmer‐Smith S. Population based study of late onset cerebellar ataxia in south east Wales. J Neurol Neurosurg Psychiatry 2004;75:1129–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saute JA, Donis KC, Serrano‐Munuera C, et al. Ataxia rating scales—psychometric profiles, natural history and their application in clinical trials. Cerebellum 2012;11:488–504. [DOI] [PubMed] [Google Scholar]
- 3. Schmahmann J, Gardner R, MacMore J, Vangel MG. Development of a Brief Ataxia Rating Scale (BARS) based on a modified form of the ICARS. Mov Disord 2009;24:1820–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Braga‐Neto P, Godeiro‐Junior C, Dutra LA, Pedroso JL, Barsottini OG. Translation and validation into Brazilian version of the Scale of the Assessment and Rating of Ataxia (SARA). Arq Neuropsiquiatr 2010;68:228–230. [DOI] [PubMed] [Google Scholar]
- 5. Velázquez‐Pérez L, Cerecedo‐Zapata CM, Hernández‐Hernández O, et al. A comprehensive clinical and genetic study of a large Mexican population with spinocerebellar ataxia type 7. Neurogenetics 2015;16:11–21. [DOI] [PubMed] [Google Scholar]
- 6. Hartley H, Pizer B, Lane S, et al. Inter‐rater reliability and validity of two ataxia rating scales in children with brain tumours. Childs Nerv Syst 2015;31:693–697. [DOI] [PubMed] [Google Scholar]
- 7. Brandsma R, Spits AH, Kuiper MJ, et al. Ataxia rating scales are age‐dependent in healthy children. Dev Med Child Neurol 2014;56:556–563. [DOI] [PubMed] [Google Scholar]
- 8. Bowman EA, Walterfang M, Abel L, et al. Longitudinal changes in cerebellar and subcortical volumes in adult‐onset Niemann‐Pick disease type C patients treated with miglustat. J Neurol 2015;262:2106–2114. [DOI] [PubMed] [Google Scholar]
- 9. Valis M, Masopust J, Bažant J, et al. Cognitive changes in spinocerebellar ataxia type 2. Neuro Endocrinol Lett 2011;32:354–359. [PubMed] [Google Scholar]
- 10. Gunn H, Creanor S, Haas B, Marsden J, Freeman J. Risk factors for falls in multiple sclerosis: an observational study. Mult Scler 2013;19:1913–1922. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Brief Ataxia Rating Scale Translation to Brazilian Portuguese.


