Abstract
Background
Cognitive and motor decline, along with psychiatric symptoms, have a major impact on independence, nursing home admission, caregiver burden, and mortality in Parkinson's disease (PD). The single most common genetic risk factor for developing PD is a mutation in the glucocerebrosidase (GBA) gene.
Methods
This work is a literature review regarding “GBA” and “Parkinson's disease” as conducted by PubMed search.
Results
There is a higher prevalence of cognitive decline and more rapid trajectory of disease progression in PD‐GBA carriers, compared to noncarriers. PD‐GBA carriers also have domain‐specific cognitive impairment, particularly in visual memory tasks. PD‐GBA carriers may also have a more aggressive motor phenotype than noncarriers.
Conclusions
Early identification of PD‐GBA carriers may lead to targeted therapies and development of new treatments.
Keywords: Parkinson's disease, GBA, glucocerebrosidase, genetics
At least 1 million Americans live with Parkinson's disease (PD), with a world‐wide estimate totaling 7 to 10 million people. Despite the benefit of dopaminergic therapy for the cardinal motor signs of tremor and bradykinesia in PD, the patient's disease course is typically progressive and results in significant disability. In addition, many motor features, including postural instability and freezing of gait, are dopamine resistant and may rapidly lead to mobility impairments and falls. The risk of cognitive decline and dementia in PD increases with age, disease duration, and severity.1 The incidence rate of dementia in PD is reported to be 69 to 95.3 per 1000 person‐years.2, 3 Cognitive and motor decline have a major impact on independence, psychiatric comorbidity, nursing home admission, caregiver burden, and mortality.4, 5, 6
Interestingly, reports of parkinsonism started to emerge in families of patients with Gaucher's disease (GD). Neil et al.9 reported on 2 adult children with the disease who also had a mother with a “Parkinson syndrome.” Furthermore, screening of patients with PD has led to the identification of mutations in the glucocerebrosidase (GBA) gene as the single most common risk factor for the development of PD, with a reported prevalence of 3% to 7% in the general PD population worldwide.7, 8 The GBA gene is located at chromosome 1q21 and it codes for the beta‐glucocerebrosidase enzyme. Mutations in GBA result in reduction or elimination of beta‐glucocerebrosidase activity, causing glucocerebrosidase to accumulate in lysosomes of affected organs. Homozygotes for GBA develop GD, a multiorgan disease that can range from a lethal perinatal disorder to an asymptomatic type. Heterozygotes for GBA do not develop GD, but are still at an increased risk for PD. It should be noted that not all GBA mutation carriers develop PD. Thus, there is an association between the GBA mutation and PD, but the exact pathophysiological mechanism behind GBA‐associated PD remains unknown.
Understanding the phenotype of PD patients who carry the GBA mutation (PD‐GBA carriers) is important clinically given that these patients have a higher frequency of baseline cognitive impairment and earlier onset of motor disability, along with a more aggressive disease course. We aim to review the neuropsychiatric and motor profile of PD‐GBA carriers.
Methods
To identify relevant publications, a PubMed search using the terms “GBA and Parkinson” and “glucocerebrosidase and Parkinson” was conducted. The search engine generated 195 publications. Epidemiological studies and case series with greater than 10 subjects were included and reviewed. All journal articles reviewed were written in English.
Neuropsychiatric Profile of PD‐GBA Carriers
Global Cognitive Function
PD‐GBA carriers are often cognizant of, and able to report, their deficits in cognitive function. Alcalay et al.10 administered the UPDRS‐I, a self‐report of mentation, behavior, and mood, to PD‐GBA carriers and subjects with other genetic forms of PD. More PD‐GBA carriers reported cognitive impairment (score of ≥2) than carriers of other genetic forms of PD (21.9% vs. 14.2%, respectively; P < 0.001).10
Cognitive screening tests have also been used to evaluate global cognitive function in PD‐GBA carriers. Alcalay et al.11 showed that PD‐GBA carriers (n = 24), compared to noncarriers (n = 47), performed more poorly on the Mini–Mental Status Examination (MMSE; 27.1 ± 4.9 vs. 28.9 ± 1.3, respectively; P = 0.035). The Montreal Cognitive Assessment (MoCA), a test of global cognitive function which may be more sensitive than the MMSE at detecting cognitive impairment in PD,12 was used by Brockmann et al.13 to compare global cognition in PD‐GBA carriers and noncarriers, with a cutoff score of <26 to indicate cognitive impairment. Subjects were matched for sex and disease duration between the 2 groups. Cognitive impairment was more frequent (45% vs. 30%; respectively) and severe (22.53 vs. 26.53 points, respectively; P = 0.02) in PD‐GBA carriers, compared to noncarriers. This pattern of greater cognitive impairment in PD‐GBA carriers was also shown using the Clinical Dementia Rating scale (CDR), a clinically based tool that is independent of psychometric test scores. Alcalay et al.11 found that 30.8% of PD‐GBA carriers met criteria for a diagnosis of dementia (CDR ≥1), compared to 21.1% of noncarriers, a finding that has also been supported by other groups.14, 21 It is important to recognize that Alcalay et al. included GBA carriers with early‐onset PD (average age of onset: 42.9 ± 5.2 yr), whereas Brockmann et al. included GBA carriers with later‐onset PD (average age of onset: 52.8 ± 9.2 yr). In both studies, subjects were age matched to noncarriers. Despite the age differences between GBA groups in these studies, global cognitive function was more impaired in GBA carriers compared to noncarriers.
In addition to a higher prevalence of cognitive decline, PD‐GBA carriers may also have a more rapid trajectory of cognitive decline compared to noncarriers. Brockmann et al.13 evaluated 20 PD‐GBA carriers and 27 noncarriers longitudinally over a 3‐yr period using the MoCA. The groups did not differ at baseline (MoCA, P = 0.56), but longitudinal intragroup analyses showed a significantly more rapid progression of cognitive impairment in PD‐GBA carriers (P = 0.04).13 Additionally, natural history analysis has shown that PD‐GBA carriers (n = 4) progress from normal cognition to dementia (MMSE score <24) over a median time of 46 mo (95% confidence interval: 14.9, 77.1), whereas fewer than half of noncarriers developed dementia over a median time of 82 mo.9 Overall, PD‐GBA status has been shown to be a significant predictor of progression to dementia.9
Decline in global cognitive function may be seen as a prodromal feature of PD in GBA carriers, as shown by Beavan et al. in a longitudinal study evaluating a cohort of GBA hetero‐ and homozygotes over a 2‐yr period. The GBA‐positive cohort declined significantly from baseline, compared to the control group (P < 0.05), indicating that GBA‐positive individuals show deterioration in clinical markers consistent with the prodrome of PD.
Domain‐Specific Impairment
Despite being informative regarding the higher prevalence and more rapid trajectory of cognitive decline in PD‐GBA carriers, global measures of cognition lack the sensitivity and specificity to distinguish individual PD‐GBA carriers from noncarriers. Impairment on specific cognitive domains would provide more‐informative markers to identify individuals who are GBA carriers. Although impairment on a variety of cognitive domains has been described in PD‐GBA carriers,11 visual memory testing has shown to be the most promising for identifying this subset of patients thus far.11, 15 Zokaei et al.15 used a serial order task to assess visual short‐term memory impairments in PD‐GBA carriers, GBA carriers without PD, and GBA noncarriers with and without PD. Participants observed 4 bars, each of a different color and orientation, presented sequentially at the center of a screen and were subsequently asked to match the orientation of a colored probe with the orientation of the same colored bar in the sequence (Fig. 1). Two types of errors were noted: (1) misbinding errors: incorrect conjunction of color and orientation, and (2) errors owing to random responses. PD‐GBA carriers made both types of errors, whereas PD‐GBA noncarriers made errors only owing to random responses. Interestingly, GBA carriers without PD made primarily misbinding errors. This suggests that PD‐GBA carriers may be specifically identified by this “double‐hit” in errors made (both misbinding errors and errors owing to random responses), which is related to their gene status and the presence of PD. Further work is needed to determine the predictive value of using domain‐specific testing to identify PD‐GBA carriers and whether this pattern is truly observed exclusively in PD‐GBA.
Figure 1.
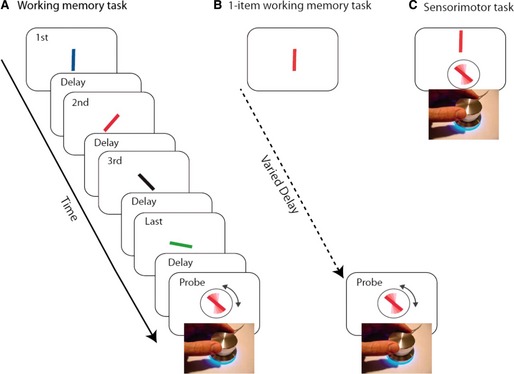
(A) A sequence of 4 colored oriented bars were presented sequentially. Any of the bars could be probed by color of the response stimuli, and participants were asked to adjust the orientation of the response stimuli to the orientation of the bar with the same color. (B) An example of the 1‐item working memory task. A rotating dial is used to orient the probe bar (surrounded by circle) to match the orientation of the target bar presented after a delay. (C) An example of a sensorimotor task. A rotating dial is used to orient the probe bar (surrounded by circle) to match the orientation of the target bar presented above the probe. Figure taken from Zokaei et al.15 This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0/), which permits unrestricted reuse, distribution, and reproduction in any medium, provided the original work is properly cited.
Other tests have also been used to examine visual memory in PD‐GBA carriers, including the Wechsler Memory Scale‐Revised (WMS‐R) Visual Reproduction I and II, as well as the Benton Visual Retention Test‐Revised (BVRT).16, 17 Twenty‐one GBA carriers performed significantly worse than 46 noncarriers using the WMS‐R Visual Reproduction I (P = 0.001) and II (P < 0.001), as well as the BVRT (P = 0.009). This highlights the domain‐specific impairment in visual memory tasks in GBA carriers.
Neurobehavioral/Psychiatric Features
The neurobehavioral and psychiatric features of PD‐GBA carriers remains to be fully elucidated. It has been suggested that psychiatric symptoms occur more frequently in PD‐GBA carriers, compared to noncarriers.18, 19, 20 In a study by Brockmann et al.,18 the psychiatric profile of 20 PD‐GBA carriers and 20 noncarriers was examined using the Neuropsychiatric Inventory (NPI) and the Beck Depression Inventory (BDI). The NPI was carried out as a personal interview between the neurologist and caregiver. PD‐GBA carriers had higher individual item scores for depression (P = 0.013), anxiety disorder (P = 0.007), and apathy and indifference (P = 0.043), when compared to noncarriers. PD‐GBA carriers also scored higher on the BDI, compared to noncarriers (12.05 vs. 7.10; P = 0.031) and more frequently scored above the cutoff indicating depression (70% vs. 25%; P = 0.004).18 However, Alcalay et al. failed to show a clear pattern of depressive symptoms in the PD‐GBA population utilizing the BDI,11 though this was in an early‐onset PD population (average age of onset: 42.9 ± 5.2).13 Tests such as the Hamilton Depression Scale19 and the Center for Epidemiologic Studies Depression scale20 have also failed to show a higher prevalence of depression in PD‐GBA carriers, compared to noncarriers.
Similarly, it is unclear whether anxiety is more common in PD‐GBA carriers, compared to noncarriers. Anxiety has been reported to be more common in PD‐GBA carriers, compared to noncarriers, in several case series,14, 21, 22, 23 but larger studies have not validated this finding.20 Further work is needed to understand whether depression and anxiety are truly part of the PD‐ GBA profile.
In addition to mood symptoms, behavioral disturbances are suggested to occur at a higher frequency in PD‐GBA carriers, compared to noncarriers. Li et al.24 found a greater percentage of hallucinations (41.2 vs. 17.7; P = 0.009), delusions (23.5 vs. 5.3; P = 0.004), and other psychoses (35.3 vs. 9.7; P = 0.0009) in PD‐GBA carriers, compared to noncarriers. Visual hallucinations have been reported to be as high as 45.16% in PD‐GBA carrier patients.22
Motor Profile of PD‐GBA Carriers
Numerous studies have shown that PD‐GBA carriers present with motor signs of PD at an earlier age than noncarriers. Wang et al.20 reported that PD‐GBA carriers (n = 49) had an average age of onset of motor symptoms 6.5 to 7.0 yr earlier than noncarriers (n = 1366; mean age: 51.4 ± 9.1 vs. 58.4 ± 11.0, respectively; P < 0.006). Other groups have reported similar findings, with PD‐GBA carriers typically manifesting motor symptoms ranging between 5 and 10 yr earlier than noncarriers.13, 22, 25
PD‐GBA carriers typically present with similar parkinsonian symptoms at onset as noncarriers,20, 25 but the pattern of motor dysfunction may be atypical. Gan‐Or et al.26 and Lesage et al.27 reported more‐severe bradykinesia (P = 0.021 and P < 0.014, respectively) at initial diagnosis in PD‐GBA carriers, compared to noncarriers, and Sidransky et al.7 reported a lower frequency of asymmetric onset of resting tremor (P < 0.0001).
Studies investigating the nature and severity of motor symptoms and progression in PD GBA carriers versus noncarriers are conflicting. Specific PD subtypes and symptom profiles were similar in PD‐GBA carriers and noncarriers.13, 28 However, Sidransky et al.7 reported significantly lower frequency of bradykinesia (P = 0.0001), resting tremor (P = 0.0298), and rigidity (P < 0.0001) in PD‐GBA carriers, compared to noncarriers. Other studies suggest that PD‐GBA carriers may develop more‐severe signs of parkinsonism earlier in their disease course, compared to noncarriers.26, 27 Wang et al.20 found that PD‐GBA carriers had higher total UPDRS scores (40.3 ± 18.7 vs. 35.9 ± 20.2; P = 0.024) and UPDRS‐II activities of daily living scores (11.78 ± 5.46 vs. 10.49 ± 6.22; P = 0.015) than noncarriers. In a prospective 3‐yr longitudinal study, PD‐GBA carriers had more‐rapid progression of motor deficits, as assessed by UPDRS‐III (P = 0.03) and H & Y staging (P < 0.001).13 Also, the PD‐GBA carriers versus noncarriers required more‐rapid levodopa equivalent dose escalation (950 ± 714.1 mg vs. 687.3 ± 338.2 mg; p = 0.01) and had higher mortality rates (25% vs. 0%, respectively; P = 0.01).13 In line with a more severe disease phenotype, PD‐GBA carriers were found to have developed motor fluctuations and dyskinesia earlier in their disease course.20 It is unclear whether this is related to disease progression, rapid l‐dopa titration, or a combination of both. Further work is needed to clarify the pattern of motor dysfunction in PD‐GBA carriers and determine whether it is truly different from those with idiopathic PD.
Discussion
PD is a neurodegenerative disorder with significant phenotypic variability. PD patients differ in their degree of motor disability, rate of progression, response to medication, and level of cognitive impairment. This heterogeneity is likely owing to a combination of specific genetic mutations with superimposed environmental contributions. In this review, we have highlighted studies that show PD‐GBA carriers to be at higher risk for: (1) PD with a cognitive impairment phenotype; (2) neuropsychiatric and behavioral disturbances; and (3) a more aggressive clinical course from both a cognitive and motor standpoint.
GBA has also been found to be a risk factor for dementia with Lewy bodies, further supporting the link between GBA and cognitive dysfunction.29 It should be noted that a large variety of mutations are implicated in PD‐GBA carriers, with heterogeneity in clinical phenotype based on the specific mutation(s) present. Gan‐Or et al.30 recently showed the differential effects of severe versus mild GBA mutations on PD, reporting that carriers of severe mutations had a roughly 3‐ to 4‐fold higher risk and around 5 yr younger average age of PD onset than carriers of mild mutations. This has been supported by additional studies.31, 32 However, the mechanism underlying GBA‐associated PD remains to be elucidated. Loss‐of‐function mechanisms or toxic gain‐of‐function mechanisms have been suggested.33 The loss‐of‐function hypothesis is gaining more ground owing to evidence that PD‐GBA carriers have significantly reduced production of the glucocerebrosidase protein.30 This may result in α‐synuclein accumulation and inhibition of glucocerebrosidase trafficking into the lysosome, ultimately resulting in a pathogenic positive feedback loop.33, 34 However, additional studies are needed to determine the exact mechanism causing this α‐synuclein accumulation and PD.
It is critical to identify these patients early on not only for genetic counseling and prognosis, but also so that new disease specific treatments can be explored given that PD‐GBA carriers may have a distinctive pattern of impairment on specific cognitive domains early on. By the time there is evidence of clinical dementia, PD‐GBA carriers no longer exhibit a distinctive pattern of cognitive impairment and are thus indistinguishable from noncarriers.11 Therefore, it is imperative to identify these patients early in the disease course when dementia is not yet fully manifest, and it may be possible to do so by utilizing testing that involves visual memory tasks. This could be achieved by more‐widespread utilization of the diagnostic criteria for PD with mild cognitive impairment as proposed by the International Parkinson and Movement Disorder Society Task Force.35
Furthermore, the prevalence of PD‐GBA mutations is likely underestimated. Although GBA mutations have been reported to occur at a frequency of 3.5% in the general PD population,8 frequencies as high as 7% have been found worldwide when the gene is fully sequenced, regardless of ethnic background.36 This is important to recognize given that mutation status may eventually be a useful clinical tool for therapeutic decision making. As costs for genetic testing decline and additional insights into genotype‐phenotype relationships are gained, clinicians may be able to tailor treatments to particular subgroups based on their mutation status.
Summary Points.
PD‐GBA carriers: Cognitive and psychiatric profile
Higher prevalence of cognitive decline compared to noncarriers
More rapid progression of cognitive decline compared to noncarriers
More likely to have domain‐specific impairment in visual memory tasks
May have more psychiatric symptoms, but this remains unclear
PD‐GBA carriers: Motor profile
Earlier age of motor signs of parkinsonism compared to noncarriers
May be more‐symmetric parkinsonism signs at onset compared to noncarriers
May have more rapid progression of motor decline compared to noncarriers
Likely to have earlier motor fluctuations and dyskinesia
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
G.P.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
E.R.: 1C, 2A, 2B, 2C, 3A, 3B
J.O.: 1C, 2A, 2B, 2C, 3A, 3B
D.H.: 1A, 1B, 1C, 2A, 2B, 2C, 3B
Disclosures
Funding Sources and Conflicts of Interest: The authors received funding from the Department of Neurological Sciences at Rush University. The authors report no conflicts of interest.
Financial Disclosures for previous 12 months: D.H. reports research funding from NIH, Shapiro Foundation, and Pfizer.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Alcalay RN, Dinur T, Quinn T, et al. Comparison of Parkinson risk in Ashkenazi Jewish patients with Gaucher disease and GBA heterozygotes. JAMA Neurol 2014;71:752–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mayeux R, Chen J, Mirabello E, et al. An estimate of the incidence of dementia in idiopathic Parkinson's disease. Neurology 1990;40:1513–1517. [DOI] [PubMed] [Google Scholar]
- 3. Aarsland D, Andersen K, Larsen JP, Lolk A, Nielsen H, Kragh‐Sørensen P. Risk of dementia in Parkinson's disease: a community‐based, prospective study. Neurology 2001;56:730–736. [DOI] [PubMed] [Google Scholar]
- 4. Aarsland D, Brønnick K, Ehrt U, De Deyn PP, Tekin S, Emre M, Cummings JL. Neuropsychiatric symptoms in patients with Parkinson's disease and dementia: frequency, profile and associated care giver stress. J Neurol Neurosurg Psychiatry 2007;78:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bronnick K, Ehrt U, Emre M, De Deyn PP, Wesnes K, Tekin S, Aarsland D. Attentional deficits affect activities of daily living in dementia‐associated with Parkinson's disease. J Neurol Neurosurg Psychiatry 2006;77:1136–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levy G, Tang MX, Louis ED, et al. The association of incident dementia with mortality in PD. Neurology 2002;59:1708–1713. [DOI] [PubMed] [Google Scholar]
- 7. Sidransky E, Nalls MA, Aasly JO, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N Engl J Med 2009;361:1651–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Winder‐Rhodes SE, Evans JR, Ban M, et al. Glucocerebrosidase mutations influence the natural history of Parkinson's disease in a community‐based incident cohort. Brain 2013;136:392–399. [DOI] [PubMed] [Google Scholar]
- 9. Neil JF, Glew RH, Peters SP. Familial psychosis and diverse neurologic abnormalities in adult‐onset Gaucher's disease. Arch Neurol 1979;36:95–99. [DOI] [PubMed] [Google Scholar]
- 10. Alcalay RN, Mejia‐Santana H, Tang MX, et al. Self‐report of cognitive impairment and mini‐mental state examination performance in PRKN, LRRK2, and GBA carriers with early onset Parkinson's disease. J Clin Exp Neuropsychol 2010;32:775–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alcalay RN, Caccappolo E, Mejia‐Santana H, et al. Cognitive performance of GBA mutation carriers with early‐onset PD: the CORE‐PD study. Neurology 2012;78:1434–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Steenoven I, Aarsland D, Hurtig H, et al. Conversion between Mini‐Mental State Examination, Montreal Cognitive Assessment, and Dementia Rating Scale‐2 scores in Parkinson's disease. Mov Disord 2014;29:1809–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brockmann K, Srulijes K, Pflederer S, et al. GBA‐associated Parkinson's disease: reduced survival and more rapid progression in a prospective longitudinal study. Mov Disord 2015;30:407–411. [DOI] [PubMed] [Google Scholar]
- 14. Setó‐Salvia N, Pagonabarraga J, Houlden H, et al. Glucocerebrosidase mutations confer a greater risk of dementia during Parkinson's disease course. Mov Disord 2012;27:393–399. [DOI] [PubMed] [Google Scholar]
- 15. Zokaei N, McNeill A, Proukakis C, et al. Visual short‐term memory deficits associated with GBA mutation and Parkinson's disease. Brain 2014;137:2303–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Benton AL. A visual retention test for clinical use. Arch Neurol Psychiatry 1945;54:212–216. [DOI] [PubMed] [Google Scholar]
- 17. Duchaine BC, Weidenfeld A. An evaluation of two commonly used tests of unfamiliar face recognition. Neuropsychologia 2003;41:713–720. [DOI] [PubMed] [Google Scholar]
- 18. Brockmann K, Srulijes K, Hauser AK, Schulte C, Csoti I, Gasser T, Berg D. GBA‐associated PD presents with nonmotor characteristics. Neurology 2011;77:276–280. [DOI] [PubMed] [Google Scholar]
- 19. Malec‐Litwinowicz M, Rudzińska M, Szubiga M, Michalski M, Tomaszewski T, Szczudlik A. Cognitive impairment in carriers of glucocerebrosidase gene mutation in Parkinson disease patients. Neurol Neurochir Pol 2014;48:258–261. [DOI] [PubMed] [Google Scholar]
- 20. Wang C, Cai Y, Gu Z, et al. Clinical profiles of Parkinson's disease associated with common leucine‐rich repeat kinase 2 and glucocerebrosidase genetic variants in Chinese individuals. Neurobiol Aging 2014;35:725.e1‐6. [DOI] [PubMed] [Google Scholar]
- 21. Goker‐Alpan O, Lopez G, Vithayathil J, Davis J, Hallett M, Sidransky E. The spectrum of parkinsonian manifestations associated with glucocerebrosidase mutations. Arch Neurol 2008;65:1353–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Neumann J, Bras J, Deas E, et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson's disease. Brain 2009;132:1783–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saunders‐Pullman R, Hagenah J, Dhawan V, et al. Gaucher disease ascertained through a Parkinson's center: imaging and clinical characterization. Mov Disord 2010;25:1364–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li Y, Sekine T, Funayama M, et al. Clinicogenetic study of GBA mutations in patients with familial Parkinson's disease. Neurobiol Aging 2014;35:935.e3‐8. [DOI] [PubMed] [Google Scholar]
- 25. Asselta R, Rimoldi V, Siri C, et al. Glucocerebrosidase mutations in primary parkinsonism. Parkinsonism Relat Disord 2014;20:1215–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gan‐Or Z, Bar‐Shira A, Mirelman A, Gurevich T, Kedmi M, Giladi N, Orr‐Urtreger A. LRRK2 and GBA mutations differentially affect the initial presentation of Parkinson disease. Neurogenetics 2010;11:121–125. [DOI] [PubMed] [Google Scholar]
- 27. Lesage S, Anheim M, Condroyer C, et al. Large‐scale screening of the Gaucher's disease‐related glucocerebrosidase gene in Europeans with Parkinson's disease. Hum Mol Genet 2011;20:202–210. [DOI] [PubMed] [Google Scholar]
- 28. Aharon‐Peretz J, Badarny S, Rosenbaum H, Gershoni‐Baruch R. Mutations in the glucocerebrosidase gene and Parkinson disease: phenotype‐genotype correlation. Neurology 2005;65:1460–1461. [DOI] [PubMed] [Google Scholar]
- 29. Mata IF, Samii A, Schneer SH, et al. Glucocerebrosidase gene mutations: a risk factor for Lewy body disorders. Arch Neurol 2008;65:379–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gan‐Or Z, Amshalom I, Kilarski LL, et al. Differential effects of severe vs mild GBA mutations on Parkinson disease. Neurology 2015;84:880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guimaraes Bde C, Pereira AC, Rodrigues Fda C, et al. Glucocerebrosidase N370S and L444P mutations as risk factors for Parkinson's disease in Brazilian patients. Parkinsonism Relat Disord 2012;18:688–689. [DOI] [PubMed] [Google Scholar]
- 32. Kumar KR, Ramirez A, Gobel A, et al. Glucocerebrosidase mutations in a Serbian Parkinson's disease population. Eur J Neurol 2013;20:402–405. [DOI] [PubMed] [Google Scholar]
- 33. Mazzulli JR, Xu YH, Sun Y, et al. Gaucher disease glucocerebrosidase and alpha‐synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell 2011;146:37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Beavan M, McNeill A, Proukakis C, Hughes DA, Mehta A, Schapira AH. Evolution of prodromal clinical markers of Parkinson disease in a GBA mutation‐positive cohort. JAMA Neurol 2015;72:201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Litvan I, Goldman JG, Tröster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord 2012;27:349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sidransky E, Lopez G. The link between the GBA gene and parkinsonism. Lancet Neurol 2012;11:986–998. [DOI] [PMC free article] [PubMed] [Google Scholar]


