Belly dancer's dyskinesia (BDD) is described as undulating rhythmical movements of the abdominal wall, an appearance that resembles belly dancing. It would be possible to mimic these movements voluntarily, and hence the disorder could be functional. Here, we report on the first case of functional BDD with supporting clinical neurophysiology.
Case Description
A 51‐year‐old right‐handed woman presented with abnormal abdominal movements for 9 years. She was in good health until she suddenly felt extreme pain in the middle of her back and the right leg during Pilates class. A few days later, she developed constipation, bloating, and severe pain in abdominal muscles. In 3 months, she started noticing slow writhing and undulating belly movements. Her movements were continuous during the day, but it was not clear whether it persisted during sleep. She started seeking medical attention and was found to have left ninth rib fracture. She underwent a rib repair surgery. She also received fundoplasty for acid reflux, which did not abate the symptoms. Because of chronic pain, she had CT‐guided T8/T9 nerve root block followed by intercostal nerve block at T8/9 and T12 level, which resulted in pneumothorax without benefit. Her movements became more intense and disabling along the course. Six years after the onset of her symptoms, she was diagnosed with BDD by a movement disorder specialist. She was started on clonazepam, baclofen, and gabapentin as well as botulinum neurotoxin injection in bilateral rectus abdominus, abdominal external oblique (AEO), and left thoracic paraspinal muscles, which was slightly helpful.
Neurological exam showed slow abdominal contraction that caused either undulating or circular umbilical movements (see Video 1). She could stop the movement by holding her breath when suggested to do so. The movements were neither distractible nor entrainable to hand tapping and alternating hand movement.
MRI of her brain and spine were normal. Surface electromyography (EMG) showed irregular EMG bursts with variable duration of 1,000 to 3,000 ms and cocontraction in bilateral AEO. Frequency varied from 0.3 to 1 Hz (Fig. 1A). The movements completely disappeared with sleep on EEG‐EMG recording (Fig. 1B). Jerk‐locked back‐averaging of EEG‐EMG was performed by placing the marker at the onset of EMG activity of AEO and back‐averaging EEG in central leads (Fz, Cz, F3, F4, C3, and C4) for −3,000 ms to +1,000 ms from the marker. A slowly rising negative potential was observed in central leads, starting around 1000 ms preceding movement onset consistent with a Bereitschaftspotential (BP) (Fig. 1C). The diagnosis of functional BDD was made based on presence of BP, in addition to clinical features of suggestibility and variability of the movement.
Figure 1.
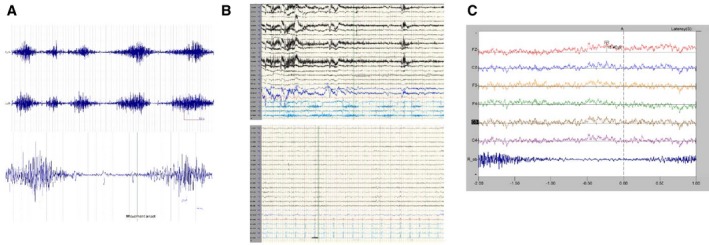
(A) EMG tracing showing variability of the duration and frequency of EMG bursts. Lower panel demonstrates how the movement onset was marked for back‐averaging. R_ob, right abdominal external oblique muscle; L_ob, left abdominal external oblique muscle. (B) EEG‐EMG tracing during awake (upper panel) and sleep (lower panel). (C) Back‐averaged EEG eliciting normal‐looking BP.
Discussion
BDD is a rare movement disorder of undulating or circular abdominal contraction that has been related to number of possible etiologies. It has been reported in patients with spinal cord or basal ganglia lesions.1, 2 In cases of spinal cord pathology, the movements would generally continue during sleep. In our patient, the movement disappeared with sleep, which argued against peripheral or spinal cord origin. She also had normal brain and spine MRI. BDD also has been linked to levodopa or antipsychotic exposure,3 which was not present in our patient. BDD has been reported after local trauma, such as abdominal surgery.4 There is a great deal of debate whether trauma can cause this movement disorder or whether trauma is a trigger for a functional disorder.5, 6
Physiological study with EMG can be helpful in differential diagnosis. BDD can be distinguished from propriospinal myoclonus, wherein EMG activity has a specific temporospatial distribution. EMG can also be helpful in identifying functional movement disorders. There are suggested electrophysiological test criteria for diagnosis of functional myoclonus and tremor7; however, they may be difficult to apply to dyskinesia or dystonia. Nonetheless, they share general hallmarks of being functional, including suggestibility, entrainability, and distractibility. Back‐averaging of EEG‐EMG is a useful diagnostic tool to identify FMD in patients with suitable symptoms. BP cannot be recorded in patients with continuous abnormal movements because it is necessary to have multiple discrete onsets that would serve as points from which to back‐average. Our patient had breaks in her abnormal movements, which allowed the recording of the BP. A BP preceding movement indicates involvement of the premotor/supplementary motor cortex in the preparation for movement.8 This potential can be recorded in patients with functional myoclonus, but not with organic myoclonus.9 In a large cohort of propriospinal myoclonus patients, approximately 50% clinically diagnosed as organic propriospinal myoclonus by a movement disorder expert turned out to be functional based on presence of BP and EMG characteristics.10 This observation can now be extended to BDD. The presence of a BP in our case indicates that BDD can be functional. Therefore, we suggest that looking for BP should be considered for differential diagnosis of abnormal axial movements after excluding identifiable underlying causes.
Author Roles
(1) Research project: A. Conception, B. Organization, C. Execution; (2) Manuscript: A. Writing of the First Draft, B. Review and Critique.
H.J.S.C.: 1A, 1B, 1C, 2A, 2B
P.P.: 1B, 1C, 2B
P.S.: 1B, 1C, 2B
M.H.: 1A, 1C, 2B
Disclosures
Funding Sources and Conflicts of Interest: The authors report no sources of funding and no conflicts of interest.
Financial Disclosures for previous 12 months: Dr. Cho has received a research grant from the Dystonia Medical Research Foundation. Dr. Hallett serves as chair of the medical advisory board for and receives honoraria and funding for travel from the Neurotoxin Institute. He may accrue revenue on US Patent #6 780 413 B2 (Issued: 24 August 2004): Immunotoxin (MAB‐Ricin) for the treatment of focal movement disorders, and US Patent #7 407 478 (Issued: 5 August 2008): Coil for Magnetic Stimulation and methods for using the same (H‐coil); in relation to the latter, he has received license fee payments from the National Institutes of Health (NIH; from Brainsway) for licensing of this patent. He is on the editorial board of 20 journals and received royalties and/or honoraria from publishing from Cambridge University Press, Oxford University Press, John Wiley & Sons, Wolters Kluwer, Springer, and Elsevier. He has received honoraria for lecturing from Columbia University. Dr. Hallett's research at the NIH is largely supported by the NIH Intramural Program. Supplemental research funds have been granted by the Kinetics Foundation for studies of instrumental methods to monitor Parkinson's disease, BCN Peptides, S.A. for treatment studies of blepharospasm, Medtronics, Inc., for studies of DBS, Parkinson Alliance for studies of eye movements in Parkinson's disease, Merz for treatment studies of focal hand dystonia, and Allergan for studies of methods to inject botulinum toxins.
Supporting information
A video accompanying this article is available in the supporting information here.
Video 1. A 51‐year‐old woman with continuous slow undulating movements of the abdomen resembling a belly dance.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Lobsien E, Gruber D, Schneider GH, Kuhn AA, Kupsch A. Iatrogenic belly dancer syndrome following quadruple deep brain stimulation in a patient with myoclonus dystonia (DYT11). Mov Disord 2010;25:2692–2693. [DOI] [PubMed] [Google Scholar]
- 2. Shamim EA, Hallett M. Intramedullary spinal tumor causing “belly dancer syndrome”. Mov Disord 2007;22:1673–1674. [DOI] [PubMed] [Google Scholar]
- 3. Carecchio M, Collini A, Comi C, Cantello R, Bhatia KP, Monaco F. Levodopa‐induced belly dancer's dyskinesias in Parkinson's disease: report of one case. Mov Disord 2010;25:1760–1762. [DOI] [PubMed] [Google Scholar]
- 4. Iliceto G, Thompson PD, Day BL, Rothwell JC, Lees AJ, Marsden CD. Diaphragmatic flutter, the moving umbilicus syndrome, and “belly dancer's” dyskinesia. Mov Disord 1990;5:15–22. [DOI] [PubMed] [Google Scholar]
- 5. Hawley JS, Weiner WJ. Psychogenic dystonia and peripheral trauma. Neurology 2011;77:496–502. [DOI] [PubMed] [Google Scholar]
- 6. van Rooijen DE, Geraedts EJ, Marinus J, Jankovic J, van Hilten JJ. Peripheral trauma and movement disorders: a systematic review of reported cases. J Neurol Neurosurg Psychiatry 2011;82:892–898. [DOI] [PubMed] [Google Scholar]
- 7. Schwingenschuh P, Katschnig P, Seiler S, et al. Moving toward “laboratory‐supported” criteria for psychogenic tremor. Mov Disord 2011;26:2509–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shibasaki H, Hallett M. What is the Bereitschaftspotential? Clin Neurophysiol 2006;117:2341–2356. [DOI] [PubMed] [Google Scholar]
- 9. Terada K, Ikeda A, Van Ness PC, et al. Presence of Bereitschaftspotential preceding psychogenic myoclonus: clinical application of jerk‐locked back averaging. J Neurol Neurosurg Psychiatry 1995;58:745–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Erro R, Bhatia KP, Edwards MJ, Farmer SF, Cordivari C. Clinical diagnosis of propriospinal myoclonus is unreliable: an electrophysiologic study. Mov Disord 2013;28:1868–1873. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A video accompanying this article is available in the supporting information here.
Video 1. A 51‐year‐old woman with continuous slow undulating movements of the abdomen resembling a belly dance.


