Abstract
Background
Prediction models for venous thromboembolism recurrence will likely be improved by adding levels of coagulation factors. Risk assessment is ideally performed during anticoagulant treatment, however, the influence of direct oral anticoagulants on coagulation factors is uncertain.
Objective
To assess the influence of rivaroxaban and apixaban on several coagulation factor levels.
Methods
In two crossover trials we assessed the influence of rivaroxaban and apixaban intake on factor (F)VIII, FXI and FXII‐activity and fibrinogen, von Willebrand factor (VWF:Ag), and d‐dimer levels. At three sessions with a washout period in between, blood was taken from 12 healthy male individuals immediately before intake of rivaroxaban 15 mg twice daily (n = 6) or apixaban 10 mg twice daily (n = 6) and three hours after the last intake.
Results
Overall, measured levels were lower after rivaroxaban/apixaban intake. The paired mean difference after rivaroxaban intake was −38 IU/dL (95% CI −43; −33) for FVIII:C, −29 U/dL (95% CI −45; −12) for FXI:C, −22 IU/dL (95% CI −43; −1) for FXII:C, −0.11 g/L (95% CI −0.25; 0.03) for fibrinogen, −7 IU/dL (95% CI −18; 3) for VWF:Ag, −27 ng/mL (95% CI −50; −4) for d‐dimer and −0.36 (95% CI −0.57; −0.15) for Ln d‐dimer. After apixaban intake this was −29 IU/dL (95% CI −38; −21) for FVIII:C, −29 IU/dL (95% CI −36; −22) for FXI:C, −19 IU/dL (95% CI −24; −15) for FXII:C, −0.18 g/L (95% CI −0.33; 0.03) for fibrinogen, −52 ng/mL (95% CI −100; −4) for d‐dimer, 0.25 (−0.60; 0.09) for Ln d‐dimer and 1 IU/dL (95% CI −7; 9) for VWF:Ag.
Conclusion
FVIII:C, FXI:C, FXII:C, and d‐dimer measurements were influenced by rivaroxaban/apixaban intake, while fibrinogen and VWF:Ag were not.
Keywords: apixaban, blood coagulation tests, coagulants, factor Xa inhibitors, risk assessment, rivaroxaban
Essentials.
It is uncertain whether direct factor Xa inhibitors influence levels of coagulation factors.
In two trials we assessed the influence of rivaroxaban and apixaban on coagulation factor levels.
Factors VIII, XI, XII, and d‐dimer measurements were influenced by rivaroxaban/apixaban intake.
Fibrinogen and von Willebrand factor measurements were not affected by rivaroxaban/apixaban use.
1. BACKGROUND
Venous thromboembolism, the composite of deep vein thrombosis and pulmonary embolism, can be prevented and treated effectively with anticoagulation therapy. Unfortunately, all currently available anticoagulant therapies carry the burden of increased bleeding risk. After initial treatment of an episode of venous thromboembolism (typically 3‐6 months), the decision to prolong anticoagulant treatment must be weighed against the bleeding risk.1 Ideally, persons with a high recurrence risk are identified and anticoagulant treatment prolonged, whereas in patients with a low recurrence risk, treatment can be stopped safely. Several prediction models that estimate the risk of venous thromboembolism recurrence have been developed, most of which perform moderately.2 The accuracy and discriminative performance of these models can potentially be improved by adding coagulation factors as predictors. By preference such a predictor is not influenced by use or type of anticoagulants, optimizing the applicability of the assessment model. Candidate coagulation factors are factor (F)VIII, von Willebrand factor (VWF), and d‐dimer for which higher levels are associated with increased recurrence risk.3, 4, 5, 6, 7 Preferably, risk assessment and herewith the measurement of these coagulation factors is performed while the patient is still on anticoagulant treatment. By doing so, persons at high recurrence risk are not exposed to an anticoagulation‐free interval and remain protected during risk assessment. This approach also seems more patient‐friendly as only one (outpatient) contact moment, which can be combined with blood draw, is necessary. This was for example done in the derivation and in the majority of patients in the validation study of the HERDOO2 model, in which d‐dimer levels were measured on vitamin K antagonist (VKA) treatment.8, 9 Treatment with VKA influences d‐dimer levels, probably due to its anticoagulant effect.10 Whether d‐dimer test levels are affected by direct oral anticoagulants (DOACs) is not well known. In contrast, FVIII levels are non‐vitamin K dependent and seem only marginally influenced by VKAs, but may be affected by DOACs11, 12, 13 which are now suggested as first treatment of choice for venous thromboembolism.14 Several previous studies that investigated the effect of DOACs on the measurement of coagulation factors investigated spiked ex vivo samples and found varying results according to the combinations of reagents and drugs.15, 16, 17, 18, 19 For instance, FVIII:C levels seem to be effected by concurrent DOAC use, while d‐dimer, fibrinogen, and VWF:Ag may not.18, 19 As DOACs have been rapidly implemented in clinical practice, it is of importance to study whether commonly used measurements of coagulation are influenced by DOAC intake.
In two crossover trials, we determined whether levels of FVIII:C, FXI:C, FXII:C, VWF, fibrinogen, and d‐dimer are influenced by the direct FXa inhibitors rivaroxaban and apixaban.
2. METHODS
2.1. Study design
We performed a post hoc analysis in two crossover trials of which the main results were published previously by Barco et al20 and Cheung et al21; both studies are registered in the Dutch Trial Registry (http://www.trialregister.nl; NTR3559). In the study by Barco et al, six healthy male volunteers received a twice daily rivaroxaban 15 mg bid for 2.5 days at three different sessions. The main aim of that study was to assess reversal of treatment with prothrombin complex concentrate in two different dosages compared with placebo. Hence, volunteers repeated the study two times with a washout period of at least 15 days. The design from the Cheung et al study was similar to the Barco et al study. Here, six healthy male volunteers received twice‐daily apixaban 10 mg for 3.5 days followed by a single bolus of prothrombin complex concentrate in two different dosages at three (one with placebo) different sessions. All participants provided written informed consent and both studies were approved by the Medical Ethical Committee of the Academic Medical Center in Amsterdam, the Netherlands. For the current study, only the blood samples drawn just before DOAC intake and three hours after the last intake were used (eg, before the administration of prothrombin complex concentrate), in total there were two studies, six participants, three sessions, for a total of 36 blood samples.
2.2. BLOOD SAMPLES
For each of the three sessions, blood samples were drawn immediately before the first intake of rivaroxaban/apixaban (T = 0, 0′ and 0′′) and three hours after the last dose of rivaroxaban/apixaban (T = 1, 1′ and 1′′), in vacuum tubes containing 0.105 mol/L sodium citrate and centrifuged twice at 2500 g for 10 minutes at 18°C within two hours after venipuncture. After aliquoting, the samples were stored at −80°C.
2.3. LABORATORY MEASUREMENTS
Activity (:C) of FVIII, FXI, FXII (one stage‐clotting), and VWF antigen (VWF:Ag) (imunoturbimetric), fibrinogen (Clauss method) and d‐dimer (imunoturbimetric) levels were measured using the ACL TOP 700 analyzer (Werfen Instrumentation Laboratory, Barcelona, Spain), using HemosIL insertions (Werfen Instrumentation Laboratory), FVIII deficient plasma, FXI deficient plasma, FXII deficient plasma, VWF antigen, Thrombin (Bovine), and d‐Dimer HS 500, respectively. Samples were not diluted before the analysis. Measurement of FVIII, FXI, FXII, and fibrinogen by the ACL TOP 700 involves a 1:10 dilution step. Measurement of VWF:Ag and d‐dimer did not involve a dilution step. The corresponding manufacturer reference ranges were, FVIII: 50‐150 IU/dL (%), FXI: 65‐150 IU/dL (%), FXII: 50‐150 IU/dL (%), fibrinogen: 2.0‐3.93 g/L, d‐dimer: <500 ng/mL fibrinogen equivalent units (FEU), VWF:Ag: 42.0‐140.8 IU/dL (%) for blood group O and 66.1‐176.3 IU/dL (%) for non‐O blood groups.
Laboratory technicians were blinded to time point and agent corresponding to each sample. All coagulation factor levels were determined within one batch. All coagulation factors, except for d‐dimer levels were measured in duplicate.
2.4. STATISTICAL ANALYSIS
Differences in coagulation factor levels before and after rivaroxaban/apixaban intake were plotted for every participant and for every factor at the three sessions. We estimated the mean difference with 95% confidence intervals (CIs) in levels of the coagulation factors (before and after the intake of rivaroxaban/apixaban) for every participant at the three different sessions (within pair comparison). The observed mean of these paired differences are presented both as absolute differences and as percentages. d‐dimer was also assessed on a natural logarithmic (Ln) scale as the distribution of d‐dimer is slightly skewed.
For a post hoc sample size calculation, assuming an alpha of 0.05 and a beta of 0.80, we would need a sample size of 11 paired measurements for both rivaroxaban and apixaban separately to detect a paired mean difference of 10 IU/dL or more in FVIII levels with a (conservative) standard deviation (SD) of 10 IU/dL.
3. RESULTS AND DISCUSSION
The clinical characteristics of the participants and the mean levels of the coagulation factors at the start of each session are shown in Table 1. In both the rivaroxaban and apixaban trial six healthy male participants were enrolled and all 12 completed the trial. The mean age in the rivaroxaban trial was 27 (SD 12) years and the mean weight was 83 (SD 14) kg, this was 26 (SD 7) years and 75 (SD 12) kg in the apixaban trial.
Table 1.
Clinical characteristics and levels of the coagulation factors at start of each session
| Rivaroxaban | Apixaban | |
|---|---|---|
| Participants, n | 6 | 6 |
| Age, mean (SD) | 27 (12) | 26 (7) |
| Weight in kg, mean (SD) | 83 (14) | 75 (12) |
| Mean levels (SD) at T0, start of each session before rivaroxaban/apixaban intake | ||
| FVIII:C (IU/dL) | ||
| Session 1 | 113 (25) | 122 (16) |
| Session 2a | 115 (22) | 105 (14) |
| Session 3a | 107 (16) | 114 (14) |
| FXI:C (IU/dL) | ||
| Session 1 | 109 (20) | 122 (9) |
| Session 2a | 110 (14) | 117 (13) |
| Session 3a | 106 (12) | 121 (13) |
| FXII:C (IU/dL) | ||
| Session 1 | 120 (24) | 104 (36) |
| Session 2a | 122 (18) | 103 (36) |
| Session 3a | 123 (14) | 105 (36) |
| Fibrinogen (g/L) | ||
| Session 1 | 2.87 (0.98) | 3.08 (0.67) |
| Session 2a | 2.79 (1.00) | 2.25 (0.38) |
| Session 3a | 2.59 (0.86) | 2.35 (0.34) |
| VWF:Ag (IU/dL) | ||
| Session 1 | 99 (28) | 123 (23) |
| Session 2a | 105 (36) | 100 (15) |
| Session 3a | 92 (25) | 104 (16) |
| d‐dimer (FEU ng/mL) | ||
| Session 1 | 116 (60) | 208 (141) |
| Session 2a | 110 (57) | 147 (104) |
| Session 3a | 126 (48) | 136 (82) |
FEU, fibrinogen equivalent units; SD, standard deviation.
After a washout period of at least 15 days after previous session.
Figure 1 depicts the difference in coagulant factor levels between T0 (before) and T1 (after rivaroxaban/apixaban intake) for every participant at all three sessions. FVIII:C, FXI:C, FXII:C, d‐dimer levels were mostly lower after rivaroxaban/apixaban intake and fibrinogen and VWF:Ag levels changed only marginally if at all.
Figure 1.
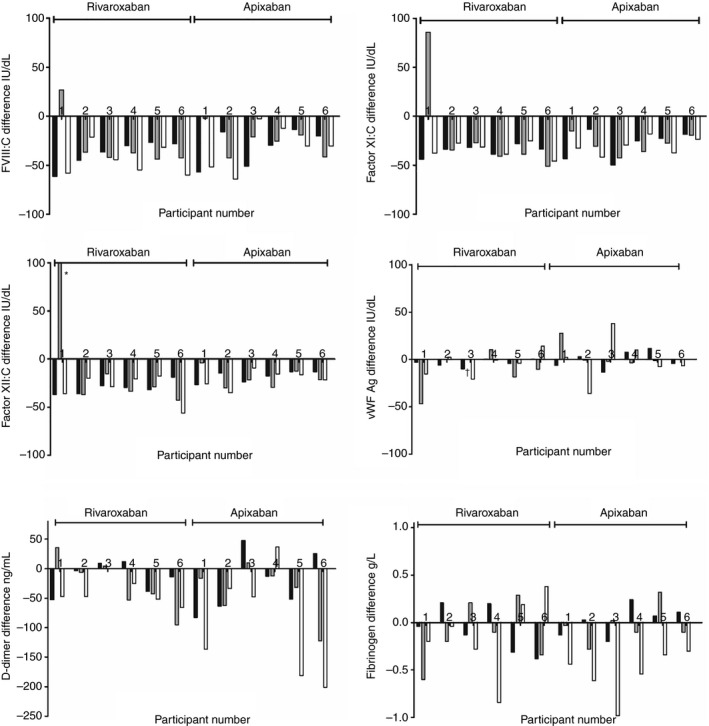
Difference in coagulation factor levels before (T0) and after (T1) rivaroxaban/apixaban intake for every participant at all three sessions. Black bars indicate the difference in the factor at the first session, the grey and white bar the second and third session, respectively. Bars pointing down indicate a lower factor level after rivaroxaban/apixaban intake, bars pointing up indicate higher levels. The order of participants is the same in all figures. Please note that the y‐axis (and unit) is different for d‐dimer (in fibrinogen equivalent units) and fibrinogen.*124 IU/dL. †Measurement missing
The average paired mean differences in coagulation factors levels after rivaroxaban/apixaban intake are shown in Table 2. The differences in levels after rivaroxaban intake were −38 IU/dL (95% CI −43; −33) for FVIII:C, −29 IU/dL (95% CI −45; −12) for FXI:C, −22 IU/dL (95% CI −43; −1) for FXII:C, −0.11 g/L (95% CI −0.25; 0.03) for fibrinogen, −7 IU/dL (95% CI −18; 3) for VWF:Ag, −27 FEU ng/mL (95% CI −50; −4) for d‐dimer and −0.36 (95% CI −0.57; −0.15) for Ln d‐dimer. This corresponds to a relative difference in levels of −33.9% for FVIII:C, −26.9% for FXI:C, and −18.0% for FXII:C. This was −23.1% for d‐dimer, −7.8% for Ln d‐dimer, −4.0% for fibrinogen, and ‐8.1% for VWF:Ag levels.
Table 2.
Effects of rivaroxaban and apixaban on coagulation factor measurements
| Mean (SD) | FVIII:C | FXI:C | FXII:C | Fibrinogen | VWF:Ag | d‐dimer | Ln d‐dimer |
|---|---|---|---|---|---|---|---|
| Rivaroxaban | |||||||
| T = 0 | 112 (20) | 108 (15) | 122 (18) | 2.75 (0.92) | 99 (28) | 117 (54) | 4.59 (0.78) |
| T = 1 | 74 (19) | 79 (11) | 100 (23) | 2.64 (0.93) | 91 (27) | 90 (36) | 4.23 (0.94) |
| Mean differencea | −38 (−43 to −33) | −29 (−45 to −12) | −22 (−43 to −1) | −0.11 (−0.25 to 0.03) | −7 (−18 to 3) | −27 (−50 to −4) | −0.36 (−0.57 to −0.15) |
| Apixaban | |||||||
| T = 0 | 114 (12) | 120 (11) | 104 (36) | 2.56 (0.36) | 109 (16) | 164 (103) | 4.84 (0.78) |
| T = 1 | 84 (6) | 91 (14) | 84 (33) | 2.38 (0.25) | 110 (12) | 112 (65) | 4.58 (0.54) |
| Mean differencea | −29 (−38 to −21) | −29 (−36 to −22) | −19 (−24 to −15) | −0.18 (−0.33 to 0.03) | 1 (−7 to 9) | −52 (−100 to −4) | −0.25 (−0.60 to 0.09) |
Fibrinogen in g/L, d‐dimer in fibrinogen equivalent units ng/mL, other variables n IU/dL. SD, standard deviation; VWF, von Willebrand factor.
Within pair comparison with (95% confidence interval).
In the apixaban trial the paired mean differences were −29 IU/dL (95% CI −38; −21) for FVIII:C, −29 IU/dL (95% CI −36; −22) for FXI:C, −19 IU/dL (95% CI −24; −15) for FXII:C, −0.18 g/L (95% CI −0.33; 0.03) for fibrinogen, −52 FEU ng/mL (95% CI −100; −4) for d‐dimer, −0.25 (−0.60; 0.09) for Ln d‐dimer, and 1 IU/dL (95% CI −7; 9) for VWF:Ag. This corresponded to a relative difference of ‐26.3% for FVIII:C, −24.2% for FXI:C, and ‐19.2% for FXII:C. This was −31.7% for d‐dimer, ‐5.4% for Ln d‐dimer, −7.0% for fibrinogen, and +0.9% for VWF:Ag levels.
Finally, we assessed whether there was a linear relationship between measured FVIII:C and d‐dimer levels before and after rivaroxaban/apixaban intake. As depicted in Figure 2A, we observed a linear association between FVIII:C levels before rivaroxaban/apixaban intake and the decrease in levels after intake. For d‐dimer levels there also appeared to be a linear association, although levels were low overall (Figure 2B). This is relevant as a linear association would make these measurements still usable as a predictor, whereas no association (eg, random influence of the anticoagulant intake on the measurement) could render the predictor unusable.
Figure 2.
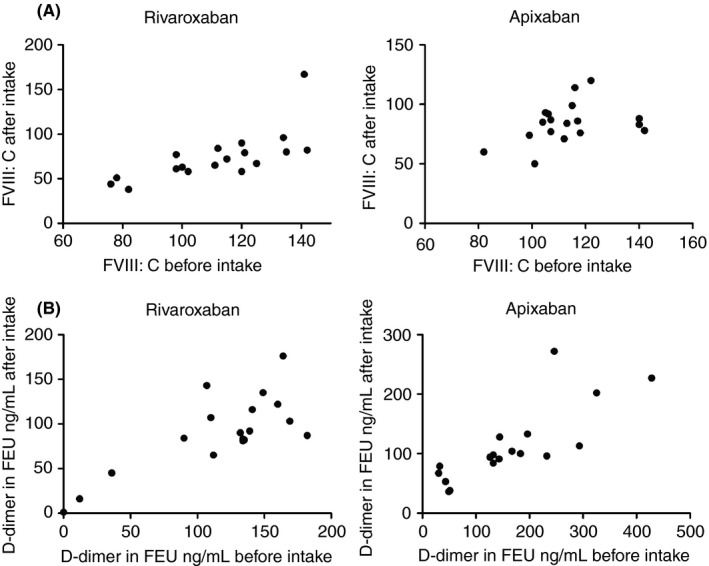
Association between (A) FVIII:C and (B) d‐dimer levels in fibrinogen equivalent units (FEU) ng/mL before and after intake of rivaroxaban/apixaban. Results are shown for rivaroxaban and apixaban
As also reported by Tichelaar and colleagues,11 FVIII:C measurements seem to be affected by rivaroxaban intake. We observed the same effect for FXI:C and FXII:C measurements for both rivaroxaban and apixaban intake. As was also reported by Mani et al, VWF:Ag measurements did not seem to be affected by a low dose of rivaroxaban (10 mg daily) intake, although in their study the average VWF:Ag study levels were high, likely due to the postsurgical status of the participants and no measurement was done before rivaroxaban intake.12 In addition, both VWF:Ag as well as VWF activity measurements were not affected in an in vitro study with drug enriched samples of rivaroxaban and apixaban.19 In other studies in which factor Xa inhibitors were added to human plasma ex vivo, fibrinogen measurements were reported not to be affected.17, 18, 19 Our study confirm these findings in healthy men on therapeutic doses of rivaroxaban/apixaban and by comparing coagulation factor levels before and after intake. Intake of rivaroxaban or apixaban could influence these coagulation factor measurements either due to a direct effect on the coagulant factors (eg, the actual in vivo levels are lower due to the effects of the agent) or due to interference with the assay (eg, the actual in vivo levels are unchanged, only the measurement is influenced). In previous studies sample plasma dilutions partially resolved the difference in measured levels after spiking with DOAC, indicating that interference with the assay is the most feasible explanation.11, 12
A strength of this study is its controlled design, where at three sessions (with a washout period in between) blood was taken immediately before rivaroxaban/apixaban intake and three hours after intake. Where most previous studies were limited to ex vivo or in vitro settings, we investigated the effects of rivaroxaban/apixaban in human volunteers on therapeutic doses of the agents. A limitation of this study is that it was performed in healthy individuals with generally low d‐dimer levels (often below the official detection limit of 203 ng/mL FEU), and this finding might therefore not be directly extrapolated to the anticoagulant using patient population. In an in vitro study d‐dimer measurements were not influenced by enriching samples with rivaroxaban or apixaban.19 In addition, all participants were men hereby limiting the generalizability of the results to women.
The results of our study are relevant to clinical practice: d‐dimer, FVIII:C, FXI:C, and FXII:C measurements in patients using rivaroxaban or apixaban should be interpreted with caution as they are probably different from as when measured without rivaroxaban/apixaban use. As there was a linear association in the decrease of FVIII:C measurement after intake of rivaroxaban/apixaban, FVIII:C might still be a useful predictor while using rivaroxaban or apixaban, however, this requires further validation. VWF:Ag was hardly, if at all, influenced by the treatment and was previously found to have a similar predictive performance to FVIII:C in the MEGA follow‐up study.5 In this study, the risk of a venous thromboembolism recurrence increased stepwise with the highest risk observed for FVIII levels >225 IU/dL: hazard ratio (HR) 4.6 (95% CI 2.9‐7.5), 100 IU/dL as reference. This pattern was similar for VWF:Ag levels, where the observed risk was also highest at levels >225 IU/dL: HR 4.1 (95% CI 2.6‐6.5), <100 IU/dL as reference.5 Thus, VWF:Ag might have a role in future prediction models for venous thromboembolism recurrence for both patients off anticoagulant therapy and patients using a factor Xa inhibitor (at least for rivaroxaban and apixaban).
In conclusion, FVIII:C, FXI:C, FXII:C, and d‐dimer measurements were influenced and decreased by rivaroxaban or apixaban intake. Fibrinogen and VWF:Ag measurements were not affected by rivaroxaban/apixaban intake.
RELATIONSHIP DISCLOSURE
L.J.J. Scheres, W.M. Lijfering, and S.C. Cannegieter report no conflict of interest. S. Middeldorp reports grants and personal fees from GSK, Aspen, Daiichi Sankyo, fees from Bayer, Boehringer Ingelheim and Sanofi, outside the submitted work. Y.W. Cheung reports travel grants from from Sanquin Blood Supply and Bristol‐Myers Squibb. S. Barco reports congress and travel payments from Daiichi Sankyo and Bayer and financial support for the printing costs of his PhD thesis from Pfizer, CSL Behring, Sanquin Plasma Products, Boehringer Ingelheim, Aspen, and Bayer, outside of the submitted work. M. Coppens reports fees paid to his Institution from Boehringer Ingelheim, Bayer, Daiichi Sankyo, Pfizer, and CSL Behring, and research support from Daiichi Sankyo and Sanquin Blood Supply, outside of the submitted work.
AUTHOR CONTRIBUTION
Y. W. Cheung, S. Barco, M. Coppens, and S. Middeldorp designed and performed the original trials. L.J.J. Scheres, W.M. Lijfering, S.C. Cannegieter, and S. Middeldorp designed the current study. L.J.J. Scheres and W.M. Lijfering performed the analyses. L.J.J. Scheres, W.M. Lijfering, and S.C. Cannegieter interpreted the data and drafted the manuscript. Y. W. Cheung, S. Barco, M. Coppens, and S. Middeldorp critically revised the manuscript. All authors approved the final version of the manuscript.
ACKNOWLEDGMENTS
We are grateful to the volunteers for their participation in the study. We would like to thank Petra Noordijk, Annelies Hoenderdos, and Lejla Mahic for performing the laboratory analyses.
Scheres LJJ, Lijfering WM, Middeldorp S, et al. Measurement of coagulation factors during rivaroxaban and apixaban treatment: Results from two crossover trials. Res Pract Thromb Haemost. 2018;2:689–695. 10.1002/rth2.12142
Funding Information
The original rivaroxaban study by Barco et al was funded the Netherlands Organisation for Health Research and Development (project number 836021017) and Sanquin Blood Supply. The original apixaban study by Cheung et al was funded by the Netherlands Organisation for Health Research and Development (project number 836021017), Sanquin Blood Supply, and the Bristol‐Myers Squibb Pfizer alliance. L.J.J. Scheres is a PhD candidate supported by the Netherlands Heart Foundation, CREW project (2013T083). S. Barco is supported by the German Federal Ministry of Education and Research (BMBF 01EO1003 and 01EO1503).
Contributor Information
Luuk J. J. Scheres, Email: l.j.j.scheres@lumc.nl, https://twitter.com/Scheresluuk.
Saskia Middeldorp, https://twitter.com/MiddeldorpS.
Stefano Barco, https://twitter.com/stebarco.
Suzanne C. Cannegieter, https://twitter.com/scannegieter.
REFERENCES
- 1. Di Nisio M, van Es N, Buller HR. Deep vein thrombosis and pulmonary embolism. Lancet. 2016;388:3060–73. [DOI] [PubMed] [Google Scholar]
- 2. Ensor J, Riley RD, Moore D, Snell KI, Bayliss S, Fitzmaurice D. Systematic review of prognostic models for recurrent venous thromboembolism (VTE) post‐treatment of first unprovoked VTE. BMJ Open. 2016;6:e011190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Palareti G, Legnani C, Cosmi B, Guazzaloca G, Pancani C, Coccheri S. Risk of venous thromboembolism recurrence: high negative predictive value of d‐dimer performed after oral anticoagulation is stopped. Thromb Haemost. 2002;87:7–12. [PubMed] [Google Scholar]
- 4. Bjori E, Johnsen HS, Hansen JB, Braekkan SK. d‐dimer at venous thrombosis diagnosis is associated with risk of recurrence. J Thromb Haemost. 2017;15(5):917–24. [DOI] [PubMed] [Google Scholar]
- 5. Timp JF, Lijfering WM, Flinterman LE, et al. Predictive value of factor VIII levels for recurrent venous thrombosis: results from the MEGA follow‐up study. J Thromb Haemost. 2015;13:1823–32. [DOI] [PubMed] [Google Scholar]
- 6. Cristina L, Benilde C, Michela C, Mirella F, Giuliana G, Gualtiero P. High plasma levels of factor VIII and risk of recurrence of venous thromboembolism. Br J Haematol. 2004;124:504–10. [DOI] [PubMed] [Google Scholar]
- 7. Kyrle PA, Minar E, Hirschl M, et al. High plasma levels of factor VIII and the risk of recurrent venous thromboembolism. N Engl J Med. 2000;343:457–62. [DOI] [PubMed] [Google Scholar]
- 8. Rodger MA, Kahn SR, Wells PS, et al. Identifying unprovoked thromboembolism patients at low risk for recurrence who can discontinue anticoagulant therapy. CMAJ. 2008;179:417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rodger MA, Le Gal G, Anderson DR, et al. Validating the HERDOO2 rule to guide treatment duration for women with unprovoked venous thrombosis: multinational prospective cohort management study. BMJ. 2017;356:j1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baglin T, Keeling D, Kitchen S. Effects on routine coagulation screens and assessment of anticoagulant intensity in patients taking oral dabigatran or rivaroxaban: guidance from the British Committee for Standards in Haematology. Br J Haematol. 2012;159:427–9. [DOI] [PubMed] [Google Scholar]
- 11. Tichelaar V, de Jong H, Nijland H, Kluin‐Nelemans H, Meijer K, Mulder A. Interference of rivaroxaban in one‐stage and chromogenic factor VIII: C assays. Thromb Haemost. 2011;106:990–2. [DOI] [PubMed] [Google Scholar]
- 12. Mani H, Hesse C, Stratmann G, Lindhoff‐Last E. Ex vivo effects of low‐dose rivaroxaban on specific coagulation assays and coagulation factor activities in patients under real life conditions. Thromb Haemost. 2013;109:127–36. [DOI] [PubMed] [Google Scholar]
- 13. Passamonti SM, Bucciarelli P, Bader R, Martinelli I. Influence of anticoagulant therapy with vitamin K antagonists on plasma levels of coagulation factor VIII. Thromb Res. 2010;126:243–5. [DOI] [PubMed] [Google Scholar]
- 14. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–52. [DOI] [PubMed] [Google Scholar]
- 15. Bonar R, Favaloro EJ, Mohammed S, et al. The effect of the direct factor Xa inhibitors apixaban and rivaroxaban on haemostasis tests: a comprehensive assessment using in vitro and ex vivo samples. Pathology. 2016;48:60–71. [DOI] [PubMed] [Google Scholar]
- 16. Tripodi A, Padovan L, Testa S, et al. How the direct oral anticoagulant apixaban affects hemostatic parameters. Results of a multicenter multiplatform study. Clin Chem Lab Med. 2015;53:265–73. [DOI] [PubMed] [Google Scholar]
- 17. Hillarp A, Baghaei F, Fagerberg Blixter I, et al. Effects of the oral, direct factor Xa inhibitor rivaroxaban on commonly used coagulation assays. J Thromb Haemost. 2011;9:133–9. [DOI] [PubMed] [Google Scholar]
- 18. Goodwin AJAD. Thrombophilia testing and venous thrombosis. N Engl J Med. 2017;377:2297–8. [DOI] [PubMed] [Google Scholar]
- 19. Gosselin R, Grant RP, Adcock DM. Comparison of the effect of the anti‐Xa direct oral anticoagulants apixaban, edoxaban, and rivaroxaban on coagulation assays. Int J Lab Hematol. 2016;38:505–13. [DOI] [PubMed] [Google Scholar]
- 20. Barco S, Whitney Cheung Y, Coppens M, Hutten BA, Meijers JC, Middeldorp S. In vivo reversal of the anticoagulant effect of rivaroxaban with four‐factor prothrombin complex concentrate. Br J Haematol. 2016;172:255–61. [DOI] [PubMed] [Google Scholar]
- 21. Cheung YW, Barco S, Hutten BA, Meijers JC, Middeldorp S, Coppens M. In vivo increase in thrombin generation by four‐factor prothrombin complex concentrate in apixaban‐treated healthy volunteers. J Thromb Haemost. 2015;13:1799–805. [DOI] [PubMed] [Google Scholar]


