Abstract
Background
The aim of this study was to determine the prevalence of neutralizing antibodies in a large cohort of long‐term treated patients with cervical dystonia (CD) still responding to repetitive injections with botulinum toxin (BoNT).
Methods
Consecutively recruited CD patients (n = 221) under long‐term BoNT treatment (≥2–21 years) underwent a clinical examination at the same time blood samples were taken for neutralizing antibody determination. Collected data included demographics, mean dose of the last 10 botulinum injections, treatment duration, Tsui score for CD severity, and patients' subjective impression of treatment effect. Blood samples were screened for antibody presence by ELISA; positive samples were further analyzed by mouse hemidiaphragm test. The two laboratories performing antibody testing were blinded to the coded samples.
Results
Antibody status could be determined for 212 patients; 39 (18.4%) were ELISA positive and 31 (14.6%) additionally positive in the mouse hemidiaphragm test. Patients with positive neutralizing antibody titers had significantly higher Tsui scores and were treated for a significantly longer time with significantly higher doses. There were no differences between male and female patients and between onabotulinumtoxinA‐ and abobotulinumtoxinA‐treated patients. When BoNT preparations had been switched during the last 10 injections, a significantly higher proportion of neutralizing antibody‐positive patients was detected.
Conclusions
Neutralizing antibody prevalence in long‐term treated, still responding CD patients is substantially higher than suggested by follow‐up studies with a shorter time frame. It should therefore be emphasized that antigenicity of BoTN preparations is still a relevant problem and should be taken into account in long‐term treatment decisions.
Keywords: neutralizing antibodies, long‐term treatment, cervical dystonia, partial treatment failure, antigenicity of botulinumtoxin preparations
Botulinum toxin (BoNT) type A (BoNT/A) preparations are the treatment of choice for cervical dystonia (CD),1 but efficacious treatment requires intramuscular injections every 10 to 16 weeks. Thus, the patient's immune system is regularly confronted with large BoNT/A molecules and its complexing proteins,2, 3 and a certain proportion of patients show an immune response not only against the complexing proteins, but also against the BoNT/A molecule.3, 4 Some of the induced antibodies neutralize the biological activity of BoNT,5, 6 leading to reduced clinical efficacy (partial secondary treatment failure; PSTF) and, in some patients, even to a complete abolishment of BoNT action (complete secondary treatment failure; CSTF). In previous years, PSTF and CSTF occurrence in BoNT/A treatment for CD was observed quite frequently.4 Improvement of BoNT/A preparations and reduction of protein content reduced the immune response considerably.2, 3 Neutralizing antibody (NAB) rates as low as 1.2% have been reported for the new onabotulinumtoxinA formulation7, 8; the rates seem to be even lower for incobotulinumtoxinA, a preparation free of complexing proteins.2, 8, 9, 10 However, these rates rely on studies determining patients' antibody status for less than 3 years and provide an estimate of NAB incidence (proportion of new NAB‐positive patients per year), rather than an estimate of NAB prevalence in a cohort of long‐term treated patients.
It has been suggested that NABs probably develop early in the course of treatment and that PSTF occurrence later in the course of treatment is quite rare.11, 12 However, the database for this hypothesis is small. The number of patients with short‐term treatment is much higher than the number of patients monitored carefully over a long time period. This might be one of the major reasons why NAB development is observed more frequently at the start of BoNT/A treatment. We previously suggested Kaplan‐Meier's survival analysis (censoring all patients in whom therapy was interrupted for whatever reason) as the adequate method to estimate NAB prevalence in long‐term treated patients.13 Studies using this method4, 13 show a much higher proportion of NABs (>20%)4 or PSTF (>15%)13 in long‐term BoNT/A‐treated CD patients than suggested by the low rate estimates between 2% and 5%.7, 8, 14, 15, 16 .
Another adequate method is the deduction of NAB prevalence in a large patient cohort from a detailed analysis of a small representative patient sample.17 Using this approach, Kranz et al. estimated up to 40% of NAB‐positive patients in their cohort of long‐term treated CD patients. However, this method heavily relies on the selection of the representative sample; a bias in the selection procedure might result in misleading findings. The investigators hypothesized that there will be a considerable proportion of patients still responding to BoNT injections, but with positive antibody titers, in a cohort of CD patients under continuous treatment.17 .
The present cross‐sectional monocentric study was designed to determine the precise NAB prevalence in a large cohort of CD patients still responding to BoNT treatment.
Patients and Methods
Patients
In 2010, all CD patients treated at our BoTN outpatient clinic with BoNT injections every 3 to 4 months for at least 10 times (without interruption during the last 2–3 years) and who still experienced a treatment effect were asked to participate in the present study. Patients who did not experience a treatment effect were excluded from the study. A total of 221 patients agreed to participate and gave their written informed consent. A general approval from the local ethics committee allows us to take blood samples and publish anonymized clinical data and results of antibody testing of patients having given informed consent.
Patients underwent a clinical examination at the same time blood samples were taken for NAB determination. Besides demographic data, BoNT preparation and mean dose of the last 10 BoNT injections, treatment duration, and CD severity (using the Tsui score18) were determined by the attending physician. Patients' subjective impression of the overall treatment effect of the last injection cycle, compared to CD severity just before start of BoNT therapy, was rated on a visual analog scale (VAS; 0–100; 0 = no more symptoms and 100 = CD severity just as bad as before start of BoNT therapy). Patients had been treated with abobotulinumtoxinA, onabotulinumtoxinA, incobotulinumtoxinA (BoNT/A preparations), or rimabotulinumtoxinB (BoNT/B). For comparison, doses were transformed to unified dose units (uDU): because most of the patients had been treated with abobotulinumtoxinA, doses of onabotulinumtoxinA and incobotulinumtoxinA were multiplied by 3, doses of rimabotulinumtoxinB were divided by 10, and abobotulinumtoxinA doses remained unchanged. The mean of the unified doses of the last 10 single injections was used for data analysis.
Determination of antibody status
Blood samples were first sent to BioProof AG (Munich, Germany) to determine the presence of antibodies using enzyme‐linked immunosorbent assay (ELISA) testing (fluoroimmunoassy). Neutralizing antibody titers of ELISA‐positive samples were then determined with the mouse hemidiaphragm assay (MHDA6) by Toxogen GmbH (Hannover, Germany). Both laboratories were blinded to the (coded) samples and did not receive any clinical information, except the time the samples were taken. Antibody status could be determined in 212 patients.
Statistical analysis
Data analysis was based on the 212 patients with known antibody status with stratification into the following subgroups: all ELISA‐negative patients (group I: n = 173); all ELISA‐positive, but MHDA‐negative, patients (group II: n = 8); and all ELISA‐ and MHDA‐positive patients (group III: n = 31). Groups II and III were combined for further analysis of all ELISA‐positive patients (n = 39). NAB prevalence and incidence were also compared according to BoNT preparation.
All statistical analyses were carried out with the commercially internationally available SPSS package (SPSS, Inc., Chicago, IL). After an analysis of variance had yielded differences among subgroups in a first step, comparisons between subgroups were then performed nonparametrically using Kendall's tau B test. Results were confirmed by t testing if group size and parameter used allowed the use of the t test. Both nonparametric and parametric testing yielded the same significant results (with slightly different levels of significance). Pearson's correlation coefficient was determined for the correlation analysis.
Results
Patients included in this analysis (mean age: 61.0 ± 11.8 years; 60.4% female) had suffered from CD for a mean of 17.7 ± 8.9 years. Table 1 summarizes demographic and clinical characteristics of the cohort stratified by antibody status. During the last 10 injection cycles, 128 of the patients had been treated exclusively with abobotulinumtoxinA, 36 with onabotulinumtoxinA, 16 with incobotulinumtoxinA, and four with rimabotulinumtoxinB. Preparations had been changed in 28 patients.
Table 1.
Demographic and clinical characteristics and mean BoNT dose of patients treated long term with BoNT for CD
| Entire Cohort | Group I (ELISA Negative) | Group II (ELISA Positive but MHDA Negative) | Group III (ELISA Positive, MHDA Positive) | Group II + III (ELISA Positive) | Significance (I vs. II + III) | |
|---|---|---|---|---|---|---|
| No. of patients | 212 | 173 | 8 | 31 | 39 | na |
| Age, years | 61.0 ± 11.8 | 59.8 ± 12.0 | 65.5 ± 13.7 | 64.4 ± 9.7 | 64.6 ± 10.4 | <0.018 |
| Female sex (%) | 128 (60.4) | 102 (59.0) | 7 (87.5) | 19 (61.3) | 26 (66.7) | ns (0.114) |
| Weight, kg | 75.2 ± 17.9 | 75.5 ± 18.3 | 75.8 ± 13.6 | 74.0 ± 15.4 | 74.4 ± 15.4 | ns (0.76) |
| Age at onset of CD, years | 43.1 ± 11.1 | 42.8 ± 11.2 | 40.6 ± 10.0 | 45.3 ± 11.0 | 44.1 ± 10.8 | ns (0.532) |
| Duration of treatment, years | 11.7 ± 5.3 | 11.2 ± 5.5 | 13.5 ± 4.8 | 13.0 ± 4.1 | 13.5 ± 4.2 | <0.022 |
| Tsui score | 4.93 ± 3.3 | 4.75 ± 3.2 | 5.25 ± 3.5 | 6.29 ± 3.8 | 6.08 ± 3.7 | <0.015 |
| Patients' rating of treatment effect (VAS 0–100) | 46.6 ± 27.9 | 46.3 ± 27.3 | 34.3 ± 30.3 | 51.8 ± 29.2 | 48.3 ± 29.8 | ns (0.696) |
| Dose per injection (uDU)a | 719 ± 146 | 704 ± 148 | 727 ± 111 | 798 ± 117 | 782 ± 119 | <0.004 |
Only patients with known antibody status were analyzed (n = 212). Data are mean ± standard deviation or number of patients (%).
OnabotulinumtoxinA/abobotulinumtoxinA/rimabotulinumtoxinB 1:3:10
na, not applicable; ns, not significant.
Clinical outcome after long‐term cd treatment with BoNT
The mean Tsui score of the entire cohort was 4.93 ± 3.3 (Table 1). Plotting of scores against treatment duration showed a completely flat trend line (Slope SL = −0.0024 Tsui point/year; r 2 = 0.00002; P = 0.879; Fig. 1A). On average, there was no difference between scores of patients having been treated for a rather short time (approximately 2–3 years) or for approximately 20 years. Patients scored treatment efficacy as 46.6 ± 27.9% (VAS 0–100; Table 1), indicating that they experienced a reduction of CD symptom severity by, on average, more than 50%. This rating decreased significantly with duration of treatment (SL = −0.7363%/year; r 2 = 0.017; P ≤ 0.04). Mean dose per injection over the last 10 injections was 719 ± 146 uDU. A significant increase (SL = 50.24 uDU/year; r 2 = 0.0313; P < 0.015) was observed with duration of treatment.
Figure 1.
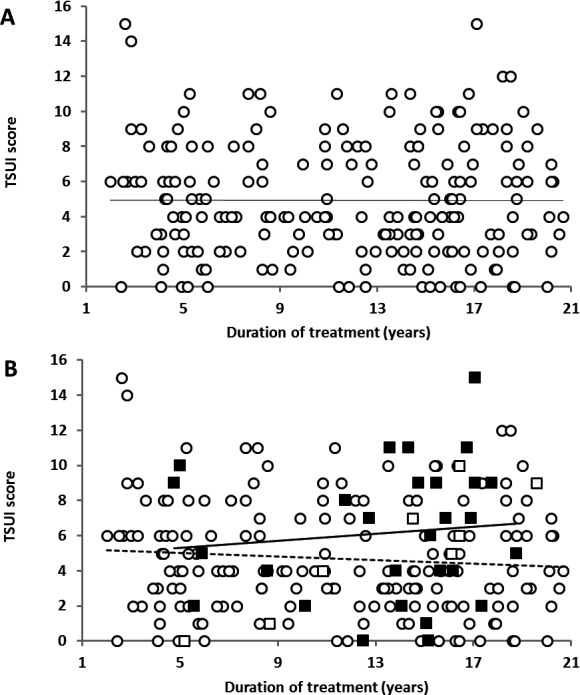
Tsui scores for different treatment duration. (A) Entire cohort (n = 212), regression line (─). (B) Stratified by antibody status. Group I, ELISA‐negative patients (○); group II, ELISA‐positive, but MHDA‐negative patients (□); group III, ELISA‐ and MHDA‐positive patients (■); regression line for group I (—), regression line for group III (─).
Prevalence of ELISA‐ and MHDA‐positive patients in the cohort
Thirty‐nine blood samples were ELISA positive (18.4%); 31 of these were also MHDA positive (14.6% of entire cohort). Because mean treatment duration of the entire cohort was 11.7 years, mean NAB incidence in our cohort was 1.26%/year. There was no influence of sex on NAB development: 19 of 128 females (14.8%) and 12 of 84 males (14.3%) had a positive MHDA test (P = 1.0).
Comparison of treatment‐related parameters in ELISA‐positive and ‐negative patients
ELISA‐positive patients (group II + III) had a significantly longer treatment duration (P < 0.022), were significantly older (P < 0.018) with a significantly worse Tsui score (P < 0.015), and received significantly larger mean BoNT doses (P < 0.004) than the ELISA‐negative patients (Table 1). There were no obvious clinical predictors for the presence of NABs. Patients' subjective scoring of treatment effect was even less sensitive than Tsui score (determined by the treating physician) or dose and did not show a significant difference between patients testing positive or negative (P = 0.696; Table 1).
Tsui scores during long‐term treatment stratified by antibody status
Figure 1B shows the Tsui scores according to treatment years stratified by antibody status. Analysis of separate trend lines for ELISA‐positive, but MHDA‐negative patients (group II; SL = −0.0497 Tsui point/year; r 2 = 0.0073; P = 0.335) and ELISA‐/MHDA‐positive patients (group III; SL = +0.1002 Tsui point/year; r 2 = 0.0112) reveals a mild improvement of Tsui scores over time in MHDA‐negative patients and a mild worsening in MHDA‐positive patients.
Clinical outcome of MHDA‐positive patients varied over an extremely wide range with Tsui scores between 0 and 15, but variability was at least as large in MHDA‐negative patients (Fig. 1B). On first glance, the values of the positive patients seemed to be randomly embedded in the range of values of the negative patients. To further investigate this, we determined the proportions of only ELISA‐positive (open bars, group II) and MHDA‐positive patients (black bars, group III) in seven successive TSUI score ranges (1–2, 3–4, …, 11–12, and 13–14 Tsui score points), nine ranges of the mean unified dose during the last 10 injections (100–200, 200‐300, …, 800‐900, >900 unified dose units), and 10 time periods of treatment duration (0–2, 2–4, …., 16–18, and >18 years; Fig. 2A–C). The proportion of MHDA positive patients increased with increasing Tsui scores, higher BoNT doses, and longer treatment duration (except for the highest ranges). These factors thus increase the risk to develop NABs. Reasons for the low proportion of ELISA‐/MHDA‐positive patients in the highest ranges will be discussed below.
Figure 2.
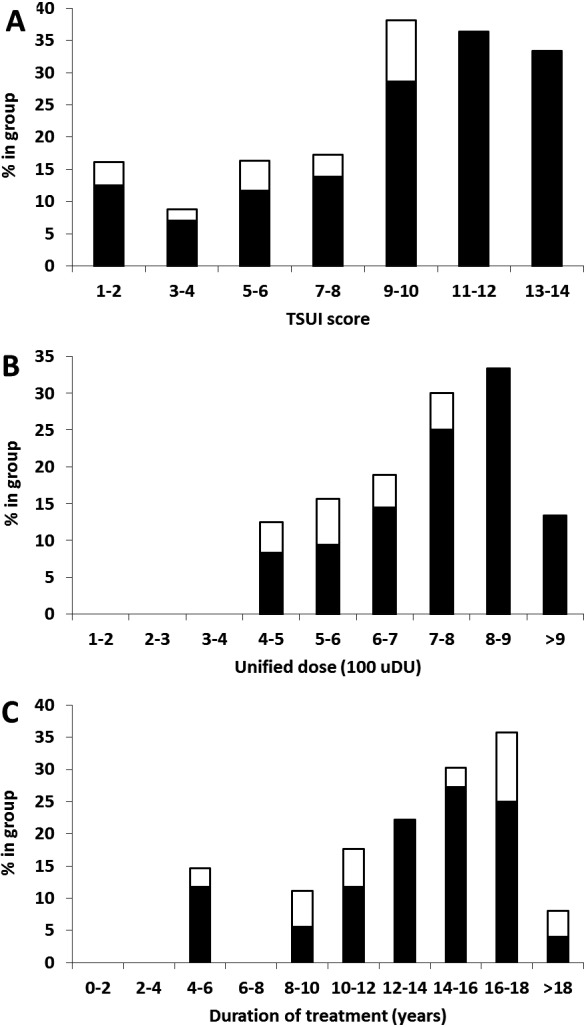
Proportion of only ELISA‐positive (□) and MHDA‐positive (■) patients according to (A) Tsui score. (B) Mean unified dose of the last 10 injections. (C) Treatment duration.
Prevalence and incidence of patients with neutralizing antibody titers according to BoNT preparation
Stratification according to BoNT preparation resulted in five subgroups: patients treated exclusively with onabotulinumtoxinA (n = 36); with abobotulinumtoxinA (n = 128); with incobotulinumtoxinA (n = 16); with rimabotulinumtoxinB (n = 4); and patients in whom BoNT preparation was changed during the last 10 treatments (switcher group: n = 28). Because of the small sample size, the incobotulinumtoxinA and rimabotulinumtoxinB groups were not analyzed separately.
Of the patients testing positive for antibodies in the ELISA assay, 4 (11.1%) received onabotulinumtoxinA, 21 (16.4%) abobotulinumtoxinA, and 10 (35.7%) belonged to the switcher group. There was no significant difference between onabotulinumtoxinA and abobotulinumtoxinA groups (P = 0.601), but prevalence of ELISA‐positive patients was significantly higher in the switcher subgroup compared to the two others (P < 0.014). Among the ELISA‐positive patients, 17 (13.3%) in the abobotulinumtoxinA, 2 (5.6%) in the onabotulinumtoxinA, and 8 (28.6%) in the switcher group tested positive in the MHDA assay, with no significant difference between onabotulinumtoxinA and abobotulinumtoxinA groups (P = 0.254). The switcher subgroup had a significantly higher proportion of MHDA‐positive patients (P < 0.02).
Mean treatment duration in the onabotulinumtoxinA group was 9.5 ± 3.3 years resulting in an estimate of mean NAB incidence of 0.59%/year. Mean treatment duration in the abobotulinumtoxinA group was significantly higher (12.6 ± 5.3 years; P < 0.001) resulting in a mean incidence of 1.05%/year. In the switcher group, treatment duration was comparable to the onabotulinumtoxinA group (9.6 ± 6.4 years); the estimated mean incidence in this subgroup was 2.98%/year, which is approximately 5 times higher than in the onabotulinumtoxinA group.
Discussion
Lack of predictors for the presence of neutralizing antibodies
The present study presents data on CD patients effectively treated long term with intramuscular BoNT injections for up to 21 years. There is no evidence for a systematic loss of efficacy of BoNT injections or that patients treated over decades respond not as well to BoNT than those treated for only a few years. This was also reported in other publications on long‐term BoNT treatment in CD.15, 16, 19, 20 When we plotted the Tsui score against treatment duration, a completely flat regression line resulted. Patients' scoring of treatment effect even indicated a continuous improvement with duration of therapy. There were patients reporting little clinical effect of BoNT treatment who did not develop NABs, whereas others responding well to treatment developed NABs. Thus, neither objective Tsui scores nor subjective patients' efficacy scoring are good parameters to reliably predict NAB presence. Increased proportions of patients with a positive NAB test with high CD severity, with higher BoNT doses, and with long treatment duration underline the well‐known fact that these are obvious risk factors for NAB development.21 However, there is no obvious, good clinical criterion clearly predicting the presence of NABs.
Partial secondary treatment failure and switching of BoNT preparation as a predictor for NAB presence
So far, there is no precise definition of partial secondary treatment failure in the literature. Without a clear‐cut formal PSTF criterion based on CD severity, a blood test for NAB presence ordered when treating physician and/or patient think that treatment is unsuccessful, may show surprisingly few positive test results: Among 362 samples of CD patients with treatment failure collected from 65 centers across Germany, only 175 (48.3%) were MHDA positive.12 Similarly, only 4 in 22 (18.2%) patients with “a less than satisfactory response” tested positive in the mouse protection assay.20 Furthermore, only 4 of 8 CD patients “who reported a less than satisfactory response to their BoNT injections on two consecutive visits” tested positive in the MHDA.16 A similar low percentage (48.3%) of NAB‐positive patients among their secondary nonresponders was reported by Kessler et al.14 .
Using a formal PSTF criterion based on a systematic worsening of at least 2 Tsui score points over two treatment cycles, 31 PSTF patients were detected and analyzed for the presence of NABs (these patients are not included in the present study cohort).13 In 25 of them (80.6%), MHDA was positive (manuscript submitted). Therefore, PSTF is a strong predictor of NAB presence: The stronger the clinical criteria for PSTF, the higher the probability of NAB presence.
Patients switched from one BoNT preparation to another are also high‐risk subjects for the presence of an immune response.22 In our switcher subgroup, a high proportion (>28%) of NAB‐positive patients was found. Thus, switching of BoNT preparation may be a further predictor for PSTF development and NAB induction.
Patients with a high Tsui score receiving high BoNT doses are highly likely to not experience a clear clinical effect, a criterion necessary for inclusion into the present study. These patients are at a high risk to develop PSTF. Some of these patients discontinued BoNT therapy; several others were referred to DBS.23, 24 This is probably the reason why the groups with the highest Tsui score and the highest doses did not have the highest NAB prevalence in the present study (compare Fig. 2A–C).
High prevalence and low incidence of NAB induction in long‐term CD treatment
At first glance, a prevalence of 14.6% of NAB‐positive patients in our cohort seems high (although a similar proportion of 16.6% was previously reported19). However, mean treatment duration in our study was 11.7 years. This results in a NAB incidence of 1.26%/year, which concurs with Kaplan‐Meier's analysis of a (retrospective) study in which a PSTF incidence of 1.65%/year was estimated.13 Taking into account that around 80% of PSTF patients (identified as PSTF with our formal definition) presented with NABs (manuscript submitted), an incidence for NAB induction of 1.32%/year (1.65 × 0.8) can be expected from the retrospective study,13 which is very close to the 1.26%/year in the present study.
Our data stratified by BoNT preparation also match data in the literature. We estimated a NAB incidence of 0.59%/year for onabotulinumtoxinA, which compares well to the study by Brin et al.7 Their mean treatment duration was 2.5 years, yielding an incidence of at least 0.49%/year. For abobotulinumtoxinA, slightly higher incidences between 0.9%/year25 and 2.2%/year26 have been reported compared to our estimate of 1.05%/year. Because only a few patients had been exclusively treated with incobotulinumtoxinA or rimabotulinumtoxinB, the small sample size did not permit separate analyses for the incobotulinumtoxinA and the rimabotulinumtoxinB subgroups. Recent literature suggests that NAB incidence for incobotulinumtoxinA is even lower than that of onabotulinumtoxinA,3, 9, 10, 27, 28 and that it is much higher for rimabotulinumtoxinB than for all BoNT/A preparations.5, 29, 30 The relevant factor for these differences is probably the different antigenic protein load of the different BoNT preparations.3, 5
In summary, the estimates of NAB incidence in the literature seem to be consistent. Given that induction or boostering of NABs may occur at any time during the course of BoNT treatment, high NAB prevalence has to be expected with long duration of treatment despite low NAB incidence. The present cross‐sectional study strongly supports this expectation and indicates that despite low NAB incidences in CD, the problem of neutralizing antibodies for long‐term treatment is underestimated.
Avoidance of high NAB titers and CSTF in long‐term CD treatment
High Tsui scores, need of high doses, and long treatment duration are obvious risk factors for NAB induction and PSTF development. When injections are performed on a regular basis every 3 months, PSTF usually begins with a reduction of effect duration,11 which corresponds to a systematic worsening of CD severity at the end of the injection intervals.13 Therefore, careful monitoring of patients' CD severity helps to detect PSTF early.
Limitations of the study
In this cross‐sectional study, clinical and NAB testing were performed only once. Therefore, no information on the temporal development of NABs, which may be very complex,23 and on the clinical course in parallel to NAB development, especially in BoNT switchers, can be derived from the present study. Furthermore, differences in subgroup sample size and duration of treatment did not allow to test for differences in antigenicity of the different BoNT preparations.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
H.H.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
D.R.: 1B, 1C, 2A, 2B, 2C, 3B
M.M.: 1A, 1B, 1C, 2A, 2B, 2C, 3B
Disclosures
Funding Sources and Conflicts of Interest: All costs associated with the publishing of the present article were met by Merz Pharmaceuticals GmbH (Frankfurt, Germany). Merz Pharmaceuticals was not involved in study design, data collection, and data analysis and interpretation. The authors report no conflicts of interest.
Financial Disclosures for previous 12 months: H.H. participated in research studies for which unrestricted grants were provided by Allergan, Merz, and Ipsen. He has been the recipient of honoraria and fees for presentations at meetings and conferences and for participating in advisory boards from Allergan, Ipsen, and Merz. M.M. received honoraria and fees for presentations at meetings and conferences.
Acknowledgments
Editorial assistance was provided by Dr. Elke Grosselindemann and was paid for by Merz Pharmaceuticals, Frankfurt, Germany.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Albanese A, Asmus F, Bhatia KP, et al. EFNS guidelines on diagnosis and treatment of primary dystonias. Eur J Neurol 2011;18:5–18. [DOI] [PubMed] [Google Scholar]
- 2. Frevert J. Content of botulinum neurotoxin in Botox(R)/Vistabel(r), Dysport(r)/Azzalure(r), and Xeomin(r)/Bocouture(r). Drugs R D 2010;10:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Frevert J, Dressler D. Complexing proteins in botulinum toxin type A drugs: a help or a hindrance? Biologics 2010;4:325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Greene P, Fahn S, Diamond B. Development of resistance to botulinum toxin type A in patients with torticollis. Mov Disord 1994;9:213–217. [DOI] [PubMed] [Google Scholar]
- 5. Dressler D, Hallett M. Immunological aspects of Botox®, Dysport® and Myobloc™/NeuroBloc® . Eur J Neurol 2006;11(Suppl 1):11–15. [DOI] [PubMed] [Google Scholar]
- 6. Göschel H, Wohlfarth K, Frevert J, Dengler A, Bigalke H. Botulinum A toxin therapy: neutralizing and nonneutralizing antibodies—therapeutic consequences. Exp Neurol 1997;147:96–102. [DOI] [PubMed] [Google Scholar]
- 7. Brin MF, Comella CL, Jankovic J, Lai F, Naumann M; for the CD‐017 BoNTA Study Group . Long‐term treatment with botulinum toxin type A in cervical dystonia has low immunogenicity by mouse protection assay. Mov Disord 2008;23:1353–1360. [DOI] [PubMed] [Google Scholar]
- 8. Naumann M, Carruthers A, Carruthers J, et al. Meta‐analysis of neutralizing antibody conversion with onabotulinumtoxinA (BOTOX®) across multiple indications. Mov Disord 2010;25:2211–2218. [DOI] [PubMed] [Google Scholar]
- 9. Jost WH, Blümel J, Grafe S. Botulinum neurotoxin type A free of complexing proteins (Xeomin®) in focal dystonia. Drugs 2007;67:669–683. [DOI] [PubMed] [Google Scholar]
- 10. Evidente VG, Fernandez HH, LeDoux MS, et al. A randomized, double‐blind study of repeated incobotulinumtoxinA (Xeomin(®)) in cervical dystonia. J Neural Transm 2013;120:1699–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dressler D. Clinical presentation and management of antibody‐induced failure of botulinum toxin therapy. Mov Disord 2004;19(Suppl 8):S92–S100. [DOI] [PubMed] [Google Scholar]
- 12. Lange O, Bigalke H, Dengler R, Wegner F, deGroot M, Wohlfarth K. Neutralizing antibodies and secondary therapy failure after treatment with botulinum toxin type A: much ado about nothing? Clin Neuropharmacol 2009;32:213–218. [DOI] [PubMed] [Google Scholar]
- 13. Hefter H, Spiess C, Rosenthal D. Very early reduction in efficacy of botulinum toxin therapy for cervical dystonia in patients with subsequent secondary treatment failure—a retrospective analysis. J Neural Transm 2014;121:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kessler KR, Skutta M, Benecke R. Long‐term treatment of cervical dystonia with botulinum toxin A: efficacy, safety, and antibody frequency. J Neurol 1999;246:265–274. [DOI] [PubMed] [Google Scholar]
- 15. Haussermann P, Marczoch S, Klinger C, Landgrebe M, Conrad B, Ceballos‐Baumann A. Long‐term follow‐up of cervical dystonia patients treated with botulinum toxin A. Mov Disord 2004;19:303–308. [DOI] [PubMed] [Google Scholar]
- 16. Mohammadi B, Buhr N, Bigalke H, Krampfl K, Dengler R, Kollewe K. A long‐term follow‐up of botulinum toxin A in cervical dystonia. Neurol Res 2009;31:463–466. [DOI] [PubMed] [Google Scholar]
- 17. Kranz G, Sycha T, Voller B, Kranz GS, Schnider P, Auff E. Neutralizing antibodies in dystonic patients who still respond well to botulinum toxin type A. Neurology 2008;70:133–136. [DOI] [PubMed] [Google Scholar]
- 18. Tsui JK, Eisen A, Stoessl AJ, Calne S, Calne DB. Double‐blind study of botulinum toxin in spasmodic torticollis. Lancet 1986;2:245–247. [DOI] [PubMed] [Google Scholar]
- 19. Hsiung GY, Das SK, Ranawaya R, Lafontaine AL, Suchowersky O. Long‐term efficacy of botulinum toxin A in treatment of various movement disorders over a 10‐year‐period. Mov Disord 2002;17:1288–1293. [DOI] [PubMed] [Google Scholar]
- 20. Mejia NI, Vuong KD, Jankovic J. Long‐term botulinum toxin efficacy, safety, and immunogenicity. Mov Disord 2005;20:592–597. [DOI] [PubMed] [Google Scholar]
- 21. Moore P, Naumann M. General and clinical aspects of treatment with botulinum toxin In: Moore P, Naumann M, eds. Handbook of Botulinum Toxin Treatment, 2nd ed Malden, MA: Blackwell Science; 2003:28–75. [Google Scholar]
- 22. Oshima M, Deitiker PR, Jankovic J, Duane DD, Aoki KR, Atassi MZ. Human T‐cell responses to botulinum neurotoxin. Responses in vitro of lymphocytes from patients with cervical dystonia and/or other movement disorders treated with BoNT/A or BoNT/B. J Neuroimmunol 2011;240–241:121–128. [DOI] [PubMed] [Google Scholar]
- 23. Hefter H, Hartmann C, Kahlen U, Moll M, Bigalke H. Prospective analysis of neutralising antibody titres in secondary non‐responders under continuous treatment with a botulinumtoxin type A preparation free of complexing proteins—a single cohort 4‐year follow‐up study. BMJ Open 2012;2:e000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hung SW, Hamani C, Lozano AM, et al. Long‐term outcome of bilateral pallidal deep brain stimulation for primary cervical dystonia. Neurology 2007;68:457–459. [DOI] [PubMed] [Google Scholar]
- 25. Truong D, Brodsky M, Lew M, et al. Long‐term efficacy and safety of botulinum toxin type A (Dysport) in cervical dystonia. Parkinsonism Relat Disord 2010;16:316–323. [DOI] [PubMed] [Google Scholar]
- 26. Coleman C, Hubble J, Schwab J, Beffy JL, Picaut P, Morte C. Seroconversion in cervical dystonia patients after treatment with abobotulinumtoxin A. Neurology 2010;74(Suppl 2):A88–A89. [DOI] [PubMed] [Google Scholar]
- 27. Benecke R, Jost WH, Kanovsky P, Ruzicka E, Comes G, Grafe S. A new botulinum toxin type A free of complexing proteins for treatment of cervical dystonia. Neurology 2005;64:1949–1951. [DOI] [PubMed] [Google Scholar]
- 28. Benecke R. Xeomin® in the treatment of cervical dystonia. Eur J Neurol 2009;16(Suppl. 2):6–10. [DOI] [PubMed] [Google Scholar]
- 29. Jankovic J, Hunter C, Dolimbek BZ, et al. Clinico‐immunologic aspects of botulinum toxin type B treatment of cervical dystonia. Neurology 2006;67:2233–2235. [DOI] [PubMed] [Google Scholar]
- 30. Dressler D, Bigalke H. Botulinum toxin type B de novo therapy of cervical dystonia: frequency of antibody induced therapy failure. J Neurol 2005;252:904–907. [DOI] [PubMed] [Google Scholar]


