Abstract
Background and Methods
To investigate the possible efficacy of an elastic abdominal binder to control orthostatic hypotension (OH) associated with Parkinson's disease (PD), 15 patients with PD and OH were enrolled in a single‐blind crossover study with elastic abdominal versus placebo binder on two different days, separated by a 1‐day interval, followed by a 4‐week open‐label follow‐up.
Results
Intervention significantly reduced blood pressure fall upon tilting. The mean difference (standard deviation; 95% confidence intervals) between abdominal binder versus placebo was +10 mm Hg (10.2; +3.5, +16.5; P = 0.006). No significant effect on supine mean blood pressure values was observed compared to placebo (P = 0.3). Symptoms of OH decreased significantly during follow‐up (P = 0.003), as assessed by means of the Orthostatic Hypotension Questionnaire.
Conclusions
Our findings suggest that elastic abdominal binders may be a simple complementary tool to alleviate OH in PD.
Keywords: Parkinson's disease, orthostatic hypotension, abdominal binder, Orthostatic Hypotension Questionnaire (OHQ)
Orthostatic hypotension (OH) is defined as a blood pressure (BP) fall by ≥20 mm Hg systolic or ≥10 mm Hg diastolic within 3 minutes of active or passive standing.1 It can be asymptomatic or manifest with symptoms of cerebral hypoperfusion, including syncope and injurious falls.
Frequencies of OH in Parkinson's disease (PD) range from 14% in early stages to 52% in more advanced or older patients.2 OH significantly affects quality of life in PD and can be accompanied by supine hypertension in one‐third of cases. Pharmacological treatment of OH relies either on vasopressor agents (e.g., direct/indirect α1‐adrenoreceptor agonists) or on drugs expanding intravascular volume (e.g., fludrocortisone), but exacerbation of supine hypertension is a common side effect. Furthermore, with the exception of droxidopa (an indirect α1‐adrenoreceptor agonist, which recently showed short‐term efficacy on OH over 1 week in PD3, 4), currently available evidence is insufficient to conclude on safety and efficacy of the above‐mentioned drugs in the setting of PD, thus classifying their use as investigational according to a recent evidence‐based medicine review.5
Nonpharmacological approaches, such as compression stockings, physical countermaneuvers against orthostatic dizziness, as well as dietary changes including salt enrichment or fractionating meals, also have a role in the clinical management of OH. Previously published open‐label studies reported elastic abdominal binders to be effective in pediatric and adult patients with OH resulting from diabetes mellitus, pure autonomic failure, or MSA6, 7, 8 and one further study reported a combined treatment of elastic abdominal binders together with elastic leg bandages to be effective in adult patients with OH of unknown etiology.9
To investigate the effects of an elastic abdominal binder on OH in patients with PD, patients with PD and OH were enrolled in a single‐blind crossover study with elastic abdominal versus placebo binder on 2 different days, separated by a 1‐day interval, followed by a 4‐week open‐label follow‐up.
Patients and Methods
Study Design and Outcomes
An overview of the study design is provided in Figure 1. The CONSORT checklist is reported in Table S1.
Figure 1.
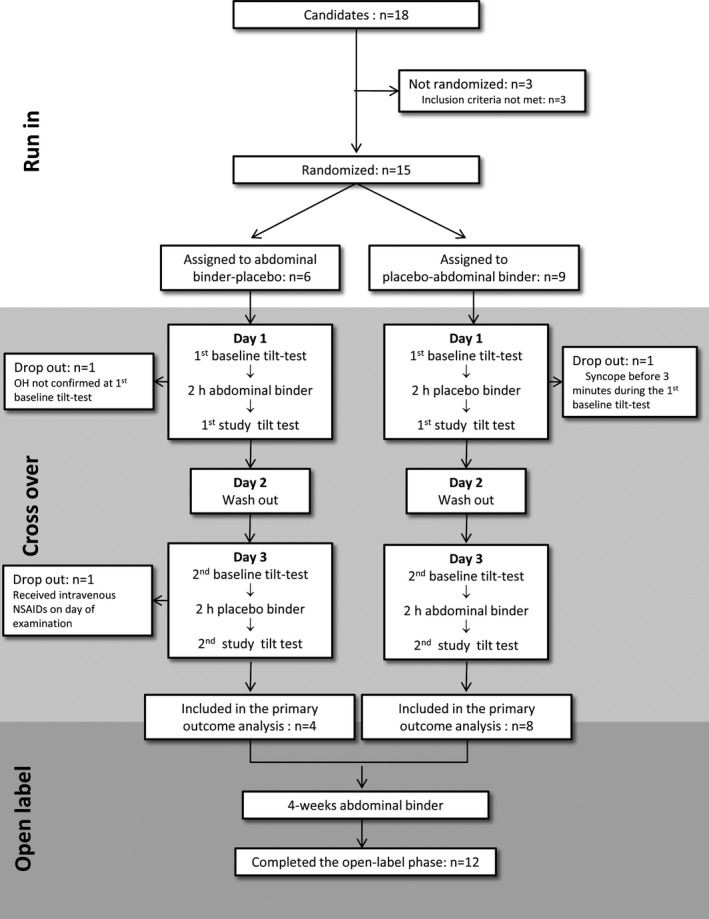
Study flow chart. Clinical demographic characteristics of the 12 patients included in the primary outcome analysis: 8 men; 69 years old (66; 75); disease duration 6 years (3; 12); H & Y stage 2 (2; 2,5); levodopa equivalent dose 555 mg/day (228; 1,690); use of antihypertensive medications n = 6; use of compression stockings n = 2; use of antihypotensive medications n = 2; MDS‐UPDRS total score = 54 (41; 66); MDS‐UPDRS part I = 12 (8; 18); item 12 (cardiovascular) of MDS‐UPDRS part I = 2.5 (1; 3); MDS‐UPDRS part II = 13 (9; 15); MDS‐UPDRS part III = 29 (19, 36); NMSS total score = 81 (50; 106); NMSS cardiovascular subscore = 8 (4; 8); SCOPA‐AUT total score = 21 (14; 29); SCOPA‐AUT cardiovascular subscore = 4 (2; 6). Quantitative variables are reported as median (1st quartile; 3rd quartile). NSAIDs, nonsteroidal anti‐inflammatory drugs.
Using a single‐blinded placebo‐controlled crossover design, 15 consecutive patients with PD and symptomatic OH1 were randomly assigned to first receive either an elastic abdominal binder (20 ± 2 mm Hg pressure on the abdominal wall, Abdo‐Syncro 3‐stripes abdominal binder; Syncro Med GmbH, Linz, Austria) or a placebo binder (3 ± 2 mm Hg pressure on the abdominal wall, Clima Care body warmer; Bort Medical GmbH, Weinstadt‐Benzach, Germany) on 2 different days, separated by an interval of 1 day, to compare their efficacy on tilt‐test examinations. Exclusion criteria included other major neurological, psychiatric or cardiac diseases, untreated diabetes mellitus with clinical features of peripheral neuropathy, varicose veins, known or suspected pregnancy, breast feeding, H & Y stage ≥4, or changes of the pharmacological therapy in the 6 weeks preceding enrollment.
The following scales were administered at baseline: International Parkinson and Movement Disorder Society‐UPDRS (MDS‐UPDRS); Non‐Motor Symptoms Scale (NMSS); Autonomic Scale for Outcomes in PD (SCOPA‐AUT); and Orthostatic Hypotension Questionnaire (OHQ). On study day 1, all patients underwent a first baseline tilt‐test examination. After, they were asked to wear the assigned binder (blinded to which type of). Two hours later, the first study tilt test was performed. The binder was subsequently taken off. After an interval on day 2, aimed at avoiding any hemodynamic carryover effect, on study day 3, patients were crossed over to the other type of binder and underwent the same protocol as on day 1. Patients were invited not to drink any coffee, tea, or taurine‐containing beverages on examination days, to have their meals at least 2 hours before the scheduled examinations, and to take their medication regularly for the whole study period. All the tilt tests were performed in a quiet setting, with mean 22°C room temperature, between 9 am and 12 pm under continuous noninvasive heart rate and BP monitoring (Task Force Monitor; CNSystems Medizintechnik AG, Graz, Austria). The following protocol was applied: 10 minutes supine; 10 minutes 60‐degree head‐up tilt; 5 minutes supine; and 5 minutes active standing. An in‐house‐created software calculated systolic, diastolic, and mean BP values at 10th minute supine, 3rd and 10th minutes tilt, fifth minute supine; and third and fifth minutes standing by averaging 15 values of the continuous BP recording at the above given time points. If continuous BP recording failed, oscillometric BP records at the same time points were used for data analysis.
Once the crossover phase was over, each patient was invited to enter an open‐label phase and wear the elastic abdominal binder every day during daytime over 4 weeks, as an add‐on to previously adopted antihypotensive strategies (either pharmacological or nonpharmacological). At the end of this period, a phone interview took place, in which the patient's compliance was assessed and the OHQ repeated.
Primary outcome of the study was the effect of the elastic abdominal binder versus placebo on mean BP changes after 3 minutes of head‐up tilt. Effect of the elastic abdominal binder versus placebo on systolic and diastolic BP changes after 3 minutes of head‐up tilt, mean BP change after 3 minutes of standing test, and mean supine BP as well as changes of the OHQ score after 4‐week open‐label follow‐up were assessed as secondary outcomes.
The study was approved by the local ethical committee and was performed according to the Declaration of Helsinki. After giving written informed consent, each patient underwent thorough neurological and cardiologic examinations.
Statistical Analysis
Normal distribution of data was checked using Shapiro‐Wilk's test. Parametric or nonparametric tests were used for group comparisons depending on the scale type of the variables (see Table 1). Paired t tests were applied to evaluate differences in BP changes between the abdominal binder and the placebo binder, as summarized in Table 1. OHQ score changes after 4‐week open‐label follow‐up were compared to baseline visit with Wilcoxon's signed‐rank test (see Table 1). Depending on the scale type of the variables, study outcome measures are reported as mean (standard deviation [SD]; 95% confidence interval [CI]) or median (mean; SD).
Table 1.
Study outcome measures
| Single‐Blind Crossover Study (Outcome Measures Obtained on Two Different Days, Separated by a 1‐Day Washout) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Outcome Measures | Placebo Binder | Abdominal Binder | Difference Between Δb | P Value | ||||
| Baseline | Placebo Binder | Δ Placebo Bindera | Baseline | Abdominal Binder | Δ Abdominal Bindera | |||
| Primary outcome | ||||||||
| Δ3rd minute tilt mean BP (mm Hg); n = 12c | −6.6 (±14.9; −16, +2.9) | −8.9 (±13.6; −17.5, −0.2) | −2.3 (±9.6; −8.4, +3.8) | −10 (±13.5; −18.6, −1.4) | −2.3 (±10.2; −8.8, +4.2) | +7.7 (±6.6; +3.5, +11.9) | +10.0 (±10.2; +3.5, +16.5) | 0.006 |
| Secondary outcomes | ||||||||
| 10th minute supine mean BP (mm Hg); n = 12 | 93.1 (±13.2; 84.7, 101.5) | 91 (±13.5; 82.3, 99.5) | −2.2 (±12.8; −10.3, +5.9) | 93.1 (±16.3; 82.8, 103.5) | 95.8 (±18.7; 83.8, 107.7) | +2.6 (±13.7; −6.1, +11.3) | +4.8 (±15.3; −4.9, +14.5) | 0.3 |
| Δ3rd minute tilt systolic BP (mm Hg); n = 12c | −14.8 (±19.5; −27.2, −2.3) | −21 (±19.2; −33.2, −8.8) | −6.2 (±14.7; −15.6, +3.1) | −17.7 (±16.4; −28.1, −7.3) | −8.4 (±15.4; −18.2, +1.4) | +9.3 (±5.6; +5.7, +12.8) | +15.5 (±15.1; +5.9, +25.1) | 0.005 |
| Δ3rd minute tilt diastolic BP (mm Hg); n = 12c | −2.5 (±13.1; −10.9, +5.9) | −2.8 (±11.2; −10, +4.3) | −0.3 (±8.2; −5.6, +4.9) | −6.1 (±12.4; −14.1, +1.7) | +0.8 (±8.2; −4.5, +6) | +6.9 (±8.2; +1.7, +12.1) | +7.3 (±9.9; +1, +13.5) | 0.027 |
| Δ3rd minute standing mean BP; n = 10 (mm Hg)d | −0.4 (±10.2; −7.7, +7) | −6.8 (±15.3; −17.7, +4.1) | −6.4 (±12.8; −15.6, +2.7) | −5.3 (±12.1; −14; +3.3) | +1.2 (±11.0; −6.7, +9.1) | +6.5 (±7.9; +0.9, +12.2) | +13 (±10.2; −0.3, +26.2) | 0.054 |
| Other tilt‐test parameters | ||||||||
| 10th minute supine systolic BP (mm Hg); n = 12 | 121 (±17.3; 106.7, 133.9) | 121.4 (±18.1; 107.2, 135.8) | 122.8 (±21.4; 105.3, 138.5) | 122.0 (±24.3; 101.3, 138.5) | ||||
| 10th minute supine diastolic BP (mm Hg); n = 12 | 79.3 (±13; 68.0, 88.0) | 75.7 (±11.8; 65.9, 84.3) | 78.3 (±15.0; 65.9, 88.7) | 82.6 (±16.1; 68.9, 93.7) | ||||
| 3rd minute tilt mean BP (mm Hg); n = 12 | 86.6 (±14.1; 77.5, 98.9) | 82 (±15.6; 74.7, 95.7) | 83.1 (±16.8; 72.9, 97.5) | 93.4 (±18.0; 81.1, 107.9) | ||||
| 3rd minute tilt systolic BP (mm Hg); n = 12 | 106.2 (±19.3; 94.3, 123.1) | 100.4 (±22.3; 90.3, 120.0) | 105.1 (±24.9; 89.1, 126.3) | 113.6 (±25.6; 95.9, 134.7) | ||||
| 3rd minute tilt diastolic BP (mm Hg); n = 12 | 76.8 (±12.5; 68.3, 87.5) | 72.8 (±12.7; 66.5, 84.0) | 72.2 (±14.4; 63.5, 84.5) | 83.3 (±14.6; 73.4, 94.9) | ||||
| 5th minute supine mean BP (mm Hg); n = 10 | 91.5 (±11.3; 82.5, 99.4) | 97.3 (±17.8; 83.8, 110.6) | 95.9 (±14.5; 84.1, 104.3) | 99.2 (±17.1; 85.7, 111.1) | ||||
| 3rd minute standing mean BP (mm Hg); n = 10 | 90.6 (±15.3; 79.6, 101.6) | 90.4 (±14.4; 80.1, 100.7) | 88.9 (±14.5; 78.5, 99.2) | 99.6 (±21; 84.6, 114.6) | ||||
| 4‐Week Open‐Label Follow‐up Study | ||||
|---|---|---|---|---|
| Outcome Measures | Baseline | Follow‐up | Change From Baseline to Follow‐up | P value |
| OHQ score; n = 12 | 5.2 (5.2 ± 1.6) | 2.4 (2.7 ± 1.5) | −2.2 (−2.5 ± 1.5) | 0.003 |
| OHSAs ubscore; n = 12 | 5.0 (4.8 ± 1.6) | 3.2 (2.8 ± 1.3) | −1.7 (−2.1 ± 1.5) | 0.003 |
| OHDAS subscore; n = 12 | 5.0 (5.6 ± 2.0) | 2.1 (2.2 ± 1.6) | −3.9 (−3.4 ± 2.6) | 0.007 |
Study outcome measures of the single‐blind crossover study are summarized in the first part of the table as mean (±SD; 95% CI). Study outcome measures of the 4‐week open‐label follow‐up study are reported in the second part of the table as median (mean ± SD).
Δ placebo binder = (BP with placebo binder) − (BP at respective baseline examination without any binder); Δ abdominal binder = (BP with abdominal binder) − (BP at respective baseline examination without any binder).
i.e. = (Δ abdominal binder) − (Δ placebobinder).
Δ3rd minute tilt mean/systolic/diastolic BP (mm Hg): calculated by subtracting 10th minute supine mean/systolic/diastolic BP (mm Hg) from mean/systolic/diastolic BP (mm Hg) at third minute of head‐up tilt.
Δ3rd minute standing mean BP (mm Hg): calculated by subtracting fifth minute supine mean BP from mean BP at third minute standing.
Statistical analysis was performed using SPSS software (version 20.0; SPSS, Inc., Chicago, IL). P values <0.05 were considered statistically significant.
Results
Fifteen patients were randomized. Three patients dropped out during the crossover phase (1 from the placebo‐abdominal binder group and 2 from the abdominal binder‐placebo group), and data from 12 patients were available for the primary outcome analysis (Δ3rd minute tilt mean BP). Two further patients experienced syncope shortly after standing up during baseline standing test and were excluded from the secondary outcome analysis of Δ3rd minute standing mean BP because of missing baseline value.
All primary and secondary outcome measures are reported in Table 1. Compared to the placebo binder, the abdominal binder was associated with an increase of third minute tilt mean BP by 10 (±10.2 mm Hg; +3.5, +16.5; P = 0.006 for primary outcome measure). Moreover, there was an increase of third minute diastolic (P = 0.027) and systolic (P = 0.005) tilt BP with the abdominal binder compared to the placebo binder. No significant change of supine mean BP was observed with the abdominal binder compared to placebo; the difference was +4.8 mm Hg (±15.3; −4.9, +14.5; P = 0.3). A trend toward increase of mean BP at third minute standing was observed with the abdominal binder versus placebo (P = 0.054; n = 10).
During the open‐label phase, 12 patients agreed to wear the abdominal binder an average of 5.6 ± 0.6 days/week, 50% to 75% of daytime. At 4‐week follow‐up, the OHQ score decreased by −2.2 (−2.5 ± 1.5) points (P = 0.003), the OH Symptom Assessment (OHSA) subscore by −1.7 (−2.1 ± 1.5) points (P = 0.003) and the OH Daily Activity Scale (OHDAS) by −3.9 (−3.4 ± 2.6; P = 0.007).
No side effect occurred during the crossover phase. One patient with a history of gastro‐oesophageal reflux reported a mild exacerbation of symptoms during the open‐label follow‐up.
Discussion
In the present study, use of elastic abdominal binders was associated with a significant reduction of OH upon tilting in patients with PD. This is in agreement with other conditions, in which abdominal binders have been also reported to reduce OH.7, 8, 9 In addition, we observed a clinically relevant10 reduction of OH symptoms during extended open‐label use. Importantly, wearing the binder was not associated with significant changes of supine mean BP levels, which is in contrast to alpha‐adrenergic drugs, which may induce or worsen supine hypertension.
The binder works by reducing splanchnic venous pooling under orthostatic stress.7 Its use for the management of OH may have several advantages: not inducing pharmacological interactions, it can be preferable in patients with complex therapeutic schedules; not increasing supine BP, it may be preferred in case of overt supine hypertension; and being less cumbersome to wear than compression stockings, it may achieve higher compliance in advanced stages.
Some limitations of our study need to be addressed: first, the investigator performing the tilt tests was not blinded, because such examinations need to be performed bare‐chested for safety reasons. To minimize the risk of bias, however, outcome measures were collected in an automated way. Second, patients with varicose veins were excluded. Although previous studies reported no change of femoral vein diameter from a 40‐mm‐Hg pressure on the abdominal wall,7 we cannot exclude that abdominal binders worsen leg venous insufficiency in the long term. We also cannot exclude that the exacerbation of gastroesophageal reflux reported from one patient during follow‐up was related to the abdominal binder. Finally, the positive effects of the abdominal binder on symptomatic burden of OH in daily living were assessed in an open‐label fashion only. In conclusion, abdominal binders are an effective tool in reducing OH on tilt‐table testing (class I evidence). Moreover, our data provide class III evidence that abdominal binders reduce overall symptomatic burden of OH in PD.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
A.F.: 1A, 1B, 1C, 2A, 2B, 2C, 3A
G.G.: 1B, 2A, 2B, 2C, 3B
B.M.: 1A, 1C, 3B
F.S.: 1B, 3B
W.P.: 1A, 1B, 3B
G.K.W.: 1A, 1C, 2A, 2B, 2C, 3B
K.S.: 1A, 1B, 1C, 2A, 2B, 2C, 3B
Disclosures
Funding Sources and Conflicts of Interest: The authors report no sources of funding and no conflicts of interest.
Financial Disclosures for previous 12 months: Dr. Fanciulli received royalties from Springer Verlag and from the Massachusetts Medical Society outside of the submitted work. Prof. Poewe reports personal fees from AbbVie, AstraZeneca, Teva, Novartis, GlaxoSmithKline/GSK, Boehringer Ingelheim, UCB, Orion Pharma, Merck Serono, and Merz Pharmaceuticals (consultancy and lecture fees in relation to clinical drug development programmes for PD). Prof. Wenning reports personal fees from Affiris, Astra, Lundbeck, TEVA, and UCB. Prof. Seppi reports grants from Oesterreichische Nationalbank, FWF Austrian Science Fund, Michael J. Fox Foundation, and International Parkinson and Movement Disorder Society (MDS) and personal fees from MDS, Boehringer Ingelheim, UCB, Lundbeck, and AOP Orphan Pharmaceuticals AG outside the submitted work.
Supporting information
Figure S1. Elastic abdominal binder.
Table S1. CONSORT statement.
Acknowledgments
The authors thank the patients who took part in the present study. The authors are grateful to Jean Pierre Ndayisaba for programming the BP calculating software. This was an academic project. No external financial support was allotted.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res 2011;21:69–72. [DOI] [PubMed] [Google Scholar]
- 2. Velseboer DC, de Haan RJ, Wieling W, Goldstein DS, de Bie RM. Prevalence of orthostatic hypotension in Parkinson's disease: a systematic review and meta‐analysis. Parkinsonism Relat Disord 2011;17:724–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hauser RA, Isaacson S, Lisk JP, Hewitt LA, Rowse G. Droxidopa for the short‐term treatment of symptomatic neurogenic orthostatic hypotension in Parkinson's disease (nOH306B). Mov Disord 2015;30:646–654. [DOI] [PubMed] [Google Scholar]
- 4. Kaufmann H, Freeman R, Biaggioni I, et al. Droxidopa for neurogenic orthostatic hypotension: a randomized, placebo‐controlled, phase 3 trial. Neurology 2014;83:328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seppi K, Weintraub D, Coelho M, et al. The Movement Disorder Society Evidence‐Based Medicine Review Update: treatments for the non‐motor symptoms of Parkinson's disease. Mov Disord 2011;26(Suppl 3):S42–S80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Denq JC, Opfer‐Gehrking TL, Giuliani M, Felten J, Convertino VA, Low PA. Efficacy of compression of different capacitance beds in the amelioration of orthostatic hypotension. Clin Auton Res 1997;7:321–326. [DOI] [PubMed] [Google Scholar]
- 7. Smit AA, Wieling W, Fujimura J, et al. Use of lower abdominal compression to combat orthostatic hypotension in patients with autonomic dysfunction. Clin Auton Res 2004;14:167–175. [DOI] [PubMed] [Google Scholar]
- 8. Tanaka H, Yamaguchi H, Tamai H. Treatment of orthostatic intolerance with inflatable abdominal band. Lancet 1997;349:175. [DOI] [PubMed] [Google Scholar]
- 9. Podoleanu C, Maggi R, Brignole M, et al. Lower limb and abdominal compression bandages prevent progressive orthostatic hypotension in elderly persons: a randomized single‐blind controlled study. J Am Coll Cardiol 2006;48:1425–1432. [DOI] [PubMed] [Google Scholar]
- 10. Kaufmann H, Malamut R, Norcliffe‐Kaufmann L, Rosa K, Freeman R. The Orthostatic Hypotension Questionnaire (OHQ): validation of a novel symptom assessment scale. Clin Auton Res 2012;22:79–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Elastic abdominal binder.
Table S1. CONSORT statement.


