Abstract
Background
Patients with von Willebrand disease (VWD) type 2A or acquired von Willebrand syndrome (aVWS) as a consequence of implantation of left ventricular assist devices (LVAD) are both characterized by a loss of von Willebrand factor (VWF) function. Loss of VWF function is however more severe in VWD type 2A than in LVAD patients.
Objectives
To compare VWF function in patients with VWD type 2A and LVAD‐induced aVWS to highlight the differences in VWF activity and to stress the importance of VWF multimer analysis for correct diagnosis of aVWS in LVAD patients.
Patients/Methods
Plasma samples from nine VWD type 2A, nine LVAD patients, and 20 healthy donors (HD) were analyzed for VWF function (VWF:CB/VWF:Ag and VWF:RCo/VWF:Ag) and loss of high molecular weight (HMW) VWF multimers.
Results
A severely impaired VWF function was indeed confirmed in all VWD 2A patients. HMW VWF multimers were severely reduced compared to HD (0% [0, 12.29] vs 34.19% [31.68, 38.88] for HD, P < 0.001) and this loss was reflected by VWF:CB/VWF:Ag and VWF:RCo/VWF:Ag ratios <0.7. In contrast, VWF function was less affected in LVAD patients. Although HMW VWF multimers were reduced in all patients (20.31% [15.84, 21.71], vs 34.19% [31.68, 38.88] for HD, P < 0.001), six out of nine LVAD patients had normal VWF:CB/VWF:Ag or VWF:RCo/VWF:Ag ratios (>0.7).
Conclusions
VWF:CB/VWF:Ag or VWF:RCo/VWF:Ag analysis allows detection of impaired VWF function in VWD type 2A but not always in LVAD‐induced aVWS patients. In contrast, VWF multimeric analysis allows detection of the loss of HMW VWF multimers in both groups of patients. Hence, performing VWF multimer analysis is crucial to detect aVWS in LVAD patients.
Keywords: heart failure, laboratory diagnosis, ventricular assist device, von Willebrand disease, von Willebrand factor
Essentials.
VWF is defective in VWD type 2A and LVAD‐induced aVWS patients.
The difference in VWF function and multimers were studied in these two groups.
VWF multimers were decreased in all VWD type 2A and LVAD patients in contrast to VWF function.
Hence, VWF multimer analysis is the gold standard for diagnosis of aVWS in LVAD patients.
1. Introduction
Patients with the bleeding disorder von Willebrand disease (VWD) type 2A have a severe defect in von Willebrand factor (VWF). Laboratory diagnosis is based on the detection of an impaired VWF function reflected in VWF collagen binding and VWF ristocetin cofactor activities over VWF antigen ratios (VWF:CB/VWF:Ag and VWF:RCo/VWF:Ag) below 0.7 and a decrease (severe or mild) or lack of the high molecular weight (HMW) VWF multimers, depending on the location of the mutation.1, 2 Patients implanted with left ventricular assist devices (LVAD) suffer from acquired von Willebrand syndrome (aVWS).3, 4, 5, 6, 7, 8, 9, 10 However, the impaired VWF function is not always detected in the VWF:CB/VWF:Ag and VWF:RCo/VWF:Ag ratios but is reflected by a loss of HMW VWF multimers.5, 6, 8, 11, 12
The aim of this study was to perform an analysis of VWF function in VWD type 2A and LVAD‐induced aVWS patients to highlight to non‐experts in the field that VWF function in LVAD patients is less impaired than in VWD type 2A patients and that VWF multimer analysis is the gold standard to detect aVWS in LVAD patients.
2. Patients and Methods
2.1. Patients
Sodium citrated plasma samples, stored at −80°C, of nine patients diagnosed with von Willebrand disease type 2A (mutations are located in the VWF A2 domain [exon 28]) were available and samples of nine end‐stage heart failure patients with 4‐6 months of LVAD support receiving standard anticoagulation therapy with oral anticoagulants (INR 2.5) were collected. The nine LVAD patients were all in end‐stage heart failure, NYHA class IV, INTERMACS > grade 5 at time of implant. Heart failure was due to ischemic cardiomyopathy in four, idiopathic dilated cardiomyopathy in four, and hypertrophic cardiomyopathy in one patient. None of them had a previous history of bleeding. Mean left ventricular ejection fraction was 16 ± 7%, mean age at time of implant was 48 ± 11 years. Patients were followed up to 3 years after LVAD implantation. Blood samples taken between 4 and 6 months after LVAD implantation were used in this study. Bleedings were observed in five of the nine LVAD patients (time to bleeding from LVAD was 1 day, 20 days, 8 months, 2 years and 4 months and 2 years and 11 months). All patients provided informed written consent and the study was approved by the local ethical committee.
2.2. Laboratory diagnostics
von Willebrand factor antigen (VWF:Ag) and VWF ristocetin cofactor activity (VWF:RCo) were determined via standard assays. VWF:Ag was measured via a latex‐based von Willebrand factor antigen kit (HemosIL, Instrumentation Laboratory, Bedford, MA, USA) on a ACLTOP (Instrumentation Laboratory) and the Sta Liatest (Stago, Asniéres sur Seine Cedex, France) in LVAD‐induced aVWS patients and VWD type 2A patients, respectively. VWF:RCo was performed via an immunoturbidimetric latex particle assay (GPIb binding assay, HemosIL, Instrumentation Laboratory) for LVAD‐induced aVWS patients or via the BC von Willebrand reagent kit (Siemens Healthcare Diagnostics) for VWD type 2A patients. Both automated assays showed to be in good correlation with standard aggregometry.13, 14 VWF collagen binding activity (VWF:CB) was determined by coating human collagen type III (Sigma‐Aldrich, Saint‐Louis, MO, USA) on a microtiter plate, adding the different plasmas and detecting bound VWF using HRP‐labelled polyclonal anti‐VWF antibodies.15, 16 A normal human plasma pool (NHP) was used to set up a calibration curve and undiluted plasma was set at 100% VWF:CB. Next, the VWF:CB/VWF:Ag and VWF:RCo/VWF:Ag ratios were calculated. VWF multimers were separated on a 1.2% sodium dodecyl sulphate (SDS) 1.5% isoelectric focusing (IEF) agarose gel and fixed on a Gelbond (Cambrex Bio Science Rockland Inc., Rockland, ME, USA).17, 18 VWF was detected with an alkaline phosphatase (AP) labelled anti‐human VWF antibody and an AP conjugate substrate kit (BioRad, Hercules, CA, USA). Densitometric analysis was performed using ImageJ software (version 1.47, NIH, Bethesda, MD, USA). The lowest (band 1‐5, low molecular weight, LMW), the medium (band 6‐10, medium molecular weight, MMW), and high molecular weight (HMW band >10) multimers were selected and the density of the HMW multimers relative to the total multimer density was calculated as a percentage.
2.3. Statistical analysis
Significance between datasets was assessed with the one‐way ANOVA test with corrections for multiple comparisons (Prism Version 6, GraphPad). P values were calculated comparing median values using P < 0.05 as a cut‐off for significance. Data are represented as median with interquartile ranges.
3. Results/Discussion
In this study, we wanted to highlight the differences in VWF function between VWD type 2A patients and LVAD‐induced aVWS patients to better explain why VWF multimer analysis is needed to diagnose an LVAD patient with aVWS. Differences in VWF function in VWD type 2A versus LVAD‐induced aVWS were demonstrated via routine tests for VWF activity (VWF:CB, VWF:RCo) and VWF antigen (VWF:Ag).5 The loss of HMW VWF multimers was visualized via VWF multimeric analysis. This study confirmed that VWF function was indeed severely impaired in all VWD type 2A patients. The HMW VWF multimers were severely reduced compared to healthy individuals (0% [0, 12.29] vs 34.19% (31.68, 38.88) in healthy individuals, P < 0.001) and were even absent in six out of nine patients (Figure 1A and G). This severe loss of HMW VWF multimers was reflected in the VWF activity assays. In all VWD type 2A patients, VWF:CB/VWF:Ag and VWF:RCo/VWF:Ag ratios were significantly decreased compared to healthy individuals and were well below 0.7 (0 [0, 0.29] and 0.30 [0.18, 0.34] vs 1.05 [0.97, 1.13] and 0.88 [0.86, 0.92] in healthy individuals, respectively, P < 0.001, Figure 1B and C). Values of VWF:Ag, VWF:CB, and VWF:RCo were significantly decreased when compared to healthy individuals and are given in Figure 1D, E, and F, respectively (46% [32.00, 85.80], 0% [0, 20.12] and 12% [8.50, 24] vs 108.5% [95.90, 117.3], 79.94% [74.95, 114.9], and 98.10% [82.73, 104.2] in healthy individuals, P = 0.01, P < 0.001, and P < 0.001, respectively).
Figure 1.
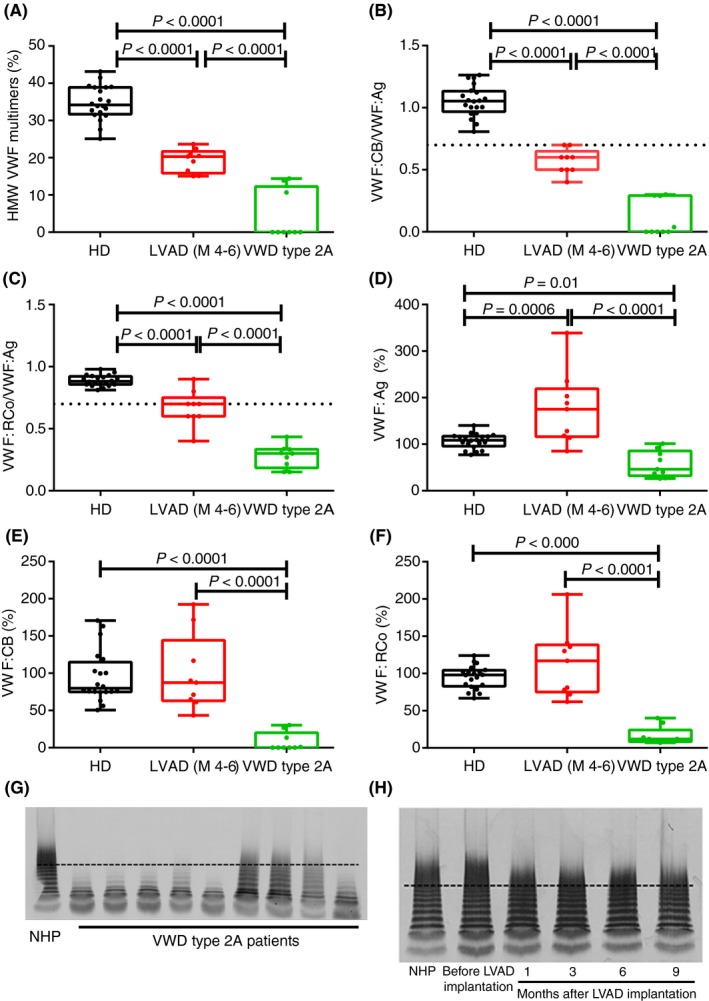
HMW VWF multimers, VWF antigen, VWF collagen binding activity and VWF ristocetin cofactor activity in VWD type 2A and LVAD‐induced aVWS patients. HMW VWF multimers, VWF antigen (VWF:Ag), VWF collagen binding activity (VWF:CB) and VWF ristocetin cofactor activity (VWF:RCo) were determined in healthy donors (HD, n = 20), patients with VWD type 2A (n = 9) and in LVAD‐induced aVWS patients (n = 9). (A) HMW VWF multimers were severely reduced in VWD type 2A patients and decreased in LVAD‐induced aVWS patients at 4 to 6 (M 4‐6) months after LVAD implantation. HD were used as control samples. (B) The ratio of VWF:CB over VWF:Ag (VWF:CB/VWF:Ag) and (C) VWF:RCo over VWF:Ag (VWF:RCo/VWF:Ag) was severely decreased in all VWD type 2A patients but not in LVAD‐induced aVWS patients. VWF:Ag (D), VWF:CB (E) and VWF:RCo (F) was decreased in VWD type 2A patients but not in LVAD‐induced aVWS patients compared to HD. Representative VWF multimeric pattern of (G) VWD type 2A patients and NHP (normal human plasma) and (H) an LVAD‐induced aVWS patient before LVAD implantation and 1,3,6 and 9 months after implantation of the LVAD and NHP (the proportion of HMW VWF multimers are situated above the dashed line). Percentages and ratios are represented as a boxplot with the median and interquartile ranges (25th and 75th percentile). LVAD, left ventricular assist devices; NHP, normal human plasma; VWF, von Willebrand factor; VWS, von Willebrand syndrome
In contrast, VWF activity was less impaired in LVAD‐induced aVWS patients compared to VWD type 2A patients. Although the loss of HMW VWF multimers in all LVAD patients was clear, HMW VWF multimers were less severely reduced compared to VWD type 2A patients (20.31% [15.84, 21.71] vs 0% [0, 12.29] in VWD type 2A patients, P < 0.001, Figure 1A and H), but were still significantly lower than healthy individuals (34.19% [31.68, 38.88], P < 0.001, Figure 1A). Accordingly, VWF:CB/VWF:Ag and VWF:RCo/VWF:Ag ratios were higher than those in VWD type 2A patients (0.60 [0.50, 0.65] and 0.70 [0.60, 0.75] vs 0 [0, 0.29] and 0.30 [0.18, 0.34] for VWD type 2A patients, P < 0.001, respectively, Figure 1B and C). However, in contrast to the VWD type 2A patients, two out of nine LVAD patients had a VWF:CB/VWF:Ag ratio that was normal (=0.7) (Figure 1B) and five out of nine LVAD patients, had normal VWF:RCo/VWF:Ag ratios (= or >0.7) (Figure 1C). Hence, by performing a comparison of VWF function in VWD type 2A and LVAD patients, we stressed that the defect in VWF function in LVAD patients is indeed less severe than in VWD type 2A patients. VWD type 2A patients lack their HMW and even medium and low molecular weight multimers and the severe defect in VWF function is also reflected in laboratory VWF activity assays where VWF:CB/VWF:Ag and VWF:RCo/VWF:Ag ratios are clearly below 0.7.1, 2 In LVAD patients however, HMW VWF multimers are mildly decreased which is reflected in a moderate defect in VWF activity. Indeed, we and others8, 12 show that VWF:CB/VWF:Ag and VWF:RCo/VWF:Ag ratios are not always below 0.7 in LVAD patients. As a consequence, VWF multimer analysis is the gold standard to detect aVWS in these patients.5, 19 Comparable findings were obtained for aortic stenosis patients, in which aVWS has also been frequently detected.20, 21 As multimer analysis is a time‐consuming technique, an ELISA‐based method was proposed as an alternative to detect VWF proteolysis.7 We also determined if there was a correlation between the ratios of VWF parameters or the loss of HMW VWF multimers and the occurrence of bleeding, but no relationship was found. None of the patients experienced bleedings between four and six months after implantation of the device despite the loss of HMW VWF multimers or decreased VWF:CB/VWF:Ag and VWF:RCo/VWF:Ag ratios. These findings are in line with what is generally described in literature4, 6, 22 suggesting that the loss of HMW VWF multimers alone does not account for the bleeding complications observed in the LVAD patients.
4. Conclusion
In conclusion, we want to emphasize that VWF function in heart failure patients on LVAD support is much less impaired compared to VWD type 2A patients and that multimer analysis should always be performed to detect aVWS in LVAD patients.
Relationship Disclosure
The authors report nothing to disclose.
Author Contributions
S. Deconinck designed research, performed the experiments, analyzed data, and wrote and reviewed the manuscript. C. Tersteeg analyzed the data and reviewed the manuscript. E. Bailleul acquired data and reviewed the manuscript. L. Delrue, N. Vandeputte, and I. Pareyn acquired data. N. Itzhar‐Baikian acquired and analyzed data and reviewed the manuscript. H. Deckmyn and S.F. De Meyer reviewed the manuscript. M. Vanderheyden acquired data and reviewed the manuscript. K. Vanhoorelbeke supervised, designed research, analyzed the data, wrote and reviewed the manuscript. All authors approved the final manuscript.
Deconinck S, Tersteeg C, Bailleul E, et al. Differences in von Willebrand factor function in type 2A von Willebrand disease and left ventricular assist device‐induced acquired von Willebrand syndrome. Res Pract Thromb Haemost. 2018;2:762–766. 10.1002/rth2.12150
Funding information
This work was supported by the “Fund for scientific research Flanders (FWO Vlaanderen)” (1S60917N) awarded to S.D. and the KU Leuven (PF/10/014, C32/17/012) awarded to K.V.
References
- 1. Budde U, Pieconka A, Will K, Schneppenheim R. Laboratory testing for von Willebrand disease: contribution of multimer analysis to diagnosis and classification. Semin Thromb Hemost. 2006;32(5):514‐21. [DOI] [PubMed] [Google Scholar]
- 2. Sadler JE, Budde U, Eikenboom JCJ, et al. Update on the pathophysiology and classification of von Willebrand disease: a report of the Subcommittee on von Willebrand Factor. J Thromb Haemost. 2006;4(10):2103‐14. [DOI] [PubMed] [Google Scholar]
- 3. Geisen U, Heilmann C, Beyersdorf F, et al. Non‐surgical bleeding in patients with ventricular assist devices could be explained by acquired von Willebrand disease. Eur J Cardiothorac Surg. 2008;33(4):679‐84. [DOI] [PubMed] [Google Scholar]
- 4. Crow S, Chen D, Milano C, et al. Acquired von Willebrand syndrome in continuous‐flow ventricular assist device recipients. Ann Thorac Surg. 2010;90(4):1263‐9. [DOI] [PubMed] [Google Scholar]
- 5. Tiede A. Diagnosis and treatment of acquired von Willebrand syndrome. Thromb Res. 2012;130(Suppl 2):S2‐6. [DOI] [PubMed] [Google Scholar]
- 6. Meyer AL, Malehsa D, Budde U, Bara C, Haverich A, Strueber M. Acquired von Willebrand syndrome in patients with a centrifugal or axial continuous flow left ventricular assist device. JACC Heart Fail. 2014;2(2):141‐5. [DOI] [PubMed] [Google Scholar]
- 7. Rauch A, Caron C, Vincent F, et al. A novel ELISA‐based diagnosis of acquired von Willebrand disease with increased VWF proteolysis. Thromb Haemost. 2016;115(5):950‐9. [DOI] [PubMed] [Google Scholar]
- 8. Reich HJ, Morgan J, Arabia F, et al. Comparative analysis of von Willebrand factor profiles after implantation of left ventricular assist device and total artificial heart. J Thromb Haemost. 2017;15(8):1620‐4. [DOI] [PubMed] [Google Scholar]
- 9. Heilmann C, Trummer G, Beyersdorf F, et al. Acquired von Willebrand syndrome in patients on long‐term support with HeartMate II. Eur J Cardiothorac Surg. 2017;51(3):587‐90. [DOI] [PubMed] [Google Scholar]
- 10. Nascimbene A, Neelamegham S, Frazier OH, Moake JL, Dong JF. Acquired von Willebrand syndrome associated with left ventricular assist device. Blood. 2016;127(25):3133‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen D, Tange JI, Meyers BJ, Pruthi RK, Nichols WL, Heit JA. Validation of an automated latex particle‐enhanced immunoturbidimetric von Willebrand factor activity assay. J Thromb Haemost. 2011;9(10):1993‐2002. [DOI] [PubMed] [Google Scholar]
- 12. Tiede A, Rand JH, Budde U, Ganser A, Federici AB. How I treat the acquired von Willebrand syndrome. Blood. 2011;117(25):6777‐85. [DOI] [PubMed] [Google Scholar]
- 13. Lawrie AS, Mackie IJ, Machin SJ, Peyvandi F. Evaluation of an automated platelet‐based assay of ristocetin cofactor activity. Haemophilia. 2011;17(2):252‐6. [DOI] [PubMed] [Google Scholar]
- 14. De Vleeschauwer A, Devreese K. Comparison of a new automated von Willebrand factor activity assay with an aggregation von Willebrand ristocetin cofactor activity assay for the diagnosis of von Willebrand disease. Blood Coagul Fibrinolysis. 2006;17(5):353‐8. [DOI] [PubMed] [Google Scholar]
- 15. Favaloro EJ, Grispo L, Exner T, Koutts J. Development of a simple collagen based ELISA assay aids in the diagnosis of, and permits sensitive discrimination between Type I and Type II, von Willebrands disease. Blood Coagul Fibrinolysis. 1991;2(2):285‐92. [DOI] [PubMed] [Google Scholar]
- 16. Vanhoorelbeke K, Cauwenberghs N, Vandecasteele G, Vauterin S, Deckmyn H. A reliable von Willebrand factor: ristocetin cofactor enzyme‐linked immunosorbent assay to differentiate between type 1 and type 2 von Willebrand disease. Semin Thromb Hemost. 2002;28(2):1610‐16. [DOI] [PubMed] [Google Scholar]
- 17. De Meyer SF, Vandeputte N, Pareyn I, et al. Restoration of plasma von willebrand factor deficiency is sufficient to correct thrombus formation after gene therapy for severe von willebrand disease. Arterioscler Thromb Vasc Biol. 2008;28(9):1621‐6. [DOI] [PubMed] [Google Scholar]
- 18. Tersteeg C, Roodt J, Van Rensburg WJ, et al. N‐acetylcysteine in preclinical mouse and baboon models of thrombotic thrombocytopenic purpura. Blood. 2017;129(8):1030‐8. [DOI] [PubMed] [Google Scholar]
- 19. Tiede A, Priesack J, Werwitzke S, et al. Diagnostic workup of patients with acquired von Willebrand syndrome: a retrospective single‐centre cohort study. J Thromb Haemost. 2008;6(4):569‐76. [DOI] [PubMed] [Google Scholar]
- 20. Kellermair J, Ott HW, Spannagl M, et al. Characterization of von Willebrand factor multimer structure in patients with severe aortic stenosis. Clin Appl Thromb Hemost. 2018;24(3):496‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sucker C, Feindt P, Zotz RB, Stockschlaeder M, Scharf RE. Functional von Willebrand factor assays are not predictive for the absence of highest‐molecular weight von Willebrand factor multimers in patients with aortic‐valve stenosis. Thromb Haemost. 2005;94(2):465‐6. [PubMed] [Google Scholar]
- 22. Uriel N, Pak SW, Jorde UP, et al. Acquired von Willebrand syndrome after continuous‐flow mechanical device support contributes to a high prevalence of bleeding during long‐term support and at the time of transplantation. J Am Coll Cardiol. 2010;56(15):1207‐13. [DOI] [PubMed] [Google Scholar]


