Abstract
Background
Pain is a significant burden for patients with Parkinson's disease (PD) with a high impact on quality of life. The present article aims at summarizing epidemiological, pathophysiological, clinical, and neurophysiological data regarding pain in PD.
Methods
In this domain, a procedure of systematic assessment is still lacking for the syndromic diagnosis and should take into account pain characteristics, effects of dopaminergic treatment, motor fluctuations, and non‐PD‐associated pain.
Findings
We propose an original questionnaire addressing an algorithm suitable for daily clinical practice. The questionnaire is based on a three‐step approach addressing first the relationship between pain and PD (including temporal relationship with the course of the disease, association with motor fluctuations, and impact of antiparkinsonian treatment), before classifying pain into one of three main syndromes (i.e., musculoskeletal pain, psychomotor restlessness pain, and neuropathic pain).
Conclusions
The proposed questionnaire allows the characteristics of each pain type to be determined according to its relationship with the disease and its treatment. The validation of the clinical use of this questionnaire will be the goal of a forthcoming work.
Keywords: Parkinson's disease, pain, diagnosis, questionnaire
Pain is classified among the nonmotor symptoms of Parkinson's disease (PD)1, 2 and has a major negative impact on the quality of life of many patients with PD.3, 4 Despite a large body of evidence on its high prevalence, this symptom is frequently underestimated, probably owing to the lack of objective assessment tools for its diagnosis and classification. The aim of the present review was to analyze the current data on the prevalence, pathophysiology, clinical scales, and methods of assessment of pain in PD in order to propose a new algorithm for its syndromic diagnosis.
Prevalence of Pain in PD Patients, According to Pain Characteristics
Depending on the population assessed (in‐ vs. outpatients) and the criteria employed to define the presence of pain, its prevalence ranges from 40% to 83% of PD patients.5, 6, 7, 8, 9 In a case‐control study, pain was present in at least one quarter of PD patients before any treatment6 and in almost 40% of patients early in the course of the disease.10 In a recent cohort study, pain has been considered a premotor symptom.11 Pain occurrence further increases with disease progression, particularly associated with the development of therapy‐dependent motor fluctuations.9
Most studies distinguish between PD‐related pain, pain related to PD treatment, pain indirectly associated with PD, and pain unrelated to PD.7, 8 According to the classification proposed by Ford,12 PD‐related pain can be further subdivided into different subtypes, including musculoskeletal pain, dystonia‐associated pain, radicular or peripheral pain, central pain, and akathisia (Appendix S1). Using this classification, pain was assessed in a British series of inpatients and in French and Norwegian series of outpatients.5, 7, 8 In the British study, PD‐related pain was considered as responsive to dopaminergic treatment, prominent on the body side most affected by PD and with no specific etiology other than PD.7 In this study, most PD patients reported at least two concomitant pain syndromes (85%), including PD‐related pain in 63% of patients and non‐PD‐related pain in 64% of patients, whereas pain related to PD treatment and pain indirectly associated with PD were reported as only seldom (8% and 1%, respectively). The researchers further claimed that non‐PD‐related pain was more severe and constant over time compared to PD‐related pain, whereas no interaction between both pain types was found.
A second study, performed in French outpatients, distinguished between non‐PD‐ and PD‐related pain, including pain syndromes directly or indirectly caused by PD.8 In this series, 60% of patients suffered from chronic pain, which was related to the disease in 60% and unrelated in 40%. Finally, in the Norwegian study, 83% of patients had PD‐related pain, 70% with musculoskeletal pain, 40% with dystonic pain, 20% with radicular or peripheral neuropathic pain, and 10% with central neuropathic pain.5
Chronic pain may contribute to the development or aggravation of previous depressive symptoms, as described in the elderly population in general.13 Only a few studies have addressed this issue. In another Norwegian series of 227 PD patients, 67% had pain and patients with pain had more‐severe depression.14 They also had more‐severe motor impairment, lower cognitive performance, and longer disease duration. The finding of an association between depression and pain in PD was replicated.15, 16 However, the direction of causality between pain and depression cannot be determined with certainty.
Pathophysiology of Pain in PD
The basal ganglia and connected structures play a pivotal role in the pathophysiology of pain in PD.17 The classical pathophysiological model of PD motor symptoms consists of a reduced activation of the D1 receptor‐mediated direct striatopallidal pathway and a reduced inhibition of the D2 receptor‐mediated indirect striatopallidal pathway resulting from dopaminergic neuron loss within the pars compacta of the SN.18 This model explains a reduced activation of the thalamocortical motor drive and the beneficial effects of dopaminergic treatment or DBS of the internal pallidum or STN.19 However, the influence of dopamine depletion on the emergence of nonmotor parkinsonian symptoms, such as attentional deficit, cognitive decline, depression, dysautonomia, or pain, likely involves additional pathways described in the so‐called “three‐loop model.”20, 21 Various dysfunctional motor, cognitive, and limbic networks have connections between basal ganglia and cortical regions, which potentially play an important role in pain processing. According to studies in rodents, connections with the insular cortex play a crucial role in D1 receptor‐mediated descending inhibition of pain,22 whereas antinociceptive capacity is determined by D2 receptor availability within the striatum and the right medial temporal cortex of healthy volunteers.23 A careful evaluation of the characteristics of a pain syndrome in its sensory, motor, cognitive, and autonomic components in each patient may provide further information on the underlying pathophysiology.
An original model has recently been proposed in order to explain primary central pain in PD, which may also apply to other types of PD‐related pain.24 This model assumed that dopamine depletion leads to an intrastriatal amplification of sensory inputs from corticostriatal projections. Consistent with this model, the amplitude of laser‐evoked potentials (LEPs), reflecting cortical processing of nociceptive stimuli, was greater during the off period in PD patients with primary central pain than in PD patients without pain or controls.25 However, LEP amplitude returned to normal values during the on period, supporting a dopaminergic modulation of primary central pain. This study also showed a reduced habituation of laser‐evoked sympathetic skin responses, suggesting an over‐reaction of the autonomic nervous system to nociceptive stimuli in PD patients with primary central pain.
Other mechanisms were proposed for dyskinesia‐associated pain.26 The analgesic effect of levodopa is more pronounced in fluctuators with dyskinesia than in stable responders, as revealed by pain threshold increase compared to the off period in dyskinetic patients. Limbic and associative brain structures, which are overactivated in dyskinetic patients, could be involved, as well as the reward system.26 In particular, the mesolimbic pain inhibitory system is thought to play a role, through dopaminergic projections from the ventral tegmental area to the nucleus accumbens.
Pathological changes in PD also include Lewy body aggregation within the lamina I of the dorsal horn, presumably contributing to increased temporal summation of sensory stimuli and enhanced nociception at the spinal level in PD patients.27, 28, 29, 30 Finally, there could also be alterations in the peripheral nervous system owing to alpha‐synuclein accumulation within the sensory afferents31 or to an interaction of l‐dopa medication with cobalamin metabolism.32, 33 These alterations contribute the occurrence of peripheral neuropathies in PD, possibly at the origin of neuropathic pain in the limbs.32, 33
Musculoskeletal pain shares with other causes of PD‐related pain some sensitivity to dopaminergic treatment. Rigidity or abnormal posture may affect muscles and joints, favoring musculoskeletal disorders, such as osteoarthrosis. This condition may also be associated with peripheral neuropathic pain, especially radicular pain, as shown in a group of PD patients with mechanical low back pain.34 In contrast to what is observed in PD patients with central pain, LEP amplitude can be decreased in PD patients with pain resulting from peripheral nerve fiber lesion. In parallel, nociceptive spinal reflexes can be enhanced through a reduced descending inhibitory control of pain and this may contribute to the occurrence of referred pain and secondary hyperalgesia.35
Experimentally Induced Pain
Various methodological approaches have been employed to experimentally provoke pain in PD patients. The first studies were performed in on drug conditions, and one study showed that l‐dopa could modulate heat pain sensitivity.36 More‐recent studies include off drug conditions, that is, 12‐hour (overnight) dopaminergic medication withdrawal, although this condition cannot be considered completely free of dopaminergic influence. Sensitivity to nociceptive stimuli is increased during the off state28, 29, 30, 37, 38, 39 and decreased during the on state.31 The increase in provoked pain correlates to the intensity of spontaneous pain and to the body side most affected by PD in some studies38, 39 but not in others, likely owing to a heterogeneous recruitment of patients.
Patients at an early stage of the disease also tend to have increased responses to nociceptive stimuli (e.g., enhanced spinal nociceptive reflexes), but unaltered pain sensory discrimination (e.g., subjective estimation of provoked pain).39 Enhanced sensitivity to nociceptive stimuli was attributed to functional changes at the spinal level27 and within the pain matrix, mainly the medial pain pathway, as shown by PET.28, 29, 37 These changes are reversed by dopaminergic treatment, showing a major role of dopamine depletion. In contrast, PD patients did not differ from controls regarding modulation of experimentally provoked pain through opioidergic, serotonergic, and adrenergic mechanisms, involved in descending nociceptive inhibitory controls.29, 40
Pain Assessment
In PD patients, pain can be assessed by various questionnaires, including the UPDRS, for example, item 17 of UPDRS part II (sensory complaints).41, 42, 43 However, these questionnaires cannot distinguish between different pain syndromes encountered in PD and do not provide information to support their classification and treatment. No specific scales or questionnaires have been developed to characterize pain in PD, and a few studies have employed the whole battery of questionnaires currently used to assess chronic pain syndromes. The visual analog scale is the most frequently used tool in PD studies to date,44, 45, 46, 47 but it may be inappropriate to assess intermittent pain, such as pain that worsens during off periods.12 The short form of the Brief Pain Inventory allows for the quantification of pain intensity and pain interference in daily activities. It has also been used in PD.5, 48, 49 The McGill Pain Questionnaire (MPQ), in its short form,50, 51, 52 allows for the quantification of the sensory‐discriminative, affective, and evaluative aspects of pain.48, 49, 53 In a recent study, for example, it has been shown that STN‐DBS improved sensory and affective aspects of pain postsurgery measured by the MPQ in PD patients.54 The same study showed that among 41 patients with PD, only 2 (4.5%) had neuropathic pain when the Douleur Neuropathique questionnaire (DN‐4), which has a relatively high sensitivity and specificity for the diagnosis of neuropathic pain,55 was employed.54 Interestingly, in this study, whereas STN‐DBS decreased pain in general in PD, its effect was more robust to control musculoskeletal than neuropathic pain symptoms. PainDETECT56 is another neuropathic pain screening tool that has been used in PD.57 Finally, the Neuropathic Pain Symptoms Inventory58, 59 is used in the follow‐up of neuropathic pain and enables the characterization of clusters of symptoms (i.e., spontaneous, evoked, or paroxysmal pain) and was also applied in PD.54 The assessment of pain in PD patients may be subject to different bias owing to PD‐related fluctuations that can influence the perception and report of pain symptoms. Thus, the characterization of pain in PD must take into account the motor status, the treatment (l‐dopa and DBS) and its complications (e.g., dyskinesia and paroxysmal off stage). Thus, considering the high prevalence of chronic pain among the general population,60 it is important to know whether or not the pain is temporally related to PD. The timing of occurrence and the patient's motor and nonmotor status provide evidence for an association with PD.8 The presence of pain syndromes, such as neuropathic pain and myofascial pain syndrome, can be readily diagnosed by screening tools or at the bedside. For instance, a recent study showed that 69% of PD patients reported pain that worsened during off periods and 79% had myofascial pain syndrome.54 This is associated with referred pain and secondary hyperalgesia, spatially distant from the affected muscles and can pose diagnostic challenges.61 Based on the available data, there are no current pain scales or questionnaires that allow for the characterization of pain in PD taking into account all specific particularities.
Thus, we aimed at developing an algorithm addressing the respective pain syndromes associated or not associated with PD according to the temporal relationship with the disease, the association with motor fluctuations, and the influence of antiparkinsonian treatment. Pain syndromes that are not related to PD or its treatment should be considered according to the underlying pathophysiological mechanisms of pain.
Development of a New Approach
Different classifications have been employed for the distinction between PD‐related and PD‐unrelated pain syndromes, but motor fluctuations and the response to dopaminergic medication were not usually taken into account, except in the most recent approaches.2, 62 We here provide an original questionnaire for pain assessment in PD. It can be used in addition, or as an alternative, to the questionnaire currently being developed by the nonmotor study group of the International Parkinson and Movement Disorder Society.63, 64
Our approach is based on a three‐step approach: (1) establish a relationship with PD on the basis of a temporal association between the onset of pain and PD symptoms, whereas other causes of pain are excluded; (2) determine whether pain depends on motor fluctuations; and (3) determine whether pain depends on the antiparkinsonian treatment. At the end of this three‐step approach, pain could be classified as a PD‐related or non‐PD‐related pain. Finally, concerning PD‐related pain, the type of pain is categorized as one of three main syndromes, (i.e., musculoskeletal pain, psychomotor restlessness pain, and neuropathic pain). The proposed algorithm was converted into a pain questionnaire named Marburg‐Sao Paulo‐Créteil Questionnaire for Pain in Parkinson's disease (Appendix S2; Fig. 1).
It may be challenging, in some instances, to differentiate PD‐related from PD‐unrelated pain, but PD‐related pain can be considered when pain symptoms show a temporal relationship with the onset of PD symptoms and the clinical course of the disease, whereas no other etiology can be detected. However, given that pain may present as a nonmotor symptom preceding motor symptoms, any other symptoms possibly indicating early PD need to be assessed.6
It is important to determine whether or not pain occurring in a PD patient is related to motor fluctuations. Pain can depend on motor fluctuations present at low, intermediate or high dopaminergic levels, that is, usually related to hypo‐ or hyperkinesia (in off or on condition).In off condition (low dopaminergic level), pain can be associated with wearing‐off and/or end‐of‐dose akinesia (early morning or nocturnal akinesia and/or akinesia related with medication intake), paroxysmal off stage (unrelated to medication intake), or off dystonia (often in the early morning). This subclassification mainly derives from previous observations9, 26 underlining the influence of motor fluctuations on pain intensity, particularly for musculoskeletal and dystonia‐associated pain.9In on condition (high dopaminergic level), mainly choreatic dyskinesia is present. Choreatic dyskinesia is usually perceived as a nonpainful symptom, but dyskinetic movements may become painful in the case of additional pathological conditions (e.g., osteoarthritis). Conversely, pain relief can occur along with dyskinesia in some patients,9, 65 as revealed by increased pain thresholds. Choreatic dyskinesia includes peak on, plateau, and biphasic dyskinesia, with the latter occurring at intermediate dopaminergic levels. On dyskinesia (i.e., especially biphasic dyskinesia) can also manifest as painful dystonia in some cases. Rarely, both choreatic and dystonic biphasic dyskinesia may occur simultaneously. The influence of choreatic dyskinesia on pain sensitivity was further studied in stable responders and fluctuators.26 Fluctuators with choreatic dyskinesia (peak on or plateau dyskinesia) exhibited an l‐dopa‐dependent increase in cold pain and tolerance thresholds, which was not observed in stable responders. The researchers postulated that pain and choreatic dyskinesia may share common mechanisms and that central sensitization leads to greater analgesic and motivational responses.
The fluctuation with the dopaminergic state may give an additional hint for an association between pain and PD in more‐advanced cases. Thus, the effects of any antiparkinsonian treatment on pain should be systematically included in the diagnostic algorithm, as recently proposed.62
According to the three main issues mentioned above (temporal relationship with the course of the disease, the change with motor fluctuations, and the impact of antiparkinsonian treatment), pain features can be classified as PD related or non‐PD related. Then, three main PD‐related pain syndromes can be distinguished (i.e., musculoskeletal pain, psychomotor restlessness pain, and neuropathic pain).Musculoskeletal pain is caused by PD‐related rigidity and related to the presence of joint, tendon, or muscle soreness. It includes low back pain and frozen shoulder pain. An associated myofascial pain syndrome is frequent in this context, leading to referred pain distant from the affected muscle and an area of secondary mechanical hyperalgesia (increased pain to pinprick) that has no dermatomal distribution. The muscles more frequently involved are axial (e.g., scapular and pelvic girdle muscles).54 Musculoskeletal pain may be aggravated or not by motor off periods and usually responds well to the treatment of motor symptoms by drugs or DBS. The so‐called “coat hanger headache,” a neck pain associated with a tension‐type headache, is also considered as a type of musculoskeletal pain owing to the fact that it usually presents with similar symptoms. It results from orthostatic hypotension (either primary or secondary to treatment) and it is more common in patients with atypical parkinsonian syndromes and severe autonomic dysfunction, but it may occur also in later stages in PD patients.66Psychomotor restlessness pain comprises various pain syndromes that occur in the case of motor or emotional restlessness, including leg motor restlessness, nonmotor off‐fluctuation‐related pain, and the dopamine agonist withdrawal syndrome (DAWS).67 In this context, patients are usually experiencing various neuropsychiatric complications of l‐dopa or dopaminergic agonist withdrawal. Therefore, pain symptoms rarely occur in isolation, being rather part of a broader clinical picture, in which tachycardia, excessive sweating, anxiety, depression, and motor restlessness occur all together. Pain can be diffuse or located around the mouth, abdomen, or pelvic floor68 and may migrate from one location to another in relatively short periods of time. This may reveal as a nonmotor off fluctuation that can be relieved by l‐dopa or dopamine agonist adjustment. The term “motor restlessness” was chosen according to a recent study suggesting that leg motor restlessness, rather than RLS, occurs early in PD patients and may correspond to the formerly used term, akathisia.69 We doubt, however, that there is an independent form of akathisia in PD, but rather believe that the reported cases of akathisia in PD are owing to leg motor restless, non motor off fluctuations, and DAWS.The pain syndromes associated with these clinical presentations, especially DAWS, are difficult to differentiate from primary central pain. One of the key features of DAWS is the clear association between dopamine agonist administration and symptom improvement. Conversely, central pain only responds poorly to dopaminergic treatment.Neuropathic pain is secondary to a lesion or disease of the somatosensory system, either peripheral or central, and refers to specific questionnaires. Pain is located in a body region where negative (thermal or mechanical hypoesthesia) or positive (dynamic mechanical allodynia, hyperpathia, or cold allodynia) sensory symptoms exist, resulting from the somatosensory lesion or disease. Neuropathic features of pain include burning, electric shock‐like, and pins‐and‐needles sensations.
Pain syndromes unrelated to PD are not dependent on the course of the disease or its treatment. They should be also divided into nociceptive and neuropathic pain by using a neuropathic pain questionnaire (e.g., PainDETECT or DN‐4).55, 56 This facilitates the diagnosis of an underlying pain syndrome not attributable to PD. For example, osteoarthrosis is likely to be the most relevant pain syndrome in the elderly, which may occur regardless of PD.7 The determination of a specific cause of non‐PD‐related pain syndrome may lead to dedicated consultations for further diagnosis and treatment.
Associated nonmotor factors, such as mood and cognitive alterations, should be evaluated clinically, using respective scales (e.g., Non‐Motor Symptoms Questionnaire and the Non‐Motor Symptoms Scale, Montgomery and Asberg Depression Rating Scale, or the Montreal Cognitive Assessment).41, 70, 71, 72
Figure 1.
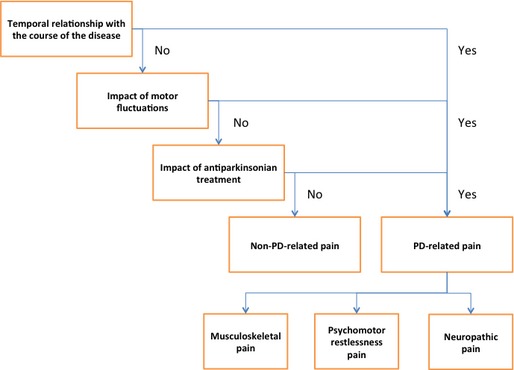
Taxonomy of pain types according to the Marburg‐Sao‐Paulo‐Créteil Questionnaire for Pain in Parkinson's Disease
Treatment: General Principles
One of the advantages of an accurate classification of the pain syndrome in PD is to facilitate its treatment. For pain associated with motor fluctuations, treatment should consist of an adequate management of these fluctuations.73 For pain occurring during wearing‐off, long‐lasting l‐dopa formulations should be administered in case of early morning akinesia and nocturnal akinesia, whereas catechol O‐methyltransferase inhibitors and shortening of dosing intervals should relieve pain associated with end‐of‐dose akinesia. Regarding pain resulting from off dystonia, probably the most painful form of dyskinesia, the treatment should be based on long‐lasting l‐dopa formulations in the evening and readily soluble l‐dopa formulations in the morning. Finally, once‐daily application of a transdermal patch of rotigotine, a dopamine agonist, was shown to produce significant pain relief related with early morning motor function and sleep quality improvement.74, 75
Beyond pharmacological treatments, DBS can be proposed in patients with advanced PD to treat motor symptoms, which are not controlled by oral pharmacotherapy (i.e., patients with paroxysmal off phases),76, 77 especially using the STN target.78 It has been shown that STN‐DBS could also produce a significant reduction of spontaneous pain intensity in 40% to over 80% of PD patients up to 24 months postsurgery.44, 46 Pain relief induced by DBS is independent from the motor effect.45, 79 In fact, the correlation between pain relief and motor improvement post‐DBS probably varies with the type of pain syndrome. For example, STN‐DBS was reported to preferentially ameliorate dystonic pain in the off drug condition.80, 81 Reduction of pain intensity by DBS was also correlated with improvement in quality of life.54 For pain symptoms that are not associated with motor fluctuations or persist after dopaminergic adjustments, the therapeutic approach should be based on the underlying mechanism, either musculoskeletal or neuropathic, for example. Regarding musculoskeletal pain, various rehabilitation programs can be relevant for improving the function of axial muscles, which play the major role in postural adjustment during gait and are the most commonly affected.54 In case of neuropathic pain, the principles of treatment should be the same as for other conditions of neuropathic pain (for review, see a previous work82). For example, duloxetine hydrochloride, a selective serotonin and noradrenaline reuptake inhibitor indicated for neuropathic pain syndrome, was found to be effective in central pain of PD patients.49 Therapeutic strategies can also be based on the reinforcement of descending inhibitory controls of pain.
Conclusion
We suggest a modification of Ford's classification regarding the types of pain occurring in PD patients, especially to take into account the different pain syndromes associated with the different types of motor fluctuations. The impact of dopaminergic therapy on pain should also be considered for diagnostic classification. Our proposed questionnaire allows the characteristics of each pain type to be determined according to its relationship with the disease and its treatment. The reliability and validity of this questionnaire will be further evaluated in a prospective clinical study.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Manuscript: A. Writing of the First Draft, B. Review and Critique.
V.M.: 1A, 1B, 1C, 2A, 2B
D.C.A.: 1A, 1B, 1C, 2A, 2B
R.G.C.: 1C, 2B
M.T.: 2B
U.E.: 2B
K.M.E.: 1C, 2B
S.B.: 2B
J.K.: 2B
M.S.: 1C, 2B
W.H.O.: 1C, 2B
J.C.M.: 1A, 1B, 1C, 2A, 2B
J.‐P.L.: 1A, 1B, 1C, 2A, 2B
Disclosures
Funding Sources and Conflicts of Interest: V.M. received a research grant of the Prof. Schmidtmann Foundation in Marburg, Germany. The authors report no conflicts of interest.
Financial Disclosures for previous 12 months: K.M.E. has served as a consultant for Schwarz Pharma Neuroscience (UCB), Desitin, Novartis, and Mundipharma. She belongs to the advisory boards of Schwarz Pharma Neuroscience (UCB), Novartis, Medtronic, Mundipharma, and Zambon. She received honoraria from Schwarz Pharma Neuroscience (UCB), Desitin, Novartis, Teva Pharma, Mundipharma, and Zambon. W.H.O. owns stocks from Roche and Medigene and is a consultant for Mundipharma and Novartis. He is a member of the advisory boards of GE Health, Mundipharma, Novartis, Schwarz Pharma Neuroscience (UCB), and Zambon. He received honoraria from AbbVie, Desitin, Mundipharma, Novartis, and Schwarz Pharma Neuroscience (UCB). He has grant support from the Charitable Hertie Foundation, the German Ministry of Education and Research, the German Research Foundation, the Michael J. Fox Foundation (MJFF), the International Parkinson Fonds, and from Novartis Pharma Germany. J.K. is a member of the data safety monitoring board of Fingolimod (Novartis). M.T. received honoraria from Allergan. M.S. received speaker and travel honoraria from Lundberg and Actelion. She had grant support from the MJFF.
Supporting information
Appendix S1. Comments on Ford's classification.
Appendix S2. Marburg‐Sao‐Paulo‐Créteil Questionnaire for Pain in Parkinson's Disease (MSPC‐PPD questionnaire).
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Chaudhuri KR, Odin P, Antonini A, Martinez‐Martin P. Parkinson's disease: the non‐motor issues. Parkinsonism Relat Disord 2011;17:717–723. [DOI] [PubMed] [Google Scholar]
- 2. Chaudhuri KR, Schapira AH. Non‐motor symptoms of Parkinson's disease: dopaminergic pathophysiology and treatment. Lancet Neurol 2009;8:464–474. [DOI] [PubMed] [Google Scholar]
- 3. Barone P, Antonini A, Colosimo C, et al. The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson's disease. Mov Disord 2009;24:1641–1649. [DOI] [PubMed] [Google Scholar]
- 4. Politis M, Wu K, Molloy S, P GB, Chaudhuri KR, Piccini P. Parkinson's disease symptoms: the patient's perspective. Mov Disord 2010;25:1646–1651. [DOI] [PubMed] [Google Scholar]
- 5. Beiske AG, Loge JH, Ronningen A, Svensson E. Pain in Parkinson's disease: prevalence and characteristics. Pain 2009;141:173–177. [DOI] [PubMed] [Google Scholar]
- 6. Defazio G, Berardelli A, Fabbrini G, et al. Pain as a nonmotor symptom of Parkinson disease: evidence from a case‐control study. Arch Neurol 2008;65:1191–1194. [DOI] [PubMed] [Google Scholar]
- 7. Lee MA, Walker RW, Hildreth TJ, Prentice WM. A survey of pain in idiopathic Parkinson's disease. J Pain Symptom Manage 2006;32:462–469. [DOI] [PubMed] [Google Scholar]
- 8. Negre‐Pages L, Regragui W, Bouhassira D, Grandjean H, Rascol O. Chronic pain in Parkinson's disease: the cross‐sectional French DoPaMiP survey. Mov Disord 2008;23:1361–1369. [DOI] [PubMed] [Google Scholar]
- 9. Tinazzi M, Del Vesco C, Fincati E, et al. Pain and motor complications in Parkinson's disease. J Neurol Neurosurg Psychiatry 2006;77:822–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Khoo TK, Yarnall AJ, Duncan GW, et al. The spectrum of nonmotor symptoms in early Parkinson disease. Neurology 2013;80:276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin CH, Wu RM, Chang HY, Chiang YT, Lin HH. Preceding pain symptoms and Parkinson's disease: a nationwide population‐based cohort study. Eur J Neurol 2013;20:1398–1404. [DOI] [PubMed] [Google Scholar]
- 12. Ford B. Pain in Parkinson's disease. Mov Disord 2010;25(suppl 1):S98–S103. [DOI] [PubMed] [Google Scholar]
- 13. Geerlings SW, Twisk JW, Beekman AT, Deeg DJ, van Tilburg W. Longitudinal relationship between pain and depression in older adults: sex, age and physical disability. Soc Psychiatry Psychiatr Epidemiol 2002;37:23–30. [DOI] [PubMed] [Google Scholar]
- 14. Ehrt U, Larsen JP, Aarsland D. Pain and its relationship to depression in Parkinson disease. Am J Geriatr Psychiatry 2009;17:269–275. [DOI] [PubMed] [Google Scholar]
- 15. Santos‐Garcia D, Abella‐Corral J, Aneiros‐Diaz A, Santos‐Canelles H, Llaneza‐Gonzalez MA, Macias‐Arribi M. Pain in Parkinson's disease: prevalence, characteristics, associated factors, and relation with other non motor symptoms, quality of life, autonomy, and caregiver burden. Rev Neurol 2011;52:385–393. [PubMed] [Google Scholar]
- 16. Wen HB, Zhang ZX, Wang H, et al. Epidemiology and clinical phenomenology for Parkinson's disease with pain and fatigue. Parkinsonism Relat Disord 2012;18(suppl 1):S222–S225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Obeso JA, Marin C, Rodriguez‐Oroz C, et al. The basal ganglia in Parkinson's disease: current concepts and unexplained observations. Ann Neurol 2008;64(suppl 2):S30–S46. [DOI] [PubMed] [Google Scholar]
- 18. West AR, Grace AA. Opposite influences of endogenous dopamine D1 and D2 receptor activation on activity states and electrophysiological properties of striatal neurons: studies combining in vivo intracellular recordings and reverse microdialysis. J Neurosci 2002;22:294–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science 1990;249:1436–1438. [DOI] [PubMed] [Google Scholar]
- 20. Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci 1990;13:266–271. [DOI] [PubMed] [Google Scholar]
- 21. Obeso JA, Rodriguez‐Oroz MC, Benitez‐Temino B, et al. Functional organization of the basal ganglia: therapeutic implications for Parkinson's disease. Mov Disord 2008;23(suppl 3):S548–S559. [DOI] [PubMed] [Google Scholar]
- 22. Burkey AR, Carstens E, Jasmin L. Dopamine reuptake inhibition in the rostral agranular insular cortex produces antinociception. J Neurosci 1999;19:4169–4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hagelberg N, Martikainen IK, Mansikka H, et al. Dopamine D2 receptor binding in the human brain is associated with the response to painful stimulation and pain modulatory capacity. Pain 2002;99:273–279. [DOI] [PubMed] [Google Scholar]
- 24. Juri C, Rodriguez‐Oroz M, Obeso JA. The pathophysiological basis of sensory disturbances in Parkinson's disease. J Neurol Sci 2010;289:60–65. [DOI] [PubMed] [Google Scholar]
- 25. Schestatsky P, Kumru H, Valls‐Sole J, et al. Neurophysiologic study of central pain in patients with Parkinson disease. Neurology 2007;69:2162–2169. [DOI] [PubMed] [Google Scholar]
- 26. Lim SY, Farrell MJ, Gibson SJ, Helme RD, Lang AE, Evans AH. Do dyskinesia and pain share common pathophysiological mechanisms in Parkinson's disease? Mov Disord 2008;23:1689–1695. [DOI] [PubMed] [Google Scholar]
- 27. Braak H, Sastre M, Bohl JR, de Vos RA, Del Tredici K. Parkinson's disease: lesions in dorsal horn layer I, involvement of parasympathetic and sympathetic pre‐ and postganglionic neurons. Acta Neuropathol 2007;113:421–429. [DOI] [PubMed] [Google Scholar]
- 28. Gerdelat‐Mas A, Simonetta‐Moreau M, Thalamas C, et al. Levodopa raises objective pain threshold in Parkinson's disease: a RIII reflex study. J Neurol Neurosurg Psychiatry 2007;78:1140–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mylius V, Engau I, Teepker M, et al. Pain sensitivity and descending inhibition of pain in Parkinson's disease. J Neurol Neurosurg Psychiatry 2009;80:24–28. [DOI] [PubMed] [Google Scholar]
- 30. Perrotta A, Sandrini G, Serrao M, et al. Facilitated temporal summation of pain at spinal level in Parkinson's disease. Mov Disord 2011;26:442–448. [DOI] [PubMed] [Google Scholar]
- 31. Nolano M, Provitera V, Estraneo A, et al. Sensory deficit in Parkinson's disease: evidence of a cutaneous denervation. Brain 2008;131:1903–1911. [DOI] [PubMed] [Google Scholar]
- 32. Toth C, Breithaupt K, Ge S, et al. Levodopa, methylmalonic acid, and neuropathy in idiopathic Parkinson disease. Ann Neurol 2010;68:28–36. [DOI] [PubMed] [Google Scholar]
- 33. Toth C, Brown MS, Furtado S, Suchowersky O, Zochodne D. Neuropathy as a potential complication of levodopa use in Parkinson's disease. Mov Disord 2008;23:1850–1859. [DOI] [PubMed] [Google Scholar]
- 34. Broetz D, Eichner M, Gasser T, Weller M, Steinbach JP. Radicular and nonradicular back pain in Parkinson's disease: a controlled study. Mov Disord 2007;22:853–856. [DOI] [PubMed] [Google Scholar]
- 35. Tinazzi M, Recchia S, Simonetto S, et al. Muscular pain in Parkinson's disease and nociceptive processing assessed with CO2 laser‐evoked potentials. Mov Disord 2010;25:213–220. [DOI] [PubMed] [Google Scholar]
- 36. Battista AF, Wolff BB. Levodopa and induced‐pain response. A study of patients with Parkinsonian and pain syndromes. Arch Intern Med 1973;132:70–74. [PubMed] [Google Scholar]
- 37. Brefel‐Courbon C, Payoux P, Thalamas C, et al. Effect of levodopa on pain threshold in Parkinson's disease: a clinical and positron emission tomography study. Mov Disord 2005;20:1557–1563. [DOI] [PubMed] [Google Scholar]
- 38. Djaldetti R, Shifrin A, Rogowski Z, Sprecher E, Melamed E, Yarnitsky D. Quantitative measurement of pain sensation in patients with Parkinson disease. Neurology 2004;62:2171–2175. [DOI] [PubMed] [Google Scholar]
- 39. Mylius V, Brebbermann J, Dohmann H, Engau I, Oertel WH, Moller JC. Pain sensitivity and clinical progression in Parkinson's disease. Mov Disord 2011;26:2220–2225. [DOI] [PubMed] [Google Scholar]
- 40. Granovsky Y, Schlesinger I, Fadel S, Erikh I, Sprecher E, Yarnitsky D. Asymmetric pain processing in Parkinson's disease. Eur J Neurol 2013;20:1375–1382. [DOI] [PubMed] [Google Scholar]
- 41. Chaudhuri KR, Martinez‐Martin P, Schapira AH, et al. International multicenter pilot study of the first comprehensive self‐completed nonmotor symptoms questionnaire for Parkinson's disease: the NMSQuest study. Mov Disord 2006;21:916–923. [DOI] [PubMed] [Google Scholar]
- 42. Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society‐sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23:2129–2170. [DOI] [PubMed] [Google Scholar]
- 43. Martinez‐Martin P, Rodriguez‐Blazquez C, Kurtis MM, Chaudhuri KR. The impact of non‐motor symptoms on health‐related quality of life of patients with Parkinson's disease. Mov Disord 2011;26:399–406. [DOI] [PubMed] [Google Scholar]
- 44. Kim HJ, Jeon BS, Lee JY, Paek SH, Kim DG. The benefit of subthalamic deep brain stimulation for pain in Parkinson disease: a 2‐year follow‐up study. Neurosurgery 2012;70:18–23; discussion 23‐14. [DOI] [PubMed] [Google Scholar]
- 45. Kim HJ, Paek SH, Kim JY, et al. Chronic subthalamic deep brain stimulation improves pain in Parkinson disease. J Neurol 2008;255:1889–1894. [DOI] [PubMed] [Google Scholar]
- 46. Oshima H, Katayama Y, Morishita T, et al. Subthalamic nucleus stimulation for attenuation of pain related to Parkinson disease. J Neurosurg 2012;116:99–106. [DOI] [PubMed] [Google Scholar]
- 47. Surucu O, Baumann‐Vogel H, Uhl M, Imbach LL, Baumann CR. Subthalamic deep brain stimulation versus best medical therapy for l‐dopa responsive pain in Parkinson's disease. Pain 2013;154:1477–1479. [DOI] [PubMed] [Google Scholar]
- 48. Ciampi de Andrade D, Lefaucheur JP, Galhardoni R, et al. Subthalamic deep brain stimulation modulates small fiber‐dependent sensory thresholds in Parkinson's disease. Pain 2012;153:1107–1113. [DOI] [PubMed] [Google Scholar]
- 49. Djaldetti R, Yust‐Katz S, Kolianov V, Melamed E, Dabby R. The effect of duloxetine on primary pain symptoms in Parkinson disease. Clin Neuropharmacol 2007;30:201–205. [DOI] [PubMed] [Google Scholar]
- 50. Ferreira KA, de Andrade DC, Teixeira MJ. Development and validation of a Brazilian version of the short‐form McGill pain questionnaire (SF‐MPQ). Pain Manag Nurs 2013;14:210–219. [DOI] [PubMed] [Google Scholar]
- 51. Menezes Costa Lda C, Maher CG, McAuley JH, et al. The Brazilian‐Portuguese versions of the McGill Pain Questionnaire were reproducible, valid, and responsive in patients with musculoskeletal pain. J Clin Epidemiol 2011;64:903–912. [DOI] [PubMed] [Google Scholar]
- 52. Voorhies RM, Jiang X, Thomas N. Predicting outcome in the surgical treatment of lumbar radiculopathy using the Pain Drawing Score, McGill Short Form Pain Questionnaire, and risk factors including psychosocial issues and axial joint pain. Spine J 2007;7:516–524. [DOI] [PubMed] [Google Scholar]
- 53. Nebe A, Ebersbach G. Pain intensity on and off levodopa in patients with Parkinson's disease. Mov Disord 2009;24:1233–1237. [DOI] [PubMed] [Google Scholar]
- 54. Cury RG, Galhardoni R, Fonoff ET, et al. Effects of deep brain stimulation on pain and other nonmotor symptoms in Parkinson disease. Neurology 2014;83:1403–1409. [DOI] [PubMed] [Google Scholar]
- 55. Bouhassira D, Attal N, Alchaar H, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 2005;114:29–36. [DOI] [PubMed] [Google Scholar]
- 56. Freynhagen R, Baron R, Gockel U, Tolle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin 2006;22:1911–1920. [DOI] [PubMed] [Google Scholar]
- 57. Gierthmuhlen J, Arning P, Binder A, et al. Influence of deep brain stimulation and levodopa on sensory signs in Parkinson's disease. Mov Disord 2010;25:1195–1202. [DOI] [PubMed] [Google Scholar]
- 58. Bouhassira D, Attal N, Fermanian J, et al. Development and validation of the Neuropathic Pain Symptom Inventory. Pain 2004;108:248–257. [DOI] [PubMed] [Google Scholar]
- 59. de Andrade DC, Ferreira KA, Nishimura CM, et al. Psychometric validation of the Portuguese version of the Neuropathic Pain Symptoms Inventory. Health Qual Life Outcomes 2011;9:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 2006;10:287–333. [DOI] [PubMed] [Google Scholar]
- 61. Riley JL III, Myers CD, Currie TP, et al. Self‐care behaviors associated with myofascial temporomandibular disorder pain. J Orofac Pain 2007;21:194–202. [PubMed] [Google Scholar]
- 62. Wasner G, Deuschl G. Pains in Parkinson disease–many syndromes under one umbrella. Nat Rev Neurol 2012;8:284–294. [DOI] [PubMed] [Google Scholar]
- 63. Rizos AM, Martinez‐Martin P, Pal S, et al. Validation of a novel Parkinson's disease pain scale (King's PD pain scale): a multicentre pilot study. Mov Disord 2014;29:510. [Google Scholar]
- 64. Rizos AM, Martinez‐Martin P, Pal S, et al. A novel Parkinson's disease pain questionnaire (King's PD pain quest): the patient's perspective. Mov Disord 2014;29:511. [Google Scholar]
- 65. Juri C, Rodriguez‐Oroz MC, Burguera JA, Guridi J, Obeso JA. Pain and dyskinesia in Parkinson's disease. Mov Disord 2010;25:130–132. [DOI] [PubMed] [Google Scholar]
- 66. Bleasdale‐Barr KM, Mathias CJ. Neck and other muscle pains in autonomic failure: their association with orthostatic hypotension. J R Soc Med 1998;91:355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nirenberg MJ. Dopamine agonist withdrawal syndrome: implications for patient care. Drugs Aging 2013;30:587–592. [DOI] [PubMed] [Google Scholar]
- 68. Rana AQ, Depradine J. Abdominal pain: a symptom of levodopa end of dose wearing off in Parkinson's disease. West Indian Med J 2011;60:223–224. [PubMed] [Google Scholar]
- 69. Gjerstad MD, Tysnes OB, Larsen JP. Increased risk of leg motor restlessness but not RLS in early Parkinson disease. Neurology 2011;77:1941–1946. [DOI] [PubMed] [Google Scholar]
- 70. Chaudhuri KR, Martinez‐Martin P, Brown RG, et al. The metric properties of a novel non‐motor symptoms scale for Parkinson's disease: results from an international pilot study. Mov Disord 2007;22:1901–1911. [DOI] [PubMed] [Google Scholar]
- 71. Mottram P, Wilson K, Copeland J. Validation of the Hamilton Depression Rating Scale and Montgommery and Asberg Rating Scales in terms of AGECAT depression cases. Int J Geriatr Psychiatry 2000;15:1113–1119. [DOI] [PubMed] [Google Scholar]
- 72. Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 73. Eggert K, Oertel WH, Reichmann H. Diagnostic and therapy of Parkinsonian Syndromes In: Diener HC, Weimar C, editors. Guidelines of the German Society of Neurology. 5 ed Stuttgart, New York: Georg Thieme Verlag; 2012; 82–112. [Google Scholar]
- 74. Kassubek J, Chaudhuri KR, Zesiewicz T, et al. Rotigotine transdermal system and evaluation of pain in patients with Parkinson's disease: a post hoc analysis of the RECOVER study. BMC Neurol 2014;14:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Trenkwalder C, Kies B, Rudzinska M, et al. Rotigotine effects on early morning motor function and sleep in Parkinson's disease: a double‐blind, randomized, placebo‐controlled study (RECOVER). Mov Disord 2011;26:90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Deuschl G, Schade‐Brittinger C, Krack P, et al. A randomized trial of deep‐brain stimulation for Parkinson's disease. N Engl J Med 2006;355:896–908. [DOI] [PubMed] [Google Scholar]
- 77. Krack P, Batir A, Van Blercom N, et al. Five‐year follow‐up of bilateral stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med 2003;349:1925–1934. [DOI] [PubMed] [Google Scholar]
- 78. Williams A, Gill S, Varma T, et al. Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson's disease (PD SURG trial): a randomised, open‐label trial. Lancet Neurol 2010;9:581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Witjas T, Kaphan E, Regis J, et al. Effects of chronic subthalamic stimulation on nonmotor fluctuations in Parkinson's disease. Mov Disord 2007;22:1729–1734. [DOI] [PubMed] [Google Scholar]
- 80. Krack P, Pollak P, Limousin P, Benazzouz A, Deuschl G, Benabid AL. From off‐period dystonia to peak‐dose chorea. The clinical spectrum of varying subthalamic nucleus activity. Brain 1999;122 (Pt 6):1133–1146. [DOI] [PubMed] [Google Scholar]
- 81. Limousin P, Krack P, Pollak P, et al. Electrical stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med 1998;339:1105–1111. [DOI] [PubMed] [Google Scholar]
- 82. Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 2010;9:807–819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Comments on Ford's classification.
Appendix S2. Marburg‐Sao‐Paulo‐Créteil Questionnaire for Pain in Parkinson's Disease (MSPC‐PPD questionnaire).


